Abstract
A cat was presented for necropsy after being found dead at home. Histologic findings suggested viral pneumonia. Polymerase chain reaction and viral typing revealed influenza A(H1N1)pdm09. This is the first report of influenza in a Canadian cat and highlights the importance of considering influenza virus in the differential diagnosis for feline respiratory distress.
Résumé
Infection par le virus de l’influenza H1N1 pandémique chez un chat canadien. Un chat a été présenté pour une nécropsie après avoir été trouvé mort à son domicile. Les résultats histologiques ont suggéré une pneumonie virale. Une amplification en chaîne par polymérase et un typage viral ont révélé l’influenza A(H1N1) pdm09. Il s’agit du premier rapport de l’influenza chez un chat canadien et il souligne l’importance de considérer le virus de l’influenza dans le diagnostic différentiel lors de détresse respiratoire féline.
(Traduit par Isabelle Vallières)
Influenza A viruses are RNA viruses in the family Orthomyxoviridae that can infect multiple species of mammals and birds, although different viral subtypes tend to be host-specific (1). These viruses have caused multiple epidemics and pandemics in human populations (2), as well as epizootics and panzootics in animals (3,4). Rapid mutation and gene reassortment of influenza A viruses lead to a high diversity of viral subtypes, and this genetic flexibility results in a propensity for between-species and cross-class transmission (1). The pandemic strain of the H1N1 influenza virus isolated in 2009 [influenza A(H1N1) pdm09] (5) contained a novel combination of genetic segments from influenza viruses affecting humans, pigs, and birds (6).
Cats are susceptible to infection by multiple influenza A virus subtypes (7); however, reports of clinical disease in cats resulting from natural infection with A(H1N1)pdm09 are relatively few (8–12). In this report we describe a case of fatal influenza A(H1N1)pdm09 infection in a cat in Canada. To our knowledge, this is the first report of influenza A(H1N1)pdm09 infection in a Canadian cat.
Case description
A 5-year-old spayed female Ragdoll-mix cat was presented for necropsy to the Diagnostic Services Unit at the University of Calgary Faculty of Veterinary Medicine in January, 2014 after being found dead at home the previous day. The cat had lived with 2 littermates and had no outdoor access. She was up-to-date with rabies and feline leukemia virus (FeLV) vaccination but overdue for feline viral rhinotracheitis/calicivirus/panleukopenia (FVRCP) vaccination at the time of death. Beginning at the age of 8 mo, the cat had experienced intermittent episodes of eosinophilic granuloma complex (EGC), which had been treated with short courses of prednisolone, 5 mg PO, q12h for 2 to 3 d.
On the day before the death of the cat described in this report, a littermate from the same home had presented to a local veterinary clinic for evaluation and treatment of respiratory distress. On presentation the littermate was in lateral recumbency, hypothermic (32.3°C), tachypneic (64 breaths/min), and was open-mouth breathing with crackles audible over all lung fields. Initial blood analysis revealed leukopenia [3.80 × 109 cells/L; reference interval (RI): 5.5 to 19.5 × 109 cells/L), characterized by a severe neutropenia (1.45% WBC; RI: 2.5% to 14% WBC). Serum total protein was mildly decreased (48 g/L; RI: 54 to 82 g/L), potassium was slightly low (3.6 mmol/L; RI: 3.7 to 5.8 mmol/L), and blood urea nitrogen was increased (12.2 mmol/L; RI: 3.6 to 10.7 mmol/L). There was a severe thrombocytosis and respiratory acidosis. Enzyme-linked immunosorbent assay (ELISA) tests for FeLV and feline immunodeficiency virus (FIV) were negative. Radiographs revealed left thoracic opacity with a mixed alveolar/bronchointerstitial pattern, cardiomegaly with enlarged pulmonary vasculature, and mild hepatomegaly. Thoracocentesis yielded no fluid and initial treatment with oxygen and furosemide, 2 mg/kg body weight (BW), PO, did not result in improvement. The cat began emitting pink fluid from the mouth and nose and was euthanized. Necropsy was declined.
