Abstract
This study investigated the relationship between treatment protocol, survival to discharge, and relapse in 46 dogs diagnosed with primary immune-mediated thrombocytopenia (ITP) at the Western College of Veterinary Medicine between 2000 and 2013. Treatment was at the discretion of the attending clinician and consisted of either a corticosteroid alone or a corticosteroid plus a secondary therapy. There was no association between survival to discharge and treatment protocol (P = 0.23). Of the surviving in-patients, 39% experienced a relapse. Our study failed to show a significant difference in survival and relapse based on treatment protocol.
Résumé
Résultat basé sur le protocole de traitement chez des patients atteints de thrombocytopénie canine primaire à médiation immunitaire : 46 cas (2000–2013). Cette étude a examiné la relation entre le protocole de traitement, la survie jusqu’au congé et la rechute chez 46 chiens diagnostiqués, entre 2000 et 2013, avec une thrombocytopénie primaire à médiation immunitaire (TPI) au Western College of Veterinary Medicine. Le traitement a été fait à la discrétion du clinicien traitant et se composait soit d’un corticostéroïde seul ou d’un corticostéroïde et d’une thérapie secondaire. Il n’y avait aucune association entre la survie jusqu’au congé et le protocole de traitement (P = 0,23). Parmi les patients hospitalisés survivants, 39 % ont eu une rechute. Notre étude n’a pas réussi à montrer une différence importante au niveau de la survie et de la rechute en fonction du protocole de traitement.
(Traduit par Isabelle Vallières)
Canine immune-mediated thrombocytopenia (ITP) is a common clinical disorder in which platelets are destroyed by antiplatelet specific antibodies in circulation or at the level of the bone marrow (1). This can lead to excessive or spontaneous bleeding when platelet counts are less than 30 000 to 50 000/μL. Immune-mediated platelet destruction can be categorized as primary when immune destruction occurs without any underlying cause or secondary when it occurs due to an underlying source including neoplasia, drug therapy, parasitic infection, or blood transfusion (1–3).
Since primary ITP is a diagnosis of exclusion, the clinical work-up can be both time-consuming and costly. Pet owners are often hesitant to pursue referral without knowledge regarding cost of diagnostic testing, what is involved in treatment, and the prognosis including the likelihood of relapse. Previous retrospective studies have reported reasonable short-term survival (74% to 97%) but with a rate of relapse of up to 58% (1,4–6).
While corticosteroids are the cornerstone for treatment of primary ITP, adjunctive therapies including other immunosuppressive drugs such as azathioprine, cyclosporine, human intravenous immunoglobulin (hIVIg), and vincristine have been given in combination with corticosteroids (7). A recent study also reported on the successful use of mycophenolate mofetil as a single agent treatment for ITP in 5 dogs, highlighting the diversity of protocols used in treating primary canine ITP (8). Since there is no standard treatment protocol, it is common to initiate therapy with a corticosteroid alone, and introduce additional agents based on the severity of the disease (i.e., extent of hemorrhage), size of dog, or poor response to corticosteroids. Many studies have assessed varying treatment protocols in attempts to improve patient outcome; however, to date no combination has been proven to significantly improve survival or prevent relapse (1,7–12).
The purpose of our study was to determine if there was a difference in outcome between use of corticosteroids alone compared to corticosteroids plus an additional treatment. Outcome was assessed based on the probability of survival to discharge and the risk of relapse. We were also interested in comparing our results with those of a previous retrospective study that was performed at our institution over 30 years ago. By evaluating the 2 sets of data, we aimed to determine if there were any changes in patient population, treatment protocol, outcome, or relapse over the last 3 decades (13).
