Abstract
Background
Acetaminophen (APAP) overdose is the leading cause of acute liver failure in the US. Although substantial progress regarding the mechanisms of APAP hepatotoxicity has been made in the past several decades, therapeutic options are still limited and novel treatments are clearly needed. c-jun N-terminal Kinase (JNK) has emerged as a promising therapeutic target in recent years.
Areas covered
Early studies established the critical role of JNK activation and mitochondrial translocation in APAP hepatotoxicity. However, this concept has also been challenged. Initial studies failed to reproduce the protection of JNK deficiency in APAP toxicity and concerns over off-target effects of JNK inhibitors and even in knock-out mice are increasing. Interestingly, recent studies have even shown that liver injury can be altered with or without effects on JNK activation. The current review addresses these discrepancies and tries to explain or reconcile some of the conflicting results.
Expert opinion
JNK is a potential therapeutic target for APAP poisoning. However, controversies still exist regarding its actual role in APAP hepatotoxicity. Future studies are warranted for more in-depth testing of specific inhibitors in well-defined preclinical models and human hepatocytes before JNK can be considered a relevant therapeutic target for APAP poisoning.
Keywords: acetaminophen, c-jun N-terminal kinase, drug hepatotoxicity, mitochondrial dysfunction
1. Introduction and Background of APAP Hepatotoxicity
Acetaminophen (APAP; paracetamol) is the most commonly used pain reliever and fever reducer in the US. It is available both as a single entity formulation and in combination with other medications. In 2008, 24.6 billion doses of APAP were sold (80% of which are in OTC products) and an estimated 48 million Americans use APAP-containing products every week [1]. Although it is considered safe at therapeutic doses, APAP overdose causes severe liver injury, contributing to around 70,000 hospitalizations and 50% of cases of acute liver failure each year in the US [2,3]. APAP alone and APAP-containing products have been listed as the fourth and sixth highest causes of poisoning-related fatalities, respectively [4,5].
2. Basic Mechanisms of APAP Hepatotoxicity
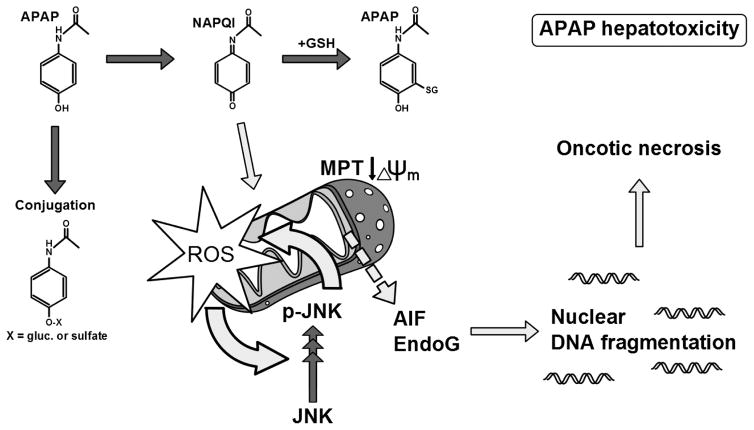
APAP hepatotoxicity results from the cytochrome P450-catalyzed formation of a reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) [6]. Although small amounts of NAPQI can be detoxified by glutathione (GSH), excess reactive metabolite formation after APAP overdose depletes hepatocellular GSH and causes protein adduct formation [7] including adducts on mitochondrial proteins [8]. Modification of mitochondrial proteins alters mitochondrial bioenergetics and causes oxidative stress, which subsequently leads to ATP depletion, opening of the mitochondrial permeability transition (MPT) pore, and release of mitochondrial intermembrane proteins, such as apoptosis-inducing factor (AIF) and endonuclease G (Endo G), and their translocation to the nucleus [9–12]. This then results in DNA fragmentation and necrotic cell death [13–14] (Figure 1). To date, N-acetylcysteine (NAC) is the only available clinical antidote against APAP poisoning [15]. Dependent on the time of NAC administration, its protective mechanisms include promoting GSH biosynthesis to scavenge NAPQI, detoxifying reactive oxygen species and peroxynitrite, and serving as a Krebs cycle intermediate to support mitochondrial energy production [11,16–18]. As a result, NAC has to be given shortly after APAP overdose, before the early metabolism phase is complete, to achieve its greatest effect (within 24h of overdose in humans) [19,20]. Since most APAP-overdose patients present during or after the peak of injury, it is urgent to develop new agents for late presenting patients [21].
Figure 1. Mechanisms of acetaminophen hepatotoxicity.
At therapeutic doses, most acetaminophen (APAP) is conjugated and excreted. A small amount is converted to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), and this can be effectively detoxified by glutathione (GSH). However, after an overdose, excess NAPQI binds to proteins. Mitochondrial protein alkylation leads to oxidative stress which activates multiple signaling pathways converging on the c-Jun N-terminal kinases 1/2 (JNK). Activated JNK (pJNK) then translocates into mitochondria and increases the oxidative stress. Eventually, the mitochondrial membrane permeability transition (MPT) occurs. Matrix swelling and lysis of the outer mitochondrial membrane, as well as translocation of Bax, facilitates release of the endonucleases apoptosis-inducing factor (AIF) and endonuclease G (EndoG) from the intermembrane space. The endonucleases then translocate to the nucleus and fragment nuclear DNA. Ultimately, the cell dies by oncotic necrosis.
3. Potential roles of JNK in APAP Hepatotoxicity
C-Jun N-terminal kinase (JNK) is a serine/threonine protein kinase that belongs to the mitogen-activated protein kinase (MAPK) superfamily. Three isoforms of JNK have been identified in mammals. JNK 1 and 2 are ubiquitously expressed, whereas JNK3 is mainly expressed in brain and testis [22,23]. JNK is activated under cell stress and has been demonstrated to be involved in various liver pathologies including inflammation, steatosis, fibrosis and hepatic cell death, and studies during the last several decades strongly support a detrimental role of sustained JNK activation in hepatocyte death and liver injury in these pathologies [24]. Interestingly, JNK activation has also been reported in both rodents and human hepatocytes after APAP overdose [25–27]. Early studies suggested that JNK signaling has a detrimental role in the toxicity by serving as a second hit to amplify the cellular stress [28–30]. It is believed that the first hit caused by GSH depletion, protein binding and oxidative stress moderately impairs mitochondrial respiration, but it is insufficient to cause cell death. However, the initial oxidative stress would sensitize mitochondria to JNK signaling and when JNK is activated, it translocates to mitochondria and provides a second hit which further amplifies the oxidant stress and leads to MPT pore formation, complete loss of the mitochondrial membrane potential, and subsequently cell necrosis [28,30,31] (Figure 1). This hypothesis has been supported by numerous studies using genetic or pharmacological intervention strategies. However, it has also been challenged by several findings, especially those from recent studies [32]. Therefore, the current review aims at addressing the conflicting findings from those studies exploring the role of JNK in APAP hepatotoxicity. By analyzing these discrepancies, we hope to provide potential explanations and future directions for studies that might be helpful to clarify these issues.