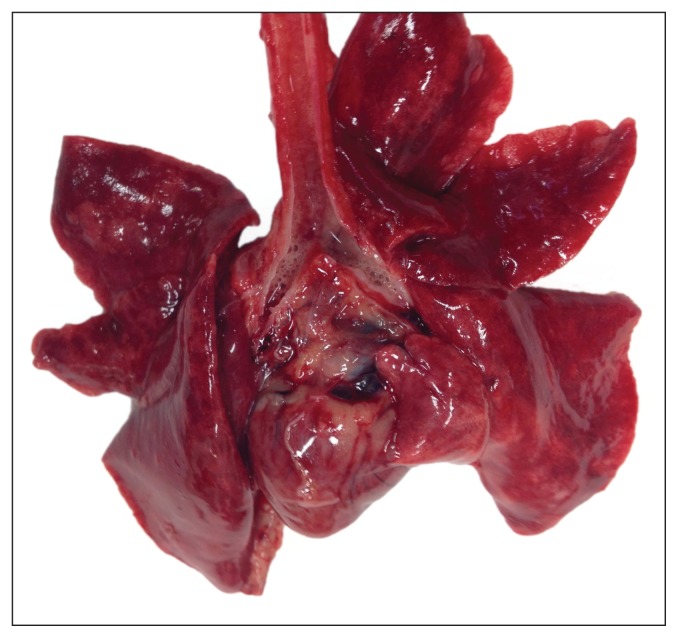
The cat described in this case report was found dead at home the day after her littermate’s death. This followed 1 d of anorexia, open-mouth breathing, and hiding, which had been attributed by the owner to distress over the littermate’s death. At necropsy both lungs were diffusely consolidated and edematous (Figure 1). There were no other gross abnormalities in any organ system. Formalin-fixed slabs of lung had prominent pale cuffs around bronchioles that, histologically, were due to severe type II alveolar pneumocyte hyperplasia (Figure 2). Other histologic changes included widespread necrosis or attenuation of bronchiolar epithelium, abundant fibrin, and numerous inflammatory cells (primarily macrophages) within bronchiolar and alveolar lumens and patchy alveolar septal fibrosis (Figure 2). A commercial diagnostic laboratory’s feline upper respiratory disease panel was negative by real-time polymerase chain reaction (RT-PCR) for feline calicivirus, Chlamydophila felis, feline herpesvirus 1, Bordetella bronchiseptica, Mycoplasma felis, and H1N1 influenza virus. However, because the gross and histologic findings were suggestive of influenza virus infection, samples of lung were submitted to the Animal Health Centre in Abbotsford, British Columbia. Polymerase chain reaction of these samples was positive for influenza virus and typing revealed that the strain was A(H1N1)pdm09.
Figure 1.
Lungs, trachea, and heart from a cat that died from influenza A(H1N1)pdm09 infection. The lungs are dark red and edematous.