This study was conducted at the Veterinary Medical Center (VMC), Western College of Veterinary Medicine at the University of Saskatchewan, a teaching hospital that provides service to both first opinion and referral cases of primary ITP. Medical records from April 2000 until October 2013 were reviewed to identify patients diagnosed with primary ITP. The inclusion criteria were a complete medical record, thrombocytopenia (≤ 75 000 platelets), and no history of recent or current treatments that have previously been documented to cause secondary ITP such as drug therapy, blood product transfusion, vaccine administration, various neoplasias, other autoimmune diseases, and infectious agents. A complete diagnostic work-up including complete blood (cell) count (CBC), manual platelet count, serum biochemistry profile, urinalysis, and diagnostic imaging (thoracic radiographs and abdominal ultrasound) were performed to rule out systemic disease in all patients included in this study. Incomplete medical records were identified as those that did not include a complete physical examination, complete diagnostic work-up as identified above, or lacked a confirmed manual platelet count.
Data collected from the medical records included signalment, presenting complaint, physical examination findings, CBC, serum biochemistry profile, urinalysis, other applicable laboratory diagnostic test results (e.g., Coombs test, clotting times, and biopsies), medical imaging findings, treatment protocol, cost associated with in-hospital treatment, survival to discharge, and documented history of relapse. Because common tick-borne diseases causing thrombocytopenia have been identified with extremely low prevalence in our geographic area (Alberta, Saskatchewan, and Manitoba, Canada) neither serologic nor molecular infectious disease testing was required for inclusion (Gaunt et al, unpublished observations).
Patients treated at the VMC as in-hospital patients or out-patients were classified into 1 of 2 groups based on the treatment protocol. Because this was a retrospective observational study, treatment protocol was determined at the discretion of the attending clinician at the time of presentation. Factors influencing treatment choice potentially included, but were not limited to, patient size, current trends in the veterinary literature, disease severity, client preference, and financial concerns. Patients in Group 1 received only a corticosteroid, including intravenous dexamethasone, oral prednisone, or intravenous dexamethasone followed by oral prednisone. Patients with vomiting and those unable to take oral medications were treated on admission with injectable dexamethasone and with oral prednisone once vomiting had resolved. Patients in Group 2 received a corticosteroid plus a second treatment. Adjunctive therapies included azathioprine, cyclosporine, vincristine, leflunomide, hIVIG, and splenectomy. Survival to discharge was only recorded for inpatients. Patients were identified as having a relapse of primary ITP if a confirmed platelet count of less than 50 000/μL was documented on a CBC after an initial response to therapy (i.e., platelet count of ≥ 200 000/μL).
Commercially available software (STATA12; StataCorp, College Station, Texas, USA) was used to compare the difference between treatment groups. Exact logistic regression was used to compare survival to discharge for inpatients and risk of relapse for those responding to initial therapy with documented follow-up between those receiving only steroids and those receiving steroids plus adjunct therapy after adjusting for age. The time to relapse was compared between treatment groups using a Mann-Whitney U-test. A P ≤ 0.05 was accepted as statistically significant.
Fifty-eight cases of primary ITP were presented to WCVM from April 2000 until October 2013. Ten cases were lost to follow-up; 2 other cases were diagnosed with primary ITP at the VMC where treatment recommendations were made but not initiated. These 12 cases were not included in the statistical analysis for survival or relapse but were included in the descriptive statistics for primary ITP cases. Thirty-one dogs were female (53%) and 27 (47%) were male. The age of affected dogs ranged from 7 mo to 14 y (median: 7 y). Most dogs were purebred (n = 35, 63%); no one breed was overrepresented in this population. Body weight ranged from 3.5 kg to 51.2 kg (median: 19.4 kg).
Serologic testing for Ehrlichia spp., Anaplasma spp., and Borrelia burgdorferi (ELISA, IDEXX SNAP® 4Dx®; IDEXX, Westbrook, Maine, USA) was reported in 12 (21%) dogs; all were negative including for canine heartworm antigen. There was no evidence of concurrent immune-mediated hemolytic anemia as defined by the presence of autoaggultination or spherocytosis in any of the 58 cases. Only 2 patients had a direct Coombs test performed, and both were negative. None of the patients were tested for antiplatelet antibodies because this test is not available at our institution and is not considered specific for primary ITP (14).