4. Evidence for JNK as a therapeutic target
4.1 Genetic interventions
Results from studies investigating the effect of JNK deficiency in APAP hepatotoxicity have been controversial. Gunawan et al. showed that JNK2- but not JNK1-deficient mice were partially protected from the APAP-induced liver injury [25]. Consistent with this, administration of JNK2 but not JNK1 anti-sense oligonucleotide also partially protected wild type (WT) mice from the toxicity [25]. These studies strongly suggested that JNK2, but not JNK1, contributes to the liver injury after APAP overdose. However, these findings were quickly challenged. Studies from several research groups failed to reproduce the protective effect in JNK2-deficient mice [26,30,32]. More surprisingly, one study even pointed out that JNK2 signaling may actually have a beneficial role in APAP hepatotoxicity, and JNK2-deficient mice had higher liver injury which may have been due to compromised tissue repair [32].
Direct comparison of these conflicting findings is difficult because of the different doses of APAP used in these studies [25,26,30,32]. Furthermore, in one study, APAP was dissolved in DMSO [25], which is well known to protect against APAP toxicity by inhibiting P450-mediated metabolic activation of APAP at even low doses [33]. Nevertheless, a follow-up study seemed to provide another explanation for these conflicting results: off-target genetic differences between the C57Bl/6J (Nnt−/−) and C57Bl/6NJ (Nnt+/+) substrains of mice [34]. It was found that the lack of protection in the later studies was actually the result of mispairing of WT C57Bl/6J (Nnt−/−) with JNK2−/− C57Bl/6NJ (Nnt+/+) substrains, and the injury could be reversed when the JNK2−/− mice were compared with WT C57Bl/6NJ (Nnt+/+) mice [34]. Despite this, the fact that JNK2-deficiency provides so little protection that it is lost when simply comparing different substrains of mice does not support the idea that JNK2 is a central mediator of APAP toxicity. Another possible reason for the lack of protection would be the potential compensatory effects of JNK1 and JNK2 isoforms in selective JNK-deficient mice, in which one isoform may compensate for the other when either one is deficient. This hypothesis is supported by the lack of protection in selective JNK1 deficient mice and a greater protection when both isoforms were knocked down [25,28]. Therefore, selective elimination of either isoform might not be sufficient to draw a convincing conclusion. Although knockout of both JNK1 and 2 might help to clarify this issue, whole body deficiency of both isoforms results in embryonic lethality [35], preventing its use in APAP study. More recently, liver specific JNK1 and JNK 2 double knockout mice have been used to assess the role of JNK in APAP-induced cell death [36]. The preliminary data suggest aggravation of APAP-induced liver injury, which appears to correlate with higher Cyp2E1 expression [36]. However, it will have to be seen if these results, which contradict many previous reports and conclusions as discussed in this review, reflect the lack of a role for JNK or off-target effects.
4.2 Direct JNK inhibitors
More powerful evidence for an important role for JNK in APAP hepatotoxicity may come from inhibitor studies (Table 1). The most commonly used JNK inhibitor is SP600125, which was discovered in 2001 [37] and subsequently introduced in studies of APAP toxicity [38]. SP600129 is a reversible ATP-competitive inhibitor of JNK and dose-dependently inhibits the phosphorylation of c-jun and JNK itself [37]. A concentration of 20 μmol/L of SP600125 was found to significantly protect primary mouse hepatocytes against APAP-induced necrosis [39]. A follow-up study in vivo confirmed the effectiveness of this inhibitor, demonstrating that a dose of 10 mg/kg completely protected mice against the toxicity when compared with vehicle controls when it was administered around the time of APAP treatment [25]. Importantly, the protection was not due to inhibition of protein binding or enhanced GSH resynthesis [25,39]. Studies from our lab and others also confirmed the protection by this inhibitor in mice [26,30,40,41], and more importantly, we demonstrated that SP600125 protects mainly by preventing mitochondrial oxidant stress and peroxynitrite formation in mice [30], and it even partially protected against APAP-induced cell death in primary human hepatocytes [27]. Its effectiveness, especially when given post-APAP, greatly raises its potential for use in late-presenting patients with APAP poisoning. Nevertheless, while these findings are encouraging, several caveats still exist when interpreting data from these studies. First, SP600125 has to be dissolved in the solvent DMSO, which is known to have numerous biological effects on cytochrome P450 enzymes [33] and inflammatory cells [42]. Although SP600125 offers greater protection in vivo than DMSO alone, various other solvent effects possibly resulting in an unrealistic model cannot be excluded. Second, very high overdoses of APAP (600 – 1000 mg/kg) were used to overcome the DMSO-mediated inhibition of protein binding and induce significant liver injury in mice [25,26,28,30,40,41]. Since intracellular signaling pathways after APAP overdose may vary depending on the dose [43,44], it is possible that JNK signaling at those high doses might not be reproduced at lower toxic doses (200 – 400 mg/kg), which are typical doses used in APAP mechanistic studies in mice. Third, although comparable GSH depletion was observed at late time points, there is a significant delay in early GSH depletion with SP600125 treatment compared to its vehicle [25,30]. This may add additional mechanisms to the protection, especially when we consider the critical role of GSH depletion in the initiation phase of APAP hepatotoxicity. Fourth, the specificity of the inhibitor is another concern. Although the specificity of SP600125 for JNK1 and JNK2 (IC50= 0.04 μM) is over 10 fold higher compared to other MAP kinases, such as MKK4 (IC50= 0.4 μM) and MKK6 (IC50= 1.0 μM) [37], we cannot exclude the possibility that inhibition of other kinases by SP600125 may contribute or even be responsible for the effect, especially since the dose of SP600125 used was ≥10 mg/kg and the actual cellular concentrations of the inhibitor in vivo were unknown [25,26,30,40,41]. An interesting finding of SP600125 was its lack of protection in HuH7 cells despite the existence of JNK activation [45]. Because mechanistic cell death signaling caused by APAP in this hepatoma cell line is clinically irrelevant due to its lack of drug metabolizing enzymes, which are indispensable to generate NAPQI and initiate the toxicity, the protection of SP600125 in mice [25,26,30,40,41] and in primary human hepatocytes [27] suggests the importance of JNK activation in these clinically relevant models.
Table I.
