Abstract
INTRODUCTION
Concomitant opioid abuse is a serious problem among patients receiving methadone maintenance treatment (MMT) for opioid use disorder. This is an exploratory study that aims to identify predictors of the length of time a patient receiving MMT for opioid use disorder remains abstinent (relapse-free).
METHODS
Data were collected from 250 MMT patients enrolled in addiction treatment clinics across Southern Ontario. The impact of certain clinical and socio-demographic factors on the outcome (time until opioid relapse) was determined using a Cox proportional hazard model.
RESULTS
History of injecting drug use behavior (hazard ratio (HR): 2.26, P = 0.042), illicit benzodiazepine consumption (HR: 1.07, P = 0.002), and the age of onset of opioid abuse (HR: 1.10, P < 0.0001) are important indicators of accelerated relapse among MMT patients. Conversely, current age is positively associated with duration of abstinence from illicit opioid use, serving as a protective factor against relapse (HR: 0.93, P = 0.003).
CONCLUSION
This study helps to identify patients at increased risk of relapse during MMT, allowing health care providers to target more aggressive adjunct therapies toward high-risk patients.
Keywords: opioid use disorder, substance abuse, methadone, opioid substitution treatment, opioid relapse
Introduction
Opioids are commonly prescribed medications for the management of pain.1 USA and Canada are the world’s highest consumers of prescription opioids.2 In Canada, there has been a 203% increase in the prescription-use of opioids between 2000 and 2010.3 According to a national survey, 16.7% of the general Canadian population aged 15 years or older were reported to use prescription opioids for pain relief in 2011.4 Despite their indication for pain relief, these medications are also highly liable for abuse and addiction.5 In 2012, 2.1 million people were estimated to suffer from opioid use disorder (OUD) secondary to prescription-use in the USA alone.6 In addition to those acquiring it through physician prescriptions, a significant number of patients with OUD obtain illicit opioids strictly from the “streets”.7 The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, defines OUD as a chronic psychiatric disorder characterized by persistent use of opioids, increased tolerance, repeated withdrawal symptoms, and sustained behavioral changes.8,9 OUD poses harmful consequences to both individuals and society, including infections (hepatitis and human immunodeficiency virus (HIV)), social adverse consequences, criminal activities, and death.10–18 The prevalence of OUD is rising worldwide.19 In 2010, 26–36 million people were estimated to suffer from OUD, leading to 9.2 million global disability-adjusted life years,16 which is a 73% increase from 1990.19 A recent investigation based on American, Australian, and Canadian data has projected that the total cost of opioid addiction hovers around €2,627 to €60,665 per-person per-year, with €21,904 per-person per-year being the most generalizable estimate accounting for the most comprehensive scope of costs.20
Methadone maintenance treatment (MMT) is the most commonly used intervention for OUD patients, consisting of supervised prescription of methadone, a long-acting synthetic opioid, to alleviate withdrawal symptoms and reduce drug-seeking behavior.21 Most studies evaluating the efficacy of MMT have focused on identifying risk factors that are negatively associated with treatment retention, including younger age and criminal justice involvement.22–30 However, relapse is a common problem among OUD patients, with many patients continuing to use illicit opioids during and after MMT, irrespective of whether this is preceded by an initial period of abstinence or not.26,31,32 In fact, concomitant use of illicit opioids in combination with MMT stands as the largest risk factor for increasing the incidence of abnormal cardiac conductivity,33,34 overdose,35–37 and death.35,37,38 In addition to its health implications, abstaining from illicit opioid use is a patient-important outcome, as many patients seek MMT in order to overcome their addiction and improve other aspects of their lives, such as by enhancing social functioning, maintaining a job, and regaining custody over their children.4 Evidently, treatment retention alone in the presence of continued opioid abuse is a limited measure of treatment response. Additionally, it has been shown that patients who continue to use illicit drugs during MMT are three times more likely to drop out of treatment and relapse post-treatment.26,39 As such, it seems reasonable that treatment should also be tailored toward lowering the risk of continued opioid abuse (relapse of OUD) during treatment, so as to improve treatment outcomes, as well as reduce the risk of detrimental side effects associated with concomitant use of illicit opioids during MMT.26,33–36,39
However, there is a paucity of research focusing on the duration of abstinence from illicit opioid use during MMT, and data from the available studies are insufficient to identify predictors of the length of time a patient remains relapsefree during MMT.2,9,40 For instance, a lower methadone dose and male sex have been associated with increased frequency of opioid-positive urine samples during MMT.2,9,40 Although this points to an association between the two variables, it does not account for the amount of time a patient remains abstinent before relapse.
Acknowledging that some patients will continue to use illicit opioids during their treatment course, since OUD is a chronic, remitting, and relapsing disorder, we seek to identify factors associated with a longer duration of abstinence from illicit opioids among relapsing patients. The objective of this exploratory study was to conduct survival analyses by evaluating key clinical and socio-demographic characteristics that serve as predictors of the length of time until opioid relapse among OUD patients on MMT. Variables assessed for inclusion in our exploratory model were current age, sex, marital status, employment, smoking status, methadone dose, age of onset of opioid abuse, source of obtaining opioids, duration of MMT, injecting drug use behavior, hepatitis C status, chronic pain, diabetes, and days of illicit benzodiazepine, cocaine, and cannabis use over the last month prior to enrollment into our study.