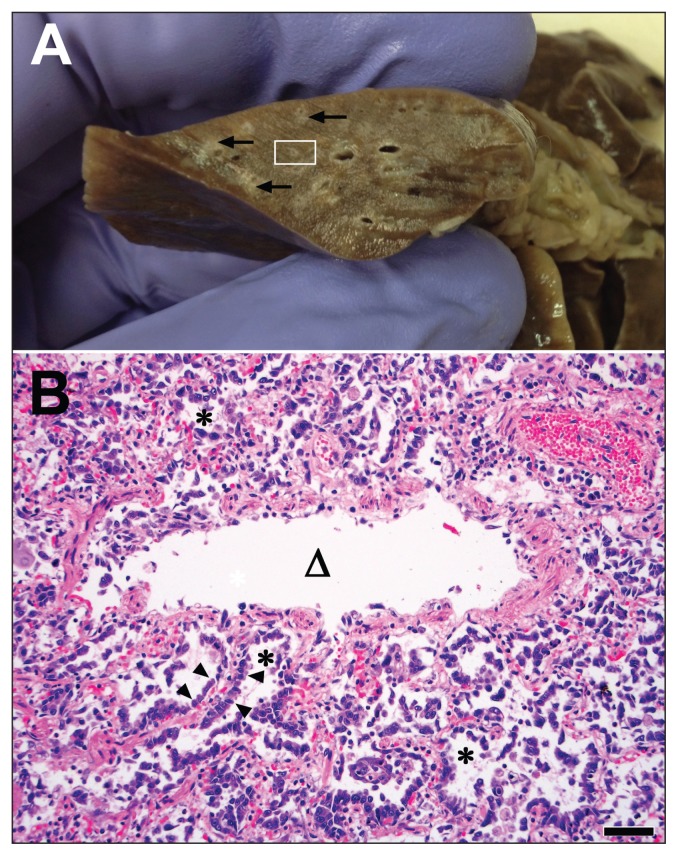
Figure 2.
Gross and microscopic pulmonary lesions in a cat that died from influenza A(H1N1)pdm09 infection. A — Transverse sections of formalin-fixed lung have prominent pale cuffs around bronchioles (arrows). The area enclosed in the white rectangle is magnified in the lower photomicrograph. B — High magnification photomicrograph of a bronchiole (triangle) and adjacent alveoli (asterisks). The bronchiolar epithelium is sloughed and the adjacent alveoli are lined by markedly hyperplastic type II pneumocytes (arrowheads). Bar = 100 μm. Hematoxylin & eosin stain.
Three separate tests were used at the Abbotsford laboratory. Initial testing was performed using a generic RT-PCR assay that targets the matrix genomic segment of influenza A viruses, including A(H1N1)pdm09, as described previously (13). This assay is used by all provincial veterinary diagnostic laboratories in Canada and follows a standard operating procedure provided by the CFIA’s National Centre for Foreign Animal Diseases in Winnipeg, Manitoba. Using this assay, the cat’s lung sample was positive for influenza A virus, with a Ct value of 32. Following initial detection, typing was then performed using a VetMAXTM-Gold Swine Influenza Virus Detection Kit (Life Technologies Corporation, Austin, Texas, USA). This commercial RT-PCR kit is validated for the detection of North American swine influenza virus subtypes H1N1, H3N2, and A(H1N1)pdm09. Using this assay the cat’s lung sample was positive for swine influenza virus H1 and N1 with Ct values 32.83 and 35.32, respectively. Finally, an in-house RT-PCR assay targeting the hemagglutinin (HA) gene of A(H1N1)pdm09 was used for further typing and confirmation of the presence of A(H1N1)pdm09 in the cat’s lung sample (forward primer: 5′-AAA ATT TAA TAT GGC TAG TT-3′; reverse primer: 5′-CTT TGT TGG TCA GCA CTA-3′; and FAM-labeled probe: 5′-FAM-TAC ATT AAT GAT AAA GGG AAA-3′). The reaction was incubated at 45°C for 10 min, 95°C for 10 min, and then amplified for 40 cycles at 94°C for 10 s, 50°C for 20 s, and 60°C for 30 s. Using this assay, the cat’s lung sample was positive, with a Ct value of 35.07. Both the swine influenza H1N1 typing PCR and the A(H1N1)pdm09 differential PCR tests were repeated using fresh lung samples and the results were consistent, confirming the presence of A(H1N1)pdm09.
Discussion
The cat described in this report died from bronchointerstitial pneumonia attributed to influenza A(H1N1)pdm09. Although the most common viruses causing bronchointerstitial pneumonia in cats are herpesvirus and calicivirus (14), these were ruled out by PCR. In addition, the first diagnostic laboratory did not detect viral sequences of any type by PCR. However, the gross and histologic lesions were nearly identical to those described previously in 2 cats that died of influenza A(H1N1) pdm09 infection (10). This prompted us to resubmit samples to a second diagnostic laboratory, which detected influenza A(H1N1)pdm09.