Studies showing evidence for or against a critical role for JNK in APAP hepatotoxicity
| Type of Intervention | Supports a role (ref.) | Does not support╪ (ref.) | Conflicting data (ref.) |
|---|---|---|---|
| Genetic | 25, 28 | 26, 30, 32, 36 | 34# |
| Inhibitor | 25–28, 30, 39–41, 46 | 47, 90 | 27* |
| Other | 72–75,77 | 79–86 |
Explained that these conflicting results might be due to off-target genetic differences between substrains of mice
JNK inhibitor protected primary human hepatocytes, but human HepaRG cells did not show JNK activation.
Some data suggest that initial JNK activation is unimportant, but late JNK activation may play a role.
Two other JNK inhibitors have also been used for APAP toxicity. Leflunomide (LEF), a disease-modifying anti-rheumatic drug, was shown to protect against APAP-induced toxicity in both immortalized human hepatocytes (HC-04) and mice via inhibition of JNK activation and JNK-mediated MPT pore opening and iNOS-induced peroxynitrite formation [40,46]. Since the study in human liver cells showed that LEF did not affect the GSH depletion and in mice LEF was administered at 4 h post-APAP, it was concluded that LEF protects without inhibition of the metabolic activation of APAP [40,46]. However, a later study investigating the protective mechanism of LEF demonstrated that inhibition of APAP bioactivation might be involved in the protection in mice, though it was not in immortalized human hepatocytes [47]. Because a very high dose of APAP (750 mg/kg) was used in the mouse study [40], it is likely that the metabolism of APAP has not finished yet at the time when LEF was administered (4 h post-APAP) and LEF could still inhibit the metabolic activation of APAP. In addition, the MAP kinase MKK4, which is upstream of JNK, was completely inhibited in LEF-treated mice. This may add to or actually be responsible for the hepatoprotection [40]. Future animal studies are clearly needed to determine LEF’s specificity for JNK and its effect on APAP bioactivation. Also, considering its immunosuppressive and hepatotoxic effects by itself [48], it is unlikely that LEF could be safely applied to APAP overdose patients who already have compromised liver function and an immunological response. Another JNK inhibitor that has been shown to protect against APAP hepatotoxicity is D-JNKI1 (JNK 1, D-stereoisomer), which is a peptide that inhibits the interaction of JNK with the substrates without affecting the formation of protein adducts [26]. Unfortunately, no further studies have been performed with this drug and mechanistic information is still very limited in terms of its potential clinical application.
4.3 Kinases and phosphatases regulating JNK activation
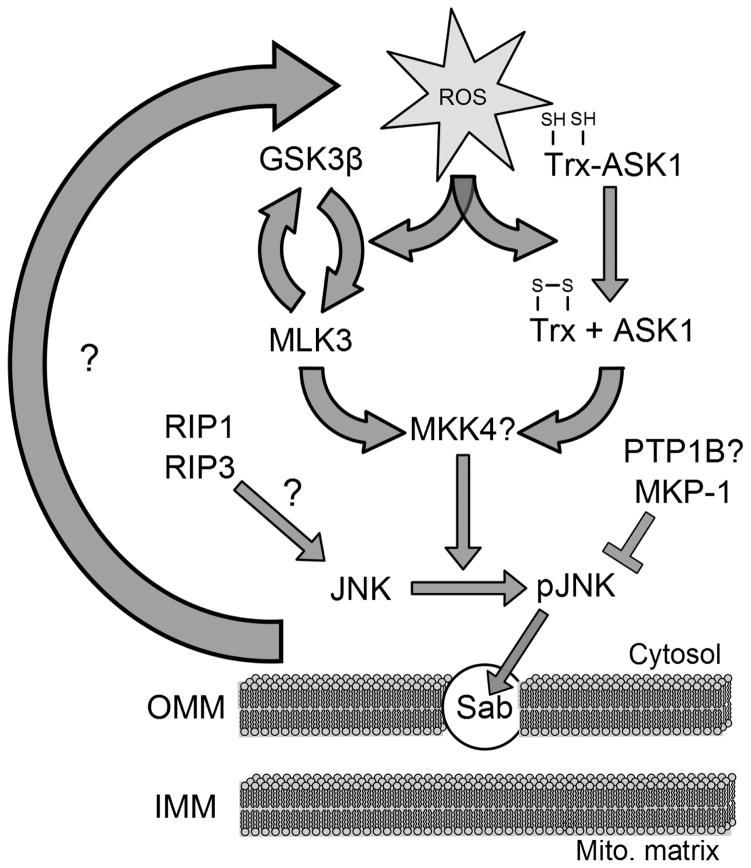
As a MAP kinase, JNK is intricately regulated by a network of kinases and phosphatases (Figure 2) and many of these modulators of JNK activation have been implicated in APAP toxicity (Table 2). The first to receive widespread attention in APAP-induced liver injury was apoptosis signal-regulating kinase 1 (ASK1) [49]. ASK1 is a mitogen-activated protein kinase kinase kinase (MAP3K) which regulates JNK and p38 pathways [50]. Under unstimulated conditions, ASK1 exists in a high-molecular-mass (HMM) complex called the signalosome which comprises an ASK1 homodimer and the reduced form of thioredoxin (Trx) [51]. In the signalosome, Trx inhibits ASK1 activity through direct binding [52]. Upon oxidant stress, thioredoxin is oxidized and dissociates from the complex, while TRAF2 and TRAF6 are recruited to facilitate ASK1 activation [53]. Due to the redox responsiveness, ASK1 is a bridge between oxidant stress and JNK activation. In APAP-induced liver injury, multiple lines of evidence have demonstrated the importance of ASK1 in maintaining sustained JNK activation and promoting cell death. ASK1 activation starts from 3 h after APAP, and genetic deletion of ASK1 protects against the toxicity in both mice and primary mouse hepatocytes [49]. In addition, either as a 30 min pretreatment or a 1.5 h posttreatment, pharmacological inhibition of ASK1 using a selective small molecule ASK1 inhibitor (GS-459679) from Gilead, Inc. provides significant protection against APAP [54]. In either ASK1 knockout mice or ASK1 inhibitor-treated mice, JNK activation in the cytosol and P-JNK translocation to mitochondria are both attenuated. Interestingly, ASK1 silencing affects prolonged (>1.5 h) but not initial (≤1.5 h) JNK activation, indicating that ASK1 may only be required for sustained JNK activation after APAP [49]. However, ASK1 inhibitor studies demonstrated the highest efficacy with pretreatment and a loss of pharmacological effect with treatment beyond 1.5 h after APAP suggesting involvement of ASK1 in early and sustained JNK activation [54]. As JNK is also involved in liver regeneration, targeting JNK therapeutically may raise the concern of inhibiting liver regeneration [55]. However, data suggest that inhibiting ASK1 does not impair the regenerative capacity of the liver after APAP, making ASK1 a potential therapeutic target in APAP toxicity [54].