Methods
This study utilized patient data collected for the Genetics of Opioid Addiction (GENOA) Research Collaborative between the Population Genomics Program at McMaster University and the Canadian Addiction Treatment Centre (CATC, formerly known as the Ontario Addiction Treatment Centre and home to the largest network of methadone clinics in North America) and the Peter Boris Centre for Addiction Research. The methods of the GENOA pilot study have been previously described.41 Briefly, the complete GENOA study is a prospective cohort investigation focusing on the genetic determinants of methadone treatment response. Participants were recruited from 13 clinical sites throughout southern Ontario, Canada. All clinical sites are managed centrally and follow the same treatment protocols. Information on medical history, methadone dose, duration of MMT, number of MMTs, and original source of opioid use were collected during a face-to-face interview with all study participants. The baseline assessment also included the M.I.N.I. International Neuropsychiatric Interview version 6.0,42 and Brief Pain Inventory to capture the severity and amount of interference pain has on a patient’s daily activities.43 Weekly urine drug screens were performed at fixed intervals throughout the study period as part of routine clinical care at the CATC recruitment sites using the iMDx™ Prep Assays.44 These assays identify mu-opioid receptor agonists and differentiate between specific types of opioids, such as synthetic (eg, oxycodone) and naturally occurring opioids (eg, heroin, detected as morphine).44
Study participants
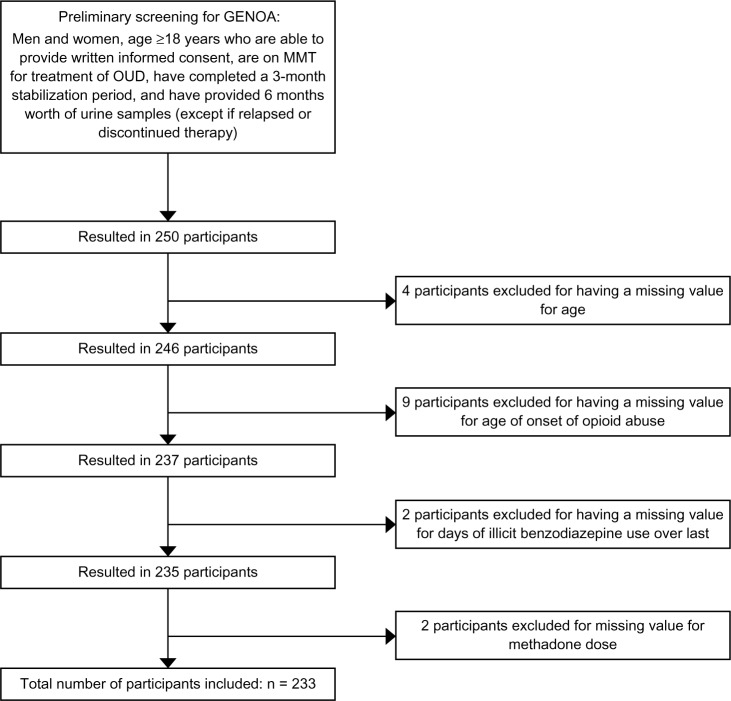
Patients presenting at 13 clinical sites throughout southern Ontario were approached consecutively by the clinical staff for involvement in our study. This study included a sample of 250 MMT patients. In order to be included in this study, participants must be 18 years of age or older, able to provide written informed consent, receiving MMT for OUD at the time of enrollment, and have completed a 3-month stabilization period on MMT. Additionally, patients were required to provide urine samples for at least 6 months, or until the point of opioid relapse, so as to allow for calculation of the primary study outcome, the time until opioid relapse. However, patients who discontinued therapy were not required to continue providing urine samples after discontinuation of MMT and were still included in the analyses. Of the 250 participants eligible for inclusion in this study, those with missing values for one or more of the covariates analyzed were dropped from the analysis (n = 17). Figure 1 shows the participant inclusion diagram.
Figure 1.
Eligibility screening and inclusion flow diagram for participant selection.
Statistical methods
We used descriptive statistics to summarize the participants’ demographic and baseline characteristics. Continuous variables were expressed using mean (standard deviation) and categorical variables using percentage. We employed t-tests (for continuous variables) and Pearson’s chi-square tests (for categorical variables) to compare participant characteristics between relapsing and nonrelapsing patients. The impact of certain factors on the survival outcome (time until opioid relapse) was determined using both visual and statistical methods. First, we compared the survival patterns of patients visually by assessing the Kaplan–Meier (KM) curves for all categorical and binary variables.45 We then compared the differences between the groups statistically by employing log-rank tests and a Cox proportional hazard (PH) model.2 We defined a relapse event as the use of illicit opioids while on MMT for the treatment of OUD. The study outcome, time until opioid relapse, was measured based on the first opioid-positive urine screen during the study’s 6-month follow-up period, from the point of entry into the study. Patients who did not experience the event (relapse) during the 6 months of follow-up or who had dropped out of therapy altogether were excluded from the analyses. It is worth noting that although patients have been on MMT for variable lengths of time at the point of inclusion, all analyses were adjusted for duration of MMT (months). Patients who were prescribed opioid medications while on MMT (for example, for management of chronic pain) were removed from all analyses to avoid bias of considering them as having relapsed, since their urine results will be positive for opioids.
We assessed 16 clinical and demographic characteristics that may serve as risk-predictors of time until opioid relapse in our exploratory model. This model was built using available clinical and demographic data, and the covariates included were as follows: current age, sex, marital status, employment, smoking status, methadone dose, age of onset of opioid abuse, source of obtaining opioids (eg, from prescription or street supply, the former referring to patients that developed OUD following an initial prescription for a pain-inducing condition, for example, following a musculoskeletal injury, where patients were first exposed to opioids and then continued use beyond the initial intended purpose of the opioid prescription), duration of MMT, injecting drug use behavior, hepatitis C status, chronic pain, diabetes, and days of illicit benzodiaz-epine, cocaine, and cannabis use over the last month prior to enrollment into our study. These are defined in the Supplementary materials. While maximum daily methadone dose (mg/day) is provided as a continuous variable, we have chosen to model it as a categorical covariate. Studies have shown better treatment responses, as measured by reduction in illicit opioid use, for instance, to be associated with a methadone dose of ≥80 mg/day.46 As such, for the sake of the analyses in this study, methadone dose was modeled as a binary predictor, split into two categories (<80 mg and ≥80 mg).
In order to build the Cox PH model, we evaluated all covariates using a backward stepwise selection with the significance level for removal from the model set at P = 0.20. We assessed the model using additional interaction terms for age and methadone dose (mg/day) (age × methadone dose), as well as age and hepatitis C status (age × hepatitis C). The interaction terms were selected as an exploratory means to evaluate the mediating effects of age on two important prognostic variables; methadone dose and hepatitis C status.47,48 Exploratory univariate Cox analyses were conducted on all included variables.
The PH assumption for the covariates selected for inclusion in the Cox PH model was evaluated using the KM and log–log survival curve visual assessments, as well as the Schoenfeld residuals tests.49 We evaluated the goodness of fit using the Cox–Snell residuals method.49 All analyses were completed using Stata 13.50
Results
Participant characteristics
A total of 233 participants were eligible for inclusion in our analyses. The mean age of participants was 38.64 years (SD = 10.9), with a mean age of onset of opioid abuse of 32.41 years (SD = 9.7). The correlation coefficient of these two variables was 0.85. Half of the participants were men (50.2%), and only 6.4% reported injecting drug use behavior in the month prior to enrollment into our study. Participants in our study had been on MMT for an average of 51.91 months (SD = 45.9). Those who relapsed were receiving MMT for a significantly shorter duration of time than participants who did not relapse (P = 0.0307). The mean methadone dose across all participants was 78.99 mg/day (SD = 39.6). The mean length of time participants remained abstinent throughout the 6 months of follow-up was 99.04 days (SD = 74.4). Use of cannabis, benzodiazepine, and cocaine over the last month was reported by 122 (48.8%), 17 (6.8%), and 17 (6.8%) participants, respectively. These illicit substances were specifically asked about (self-reported), as they are measured in the commonly used addiction assessment tool, the Maudsley addiction profile, for assessing treatment outcomes.51
Clinical and patient characteristics associated with shorter and longer opioid-free periods
The backward stepwise selection method outlined in the Statistical methods section identified current age, injecting drug use behavior, days of illicit benzodiazepine use over the last month, age of onset of opioid abuse, and the additional interaction terms for age and methadone dose (age × methadone dose) and age and hepatitis C status (age × hepatitis C) as significant prognostic characteristics impacting response to methadone treatment.