After consultation with both laboratories, several possible explanations for discrepancies in results are possible, although none can be proven. The first is that the commercial laboratory used a less sensitive PCR assay than did the provincial laboratory. Viral loads in the submitted lung samples were low (as indicated by high PCR Ct values) and a sensitive assay was required to detect A(H1N1)pdm09. Unfortunately, the performance of the 2 laboratories’ assays cannot be compared as the procedures and primers used by the commercial laboratory are proprietary. A second possible explanation is the presence of viral base changes in the regions of primer binding. If this occurred at the commercial laboratory’s primer binding sites but not at the provincial laboratory’s primer binding sites this could explain the discrepancy in detection. This could be checked by sequencing the viral genome and confirming complementarity between primers and expected binding sites. However, the commercial laboratory’s primer sequences are unavailable, making this analysis impossible. In addition, the low viral loads as detected by the provincial laboratory could make sequencing difficult. A third possible explanation is that the virus was distributed unevenly throughout the cat’s lungs and that the commercial laboratory received and tested samples that truly did not contain A(H1N1) pdm09. Uneven pulmonary distribution of influenza A virus lesions is reported in pigs (15) and is, presumably, possible in cats. A fourth possible explanation is that the lung samples tested by the commercial laboratory degraded during shipment. The samples underwent prolonged storage caused by their arrival on a weekend, whereas those received by the provincial laboratory did not. These explanations for the discrepancy between the 2 diagnostic laboratories’ results are speculative and cannot be tested. Nevertheless, if clinical signs, history (exposure to humans infected with influenza virus), gross lesions, and histologic findings are suggestive of influenza virus infection, it is reasonable to test samples in a second laboratory should the first laboratory provide a negative result for influenza A virus.
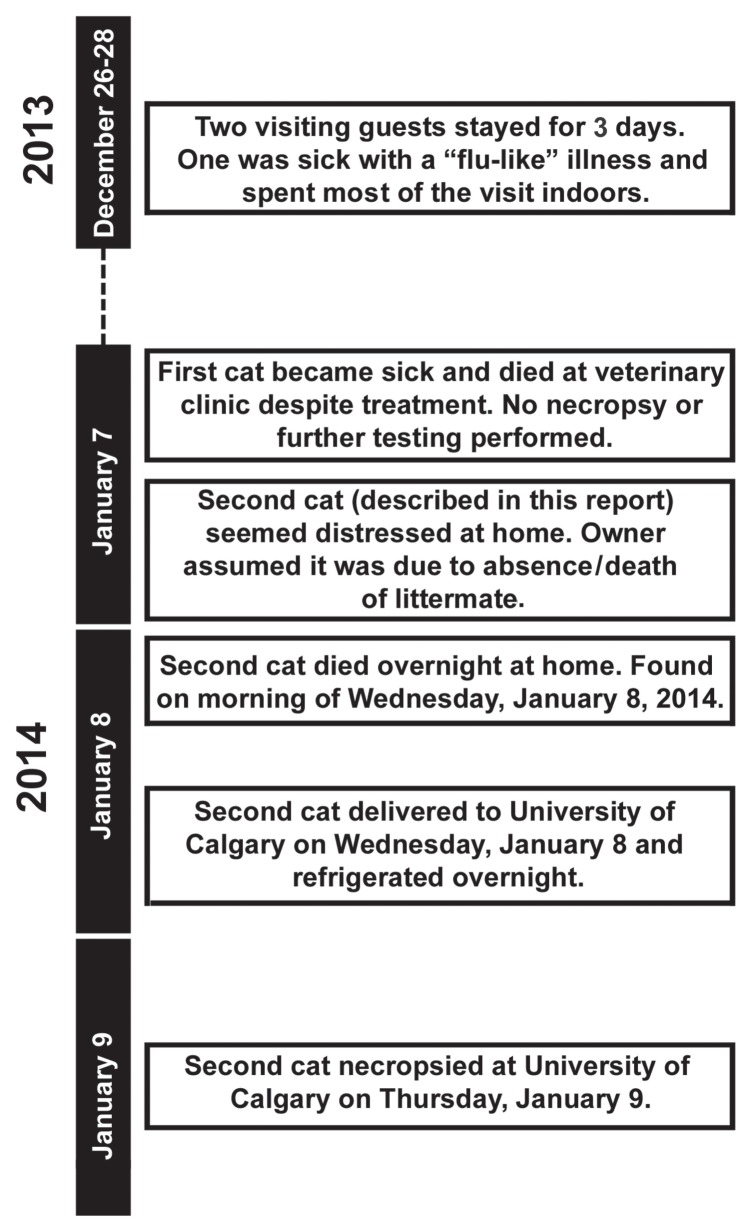
The source of this cat’s infection could not be determined. The 43-year-old owner lived alone, had received a seasonal influenza vaccination in October of 2013, and had never become ill. She reported having had 2 house guests for a 3-day period after Christmas 2013, approximately 10 to 12 d before the deaths of 2 of her cats on January 7 and 8, 2014 (Figure 3). One guest had “flu-like” symptoms and remained indoors for most of the visit. The owner reports that the sick guest likely handled the cats but would have avoided close contact such as nuzzling them because of a mild cat allergy. It is also possible that the cats slept on the sick guest’s bed with him. Although the cause of this guest’s illness was never known, the 2013 to 2014 influenza season was intense in Alberta; there