Figure 2. Mechanisms of JNK activation during acetaminophen hepatotoxicity.
Acetaminophen (APAP) overdose causes an initial oxidative stress that then activates JNK through multiple signaling pathways. In particular, reactive oxygen species (ROS) oxidize cysteine residues on thioredoxin (Trx), causing it to dissociate from apoptosis signal-regulating kinase 1 (ASK1) which is then activated and can activate JNK through a downstream kinase cascade. There is also evidence that glycogen synthase kinase 3 β (GSK3β) and mixed lineage kinase 3 (MLK3) can be activated by ROS and that GSK3β activation is dependent upon MLK3, although the details of their relationship are not yet clear. Similar to ASK1, these kinases signal downstream to activate JNK. Finally, JNK can also be activated by receptor-interacting protein kinase 1 (RIP1) and possibly by RIP3. Once activated, JNK associates with Sab in the outer mitochondrial membrane (OMM) and can feedback to enhance mitochondrial oxidative stress. Phosphatases, such as mitogen-activated protein kinase phosphatase 1 (MKP-1) and possibly protein tyrosine phosphatase 1 B (PTP1B), can regulate JNK activation through dephosphorylation.
Table II.
Other kinases and phosphatases involved in JNK signaling
| Kinase/phosphatase | Effect on JNK activation [ref.] | Upstream / Downstream of JNK |
|---|---|---|
| GSK3β | Activator (early time points?) [56] | Upstream |
| MLK3 | Activator (early time points?) [57] | Upstream |
| ASK1 | Activator (late time points) [54] | Upstream |
| RIP1 | Activator [73,77] | Upstream |
| RIP3 | Activator? [76] | Upstream? |
| Mkp-1 | Inhibitor [64] | Downstream |
| PTP1B | Inhibitor?* [69] | Downstream?* |
The expected effect is decreased JNK activation, but the published results appear to be heavily influenced by other effects.
While ASK1 is considered a regulator of late phase JNK activation after APAP, other studies identified glycogen synthase kinase-3β (GSK-3β) and mixed-lineage kinase 3 (MLK3) as early modulators of JNK activation [56,57] (Figure 2). GSK-3β was initially found to determine glycogen disposition in response to insulin by dephosphorylating glycogen synthase, and now it is recognized as a broadly influential enzyme regulating many cell functions and is associated with multiple diseases [58,59]. Importantly, previous reports demonstrated the redox-sensitivity of GSK-3β [60]. Like ASK1, MLK3 is a MAP3K and mediates ROS-dependent JNK activation in neurons [61]. In the liver, APAP treatment triggers GSK-3β activation and translocation to the mitochondria, and peak GSK-3β translocation precedes peak JNK translocation [56]. In mice and primary mouse hepatocytes, GSK-3β silencing and MLK3 deletion both significantly protect against APAP toxicity [56,57]. In both cases, JNK phosphorylation, especially in the early phase, is blocked [56,57]. Also, GSH recovery is enhanced due to elevated activities of glutamate-cysteine ligase (GCL), the rate-limiting enzyme of GSH synthesis. Moreover, loss of Mcl-1, an anti-apoptotic protein and also a substrate for GSK-3β, is prevented [56,57]. Additional experiments revealed that loss of MLK3 attenuates GSK-3β activation, indicating potential interplay between the two [57]. Although GSK-3β is upstream of JNK, there is a feed-forward mechanism between JNK and GSK-3β, as late but not early GSK-3β activation is attenuated after JNK inhibitor treatment in mice [56]. Together, GSK-3β and MLK3 work in concert to regulate initial JNK activation in an amplification loop, and the blockade of any step in the loop is sufficient to limit JNK phosphorylation and provide protection against APAP hepatotoxicity.
Phosphatases are also activated and counteract MAPK phosphorylation (Figure 2). These phosphatases include serine/threonine, tyrosine and dual-specificity phosphatases (DUSP) [62,63]. One such negative regulator of JNK activation is mitogen-activated protein kinase phosphatase (Mkp)-1, which belongs to the DUSP family [62,63]. Mkp1-deficient mice develop significantly more severe injury after APAP as shown by higher ALT levels, increased necrotic area as well as elevated mortality [64]. In parallel, loss of Mkp-1 prolongs and enhances JNK activation. Collectively, the aggravated phenotype suggests a beneficial role of Mkp-1 in APAP-induced liver injury, most likely through inhibiting JNK phosphorylation [64]. Another phosphatase is protein tyrosine phosphatase 1B (PTP1B). PTP1B is a non-transmembrane classical PTP which modulates the phosphorylation status of insulin receptors, leptin receptors, EGFR, PDGFR as well as insulin-like growth factor-I receptor (IGFIR) [65–68]. Although PTP1B is not a direct phosphatase of JNK, recent data suggest an important role of PTP1B in APAP-induced liver damage and indicates a potential crosstalk with JNK [69]. PTP1B is upregulated in liver samples from APAP overdose patients and in APAP-treated primary human hepatocytes [69]. Surprisingly, PTP1B−/− mice actually have decreased JNK activation and are protected against APAP-induced liver damage [69]. PTP1B can indirectly activate GSK3β, an upstream kinase of JNK, to initiate Nrf2 degradation. Therefore, PTP1B deficiency may lead to prolonged Nrf2 accumulation in the nucleus and enhanced antioxidant response [69]. Further studies demonstrated that JNK and PTP1B converge on insulin signaling, based on the fact that JNK and PTP1B both negatively regulate insulin signaling at least in part through insulin receptor substrate (IRS-1) after APAP [65,70,71]. Consistent with that, an insulin sensitizer rosiglitazone reduces PTP1B protein expression and inhibits JNK activation and IRS phosphorylation after APAP [70]. With that, it is possible that PTP1B modulates APAP-induced liver injury through GSK3β, IRS and JNK, suggesting an association between APAP and insulin resistance.