I. Modeling time until relapse (KM curves and log-rank test)
In this section, we present the KM curves separated by a past history of injecting drug use behavior, as it is the only categorical variable included in our final model. The vertical gap suggests that at any one point, the proportion of noninjecting drug users surviving (abstaining from illicit opioid use) was greater than that of injecting drug users. This is consistent across all time points as the KM curves do not visually cross over (Fig. 2). The log-rank test resulted in a chisquare statistic of 5.62 (P = 0.02), indicating that the difference between the survival curves is statistically significant.
Figure 2.
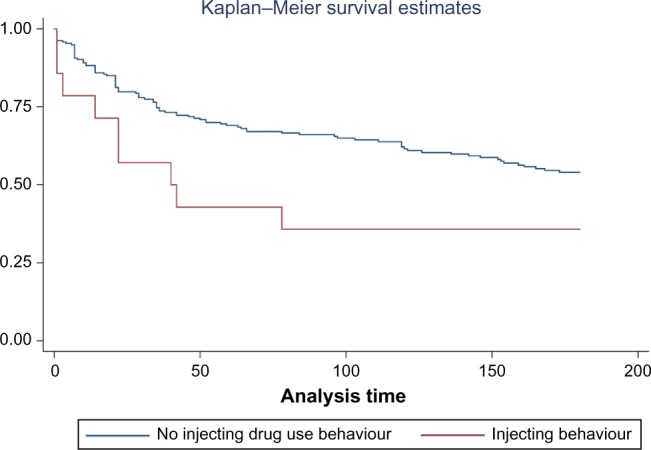
KM curve separated by past history of injecting drug use behavior, presented as probability of survival (no relapse) plotted against time until relapse in days.
Note: *The y-axis and x-axis labels are probability of survival and time until opioid relapse (days), respectively.
II. Modeling time until relapse (Cox PH model)
Results from our univariate Cox regression analysis show that illicit use of benzodiazepines 1 month prior to enrollment into the study (HR: 1.06, 95% confidence interval (CI): 1.01, 1.10, P = 0.009) and injecting drug use behavior (HR: 2.25, 95% CI: 1.12, 4.47, P = 0.022) are associated with earlier relapse.
Results from our multivariable analysis suggest that 1) participants with an injecting drug use history are at 2.26 times higher risk of relapsing during the methadone program than participants without such history (HR: 2.26, 95% CI: 1.03, 4.97, P = 0.042), 2) for every year increase in the age of onset of opioid abuse, the risk for relapse is 10% greater (HR: 1.10, 95% CI: 1.04, 1.15, P < 0.0001), 3) for every year increase in current age, the risk for relapse is 7% less (HR: 0.93, 95% CI: 0.89, 0.97, P = 0.003), and 4) for every day increase in reported illicit benzodiazepine use in the past month, there is a 7% increase in the risk for opioid relapse (HR: 1.07, 95% CI: 1.03, 1.12, P = 0.002). The significant interaction term (age × hepatitis C) suggests that the relationship between age and opioid relapse varies based on a patient's hepatitis C status (HR: 1.01, 95% CI: 1.00, 1.03, P = 0.020). We assessed the results of the stepwise backward selection, while adjusting for duration in MMT in a separate Cox regression model, and found that the results remained unchanged.
Evaluation of the KM curve for history of injecting drug use behavior (Fig. 2), the only categorical variable included in our analyses, the log–log survival curve, and the Schoenfeld residuals tests (Supplementary Fig. 1) reveal that our variables meet the PH assumption. Additionally, we evaluated the fit of the model using the Cox–Snell residuals method, wherein the model appeared to show a good fit for the data.
Discussion
Findings from this study suggest that injecting drug use behavior, illicit benzodiazepine consumption, and the age of onset of opioid abuse are important indicators for accelerated relapse among patients receiving MMT for treatment of OUD. These findings highlight clinical and socio-demographic characteristics that clinicians should consider when developing a treatment strategy for specific subpopulations of MMT patients. While acknowledging that patients will continue to abuse opioids throughout MMT is important, identification of the characteristics governing patients’ opioid abuse patterns is pivotal for proper risk stratification and treatment tailoring.
The fact that our analyses show that patients reporting injecting drug use behavior experience shorter periods of abstinence is understandable, as patients exhibiting injecting drug use behavior are typically more difficult to manage on MMT.52 This may largely be due to the fact that OUD patients who engage in injecting drug use behavior require higher levels of opioids and suffer from a more severe degree of social marginalization, which is inevitably more difficult to overcome.53,54 Patients with injecting drug use behavior are also among the highest risk patients in MMT, as they are more likely to engage in risky behavior, such as needle sharing and unsafe sex, thus increasing the probability of contracting viral infections such as HIV and hepatitis C.55,56 The nature of their risk behavior and its associated medical comorbidities, in addition to other aspects of social marginalization associated with injection drug use (such as homelessness and unemployment), are likely to contribute to the barriers encountered by these patients, when attempting to adhere to the MMT regimen.53–56 These findings are also supported by existing literature that has shown that the duration of abstinence from opioids while on treatment for OUD is shorter among those with injection drug use behavior and is inversely proportional to the frequency of injecting drug use.53,57–59
Our analyses also show that an increased use of illicit benzodiazepines in the month prior to enrollment into our study was associated with accelerated relapse. Several studies have found that benzodiazepines may be illicitly used concurrent to MMT in order to alleviate symptoms of physical (eg, tremor) and psychiatric (eg, insomnia and anxiety symptoms) comorbidities.39 In fact, those who suffer from more severe anxiety disorders have been shown to abuse longer acting benzodiazepines at higher doses and to experience more severe benzodiazepine withdrawal symptoms.60 As such, it is reasonable to believe that patients who abused benzodiazepines more frequently prior to study enrollment may suffer from more pronounced symptoms of anxiety and other comorbidities, for which they continue to self-medicate throughout MMT in order to alleviate their symptoms and ameliorate the more severe effects of withdrawal they experience.39,60 Therefore, use of benzodiazepines while receiving MMT should be carefully evaluated to ensure the identification of comorbid anxiety disorders and for providing treatment for these comorbid disorders and adjusting methadone dose accordingly (for example, to accommodate drug–drug interactions) to avoid OUD relapse.
Moreover, our study shows that an older age of onset of opioid abuse is associated with accelerated relapse. Current data studying this relationship are controversial.58,61 However, Hosseini et al have found a similar association between age of onset of opium abuse and relapse in a cohort of 100 patients receiving treatment for opium addiction (HR: 1.30, P = 0.046).61 This may be justified by the fact that patients who have experienced multiple treatment episodes are more likely to adhere to a subsequent treatment episode.62 As such, it is reasonable to believe that patients in our sample, who began abusing opioids at a younger age, may have been previously enrolled in addiction treatment programs, which may be having a positive impact on their current treatment.
