Nearly 15 million Americans 12 years of age and older were dependent on or abused alcohol in 2010.1 The high prevalence of problem alcohol use worldwide has been estimated to cause 2.5 million deaths each year,2 not to mention the exorbitant costs associated with excess morbidity3 and loss of productivity.4 The chronic and relapsing nature of alcoholism, just like that of every other substance use disorder, is one of the major obstacles to its successful treatment. This is why the search for predictive biomarkers to help clinicians select and monitor a therapeutic course of action and to help researchers evaluate new therapeutic interventions is so urgent.
It is well established that the risk for alcohol relapse is tied to static (eg, severity index, marital status, psychiatric symptoms, and genetics) and dynamic (eg, craving and stress) factors. The latter offer particularly useful metrics whose magnitudes correlate quite closely with different disease trajectories5 and can consistently predict alcohol relapse risk.6,7 Thus, a better understanding of a patient's response to stress and/or alcohol cues is bound to contribute to the design of more personalized and, therefore, effective treatment strategies. The findings reported by Seo et al8 in the current issue of JAMA Psychiatry represent an important step forward for they manage to map the relationship between relapse risk and specific neural substrates, advancing the feasibility of a brain biomarker for predicting relapse into alcohol drinking.
Using functional magnetic resonance imaging, the researchers probed the functional state of the neural circuitry underlying the behavioral response to known triggers of alcohol relapse (alcohol cues and stress). The stimulation paradigm used 3 different personalized scripts: neutral, alcohol cues, and stress. When the alcohol-dependent (AD) individuals (N = 45) were tested during the neutral script, they showed increased activity in the ventromedial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC), ventral striatum, and precuneus that was associated with a higher risk for relapse. Increased activity in these brain regions also predicted the intensity of the craving they experienced when exposed to the alcohol cues or stress conditions. In contrast, these regions were hypoactive during the stress and alcohol cue conditions, and the difference between the response of the vmPFC/ACC to the neutral vs the stress and alcohol cues was blunted compared with the response in healthy control individuals (N = 30). This net blunted differential response correlated with alcohol cue–induced and stress-induced craving among recovering patients.
The finding that various regions were associated with relapse and craving is consistent with the organization of the brain into functional networks comprising complex connections among multiple regions. It is notable that the regions with the strongest prediction for relapse (vmPFC/ACC and precuneus) are central nodes of the default mode network (DMN), which is involved with the processing of internal states (including self-monitoring) and deactivated when engaging in various behavioral tasks or when responding to the environment9 (Figure). Also, the ventral striatum, which is one of the main targets for dopamine midbrain pathways (predominantly those originating in the ventral tegmental area) and another region of hyperactivity that predicted relapse in AD patients, is functionally connected with the vmPFC/ACC and the precuneus,10 implicating it in the regulation of the DMN. Indeed, there is evidence that dopamine modulates the DMN, facilitating its deactivation11; thus, the hyperactivity in DMN regions of AD individuals is consistent with a decrease in dopamine neurotransmission in alcoholism.12 Inasmuch as increased activity in the DMN is associated with enhanced internal awareness, this could provide a substrate for the craving responses of AD individuals when exposed to stress or alcohol cues. Their increased awareness of internal states could result in greater craving and associated increased risk for relapse.
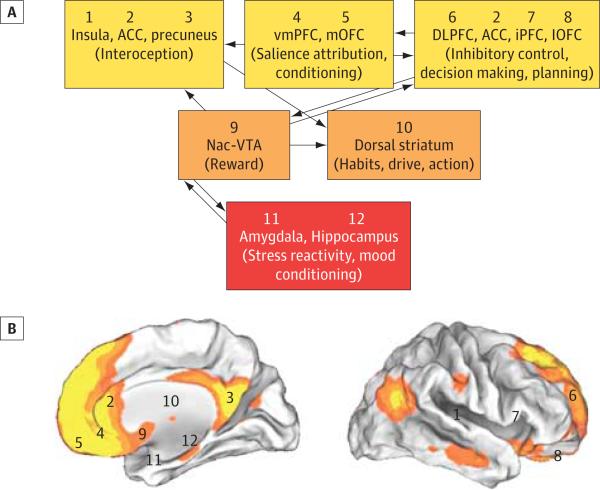
Figure. Schematic Diagram of the Neural Network Necessary for Self-Control and Its Overlap With the Default Mode Network (DMN).