4.4 Other interventions in JNK signaling
Besides direct JNK inhibitors and indirect JNK inhibition by upstream kinases or downstream phosphatases, various agents have also been reported to affect the injury by altering JNK activation. Among these, the gap junction inhibitor 2-aminoethoxy-diphenyl-borate (2-APB) was shown to moderately protect mice against the injury partially by reducing JNK activation [72]. The selective receptor-interacting protein kinase 1 (RIP1) inhibitor necrostatin-1, exogenous caspase recruitment domain (ARC) protein and the classical protein kinase C (PKC) inhibitor Go6976 also decreased JNK activation and offered significant protection [73–75]. RIP3-deficient mice are also protected against APAP and it has been suggested that RIP3 acts upstream of mitochondria and may affect JNK activation [76]. However, a recent paper, although supporting a role of RIP1 in JNK activation, argued against RIP3 signaling due to the suggestion that RIP3 is only present in non-parenchymal cells [77]. The authors also provided evidence that RIP1 translocates to the mitochondria independent of the anchor protein Sab (SH3 domain-binding protein that preferentially associates with Btk), which is a mandatory mitochondrial anchor for JNK [78]. Interestingly, allopurinol, a well-known drug used for gout and hyperuricemia, has also been reported to protect mice against APAP-induced liver injury, but only decreased late JNK activation (6 h post-APAP) without effect on early JNK activation (2 h post-APAP) [79]. This pattern of effect on JNK activation after APAP treatment was also seen in ASK1−/− mice and in female mice, which were less susceptible to the toxicity than males [41,49]. These studies suggest that the duration of JNK activation is important in APAP hepatotoxicity. However, it also needs to be considered that JNK activation is not just “on or off” but the absolute levels of P-JNK relative to its target, e.g., mitochondria, may be critical for its effect. The generally used western blot analysis of JNK and P-JNK protein levels may not be sensitive enough to capture all changes that may still have a functional impact.
Although the experimental evidence supporting the vital role of JNK in APAP hepatotoxicity seems to be quite conclusive, more and more studies have emerged in recent years to challenge the established concept. Many compounds including estradiol, broad-spectrum PKC inhibitors (Ro-31-8245, Go6983), BGP-15 (PARP inhibitor), 4-phenylbutyrate, and resveratrol have been shown to effectively protect mice against liver injury without reducing JNK activation [75,80–82]. In addition, although mice deficient in thioredoxin reductase, CCAAT-enhancer-binding protein homologous protein (CHOP), or M1 muscarinic receptors also displayed decreased injury, JNK activation was not altered [83–85]. Also, while quinone oxidoreductase 1 (NQO1) deficient mice had increased liver injury, no increased JNK activation was detected compared to WT mice [86]. Another piece of confounding evidence was that, although major mechanistic features of APAP-induced cell death in the human HepaRG cell line are the same as in rodents and humans [87], no JNK activation was seen after APAP treatment in that cell line, and a JNK inhibitor even aggravated the cell death rather than prevented it [27]. Importantly, this was not due to the fact that this cell line does not contain JNK or JNK could not be activated, since treatment with furosemide, a well-known loop diuretic, did induce JNK activation in this cell line [88]. Although we cannot rule out the possibility that signaling mechanisms in hepatoma cells might differ from normal human hepatocytes, another explanation could be that APAP-induced injury can progress independent of JNK activation. Overall, these findings are not completely unexpected. The JNK pathway is clearly an amplification loop; not all cells under all circumstances may need it. In addition, it was hypothesized that APAP overdose may induce liver injury through JNK-dependent and -independent signaling pathways [75]. In support of this, it has been reported that biological functions of JNK are context-dependent, and intracellular signaling pathways after APAP overdose can vary depending on the doses [24,43]. A dose of 300 mg/kg APAP may induce liver injury through JNK-dependent pathways in WT mice, which was supported by the close correlation between levels of JNK activation and ALT values [41]. However, under conditions of severe stress (such as 600 mg/kg APAP), mitochondrial dysfunction may be overwhelming and cell death may occur independent of JNK-mediated MPT pathway. This was exemplified by the varied effect of cyclophilin D-deficiency on the toxicity after different doses of APAP. With 200 mg/kg APAP, mice deficient of cyclophilin D, which is a key regulator of MPT pore formation, were protected with alleviated JNK activation [89]. However, under the conditions of 600 mg/kg APAP, knock-out mice displayed similar JNK activation, developed MPT and severe necrosis as WT animals [90]. The hepatoprotective effect of JNK inhibitor SP600125 was also lost, suggesting that the JNK signaling pathway may not be critically involved in this context, i.e., severe cell stress and cyclophilin D deficiency [90]. Another explanation is that the effect of JNK on APAP hepatotoxicity relies not only on its activation, but also the downstream effects of P-JNK after its binding to mitochondria. Therefore, it is possible that interventions countering the downstream events of JNK activation, such as MPT, endonuclease release or DNA fragmentation could still protect against the injury without attenuating the upstream JNK activation. Indeed, broad-spectrum PKC inhibitors (Ro-31-8245, Go6983) protected against the injury by enhancing autophagy despite sustained JNK activation [75]. Removal of damaged by mitochondria by autophagy has been shown to limit APAP-induced cell death [91]. Chemicals such as BGP-15, 4-phenylbutyrate and resveratrol protected mice against liver injury via inhibiting the downstream AIF release and DNA fragmentation without alleviating JNK activation [80–82]. Thus, JNK is needed for amplification under certain but not all conditions (dependent on dose, model system, etc). In addition, since JNK activation is not the final step in the pathophysiology, interventions can protect against downstream events without affecting JNK activation. However, this is no proof against the relevance of JNK in APAP hepatotoxicity.
5. Conclusions
Currently, NAC is the only available clinical antidote for APAP poisoning, which achieves greatest efficacy for early presenting patients. Therefore, novel therapeutic agents are clearly needed. JNK has emerged as a promising therapeutic target for APAP hepatotoxicity in recent studies. However, controversy and confusion still exist in the literature. Initial studies from mice with genetic interventions suggested a detrimental role of JNK in the toxicity. However, these results have not been consistently reproducible. Genetic differences in mouse substrains and possible compensatory effects of JNK isoforms may explain some contradictory findings. JNK inhibition (direct JNK inhibitors or upstream kinases and phosphatases) studies also support an important role of JNK in APAP hepatotoxicity. However, caveats should be noted when interpreting these data, especially possible off-target effects of the inhibitors. A few recent findings indirectly challenge the concept of JNK as pro-injury signal. This may be explained if APAP-induced liver injury can be JNK-dependent or –independent (depending upon dose used and other factors) or if initial JNK activation is less important than prolonged activation. We conclude that JNK appears to be a promising therapeutic target, but that more research is needed to better understand when JNK inhibition is beneficial before moving into the clinic.