were 35% more laboratory-confirmed cases of human influenza infection than in the previous year and the predominant circulating strain was influenza A(H1N1)pdm09 (16). Thus, the sick guest could not be ruled out as the source of this cat’s and/or her littermate’s infection. The possibility of anthroponotic transmission of influenza A(H1N1)pdm09 to cats is supported by 2 earlier reports. The first described an indoor-only cat that was infected with influenza A(H1N1)pdm09 after exposure to human family members with a non-diagnosed flu-like illness (12). The second showed that pet cats were nearly 3 times more likely to be seropositive for influenza A(H1N1)pdm09 than were free-roaming cats (17). Cat-to-cat transmission of influenza A(H1N1)pdm09 has also been documented, both experimentally (18) and in an outbreak in cats with little human contact living in a cat colony in Italy (9). Zoonotic transmission of the influenza virus from cats to humans may also occur, and the role that cats play in the epidemiology of human influenza infections needs further investigation (7). In the current case, the owner herself had been vaccinated against influenza that season, which may have protected her from infection by either her visitor or her cats.
Figure 3.
Timeline showing the dates of deaths of 2 cats in a single household, 1 confirmed to be caused by influenza A(H1N1)pdm09 infection and the other not tested. Approximately 13 d prior to the onset of clinical signs and death a visitor with a “flu-like” illness came to the house and stayed for 3 d.
There is clear evidence that domestic cats are susceptible to infection by several strains of the influenza A virus, including influenza A(H1N1)pdm09 (19). In addition, the seroprevalence of influenza A infection in cats may be higher than expected, given the infrequency of clinical disease. A recent survey of healthy and sick cats presented to the Ohio State Veterinary College over a 1-year period found that, of 400 serum samples, 22.5%, 33%, and 43.5% were seropositive against A(H1N1) pdm09, seasonal H1N1, and seasonal H3N2, respectively, and only 153 (38%) were negative for all 3 influenza subtypes tested (7). These results indicate a higher seroprevalence of influenza A infection in cats than previously reported, which the authors believe was due to their use of a more sensitive hemagglutination inhibition assay instead of the ELISA used in previous studies (7). Similarly, in 2012 and 2013, 21% of 1255 cats living in a densely populated region of northeastern China were seropositive for influenza A(H1N1)pdm09 (17). The high seroprevalence of influenza infection in domestic cats and cats’ susceptibility to both human- and avian-adapted influenza virus strains suggest that cats have the potential to act as reservoirs for influenza viruses. In addition, since cats are susceptible to multiple influenza virus strains, coinfection may allow genetic reassortment between strains and provide a potential source of new influenza pandemics (20).