The most common presenting complaints for dogs with primary ITP were lethargy or depression (46%), anorexia (34%), and melena (25%). Frequent physical examination findings included petechia or ecchymosis (66%), enlarged lymph nodes (27%), and lethargy or depression (21%); other findings were less frequent.
Anemia (36%), reticulocytosis (47%), and leukocytosis (49%) were common findings on the CBC. Thrombocytopenia with a platelet count < 10 000/μL was present in 35 dogs (76%). The average platelet count of dogs with primary ITP with platelet counts > 10 000/μL was 31 300/μL (range: 11 000/μL to 70 000/μL). Hypoproteinemia (22%), elevated urea (15%), and elevated liver enzymes [alkaline phosphatase (46%), alanine aminotransferase (31%), gamma-glutamyl transpeptidase (15%)], were common abnormalities on the serum biochemistry profile.
For the 23 dogs in which bone marrow cytology was performed, 22 (96%) had evidence of megakaryocytic hyperplasia and 1 (4%) dog had megakaryocytic hypoplasia. Findings from imaging studies included abnormalities of the liver (11%) or spleen (22%), pulmonary patterns indicative of potential hemorrhage (7%), and free abdominal or pleural fluid (18%).
Thirty-seven (80%) dogs were admitted to the VMC and treated as inpatients, and 9 (20%) were treated as outpatients. Corticosteroids alone were used for treatment of 13 patients. Corticosteroids included intravenous dexamethasone [median: 0.26 mg/kg body weight (BW), IV, q24h, range: 0.005 to 0.8 mg/kg BW, oral prednisone (median: 1.45 mg/kg BW, PO, q12h, range: 0.5 to 3.0 mg/kg BW)] or intravenous dexamethasone, followed by oral prednisone.
Corticosteroids [dexamethasone (median: 0.2 mg/kg BW, IV, q24h, range: 0.005 to 1.0 mg/kg BW) with or without prednisone (median: 2.0 mg/kg BW, PO, q12h, range: 0.5 to 2.6 mg/kg BW)] plus a second treatment were used in 33 patients. Thirteen (28%) dogs received a second oral immunosuppressive agent, 14 (30%) received a second immunosuppressive agent plus vincristine, 4 (8.7%) received vincristine, 1 received a second immunosuppressive agent plus vincristine and hIVIG (2.2%), and 1 eventually had a splenectomy due to the refractory nature of the ITP (2.2%). The secondary immunosuppressive agents used included azathioprine (n = 22), cyclosporine (n = 5), and leflunomide (n = 1). There was no difference in the odds of surviving to discharge between treatment protocols either before [OR = 3.7, 95% confidence interval (CI): 0.52 to 27, P = 0.23] or after adjusting for age in months (OR = 0.16, 95% CI: 0.19 to 8.6, P = 0.99).
In addition to treatment specifically for ITP, other therapies that were initiated on presentation included antibiotics (n = 20) such as doxycycline, amoxicillin, amikacin, gentamicin and enrofloxacin; H2 receptor blockers (n = 8); vitamin K1 (n = 2); sucralfate (n = 4); misoprostol (n = 2); opioids (n = 1); heparin (n = 1); omeprazole (n = 1); and DDAVP (n = 1). Ten dogs received a blood transfusion while hospitalized, either on presentation (n = 3) or during the course of disease (n = 7). Six of the 10 dogs that received a transfusion did not survive to discharge.