6. Expert Opinion on the role of JNK in APAP Hepatotoxicity
JNK signaling has been shown to be critical in liver injury caused by numerous etiologies, including I/R injury, fibrosis, HCC and NASH, and inhibition of JNK is emerging as an attractive therapeutic strategy for these diseases [24]. Intriguingly, various JNK inhibitors have been discovered and some of them, such as SP600125 and D-JNKI1, have demonstrated effectiveness in preclinical studies, and CC-930 has even gone into clinical trial [22,92–94]. Similar to these findings, many previous studies using JNK-deficient mice or JNK inhibitors also supported a vital role of JNK in APAP hepatotoxicity. Since currently the treatment of APAP poisoning is still limited to NAC, which is most effective in those patients presenting early, JNK has been suggested to be a therapeutic target in APAP overdose patients [25–31]. However, most of the positive findings were derived from studies with genetic or pharmaceutical interventions of JNK, and off-target effects of KO mice and JNK inhibitors have not been fully considered. The development of more specific, water soluble JNK inhibitors would certainly be helpful in clarifying these problems. In addition, many details of the JNK signaling pathway are still unknown. It was suggested that an initial oxidant stress after APAP overdose causes JNK activation in cytoplasm and consequent mitochondrial translocation. However, most of the oxidant stress in APAP hepatotoxicity develops in mitochondria, and the oxidant stress in cytoplasm is minimal [95]. Therefore, from where this initial oxidant stress comes and how it activates JNK in the cytosol is not yet known. The connection between mitochondrial P-JNK and the MPT is also not fully understood. The effect of JNK activation and translocation to the mitochondria appears to be dependent on the presence of Sab in the mitochondria, which is the binding target of JNK but not RIP1 [77,78]. Because sustained JNK activation was suggested to be necessary for the development of toxicity [28,70], while transient JNK activation is normally beneficial for cells [96,97], we also need to explore how the balance between transient activation and sustained activation is regulated in APAP toxicity. Another critical issue for JNK would be its pathophysiological relevance in APAP overdose patients. Since liver biopsies are rarely taken from APAP overdose patients in the clinic, some basic questions such as the time course of JNK activation and mitochondrial translocation still remain unknown. Primary human hepatocytes are emerging as a clinically relevant model for study of APAP hepatotoxicity that can help us to answer some of these questions [27]. Although JNK activation was maintained up to 24 h post-APAP in human hepatocytes, JNK inhibition given 3 h post-APAP was only partially effective, while the clinical antidote NAC displayed complete protection against the toxicity when administered as late as 6 h [27]. This direct comparison of their effectiveness indeed does not support the use of SP600125 in clinical trials. However, the low efficacy might due to the fact that the dose of the JNK inhibitor SP600125 used in this study was limited by its solubility [27]. Therefore, other direct or indirect interventions in JNK signaling may still be worth trying in this model.
HIGHLIGHTS.
Acetaminophen (APAP) overdose is the leading cause of acute liver failure in the US.
JNK has emerged as a promising therapeutic target for APAP poisoning in recent years.
Initial studies suggested a detrimental role of JNK in the toxicity, but this concept has also been challenged.
Controversies and contradictions in the literature are addressed and clarified.
More in-depth studies are needed before JNK can be considered as a therapeutic target for APAP poisoning.
Primary human hepatocytes are a valuable model for testing the relevance of JNK signaling in human APAP toxicity.
Acknowledgments
H Jaeschke is supported by the NIH (R01 DK102142, R01 AA12916 and 8 P20 GM103549-07) and MR McGill is supported in part by an NIH fellowship from the “Training Program in Environmental Toxicology” (T32 ES007079-26A2).
Abbreviations
- AIF
apoptosis-inducing factor
- ALT
alanine aminotransferase
- APAP
acetaminophen
- 2-APB
2-aminoethoxy-diphenyl-borate
- ARC
apoptosis repressor with caspase recruitment domain
- ASK1
apoptosis signal-regulating kinase 1
- CHOP
CCAAT-enhancer-binding protein homologous protein
- DILI
drug-induced liver injury
- DMSO
dimethyl sulfoxide
- EndoG
endonuclease G
- GSH
glutathione
- GSK-3β
glycogen synthase kinase-3β
- JNK
c-jun N-terminal kinase
- LEF
leflunomide
- MLK3
mixed-lineage kinase 3
- MPT
mitochondrial permeability transition
- MAPK
mitogen-activated protein kinase
- MKK
MAPK kinase kinase
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- Nnt
nicotinamide nucleotide transhydrogenase
- PARP
poly ADP ribose polymerase
- PHH
primary human hepatocytes
- PKC
Protein kinase C
- PTP1B
protein tyrosine phosphatase 1B
- RIP1
receptor-interacting protein kinase 1
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Krenzelok EP. The FDA Acetaminophen Advisory Committee Meeting-what is the future of acetaminophen in the United States? The perspective of a committee member Clin Toxicol. 2009;47:784–9. doi: 10.1080/15563650903232345. [DOI] [PubMed] [Google Scholar]
- 2.Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40:585–92. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Manthripragada AD, Zhou EH, Budnitz DS, et al. Characterization of acetaminophen overdose-related emergency department visits and hospitalizations in the United States. Pharmacoepidemiol Drug Saf. 2011;20:819–26. doi: 10.1002/pds.2090. [DOI] [PubMed] [Google Scholar]
- 4.Mowry JB, Spyker DA, Cantilena LR, et al. 2012 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol. 2013;51:949–1229. doi: 10.3109/15563650.2013.863906. [DOI] [PubMed] [Google Scholar]
- 5.Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2014;89:193–9. doi: 10.1007/s00204-014-1432-2. [DOI] [PubMed] [Google Scholar]
- 6.McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30:2174–87. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen SD, Pumford NR, Khairallah EA, et al. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- 8.Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3′-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9. [PubMed] [Google Scholar]
- 9.Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93:378–87. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- 10.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–9. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 11.Cover C, Mansouri A, Knight TR, et al. Peroxynitrite-induced mitochondrial and endonuclease - mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–87. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 12.Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-induced factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–25. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- 13.Bajt ML, Ramachandran A, Yan HM, et al. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.McGill MR, Sharpe MR, Williams CD, et al. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–83. doi: 10.1172/JCI59755. First evidence of mitochondrial damage in APAP hepatotoxicity in patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polson J, Lee WM AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–97. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 16.Corcoran GB, Wong BK. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. J Pharmacol Exp Ther. 1986;238:54–61. [PubMed] [Google Scholar]