In spite of the apparent high prevalence of influenza infection in domestic cats, there have been only a few reports of cats with severe clinical disease resulting from influenza virus infection (8–12). In addition, cats experimentally infected with influenza A(H1N1)pdm09 develop gross and histologic pulmonary lesions but display only mild to moderate clinical signs of disease (18). This might suggest that any cats that do develop severe clinical signs have an underlying predisposition to the disease. The Center for Disease Control reported that, in children, preexisting respiratory or nervous system conditions increased the risk of death after influenza A(H1N1)pdm09 virus infection (21). In the case described in this report, both of the affected cats in the household had a history of EGC, although neither had experienced an episode or received treatment for the disease within the previous 2 mo. The 1 surviving cat in the household did not have a history of EGC. Whether there is any relationship between EGC and immune function or susceptibility to influenza virus infection is not known.
To our knowledge, this is the first report of influenza A(H1N1)pdm09 virus infection in a cat in Canada. In 2010, a PCR-based survey of 250 cats at a shelter in Vancouver did not detect influenza A(H1N1)pdm09 in nasal swab samples (22). However, in early 2010, influenza A(H1N1)pdm09 virus was detected in 2 skunks that died on a mink farm near Vancouver (23). Our report of the death of a Canadian cat from influenza A(H1N1)pdm09 virus infection highlights the importance of including influenza in the differential diagnosis for respiratory disease in cats. It also demonstrates the importance of investigating any discrepancy between histologic findings and expected laboratory results.
Acknowledgments
The authors gratefully thank the owner of the cats discussed in this report for her continued cooperation and willingness to provide information and Dr. Amy Waterhouse for her assistance with clinical information about these cats. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pleschka S. Overview of influenza viruses. In: Richt JA, Webby RJ, editors. Current Topics in Microbiology and Immunology: Swine Influenza. Vol. 370. Berlin, Germany: Springer-Verlag; 2013. pp. 1–20. [DOI] [PubMed] [Google Scholar]
- 3.Schultz-Cherry S, Olsen CW, Easterday BC. History of swine influenza. In: Richt JA, Webby RJ, editors. Current Topics in Microbiology and Immunology: Swine Influenza. Vol. 370. Berlin, Germany: Springer-Verlag; 2013. pp. 21–28. [DOI] [PubMed] [Google Scholar]
- 4.Vijaykrishna D, Bahl J, Riley S, et al. Evolutionary dynamics and emergence of panzootic H5N1 influenza viruses. PLoS Pathog. 2008;4:10. doi: 10.1371/journal.ppat.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization [homepage on the Internet] Standardization of terminology of the pandemic A(H1N1)2009 virus. c2011. [Last accessed March 14, 2016]. Available from: http://www.who.int/influenza/gisrs_laboratory/terminology_ah1n1pdm09/en/
- 6.Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali A, Daniels JB, Zhang Y, et al. Pandemic and seasonal human influenza virus infections in domestic cats: Prevalence, association with respiratory disease, and seasonality patterns. J Clin Microbiol. 2011;49:4101–4105. doi: 10.1128/JCM.05415-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campagnolo ER, Rankin JT, Daverio SA, et al. Fatal pandemic (H1N1) 2009 influenza A virus infection in a Pennsylvania domestic cat. Zoonoses Public Health. 2011;58:500–507. doi: 10.1111/j.1863-2378.2011.01390.x. [DOI] [PubMed] [Google Scholar]