The interaction variable between current age and hepatitis C status also appears to cause a contraction of survival time, suggesting that there are differences in survival time influenced by age across hepatitis groups. This has not been previously reported and suggests that age acts as a mediator among patients depending on their hepatitis C status, such that older age will increase risk for accelerated relapse among patients with hepatitis C and that such effect depreciates in patients without hepatitis C. It is important to note, however, that according to our analyses, these effects were found to be minimal. Nevertheless, these findings are relevant and suggest that patients with hepatitis C may benefit from close monitoring, particularly within older cohorts of methadone maintenance patients.
Furthermore, our model shows that an increase in current age has a protective effect against relapse. Data investigating the association between age and continued opioid use during MMT are limited. One study showed that younger patients on MMT were more likely to use illicit methadone than older patients (odds ratio: 8.93, 95% CI: 1.60, 49.72).63 Another study by Tran et al investigated the association between older age and abstinence during MMT, although this association was not significant (adjusted odds ratio: 0.97, 95% CI: 0.94, 1.00).64 An association that has been well documented in the literature is that between older age and retention in MMT.26,29,30,34,59,65 Provided that abstinence from illicit drug use during MMT serves as a predictor of retention in treatment, a parallel trend between abstinence and retention in treatment is expected.39 The results of our study have important implications for treatment outcomes in MMT patients. Specifically, clinicians managing patients with OUD should consider stratifying treatment groups depending on the age of onset of abuse, illicit benzodiazepine use, and injecting drug use behavior at baseline interviews. In doing so, they could target additional interventions such as adjunct psychosocial therapies toward high-risk patients, including enhanced outreach counseling, contingency management, and behavioral couples therapy.66,67 Although these therapies may be needed and are beneficial for all patients receiving MMT, such therapies may be costly and time consuming.66 As such, in a setting where resources are limited, they may be considered as targeted interventions for high-risk groups, such as patients with injecting drug use behavior, patients using illicit benzodiazepines, and those having an older age of onset of opioid abuse.
It is important that we acknowledge the potential limitations of the generalizability of our study. First, we acknowledge the fact that patients’ varied duration on MMT is a limitation of the GENOA study, provided that patients beginning MMT will likely abuse substances at a higher rate and possess a different treatment characteristic profile than those at a later stage in therapy. However, it was important for the purpose of this survival analysis that we only include patients who have been stabilized on MMT (ie, patients that have completed a 3-month stabilization period), so as to exclude patients who are likely to relapse as a result of breakthrough withdrawal symptoms. Recognizing that the adequate therapeutic dose is achieved following a tapering process, evaluating relapse among patients prior to stabilization will likely confound our ability to identify predictors among patients receiving therapeutic methadone doses. Upon applying the inclusion criteria described above, we are confident that our findings highlight the important predictors of relapse among patients receiving therapeutic doses of methadone.
Additionally, as this is an observational study, our findings may be subject to selection bias, whereby patients who agree to participate in our study may not necessarily represent the target population.68 More specifically, those who agree to participate tend to be healthier than the actual population being represented by the sample, as per the healthy volunteer bias.68 This would mean that our results underestimate the relapse rates in high-risk patients and that the need for adjunctive target therapies in these more marginalized patients with OUD would be greater. Nevertheless, we sought to compare demographic characteristics of participants in the GENOA study with those of a sample of CATC patients receiving MMT from four geographically and economically diverse locations. The demographic variables compared included the following: mean age, sex, mean methadone dose, percentage of HIV-positive patients, and marital status. Our results showed that there were no statistically significant differences in any of the aforementioned variables between our study participants and the population of patients attending the CATC clinical services for the treatment of OUD, with the exception of there being more women in the GENOA study (47%) compared to nonparticipant MMT patients (33%).69 It is also important to acknowledge that we were unable to include every possible covariate that may predict relapse in our analyses, as the power of our study is limited by our sample size. For instance, our study did not take into account the status of all comorbid medical or psychiatric disorders for which opioids may help in symptom mitigation and would therefore more likely be abused sooner for self-medication reasons.39 We did, however, attempt to account for any potential biases this may pose by excluding all patients receiving prescription opioid medications from our analyses.
Moreover, information regarding use of illicit benzodiazepines in the month preceding enrollment into the study and frequency of injecting drug use was collected by self-reports from participants and was thus subject to social bias, possibly causing the reported values to underestimate the true values.68 However, we aimed to measure variables in an objective manner as much as possible. For instance, urinalysis was used to determine relapse during the study’s 6-month follow-up period and the patients’ electronic medical records were used to confirm the presence of physical comorbidities. It is also important for us to point out that all methadone administration facilities offer psychosocial services to all patients, albeit to varying degrees, potentially influencing the results. Nonetheless, confounding variables and other unknown biases may influence the study findings in keeping with the observational nature of the study. Not withstanding the limitations, this study used rigorous methods to identify predictors of relapse in a well-characterized cohort of patients with OUD receiving MMT.
Conclusion
In conclusion, results from the present study reveal that injecting drug use behavior, older age of onset of opioid abuse, increased use of illicit benzodiazepines in the month prior to enrollment, and the interaction variable between age and hepatitis C status are associated with accelerated opioid relapse in MMT patients. Conversely, current age has a protective effect against relapse. This is the first study to employ a survival analysis investigating the impact of clinical and socio-demographic factors on the length of time a patient remains abstinent from illicit opioids during MMT. There was a wide variability in the length of time patients remained opioid free throughout the 6-month duration of this study, and this is an important characteristic that aids in the categorization of patients into groups according to risk for relapse. The identification of MMT patients at high risk for opioid relapse during MMT allows for improved treatment tailoring, whereby health care providers can target more aggressive adjunct therapies within these high-risk populations. Improvement in the duration of abstinence from illicit opioid use will ultimately serve to increase treatment retention rates39 and lower the risk of comorbidities associated with concomitant use of illicit opioids during MMT, including abnormal cardiac conductivity,33,34 overdose,35–37 and death.35,37,38
Supplementary Materials
Variable definitions.
Current age (years)
Current age of GENOA Participants
This variable is measured as a continuous variable.