- 17.Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–75. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- 18.Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–54. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–62. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 20.Xie Y, McGill MR, Cook SF, et al. Time course of acetaminophen-protein adducts and acetaminophen metabolites in circulation of overdose patients and in HepaRG cells. Xenobiotica. 2015;14:1–9. doi: 10.3109/00498254.2015.1026426. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–48. doi: 10.1016/j.cld.2007.06.006. An excellent review on APAP hepatotoxicity that demonstrates the need to develop new therapeutic options for late presenting patients. [DOI] [PubMed] [Google Scholar]
- 22.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 23.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;3:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 24.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–20. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Gunawan BK, Liu ZX, Han D, et al. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–78. doi: 10.1053/j.gastro.2006.03.045. First described the potential detrimental role of JNK in a murine model of APAP hepatotoxicity. [DOI] [PubMed] [Google Scholar]
- 26••.Henderson NC, Pollock KJ, Frew J, et al. Critical role of c-jun (NH2) terminal kinase in paracetamol-induced acute liver failure. Gut. 2007;56:982–90. doi: 10.1136/gut.2006.104372. First described the existence of JNK activation in human liver with APAP hepatotoxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Xie Y, McGill MR, Dorko K, et al. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol. 2014;279:266–74. doi: 10.1016/j.taap.2014.05.010. Suggested a role of JNK in the progression of injury in primary human hepatocytes while not in HepaRG cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Hanawa N, Shinohara M, Saberi B, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–77. doi: 10.1074/jbc.M708916200. First described P-JNK translocation to mitochondria in APAP hepatotoxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplowitz N, Shinohara M, Liu ZX, Han D. How to protect against acetaminophen: don’t ask for JUNK. Gastroenterology. 2008;135:1047–51. doi: 10.1053/j.gastro.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Bourdi M, Korrapati MC, Chakraborty M, et al. Protective role of c-Jun N-terminal kinase 2 in acetaminophen-induced liver injury. Biochem Biophys Res Commun. 2008;374:6–10. doi: 10.1016/j.bbrc.2008.06.065. Challenged the previous studies regarding the detrimental role of JNK in APAP hepatotoxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–6. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 34••.Bourdi M, Davies JS, Pohl LR. Mispairing C57BL/6 substrains of genetically engineered mice and wild-type controls can lead to confounding results as it did in studies of JNK2 in acetaminophen and concanavalin A liver injury. Chem Res Toxicol. 2011;24:794–6. doi: 10.1021/tx200143x. Showed that off-target genetic differences between the mouse substrains result in the confounding data in previous studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuan CY, Yang DD, Samanta Roy DR, et al. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–76. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 36.Cubero FJ, Hu W, Zhao G, et al. Dual function of Jnk1 and Jnk2 in hepatocytes is indispensable against drug-induced liver injury (abstract) Hepatology. 2014;40(Suppl):710A. [Google Scholar]
- 37.Bennett BL, Sasaki DT, Murray BW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae MA, Pie JE, Song BJ. Acetaminophen induces apoptosis of C6 glioma cells by activating the c-Jun NH2-terminal protein kinase-related cell death pathway. Mol Pharm. 2001;60:847–56. [PubMed] [Google Scholar]
- 39.Matsumaru K, Ji C, Kaplowitz N. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology. 2003;37:1425–34. doi: 10.1053/jhep.2003.50230. [DOI] [PubMed] [Google Scholar]
- 40•.Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–21. doi: 10.1002/hep.21475. Showed the protective effect of another JNK inhibitor leflunomide in APAP hepatotoxicity. [DOI] [PubMed] [Google Scholar]
- 41.Du K, Williams CD, McGill MR, Jaeschke H. Lower susceptibility of female mice to acetaminophen hepatotoxicity: Role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol Appl Pharmacol. 2014;281:58–66. doi: 10.1016/j.taap.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masson MJ, Carpenter LD, Graf ML, Pohl LR. Pathogenic role of natural killer T and natural killer cells in acetaminophen-induced liver injury in mice is dependent on the presence of dimethyl sulfoxide. Hepatology. 2008;48:889–97. doi: 10.1002/hep.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhushan B, Walesky C, Manley M, et al. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Path. 2014;184:3013–25. doi: 10.1016/j.ajpath.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGill MR, Lebofsky M, Norris HR, et al. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269:240–9. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macanas-Pirard P, Yaacob NS. Glycogen synthase kinase-3 mediates acetaminophen-induced apoptosis in human hepatoma cells. J Pharmacol Exp Ther. 2005;313:780–9. doi: 10.1124/jpet.104.081364. [DOI] [PubMed] [Google Scholar]
- 46.Latchoumycandane C, Seah QM, Tan RC, et al. Leflunomide or A77 1726 protect from acetaminophen-induced cell injury through inhibition of JNK-mediated mitochondrial permeability transition in immortalized human hepatocytes. Toxicol Appl Pharmacol. 2006;217:125–33. doi: 10.1016/j.taap.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Tan SC, New LS, Chan EC. Prevention of acetaminophen (APAP)-induced hepatotoxicity by leflunomide via inhibition of APAP biotransformation to N-acetyl-p-benzoquinone imine. Toxicol Lett. 2008;180:174–81. doi: 10.1016/j.toxlet.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Gupta R, Bhatia J, Gupta SK. Risk of hepatotoxicity with add-on leflunomide in rheumatoid arthritis patients. Arzneimittel-Forschung. 2011;61:312–6. doi: 10.1055/s-0031-1296204. [DOI] [PubMed] [Google Scholar]
- 49•.Nakagawa H, Maeda S, Hikiba Y, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–21. doi: 10.1053/j.gastro.2008.07.006. First evidence that ASK1 is critical for JNK activation and toxicity in APAP hepatotoxicity. [DOI] [PubMed] [Google Scholar]
- 50.Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 51.Takeda K, Noguchi T, Naguro I, Ichijo H. Apoptosis signal-regulating kinase 1 in stress and immune response. Annu Rev Pharmacol Toxicol. 2008;48:199–225. doi: 10.1146/annurev.pharmtox.48.113006.094606. [DOI] [PubMed] [Google Scholar]
- 52.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noguchi T, Takeda K, Matsuzawa A, et al. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem. 2005;280:37033–40. doi: 10.1074/jbc.M506771200. [DOI] [PubMed] [Google Scholar]
- 54.Xie Y, Ramachandran A, Breckenridge DG, et al. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2015;286:1–9. doi: 10.1016/j.taap.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwabe RF, Bradham CA, Uehara T, et al. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–32. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- 56.Shinohara M, Ybanez MD, Win S, et al. Silencing glycogen synthase kinase-3β inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285:8244–55. doi: 10.1074/jbc.M109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–7. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welsh GI, Wilson C, Proud CG. GSK3: a SHAGGY frog story. Trends Cell Biol. 1996;6:274–9. doi: 10.1016/0962-8924(96)10023-4. [DOI] [PubMed] [Google Scholar]