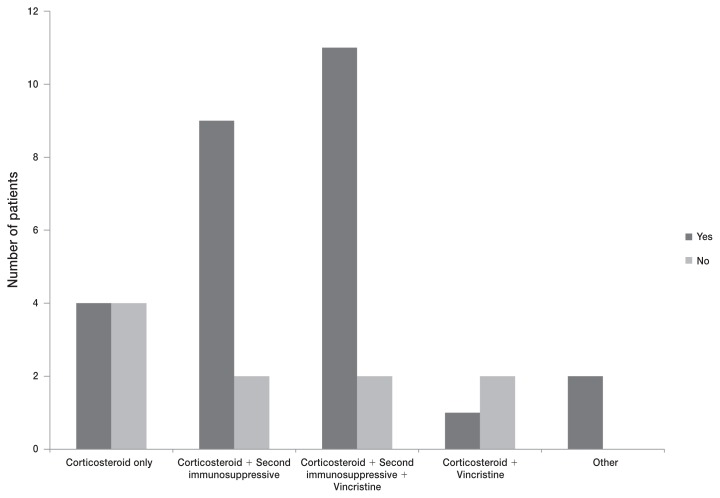
Of the 37 inpatients, 27 (73%) survived to discharge, and 10 (27%) either died (n = 4) or were euthanized (n = 6) in the hospital (Figure 1). Two of the 9 (22%) treated outpatients were known to have died as a result of ITP. The average in-hospital stay was 5.1 d, and the average time for all dogs in this study to develop a platelet count of ≥ 100 000/μL was 7.1 d. Thirty-six of 46 treated patients (78%) eventually achieved platelet counts > 200 000/μL. Of these 36 dogs, 14 (39%) experienced a documented relapse.
Figure 1.
Reported difference in survival to discharge of 37 inpatients distributed by treatment protocol for primary ITP. “Yes” indicates that the patient was discharged from the hospital. “No” indicates that the patient was not discharged from the hospital and either died or was euthanized.
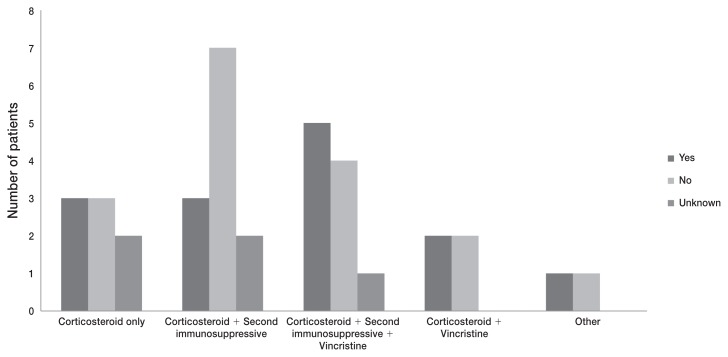
There was no difference before (OR = 1.17, 95% CI: 0.19 to 8.86, P = 0.99) or after adjusting for age (OR = 1.16, 95% CI: 0.19 to 8.86, P = 0.99) in odds that dogs would experience a relapse following initial treatment with a corticosteroid alone compared to dogs treated with a corticosteroid in combination with another treatment (Figure 2). Where the relapse status was unknown (9/46), the dogs were assumed not to have had a relapse. There was no substantial difference in the result if dogs with unknown status were considered to have had a relapse (OR = 0.75, 95% CI: 0.12 to 4.36, P = 0.99).
Figure 2.
Reported occurrence of relapse based on the treatment protocol for 36 dogs treated for ITP which survived to discharge or were treated as outpatients. “Yes” indicates those patients relapsed, and “No” indicates those that did not experience a relapse, and “Unknown” indicates that follow-up was not available.
The average time from initiating therapy to relapse in dogs receiving only corticosteroids (median: 108 d, range: 30 to 300 d, n = 4) was not significantly different than that reported for dogs receiving corticosteroids with a second treatment (median: 177 d, range: 28 to 455 d, n = 22) (P = 0.52).
Our findings are similar to those of a previous retrospective study, which also found that treatment protocol did not have a significant effect on survival to discharge (1). While a recent prospective study comparing adjunctive treatment of canine ITP with vincristine or hIVIG reported that secondary therapies were associated with rapid platelet count recovery, cessation of clinical signs, and shortened hospital stay, the study did not find a significant difference in survival to discharge (12).
