Abstract
Welwitschia mirabilis is an ancient and rare plant distributed along the western coast of Namibia and Angola. Several aspects of Welwitschia biology and ecology have been investigated, but very little is known about the microbial communities associated with this plant. This study reports on the bacterial and fungal communities inhabiting the rhizosphere of W. mirabilis and the surrounding bulk soil. Rhizosphere communities were dominated by sequences of Alphaproteobacteria and Euromycetes, while Actinobacteria, Alphaproteobacteria, and fungi of the class Dothideomycetes jointly dominated bulk soil communities. Although microbial communities within the rhizosphere and soil samples were highly variable, very few “species” (OTUs defined at a 97% identity cut-off) were shared between these two environments. There was a small ‘core’ rhizosphere bacterial community (formed by Nitratireductor, Steroidobacter, Pseudonocardia and three Phylobacteriaceae) that together with Rhizophagus, an arbuscular mycorrhizal fungus, and other putative plant growth-promoting microbes may interact synergistically to promote Welwitschia growth.
Introduction
Plants live in association with a great number of microorganisms [1], the so-called plant microbiome. The plant microbiome can have profound effects on seed germination, seedling vigour, plant growth and development, nutrition, diseases and productivity [2]. In return, plants secrete a wide range of compounds, including sugars, vitamins, amino acids, purines and nucleosides [3] that support microbial communities and influence their composition and activities [4]. The rhizosphere, the narrow zone of soil that surrounds and is influenced by plant roots, is a microbial diversity hotspot [2, 5]. Recent studies have shown that, in natural ecosystems, plant diversity [6] and the genotypes of individual plants [7] can influence the composition of their root-associated microbes. Yet, more research is still needed to better understand the plant microbiome.
Welwitschia mirabilis is an uncommon plant found in the western hyper-arid desert regions of Namibia and southern Angola, where it occurs in isolated populations ranging from 2 to more than 1000 long-living individuals [8]. It is thought that the older specimens may be more than 1500 years old [9]. The plant, which was first discovered in 1859 by the Austrian botanist Friedrich Welwitsch, has always fascinated scientists because of its primitive nature. W. mirabilis is a monogeneric and monospecific member of the family Welwitschiaceae that is grouped with the genus Ephedra and Gnetum under the plant Division Gnetophyta [10], a small group of seed plants that has intermediate characteristics between Angiosperms and Gymnosperms. In addition to its scientific significance, Welwitschia is of considerable importance to the local ecosystem because it provides refuge, shade, food, and water to many species of animals that inhabit the Namib [8]. As a result researchers have extensively studied the botany, physiology and ecology of Welwitschia plants [11]. However, very little is known about the microbial communities associated with Welwitschia (but see [12]).
In this study, we used 454 amplicon pyrosequencing to analyse the bacterial and fungal communities inhabiting the Welwitschia rhizosphere and compared them with those from the bulk soil. We asked: Are Welwitschia rhizosphere and bulk soil communities phylogenetically distinct, does W. mirabilis present a core community of rhizosphere microbes?
Given the fact that Welwitschia have co-evolved with other organisms for more than 110 million years [13], we would expect Welwitschia to have selected for a specific cohort of rhizosphere microbes.
Materials and Methods
Sample collection
Sampling of Welwitschia mirabilis (S1 Fig), a unique and protected plant (CITES Appendix II), was undertaken in September 2012 at a single location (S22°40’18.84”, E14°51’35.69”) in the Namib Desert, under the auspices of the permission granted to Swakop Uranium to transplant three Welwitschia plants, located 5–7 m apart, as part of the construction of a road system to support their mining operation. Because Welwitschia roots were found to be embedded in a matrix of calcrete, only 5 rhizosphere soil samples (i.e. soil closely adhering to the root systems, depth 20–30 cm) were obtained from the three plants (approx. 150–300 year old, visual estimation). Five bulk soil samples (i.e. unvegetated soil 10–20 cm distant from the root system, depth 20–30 cm) were also collected. Samples, consisting of ca. 20 g of soil, were stored in 50-ml Falcon tubes containing RNALater solution (Sigma-Aldrich, USA) and shipped at room temperature to the Namibian Ministry of Environment and Tourism for delivery to South Africa. All samples were processed within two weeks of collection. Samples were collected under sampling permit number 1653/2011 issued by the Namibian Ministry of Environment and Tourism.