Sex
Biological sex
This variable is measured as a dichotomous variables representing male or female categories
Marital Status
This variable identifies whether the participant is currently living with and participating in a relationship with a partner (married or common-law)
This variable intends to determine the participants current access to a social-support
This variable is measured as a dichotomous variable
Employment
Participants current employment status
This variable is measured as a dichotomous variable
Smoking Status
This variable identifies if the participant is currently an active smoker
This variable is measured as a dichotomous variable representing smokers and non-smokers
Methadone Dose
This variable identifies the patient’s methadone dose measured at baseline interview
This variable is measured as a continuous variable, in mg/day
Methadone Dose Categories
Patient methadone dose measured at baseline interview
This variable is measured in milligram per day, and is cat-egorized into a binary variable:
Category 0 (reference category: <80 mg/day)
Category 1 (≥80 mg/day)
Age of Onset of Opioid Abuse
Age when participants began to abuse opioids
Opioid abuse is defined as the persistent consumption of illicit opioids, or over consumption of prescribed opioids, to a point at which their drug-use behaviors interfered with relationships and social/physical activities of daily living
This variable is measured as a continuous variable
Origins of Opioid Abuse
This variable measures whether participants began abusing opioids secondary to the overuse of opioids prescribed to them for a medical condition
This variable is measured as a dichotomous variable
Duration of Methadone Maintenance Treatment (MMT)
This variable identifies the length of time patients have been on their current course of MMT
This variable is measured as a continuous variable, in months
Days of illicit benzodiazepine use over the last month
This variable was measured during the participants baseline interview and reports the number of days the patient used illicit benzodiazepines in the month preceding their entry to the GENOA study
This variable is measured as a continuous variable
Days of cocaine use over the last month
This variable was measured during the participants baseline interview and reports the number of days the patient used cocaine in the month preceding their entry to the GENOA study
This variable is measured as a continuous variable
Days of cannabis use over the last month
This variable was measured during the participants baseline interview and reports the number of days the patient used cannabis in the month preceding their entry to the GENOA study
This variable is measured as a continuous variable
Injecting drug use behavior
This variable was measured during the participants baseline interview and reports if the patient was injecting drugs in the month preceding their entry to the GENOA study
This variable is measured as a dichotomous variable
Hepatitis C Status
This variable identifies if participants have hepatitis C
This variable is determined using self-report and validated using participants’ electronic medical record history
This variable is measured as a dichotomous variable
Chronic pain
This variable measures whether participants have chronic pain
This variable is determined using self-report and validated using participants’ electronic medical record history
This variable is measured as a dichotomous variable, representing patients with and without chronic pain
Diabetes
This variable measures whether participants have diabetes (Type I or II)
This variable is determined using self-report and validated using participants’ electronic medical record history
This variable is measured as a dichotomous variable, representing patients with and without diabetes
Statistical data. Supplementary Figure 1. Schoenfeld Residuals Test for Main Effects Model.
Note: Results from this analysis show that all covariates passed the Schoenfeld residuals test as all P-values failed to reach statistical significance (P < 0.05). This maintains the PH assumption, as the residuals for the covariates are not related to survival time.
Table 1.
Baseline participant characteristics (total N = 250).
| PARTICIPANT CHARACTERISTIC | MEAN (SD) | P-VALUE* | |||
|---|---|---|---|---|---|
| TOTAL | RELAPSE (N = 125) | NO RELAPSE (N = 125) | |||
| Current age (years) | 38.64 (10.89) | 38.63 (10.78) | 38.65 (11.04) | 0.9874 | |
| Methadone dose (mg/day) | 78.99 (39.56) | 74.58 (35.42) | 83.43 (43.02) | 0.0787 | |
| Age of onset of opioid abuse (years) | 32.41 (9.69) | 33.61 (10.08) | 31.22 (9.16) | 0.0557 | |
| Duration of MMT (months) | 51.91 (45.93) | 45.46 (35.42) | 58.36 (53.85) | 0.0307 | |
| Days of benzodiazepine use over last month | 0.62 (3.38) | 0.91 (4.32) | 0.34 (2.04) | 0.1807 | |
| Days of cocaine use over last month | 0.62 (3.37) | 0.90 (4.31) | 0.34 (2.04) | 0.1846 | |
| Days of cannabis use over last month | 8.68 (12.44) | 7.72 (11.76) | 9.64 (13.06) | 0.2231 | |
| Length of time patient remains abstinent (days) | 99.04 (74.37) | – | – | – | |
| N (% OF TOTAL) | N (% OF RELAPSE) | N (% OF NO RELAPSE) | P-VALUE† | ||
| Sex | Women | 125 (50.0) | 68 (54.4) | 57 (45.6) | |
| Men | 125 (50.0) | 57 (45.6) | 68 (54.4) | 0.164 | |
| Marital status | Married or living with partner | 78 (31.2) | 42 (33.6) | 36 (28.8) | |
| Other | 172 (68.8) | 83 (66.4) | 89 (71.2) | 0.413 | |
| Employed | Yes | 86 (34.4) | 42 (33.6) | 44 (35.2) | |
| No | 164 (65.6) | 83 (66.4) | 81 (64.8) | 0.790 | |
| Smoking | Yes | 209 (83.9) | 107 (85.6) | 102 (81.6) | |
| No | 40 (16.0) | 18 (14.4) | 22 (17.6) | 0.473 | |
| Methadone dose | <80 mg/day | 124 (49.6) | 66 (52.8) | 58 (46.4) | |
| ≥80 mg/day | 126 (50.4) | 59 (47.2) | 67 (53.6) | 0.312 | |
| Injecting drug use | Yes | 16 (6.4) | 11 (8.80) | 5 (4.00) | |
| No | 234 (93.6) | 114 (91.2) | 120 (96.0) | 0.121 | |
| Source of opioid | From a prescription | 112 (44.8) | 62 (49.6) | 50 (40.0) | |
| Other | 138 (55.2) | 63 (50.4) | 75 (60.0) | 0.127 | |
| Hepatitis C | Yes | 57 (22.8) | 33 (26.4) | 24 (19.2) | |
| No | 193 (77.2) | 92 (73.6) | 101 (80.8) | 0.175 | |
| Chronic pain | Yes | 75 (29.6) | 34 (27.2) | 40 (32.0) | |
| No | 175 (70.0) | 91 (72.8) | 85 (68.0) | 0.406 | |
| Diabetes | Yes | 17 (6.8) | 9 (7.20) | 8 (6.40) | |
| No | 233 (93.2) | 116 (92.8) | 117 (93.6) | 0.802 | |
Notes:
T-test was used for comparing means of continuous variables.
Pearson’s chi-square test was used for comparing means of binary variables. Refer to Supplementary Data for an outline of the defnitions and measurements for each variable.
Table 2.