The risk of mortality before discharge and subsequent relapse were equivalent to or slightly higher in our study than in other studies, including a previous ITP retrospective study also performed at the WCVM almost 3 decades ago (1,2,4–6,13). In other retrospective studies, mortality rates have varied from 7% to 16% (1,6). The number of dogs which experienced a relapse episode also varied in previous studies from 9% (this study included dogs diagnosed with tick-borne disease) to 26% (1,6). Both estimates were lower than the risk of relapse (39%) reported herein.
There are many explanations for the discrepancies in survival and risk of relapse between these studies. Since primary ITP is a diagnosis of exclusion, there is a chance that dogs with a definitive cause of ITP (e.g., underlying neoplasia) were inadvertently included. It is generally thought that a diagnosis of primary ITP requires the presence of anti-platelet antibodies in combination with a lack of evidence of concurrent disease or neoplasia. However, the anti-platelet antibody test has poor specificity for identifying primary disease (14). This test was not available and was not performed on any of our patients.
The inclusion of patients with confirmed rickettsial disease in a previous study (1) might also explain differences from our findings. While there are several rickettsial diseases that are known to cause thrombocytopenia, most are rarely recognized in our area due to the extreme winter climate (Gaunt et al, unpublished observations). Treatment with doxycycline in less than 50% of our patients, rather than the 100% that would be expected in an area where these diseases are more common, is a possible although unlikely reason our risk of relapse was higher. Undiagnosed and untreated rickettsial disease such as anaplasmosis could result in relapse of ITP.
Choice of treatment protocol at our institution was at the discretion of the clinician managing each case. We acknowledge the decision to use additional therapy could have been affected by the perceived or actual severity of disease. Due to the retrospective observational nature of this study, we were unable to fully define the clinical severity of disease of patients or other factors that might have influenced treatment decisions. While it is reasonable to assume that dogs that were more stable would have been treated with a corticosteroid alone while those more severely affected received additional therapy, this cannot be determined from the available data in this study. Finally, client communication on admission and during treatment regarding prognosis, and rate of relapse could have also impacted an owner’s decision to agree to treatment or continued treatment.
A previous retrospective study performed at our institution and published in 1985 evaluated cases of canine ITP, IMHA, and concurrent ITP/IMHA (Evans syndrome) that presented to the WCVM from 1969 to 1983 (13). Seventy-three percent of dogs with thrombocytopenia were found to have primary immune disease as opposed to having a secondary cause for their thrombocytopenia. In the earlier study, female dogs were overrepresented; this was not the case in our current study. Prednisone was the most common treatment for ITP both then and now, followed by azathioprine and cyclosporine.
Survival was comparable in both studies with 52% of treated animals alive at the conclusion of the previous study, compared to 74% in the current study (both inpatients and outpatients). Forty-eight percent of the patients in the prior retrospective study died or were euthanized compared to 27% of the inpatients in our study. Over 30 y later, the time to > 100 000/μL platelets was very similar in both studies, 7.1 d in our study compared with 8 d in the previous retrospective. Relapse risk in patients treated for ITP at the VMC from 1969 to 1983 was also similar with 41% relapse from 1969 to 1983 and 39% from 2000 to 2013.