- 59.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 61.Lotharius J, Falsig J, van Beek J, et al. Progressive degeneration of human mesencephalic neuron-derived cells triggered by dopamine-dependent oxidative stress is dependent on the mixed-lineage kinase pathway. J Neurosci. 2005;25:6329–42. doi: 10.1523/JNEUROSCI.1746-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–92. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Liu Y. Regulation of innate immune response by MAP kinase phosphatase-1. Cell Signal. 2007;19:1372–82. doi: 10.1016/j.cellsig.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wancket LM, Meng X, Rogers LK, Liu Y. Mitogen-activated protein kinase phosphatase (Mkp)-1 protects mice against acetaminophen-induced hepatic injury. Toxicol Pathol. 2012;0:1095–105. doi: 10.1177/0192623312447551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salmeen A, Andersen JN, Myers MP, et al. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol Cell. 2000;6:1401–1412. doi: 10.1016/s1097-2765(00)00137-4. [DOI] [PubMed] [Google Scholar]
- 66.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, et al. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 67.Haj FG, Markova B, Klaman LD, et al. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J Biol Chem. 2003;278:739–744. doi: 10.1074/jbc.M210194200. [DOI] [PubMed] [Google Scholar]
- 68.Buckley DA, Cheng A, Kiely PA, et al. Regulation of insulin-like growth factor type I (IGF-I) receptor kinase activity by protein tyrosine phosphatase 1B (PTP-1B) and enhanced IGF-I-mediated suppression of apoptosis and motility in PTP-1B-deficient fibroblasts. Mol Cell Biol. 2002;22:1998–2010. doi: 10.1128/MCB.22.7.1998-2010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mobasher MA, González-Rodriguez A, Santamaría B, et al. Protein tyrosine phosphatase 1B modulates GSK3β/Nrf2 and IGFIR signaling pathways in acetaminophen-induced hepatotoxicity. Cell Death Dis. 2013;4:e626. doi: 10.1038/cddis.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mobasher MA, de Toro-Martín J, González-Rodríguez Á, et al. Essential role of protein-tyrosine phosphatase 1B in the modulation of insulin signaling by acetaminophen in hepatocytes. J Biol Chem. 2014;289:29406–19. doi: 10.1074/jbc.M113.539189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aguirre V, Uchida T, Yenush L, et al. The c-Jun NH (2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser (307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 72.Du K, Williams CD, McGill MR, et al. The gap junction inhibitor 2-aminoethoxy-diphenyl-borate protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes and c-jun N-terminal kinase activation. Toxicol Appl Pharmacol. 2013;273:484–91. doi: 10.1016/j.taap.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang YF, He W, Zhang C, et al. (2014) Role of receptor interacting protein (RIP) 1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicol Lett. 2014;225:445–53. doi: 10.1016/j.toxlet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 74.An J, Mehrhof F, Harms C, et al. ARC is a novel therapeutic approach against acetaminophen-induced hepatocellular necrosis. J Hepatol. 2013;58:297–305. doi: 10.1016/j.jhep.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 75•.Saberi B, Ybanez MD, Johnson HS, et al. Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and-independent signaling pathways. Hepatology. 2014;59:1543–54. doi: 10.1002/hep.26625. Showed that APAP can induce liver injury through JNK-dependent and -independent signaling pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramachandran A, McGill MR, Xie Y, et al. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dara L, Johnson H, Suda J, et al. Receptor Interacting protein kinase-1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology. 2015 doi: 10.1002/hep.27939. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Win S, Than TA, Han D, et al. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–8. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams CD, McGill MR, Lebofsky M, et al. Protection against acetaminophen-induced liver injury by allopurinol is dependent on aldehyde oxidase-mediated liver preconditioning. Toxicol Appl Pharmacol. 2014;274:417–24. doi: 10.1016/j.taap.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagy G, Szarka A, Lotz G, et al. BGP-15 inhibits caspase-independent programmed cell death in acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2010;243:96–103. doi: 10.1016/j.taap.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 81.Shimizu D, Ishitsuka Y, Miyata K, et al. Protection afforded by pre-or post-treatment with 4-phenylbutyrate against liver injury induced by acetaminophen overdose in mice. Pharmacol Res. 2014;87:26–41. doi: 10.1016/j.phrs.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 82.Du K, McGill MR, Xie Y, et al. Resveratrol prevents protein nitration and release of endonucleases from mitochondria during acetaminophen hepatotoxicity. Food Chem Toxicol. 2015;81:62–70. doi: 10.1016/j.fct.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uzi D, Barda L, Scaiewicz V, et al. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J Hepatol. 2013;59:495–503. doi: 10.1016/j.jhep.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 84.Patterson AD, Carlson BA, Li F, et al. Disruption of thioredoxin reductase 1 protects mice from acute acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. Chem Res Toxicol. 2013;26:1088–96. doi: 10.1021/tx4001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Urrunaga NH, Jadeja RN, Rachakonda V, et al. M1 muscarinic receptors modify oxidative stress response to acetaminophen-induced acute liver injury. Free Radic Biol Med. 2015;78:66–81. doi: 10.1016/j.freeradbiomed.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hwang JH, Kim YH, Noh JR, et al. The protective role of NAD (P) H: quinone oxidoreductase 1 on acetaminophen-induced liver injury is associated with prevention of adenosine triphosphate depletion and improvement of mitochondrial dysfunction. Arch Toxicol. 2014 Sep 17; doi: 10.1007/s00204-014-1340-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 87.McGill MR, Yan HM, Ramachandran A, et al. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–82. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McGill MR, Du K, Xie Y, et al. The role of the c-Jun N-terminal kinases 1/2 and receptor-interacting protein kinase 3 in furosemide-induced liver injury. Xenobiotica. 2015;45:442–449. doi: 10.3109/00498254.2014.986250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramachandran A, Lebofsky M, Baines CP, et al. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radical Res. 2011;45:156–64. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.LoGuidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology. 2011;54:969–78. doi: 10.1002/hep.24464. [DOI] [PubMed] [Google Scholar]
- 91.Ni HM, Bockus A, Boggess N, et al. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–32. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hui L, Zatloukal K, Scheuch H, et al. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118:3943–53. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Plantevin Krenitsky V, Nadolny L, Delgado M, et al. Discovery of CC-930, an orally active anti-fibrotic JNK inhibitor. Bioorg Med Chem Lett. 2012;22:1433–8. doi: 10.1016/j.bmcl.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 94.Stebbins JL, De SK, Machleidt T, et al. Identification of a new JNK inhibitor targeting the JNK-JIP interaction site. Proc Natl Acad Sci. 2008;105:16809–13. doi: 10.1073/pnas.0805677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95•.Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Toxicol. 1990;255:935–41. Demonstrated that mitochondria are the major source of oxidative stress in APAP hepatotoxicity. [PubMed] [Google Scholar]
- 96.Czaja MJ. Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis. 2007;27:378–89. doi: 10.1055/s-2007-991514. [DOI] [PubMed] [Google Scholar]
- 97.Lamb JA, Ventura JJ, Hess P, et al. JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell. 2003;11:1479–89. doi: 10.1016/s1097-2765(03)00203-x. [DOI] [PubMed] [Google Scholar]