Univariate and multivariable Cox regression analyses containing main and interaction effects of potential covariates on time until opioid relapse.
| COVARIATES | UNIVARIATE ANALYSES | MULTIVARIABLE ANALYSES* | ||
|---|---|---|---|---|
| HAZARD RATIO (95% CI) | P-VALUE | HAZARD RATIO (95% CI) | P-VALUE | |
| Current age (years) | 1.00 (0.98, 1.02) | 0.982 | 0.93 (0.89, 0.97) | 0.003 |
| Days of illicit benzodiazepine use (days) | 1.06 (1.01, 1.10) | 0.009 | 1.07 (1.03, 1.12) | 0.002 |
| Age of onset of opioid abuse (years) | 1.02 (1.00, 1.04) | 0.115 | 1.10 (1.04, 1.15) | <0.0001 |
| Injecting drug use behavior | 2.25 (1.12, 4.47) | 0.022 | 2.26 (1.03, 4.97) | 0.042 |
| Age *Methadone dose (mg/day) | – | – | 0.99 (0.98, 1.00) | 0.063 |
| Age *Hepatitis C | – | – | 1.01 (1.00, 1.03) | 0.020 |
Acknowledgments
We would like to thank all parties involved in the GENOA and CATC clinical sites that helped in making this project possible. First, we would like to thank the participants of our study for donating their time, information, and samples for analyses; without their generous contributions, this study would not have been possible. Additionally, we sincerely appreciate the GENOA and CATC clinical staff and investigative teams for their hard work and dedicated efforts in patient interviewing, participant recruitment, and data collection. We extend our special gratitude to Jackie Hudson and Sheelagh Rutherford for their commitment and dedication to the GENOA collaborative. We also extend our sincere appreciation to the undergraduate and medical students of McMaster University Faculty of Health Sciences, who dedicated their time to collect and clean the data for the GENOA project. These students include Anuja Bhalerao, Andrew Kampius, Tea Rosic, and Julia Woo.
Footnotes
ACADEMIC EDITOR: Gregory Stuart, Editor in Chief
PEER REVIEW: Seven peer reviewers contributed to the peer review report. Reviewers’ reports totaled 3056 words, excluding any confidential comments to the academic editor.
FUNDING: The study is supported by the Peter Boris Centre for Addictions Research and the CIHR Drug Safety and Effectiveness Network (DSEN) grant (grant number: 126639) and the Chanchlani Research Centre. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Ethics This study was approved by the Hamilton integrated Research Ethics Board (HiREB). The research was conducted in accordance with the principles of the Declaration of Helsinki.
Author Contributions
Conceived and designed the experiments: LN, BBD, LT, ZS. Analyzed the data: BBD, MB, LN, ZS. Wrote the first draft of the manuscript: LN, BBD, MB, ZS. Contributed to the writing of the manuscript: LN, BBD, MB, MV, JD, GP, AW, DD, CP, DCM, LT, ZS. All authors agree with manuscript results and conclusions. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Franklin GM, Rahman EA, Turner JA, Daniell WE, Fulton-Kehoe D. Opioid use for chronic low back pain: a prospective, population-based study among injured workers in Washington state, 2002–5. Clin J Pain. 2009;25(9):743–51. doi: 10.1097/AJP.0b013e3181b01710. [DOI] [PubMed] [Google Scholar]
- 2.Kosten TR, Schottenfeld R, Ziedonis D, Falcioni J. Buprenorphine versus methadone maintenance for opioid dependence. J Nerv Ment Dis. 1993;181(6):358–64. doi: 10.1097/00005053-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Centre on Substance Abuse . Canadian Drug Summary: Prescription Opioids. Ottawa, ON: Canadian Centre on Substance Abuse; 2013. [Google Scholar]
- 4.Brands B, Marsch D, Hart L, Jamieson W. Health Canada literature review-Methadone Maintenance Therapy. Ottawa, ON: Minister of public works and Government services, Canada; 2002. pp. 1–94. [Google Scholar]
- 5.Haydon E, Rehm J, Fischer B, Monga N, Adlaf E. Prescription drug abuse in Canada and the diversion of prescription drugs into the illicit drug market. Can J Public Health. 2005;96(6):459–61. doi: 10.1007/BF03405190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration . Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 7.Sproule B, Brands B, Li S, Catz-Biro L. Changing patterns in opioid addiction. Can Fam Physician. 2009;55(1):68–9. [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders Fourth Edition Text Revision DSM-IV-TR. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 9.Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug Alcohol Depend. 2000;58(1–2):55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- 10.WHO The World Health Report 2002-Reducing Risks, Promoting Healthy Life. 2002. Available at: http://www.who.int/whr/2002/en/ [DOI] [PubMed]
- 11.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 12.Zou S, Tepper M, Giulivi A. Current status of hepatitis C in Canada. Can J Public Health. 2000;91(suppl 1):S10–15. doi: 10.1007/BF03405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wall R, Rehm J, Fischer B, et al. Social costs of untreated opioid dependence. J Urban Health. 2000;77(4):688–722. doi: 10.1007/BF02344032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oppenheimer E, Tobutt C, Taylor C, Andrew T. Death and survival in a cohort of heroin addicts from London clinics: a 22-year follow-up study. Addiction. 1994;89(10):1299–308. doi: 10.1111/j.1360-0443.1994.tb03309.x. [DOI] [PubMed] [Google Scholar]
- 15.Seal KH, Kral AH, Gee L, et al. Predictors and prevention of nonfatal overdose among street-recruited injection heroin users in the San Francisco Bay Area, 1998–9. Am J Public Health. 2001;91(11):1842–6. doi: 10.2105/ajph.91.11.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutinho RA. HIV and hepatitis C among injecting drug users. BMJ. 1998;317(7156):424–5. doi: 10.1136/bmj.317.7156.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 18.Hall W, Bell J, Carless J. Crime and drug use among applicants for methadone maintenance. Drug Alcohol Depend. 1993;31(2):123–9. doi: 10.1016/0376-8716(93)90064-w. [DOI] [PubMed] [Google Scholar]
- 19.Degenhardt L, Charlson F, Mathers B, et al. The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction. 2014;109(8):1320–33. doi: 10.1111/add.12551. [DOI] [PubMed] [Google Scholar]
- 20.Disley E, Mulcah A, Pardal M, Rubin J, Ruggeri K. Development of a Framework to Estimate the Cost of Opioid Dependence. Cambridge: RAND Europe; 2013. [PMC free article] [PubMed] [Google Scholar]
- 21.The College of Physicians and Surgeons of Ontario . Methadone Maintenance Treatment Program Standards and Clinical Guidelines. Toronto, ON: The College of Physicians and Surgeons of Ontario; 2011. [Google Scholar]
- 22.Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K. Treatment needs associated with pain in substance use disorder patients: implications for concurrent treatment. Drug Alcohol Depend. 2004;73(1):23–31. doi: 10.1016/j.drugalcdep.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Dennis BB, Samaan MC, Bawor M, et al. Evaluation of clinical and inflammatory profile in opioid addiction patients with comorbid pain: results from a multicenter investigation. Neuropsychiatr Dis Treat. 2014;10:2239–47. doi: 10.2147/NDT.S72785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bawor M, Dennis BB, Samaan MC, et al. Methadone induces testosterone suppression in patients with opioid addiction. Sci Rep. 2014;4:6189. doi: 10.1038/srep06189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289(18):2370–8. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- 26.Davstad I, Stenbacka M, Leifman A, Beck O, Korkmaz S, Romelsjo A. Patterns of illicit drug use and retention in a methadone program: a longitudinal study. J Opioid Manag. 2007;3(1):27–34. doi: 10.5055/jom.2007.0036. [DOI] [PubMed] [Google Scholar]