A, The ventromedial prefrontal cortex (vmPFC)/anterior cingulate cortex (ACC), identified by Seo et al as a robust biomarker of alcohol relapse risk, modulates and, in turn, is modulated by the activity of multiple other cortical and subcortical regions whose combined output is used to orchestrate adaptive and flexible goal-directed behaviors. Current evidence suggests that information from cortical and subcortical structures converges toward a single common value representation before passing on to the choice-related motor control circuitry. Modulatory inputs play a critical role in establishing this final common representation with those inputs carrying signals related to interoception, salience attribution, conditioning, executive control, reward, habituation, and stress and mood reactivity. B, In addition, each region has been numbered and its general location tagged onto maps of the human brain (internal and external views) overlaid with orange and yellow pseudocolors that represent the resting state connectivity of the DMN. Regions (vmPFC/ACC and precuneus) that were hyperactive during the neutral conditions and that predicted relapse show significant overlap with the DMN. DLPFC indicates dorsolateral prefrontal cortex; iPFC, inferior prefrontal cortex; lOFC, lateral orbitofrontal cortex; mOFC, medial orbitofrontal cortex; Nac, nucleus accumbens; VTA, ventral tegmental area.
The finding of a loss of differential reactivity in the vmPFC/ACC between the neutral condition and the stress and alcohol cue conditions in AD individuals is also relevant because it points to a loss of flexibility (adaptability) of neuronal responses to salient stimuli. Prior studies had identified that in addiction states, the dysfunction of the orbitofrontal cortex underlies the behavioral inflexibility vis a vis reward-driven behavior as a function of changes in the environment and internal states (ie, perseverative lever pressing when the reward is no longer delivered).13 However, the mechanisms underlying the reduced flexibility in the reactivity of the vmPFC/ACC to emotional relevant states (alcohol cues and stress vs neutral cues) remain unclear. Because phasic dopamine in the mesoaccumbal pathway, which in turn is modulated by cortical, amygdalar, and thalamic glutamatergic pathways, signals saliency,14 it is possible that neuroplastic changes in glutamate neurotransmission impinging on the function of the nucleus accumbens may be involved.15 Indeed, in the study by Seo et al8, the ventral striatum (where the nucleus accumbens is located) was a region that also showed enhanced reactivity during the neutral condition.
It is noteworthy that the activation profile of the vmPFC/ACC stood out as a significant marker of relapse because hyperactivity in this area during the neutral condition boosted the chances of early relapse to heavy drinking by more than 8-fold. Based on recent studies showing that the same regions become activated in healthy subjects after the administration of alcohol,16 the authors argue reasonably that repeated administration of alcohol might have sensitized the vmPFC/ACC region to neutral-relaxing conditions, curbing its responsiveness to stress or alcohol cues. This would be consistent with the fact that alcohol administration can significantly activate the ventral striatum of social, but not heavy, drinkers.17 The situation is highly reminiscent of cocaine abusers who, if tested shortly after their last cocaine use or during drug-induced craving, display hypermetabolic activity in the vmPFC/ACC that was proportional to the intensity of their craving.18 Moreover, when cocaine addicts were administered an intravenous stimulant drug that they report to have cocaine-like effects, they activated the vmPFC/ACC, whereas nondrug-abusing control subjects deactivated it.19 Thus, this indicates that disruptions in vmPFC/ACC are not exclusive to alcoholism but may also occur in other addictions. Moreover, vmPFC activation in drug abusers seems to be associated with a greater propensity for drug craving. Indeed, purposeful inhibition of craving in cocaine abusers was shown to decrease the activity of vmPFC (and also of the ventral striatum).20 In this respect, it is noteworthy that inhibition of the equivalent region in the vmPFC in the rat with optogenetic stimulation prevents relapse into drug taking.21
Functional impairments in the vmPFC/ACC have been associated with reduced inhibitory feedback control, not just of the HPA axis22 (which would exacerbate the negative effects of the neuroendocrine loop), but also of impulsive drives generated in the subcortical circuitry and engendered by conditioned responses. Indeed, such weakened behavioral inhibitory capacity seems to characterize many different addicted states and contribute to the severe disruptions in behavioral choices associated with addiction.23 To choose between alternative options, the brain relies on a distributive system that assesses and compares their differential values. Functional neuroimaging studies have provided fundamental insights into the role of the vmPFC/ACC in decision making. These studies identify the prominence of the vmPFC/ACC as a main hub in a network that includes medial and lateral components of the orbitofrontal cortex and the nucleus accumbens and that is incharge of translating the subjective value of a reward into a common neural currency, an operation that is crucial for determining personal preferences.24 The vmPFC/ACC, as part of a node in a network necessary for self-control, modulates and, in turn, is modulated by the activity in multiple other brain regions (Figure). For example, health cues preferentially activate the left inferior frontal gyrus and 2 subregions of the left dorsolateral prefrontal cortex, which then convey their output to the vmPFC for an integrated valuation of so-called “food healthiness.”25 Also, the dorsal ACC is preferentially called on during decisions to minimize punishment, whereas the vmPFC is recruited during decisions to maximize reward.26 Moreover, the vmPFC/ACC itself is heterogeneous with regards to the computation of reward and punishment-related valuation, which results in the preferential recruitment of ventral vs dorsal neurons, respectively.27 Overall, the combined neuroimaging evidence28,29 points to the vmPFC/ACC as a central processor of overall subjective value that is then used to bias decision making.