These are interesting findings, as it is unusual to have similar retrospective studies performed at the same institution 30 y apart. These findings indicate that the population presenting for primary canine ITP at our institution has not changed significantly over the past 3 decades. It also appears that despite significant research regarding optimal treatment protocol (drugs, secondary immunosuppressive agents, or surgical interventions), mortality risk, time to > 100 000/μL platelets, and risk of relapse are also not substantially different. Whether this is a product of our unique location (i.e., lack of rickettsial disease), the most common treatment protocols utilized, or is truly representative of this disease is unknown and requires further prospective work. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.O’Marra SK, Delaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immune-mediated thrombocytopenia. J Am Vet Med Assoc. 2011;238:346–352. doi: 10.2460/javma.238.3.346. [DOI] [PubMed] [Google Scholar]
- 2.Grindem CB, Breitschwerdt EB, Corbett WT, Jans HE. Epidemiologic survey of thrombocytopenia in dogs: A report on 987 cases. Vet Clin Pathol. 1991;20:38–43. doi: 10.1111/j.1939-165x.1991.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 3.Wardrop KJ, Lewis D, Marks S, Buss M. Post transfusion purpura in a dog with hemophilia A. J Vet Intern Med. 1991;11:261–263. doi: 10.1111/j.1939-1676.1997.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 4.Williams DA, Maggio-Price L. Canine idiopathic thrombocytopenia: Clinical observations and long-term follow-up in 54 cases. J Am Vet Med Assoc. 1984;185:660–663. [PubMed] [Google Scholar]
- 5.Jans HE, Armstrong J, Price GS. Therapy of immune mediated thrombocytopenia: A retrospective study of 15 dogs. J Vet Intern Med. 1990;4:4–7. doi: 10.1111/j.1939-1676.1990.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 6.Putsche JC, Kohn B. Primary immune-mediated thrombocytopenia in 30 dogs (1997–2003) J Am Anim Hosp Assoc. 2008;44:250–257. doi: 10.5326/0440250. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura RK, Tompkins E, Bianco D. Therapeutic options for immune-mediated thrombocytopenia. J Vet Emerg Crit Care. 2012;22:59–72. doi: 10.1111/j.1476-4431.2011.00705.x. [DOI] [PubMed] [Google Scholar]
- 8.Yau VK, Bianco D. Treatment of five haemodynamically stable dogs with immune-mediated thrombocytopenia using mycophenolate mofetil as single agent. J Small Anim Pract. 2014;55:330–333. doi: 10.1111/jsap.12203. [DOI] [PubMed] [Google Scholar]
- 9.Bianco D, Armstrong PJ, Washabau RJ. Treatment of severe immune-mediated thrombocytopenia with human IV immunoglobulin in 5 dogs. J Vet Intern Med. 2007;21:694–699. doi: 10.1892/0891-6640(2007)21[694:tositw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Bianco D, Armstrong PJ, Washabau RJ. A prospective, randomized, double-blinded, placebo-controlled study of human intravenous immunoglobulin for the acute management of presumptive primary immune-mediated thrombocytopenia in dogs. J Vet Intern Med. 2009;23:1071–1078. doi: 10.1111/j.1939-1676.2009.0358.x. [DOI] [PubMed] [Google Scholar]
- 11.Rozanski EA, Callan MB, Hughes D, Sanders N, Giger U. Comparison of platelet count recovery with use of vincristine and prednisone or prednisone alone for treatment for severe immune-mediated thrombocytopenia in dogs. J Am Vet Med Assoc. 2002;220:477–481. doi: 10.2460/javma.2002.220.477. [DOI] [PubMed] [Google Scholar]
- 12.Balog K, Huang AA, Sum SO, Moore GE, Thompson C, Scott-Moncrieff JC. A prospective randomized clinical trial of vincristine versus human intravenous immunoglobulin for acute adjunctive management of presumptive primary immune-mediated thrombocytopenia in dogs. J Vet Intern Med. 2013;27:536–541. doi: 10.1111/jvim.12066. [DOI] [PubMed] [Google Scholar]
- 13.Jackson ML, Kruth SA. Immune-mediated hemolytic anemia and thrombocytopenia in the dog: A retrospective study of 55 cases diagnosed from 1979 through 1983 at the Western College of Veterinary Medicine. Can Vet J. 1985;26:245–246. 247–250. [PMC free article] [PubMed] [Google Scholar]
- 14.Kristensen AT, Weiss DJ, Klausner JS, Laber J, Christie DJ. Detection of antiplatelet antibody with a platelet immunofluorescence assay. J Vet Intern Med. 1994;8:36–39. doi: 10.1111/j.1939-1676.1994.tb03193.x. [DOI] [PubMed] [Google Scholar]