- 27.Mino A, Page D, Dumont P, Broers B. Treatment failure and methadone dose in a public methadone maintenance treatment programme in Geneva. Drug Alcohol Depend. 1998;50(3):233–9. doi: 10.1016/s0376-8716(98)00035-0. [DOI] [PubMed] [Google Scholar]
- 28.Peles E, Schreiber S, Adelson M. Factors predicting retention in treatment: 10-year experience of a methadone maintenance treatment (MMT) clinic in Israel. Drug Alcohol Depend. 2006;82(3):211–7. doi: 10.1016/j.drugalcdep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Proctor SL, Copeland AL, Kopak AM, Hoffmann NG, Herschman PL, Polukhina N. Predictors of patient retention in methadone maintenance treatment. Psychol Addict Behav. 2015;29(4):906–17. doi: 10.1037/adb0000090. [DOI] [PubMed] [Google Scholar]
- 30.Mancino M, Curran G, Han X, Allee E, Humphreys K, Booth BM. Predictors of attrition from a national sample of methadone maintenance patients. Am J Drug Alcohol Abuse. 2010;36(3):155–60. doi: 10.3109/00952991003736389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magura S, Rosenblum A. Leaving methadone treatment: lessons learned, lessons forgotten, lessons ignored. Mt Sinai J Med. 2001;68(1):62–74. [PubMed] [Google Scholar]
- 32.Raffa JD, Grebely J, Tossonian H, et al. The impact of ongoing illicit drug use on methadone adherence in illicit drug users receiving treatment for HIV in a directly observed therapy program. Drug Alcohol Depend. 2007;89(2–3):306–9. doi: 10.1016/j.drugalcdep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Krantz MJ, Kutinsky IB, Robertson AD, Mehler PS. Dose-related effects of methadone on QT prolongation in a series of patients with torsade de pointes. Pharmacotherapy. 2003;23(6):802–5. doi: 10.1592/phco.23.6.802.32186. [DOI] [PubMed] [Google Scholar]
- 34.Peles E, Bodner G, Kreek MJ, Rados V, Adelson M. Corrected-QT intervals as related to methadone dose and serum level in methadone maintenance treatment (MMT) patients: a cross-sectional study. Addiction. 2007;102(2):289–300. doi: 10.1111/j.1360-0443.2006.01668.x. [DOI] [PubMed] [Google Scholar]
- 35.Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among veterans health administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605–12. doi: 10.1097/AJP.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 36.Cao X, Wu Z, Li L, et al. National Methadone Maintenance Treatment Program Working Group Mortality among methadone maintenance clients in China: a six-year cohort study. PLoS One. 2013;8(12):e82476. doi: 10.1371/journal.pone.0082476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gagajewski A, Apple FS. Methadone-related deaths in Hennepin County, Minnesota: 1992–2002. J Forensic Sci. 2003;48(3):668–71. [PubMed] [Google Scholar]
- 38.Butler B, Rubin G, Lawrance A, Batey R, Bell J. Estimating the risk of fatal arrhythmia in patients in methadone maintenance treatment for heroin addiction. Drug Alcohol Rev. 2011;30(2):173–80. doi: 10.1111/j.1465-3362.2010.00213.x. [DOI] [PubMed] [Google Scholar]
- 39.White WL, Campbell MD, Spencer RD, Hoffman HA, Crissman B, DuPont RL. Patterns of abstinence or continued drug use among methadone maintenance patients and their relation to treatment retention. J Psychoactive Drugs. 2014;46(2):114–22. doi: 10.1080/02791072.2014.901587. [DOI] [PubMed] [Google Scholar]
- 40.Schottenfeld RS, Pakes JR, Kosten TR. Prognostic factors in buprenorphine- versus methadone-maintained patients. J Nerv Ment Dis. 1998;186(1):35–43. doi: 10.1097/00005053-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Samaan Z, Bawor M, Dennis BB, et al. Genetic influence on methadone treatment outcomes in patients undergoing methadone maintenance treatment for opioid addiction: a pilot study. Neuropsychiatr Dis Treat. 2014;10:1503–8. doi: 10.2147/NDT.S66234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 43.Basu D, Ghormode D, Madan R, Mattoo S, Nehra R, Prabhakar S. Age of onset of dependence: does it help our understanding of opioid dependence by generating meaningful categories or by acting as a useful dimension? A critical examination of the classic debate in psychiatry. Indian J Psychiatry. 2014;56(3):228–37. doi: 10.4103/0019-5545.140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NOVA Systems inventor; iMDx TM. [Accessed November 21, 2015]. Available from: http://www.novxsystems.com/novx/myweb.php?hls=10024.
- 45.Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res. 2010;1(4):274–8. doi: 10.4103/0974-7788.76794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torrens M, Castillo C, Perez-Sola V. Retention in a low-threshold methadone maintenance program. Drug Alcohol Depend. 1996;41(1):55–9. doi: 10.1016/0376-8716(96)01230-6. [DOI] [PubMed] [Google Scholar]
- 47.Bao YP, Liu ZM, Epstein DH, Du C, Shi J, Lu L. A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am J Drug Alcohol Abuse. 2009;35(1):28–33. doi: 10.1080/00952990802342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiaoli W, Lirong W, Xueliang W, Jinsong L, Hengxin L, Wei J. Risk factors of hepatitis C virus infection in drug users from eleven methadone maintenance treatment clinics in Xi’an, China. Hepat Mon. 2014;14(11):e19601. doi: 10.5812/hepatmon.19601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.David G, Kleinbaum MK. Statistics for Biology and Health. 2nd ed. New York, NY: Springer; 2005. [Google Scholar]
- 50.StataCorp . Stata Statistical Software: Release 13. College Station TX: StataCorp LP; 2013. [Google Scholar]
- 51.Marsden J, Gossop M, Stewart D, et al. The Maudsley addiction profile (MAP): a brief instrument for assessing treatment outcome. Addiction. 1998;93(12): 1857–67. doi: 10.1046/j.1360-0443.1998.9312185711.x. [DOI] [PubMed] [Google Scholar]
- 52.Ferrando SJ, Batki SL. HIV-infected intravenous drug users in methadone maintenance treatment: clinical problems and their management. J Psychoactive Drugs. 1991;23(2):217–24. doi: 10.1080/02791072.1991.10472238. [DOI] [PubMed] [Google Scholar]
- 53.Bazazi AR, Yokell M, Fu JJ, Rich JD, Zaller ND. Illicit use of buprenorphine/naloxone among injecting and noninjecting opioid users. J Addict Med. 2011;5(3):175–80. doi: 10.1097/ADM.0b013e3182034e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollini RA, Banta-Green CJ, Cuevas-Mota J, Metzner M, Teshale E, Garfein RS. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173–80. doi: 10.2147/SAR.S24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stark K, Muller R, Bienzle U, Guggenmoos-Holzmann I. Methadone maintenance treatment and HIV risk-taking behaviour among injecting drug users in Berlin. J Epidemiol Community Health. 1996;50(5):534–7. doi: 10.1136/jech.50.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaisson RE, Moss AR, Onishi R, Osmond D, Carlson JR. Human immunodeficiency virus infection in heterosexual intravenous drug users in San Francisco. Am J Public Health. 1987;77(2):169–72. doi: 10.2105/ajph.77.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langendam MW, van Brussel GH, Coutinho RA, van Ameijden EJ. Methadone maintenance and cessation of injecting drug use: results from the Amsterdam cohort study. Addiction. 2000;95(4):591–600. doi: 10.1046/j.1360-0443.2000.95459110.x. [DOI] [PubMed] [Google Scholar]