- 9.Fiorentini L, Taddei R, Moreno A, et al. Influenza A pandemic (H1N1) 2009 virus outbreak in a cat colony in Italy. Zoonoses Public Health. 2011;58:573–581. doi: 10.1111/j.1863-2378.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 10.Lohr CV, DeBess EE, Baker RJ, et al. Pathology and viral antigen distribution of lethal pneumonia in domestic cats due to pandemic (H1N1) 2009 influenza A virus. Vet Pathol. 2010;47:378–386. doi: 10.1177/0300985810368393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pigott AM, Haak CE, Breshears MA, Linklater AKJ. Acute bronchointerstitial pneumonia in two indoor cats exposed to the H1N1 influenza virus. J Vet Emerg Crit Care. 2014;24:715–723. doi: 10.1111/vec.12179. [DOI] [PubMed] [Google Scholar]
- 12.Sponseller BA, Strait E, Jergens A, et al. Influenza A pandemic (H1N1) 2009 virus infection in domestic cat. Emerg Infect Dis. 2010;16:534–537. doi: 10.3201/eid1603.091737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weingartl HM, Berhane Y, Hisanaga T, et al. Genetic and pathobiologic characterization of pandemic H1N1 2009 influenza viruses from a naturally infected swine herd. J Virol. 2010;84:2245–2256. doi: 10.1128/JVI.02118-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caswell JL, Williams KJ. Infectious respiratory diseases of cats. In: Maxie MG, editor. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, Pennsylvania: Elsevier Saunders; 2007. pp. 648–650. [Google Scholar]
- 15.Weingartl HM, Albrecht RA, Lager KM, et al. Experimental infection of pigs with the human 1918 pandemic influenza virus. J Virol. 2009;83:4287–4296. doi: 10.1128/JVI.02399-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surveillance and Assessment Branch, Alberta Health [monograph on the Internet] Edmonton, Alberta: Alberta Health; c2014. [Last accessed March 14, 2016]. Available from: http://www.health.alberta.ca/documents/Influenza-Summary-Report-2014.pdf. [Google Scholar]
- 17.Zhao FR, Liu CG, Yin X, Zhou DH, Wei P, Chang HY. Serological report of pandemic (H1N1) 2009 infection among cats in Northeastern China in 2012–02 and 2013–03. Virol J. 2014;11:4. doi: 10.1186/1743-422X-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Brand JMA, Stittelaar KJ, van Amerongen G, et al. Experimental pandemic (H1N1) 2009 virus infection of cats. Emerg Infect Dis. 2010;16:1745–1747. doi: 10.3201/eid1611.100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon SW, Webby RJ, Webster RG. Evolution and ecology of influenza A viruses. In: Compans RW, Oldstone MBA, editors. Influenza Pathogenesis and Control — Volume 1. Vol. 385. Cham, Switzerland: Springer International Publishing; 2014. pp. 359–375. [DOI] [PubMed] [Google Scholar]
- 20.Horimoto T, Gen F, Murakami S, et al. Cats as a potential source of emerging influenza virus infections. Virol Sin. 2015;30:221–223. doi: 10.1007/s12250-015-3580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shannon S, Louie J, Siniscalchi A, et al. Surveillance for pediatric deaths associated with 2009 pandemic influenza A (H1N1)-virus infection United States, April–August 2009 (Reprinted from MMWR, vol 58, pg 941–947, 2009) J Am Med Assoc. 2009;302:1855–1857. [Google Scholar]
- 22.Gourkow N, Lawson JH, Hamon SC, Phillips CJC. Descriptive epidemiology of upper respiratory disease and associated risk factors in cats in an animal shelter in coastal western Canada. Can Vet J. 2013;54:132–138. [PMC free article] [PubMed] [Google Scholar]
- 23.Britton AP, Sojonky KR, Scouras AP, Bidulka JJ. Pandemic (H1N1) 2009 in skunks, Canada. Emerg Infect Dis. 2010;16:1043–1045. doi: 10.3201/eid1606.100352. [DOI] [PMC free article] [PubMed] [Google Scholar]