The findings by Seo et al8 represent a major contribution to ongoing efforts to develop brain biomarkers to predict relapse; therefore, they need to be considered in the context of prior results from brain imaging studies that also identified regional changes associated with relapse in AD individuals and in other drug addictions. A common element across these studies is the identification of regions whose impaired function perturbs the balance between reward and executive control networks. For example, evidence of impaired communication between these critical systems has recently been documented in the form of significantly reduced resting state synchrony within both the reward and executive control networks among alcohol relapsers.30 The reduced cognitive control and enhanced reward sensitivity that has been linked to disrupted frontal white matter integrity (lower fractional anisotropy and higher radial diffusivity) among alcohol relapsers31 could help explain their poor inhibitory control of emotional-laden stimuli. Drilling down to the level of specific structures, we have also learned that likely relapsers show increased atrophy in bilateral orbitofrontal cortex and in the right medial prefrontal cortex and ACC, brain areas associated with error monitoring.32 On the functional side, many studies of AD patients revealed increased brain activity in the mesocorticolimbic system in response to alcohol-related relative to neutral cues (see review by Bühler and Mann33). For example, one such study found that cue-induced activation of the putamen, ACC, and adjacent mPFC was particularly pronounced in detoxified AD individuals who subsequently relapsed.34 The combined results strongly suggest that the imaging field is tantalizingly close to developing a reliable and practical ap proach for predicting those individuals who may be at greater risk for alcohol relapse.
As more evidence accumulates, noninvasive imaging tools, as illustrated by the study by Seo et al,8 may make it possible to develop biomarkers to predict disease trajectories and the rapeutic outcomes that are necessary for individualized medicine and optimal patient care. In the meantime, future studies designed to replicate these findings in AD patients but also assess their values in other drug addictions will allow us to determine whether abnormal function of the vmPFC/ACC and associated circuits (including the DMN) can be used as biomarkers to monitor patients afflicted by a variety of substance use disorders.
Footnotes
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration . Results from the 2011 National Survey on Drug Use and Health (NSDUH) US Department of Health and Human Services; Rockville, MD: [Google Scholar]
- 2.World Health Organization [March 20, 2013];Media Centre: alcohol fact sheet. http://www.who.int /mediacentre/factsheets/fs349/en/.
- 3.Rehm J. The risks associated with alcohol use and alcoholism. Alcohol Res Health. 2011;34(2):135–143. [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the US, 2006. Am J Prev Med. 2011;41(5):516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Witkiewitz K. Predictors of heavy drinking during and following treatment. Psychol Addict Behav. 2011;25(3):426–438. doi: 10.1037/a0022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ. Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology (Berl) 2011;218(1):121–129. doi: 10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68(9):942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo D, Lacadie CM, Tuit K, Hong K-I, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70(7):727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18(12):2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 11.Delaveau P, Salgado-Pineda P, Fossati P, Witjas T, Azulay JP, Blin O. Dopaminergic modulation of the default mode network in Parkinson's disease. Eur Neuropsychopharmacol. 2010;20(11):784–792. doi: 10.1016/j.euroneuro.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND, Wang GJ, Telang F, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27(46):12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23(22):8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35(7):1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuber GD, Hopf FW, Tye KM, Chen BT, Bonci A. Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Curr Top Behav Neurosci. 2010;3:3–27. doi: 10.1007/7854_2009_23. [DOI] [PubMed] [Google Scholar]
- 16.Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28(18):4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37(2):467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Wang GJ, Ma Y, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25(15):3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkow ND, Fowler JS, Wang GJ, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49(3):2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefanik MT, Moussawi K, Kupchik YM, et al. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18(1):50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thayer JF, Hall M, Sollers JJ, III, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J Psychophysiol. 2006;59(3):244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol. 2012;22(6):1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31(30):11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blair K, Marsh AA, Morton J, et al. Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. J Neurosci. 2006;26(44):11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monosov IE, Hikosaka O. Regionally distinct processing of rewards and punishments by the primate ventromedial prefrontal cortex. J Neurosci. 2012;32(30):10318–10330. doi: 10.1523/JNEUROSCI.1801-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 29.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Camchong J, Stenger A, Fein G. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb Cortex. doi: 10.1093/cercor/bhs190. published online July 20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorg SF, Taylor MJ, Alhassoon OM, et al. Frontal white matter integrity predictors of adult alcohol treatment outcome. Biol Psychiatry. 2012;71(3):262–268. doi: 10.1016/j.biopsych.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck A, Wüstenberg T, Genauck A, et al. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69(8):842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- 33.Bühler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35(10):1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- 34.Grüsser SM, Wrase J, Klein S, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]