- 58.Steensma C, Boivin JF, Blais L, Roy E. Cessation of injecting drug use among street-based youth. J Urban Health. 2005;82(4):622–37. doi: 10.1093/jurban/jti121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smyth BP, Barry J, Keenan E, Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010;103(6):176–9. [PubMed] [Google Scholar]
- 60.Couvee JE, Bakker A, Zitman FG. The relevance of psychiatric and somatic comorbidity in depressed chronic benzodiazepine users. Psychother Psychosom. 2002;71(5):263–8. doi: 10.1159/000064810. [DOI] [PubMed] [Google Scholar]
- 61.Hosseini S, Moghimbeigi A, Roshanaei G, Momeniarbat F. Evaluation of drug abuse relapse event rate over time in frailty model. Osong Public Health Res Perspect. 2014;5(2):92–5. doi: 10.1016/j.phrp.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nosyk B, MacNab YC, Sun H, et al. Proportional hazards frailty models for recurrent methadone maintenance treatment. Am J Epidemiol. 2009;170(6): 783–92. doi: 10.1093/aje/kwp186. [DOI] [PubMed] [Google Scholar]
- 63.Wu LT, Blazer DG, Stitzer ML, Patkar AA, Blaine JD. Infrequent illicit methadone use among stimulant-using patients in methadone maintenance treatment programs: a national drug abuse treatment clinical trials network study. Am J Addict. 2008;17(4):304–11. doi: 10.1080/10550490802138913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tran BX, Ohinmaa A, Mills S, et al. Multilevel predictors of concurrent opioid use during methadone maintenance treatment among drug users with HIV/AIDS. PLoS One. 2012;7(12):e51569. doi: 10.1371/journal.pone.0051569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saxon AJ, Wells EA, Fleming C, Jackson TR, Calsyn DA. Pre-treatment characteristics, program philosophy and level of ancillary services as predictors of methadone maintenance treatment outcome. Addiction. 1996;91(8):1197–209. doi: 10.1046/j.1360-0443.1996.918119711.x. [DOI] [PubMed] [Google Scholar]
- 66.Mayet S, Farrell M, Ferri M, Amato L, Davoli M. Psychosocial treatment for opiate abuse and dependence. Cochrane Database of Systematic Reviews. 2014;(4) doi: 10.1002/14651858.CD004330.pub3. Art. No.: CD004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delgado-Rodriguez M, Bias Llorca J. J Epidemiol Community Health. 2004;58:635–41. doi: 10.1136/jech.2003.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179–87. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 69.Dennis BB, Roshanov PS, Naji L, et al. Opioid substitution and antagonist therapy trials exclude the common addiction patient: a systematic review and analysis of eligibility criteria. Trials. 2015;16:475. doi: 10.1186/s13063-015-0942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Variable definitions.
Current age (years)
Current age of GENOA Participants
This variable is measured as a continuous variable.
Sex
Biological sex
This variable is measured as a dichotomous variables representing male or female categories
Marital Status
This variable identifies whether the participant is currently living with and participating in a relationship with a partner (married or common-law)
This variable intends to determine the participants current access to a social-support
This variable is measured as a dichotomous variable
Employment
Participants current employment status
This variable is measured as a dichotomous variable
Smoking Status
This variable identifies if the participant is currently an active smoker
This variable is measured as a dichotomous variable representing smokers and non-smokers
Methadone Dose
This variable identifies the patient’s methadone dose measured at baseline interview
This variable is measured as a continuous variable, in mg/day
Methadone Dose Categories
Patient methadone dose measured at baseline interview
This variable is measured in milligram per day, and is cat-egorized into a binary variable:
Category 0 (reference category: <80 mg/day)
Category 1 (≥80 mg/day)
Age of Onset of Opioid Abuse
Age when participants began to abuse opioids
Opioid abuse is defined as the persistent consumption of illicit opioids, or over consumption of prescribed opioids, to a point at which their drug-use behaviors interfered with relationships and social/physical activities of daily living
This variable is measured as a continuous variable
Origins of Opioid Abuse
This variable measures whether participants began abusing opioids secondary to the overuse of opioids prescribed to them for a medical condition
This variable is measured as a dichotomous variable
Duration of Methadone Maintenance Treatment (MMT)
This variable identifies the length of time patients have been on their current course of MMT
This variable is measured as a continuous variable, in months
Days of illicit benzodiazepine use over the last month
This variable was measured during the participants baseline interview and reports the number of days the patient used illicit benzodiazepines in the month preceding their entry to the GENOA study
This variable is measured as a continuous variable
Days of cocaine use over the last month
This variable was measured during the participants baseline interview and reports the number of days the patient used cocaine in the month preceding their entry to the GENOA study
This variable is measured as a continuous variable
Days of cannabis use over the last month
This variable was measured during the participants baseline interview and reports the number of days the patient used cannabis in the month preceding their entry to the GENOA study
This variable is measured as a continuous variable
Injecting drug use behavior
This variable was measured during the participants baseline interview and reports if the patient was injecting drugs in the month preceding their entry to the GENOA study
This variable is measured as a dichotomous variable
Hepatitis C Status
This variable identifies if participants have hepatitis C
This variable is determined using self-report and validated using participants’ electronic medical record history
This variable is measured as a dichotomous variable
Chronic pain
This variable measures whether participants have chronic pain
This variable is determined using self-report and validated using participants’ electronic medical record history
This variable is measured as a dichotomous variable, representing patients with and without chronic pain
Diabetes
This variable measures whether participants have diabetes (Type I or II)
This variable is determined using self-report and validated using participants’ electronic medical record history
This variable is measured as a dichotomous variable, representing patients with and without diabetes
Statistical data. Supplementary Figure 1. Schoenfeld Residuals Test for Main Effects Model.
Note: Results from this analysis show that all covariates passed the Schoenfeld residuals test as all P-values failed to reach statistical significance (P < 0.05). This maintains the PH assumption, as the residuals for the covariates are not related to survival time.