Soil DNA extraction, fragment amplification and high-throughput sequencing
Total soil DNA was extracted using the MoBio PowerSoil DNA isolation kit (MoBIO, USA) following the manufacturer’s instructions. Partial bacterial 16S rRNA gene amplicons were produced using the primers 27F (5’-AGRGTTTGATCMTGGCTCAG-3’) and 519R (5’- GTNTTACNGCGGCKGCTG-3’), targeting the V1-V3 hypervariable region, as in [14]. Partial fungal ITS amplicons were produced using the primer set ITS1F (5’-CTTGGTCATTTAGAGGAAGTAA-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’), targeting the hypervariable ITS2 region, as in [15]. PCR was performed using 50 ng soil DNA and the HotStarTaq Plus Master Mix Kit (Qiagen, USA). Amplicon products from different samples were mixed in equal concentrations and purified using Agencourt Ampure beads (Agencourt Bioscience Corporation, USA). Samples were sequenced at the Molecular Research LP next generation sequencing service (http://www.mrdnalab.com) using Roche 454 FLX titanium instruments and reagents and following manufacturer’s guidelines.
Sequence processing
Quality processing of 16S rRNA gene sequences was performed in Mothur (v.1.35.0) [16] following a previously described pipeline [17]. Briefly, the FASTA quality and flow data were extracted using the sffinfo command. Low-quality sequences were removed using trim.flows and shhh.flows, which is an implementation of the PyroNoise component of the AmpliconNoise suite of programs [18]. The data set was reduced to only unique sequences using unique.seqs. An alignment was generated using the align.seqs command by aligning the data to the SILVA bacterial database. The screen.seqs command was used to reduce the data to the overlapping region of the sequences. Chimeric sequences were removed through chimera.uchime. After quality filtering, sequences were used to construct a distance matrix and grouped into OTUs (cut-off level of 97%, species level [19]). The taxonomic affiliations of the OTUs were determined using the naive Bayesian rRNA classifier [20], at an 80% confidence level. Sequences that had the highest similarity to chloroplast sequences were removed prior to further analysis.
Pre-alignment steps for fungal ITS sequences were as described above, but we trimmed reads to a maximum length of 300 bases. Chimeras were eliminated using chimera.uchime. To cluster unaligned sequences into OTUs, we created a pairwise distance matrix using pairwise.seqs and then created clusters sharing 97% or greater sequence identity using the cluster command. Classification of sequences was performed with classify.otu using the UNITE ITS reference database (http://unite.ut.ee/repository.php).
The sequence data generated in this study were deposited in the NCBI Sequence Read Archive and are available under the project number SRP061179.
Statistical analysis
All statistical analyses were conducted in Mothur v.1.35.0 and R v.3.2.0 (R Foundation for Statistical Computing; http://www.R-project.org). Singleton sequences were removed, and each sample was subsampled with the Mothur command sub.sample to 449 reads for bacterial and 2787 for fungal OTUs, which was the minimum number of sequences remaining in a single sample. Sample rarefaction, as in [14], ensures equal sampling effort across samples. We visualised similarities in community composition using non-metric multidimensional scaling (nMDS) with weighted UniFrac distances. Differences in community structure were assessed by ANOSIM analysis using the anosim function in vegan (cran.r-project.org/package = vegan). The number of shared OTUs between communities/samples was visualized using the venn function in gplots (cran.r-project.org/package = gplots). The mean fungal and bacterial diversities were compared using paired two-tailed Student’s t-tests. The compositions of major fungal and bacterial genera were compared using UPGMA clustering on Hellinger-transformed Bray-Curtis distances together with a heatmap of abundance data created with heatmap.2 in gplots.
Results and Discussion
Here we report on the microbial community associated with Welwitschia mirabilis roots using metagenomic DNA from rhizosphere (n = 5) and bulk (n = 5) soil. The analysis, after quality filtering and removal of singletons, included a total of 31522 amplicon sequences with an average sequence length of 241 bp for the 16S rRNA gene and 283 bp for the ITS. The number of sequences was lower for the 16S rRNA gene assays (3712 sequences) than for the ITS assays (27810 sequences). 407 bacterial 16S rRNA gene OTUs and 139 ITS OTUs, both at 97% sequence identity, were included in the analysis.
Bacterial richness was significantly greater than fungal richness in both rhizosphere and bulk soil communities (S1 Table), although the number of sequences was 7-fold higher for fungi (S2 Table). A similar result was found in a recent study carried out in the rhizosphere of invasive Berberis thungerbii [7]. In general, bulk soil bacterial communities were more diverse than rhizosphere bacterial communities (S1 Table), probably due to the selection process that gradually differentiates the root microbiome from the surrounding soil biome [3]. No differences in diversity were detected between the fungal communities in the two ecosystems (S2 Table), corroborating recent findings suggesting that plant-soil feedbacks do not influence the diversity of soil fungi [21]. These results should be interpreted with caution, as rarefaction curves and Good’s coverage values indicate that we did not sample all members of the bacterial and fungal communities (S2 Table, S2 Fig). However, the goal of this study was not to obtain a full coverage of the diversity in the samples, but rather to use 454-sequencing as a tool to gain taxonomic information and assess beta-diversity patterns.
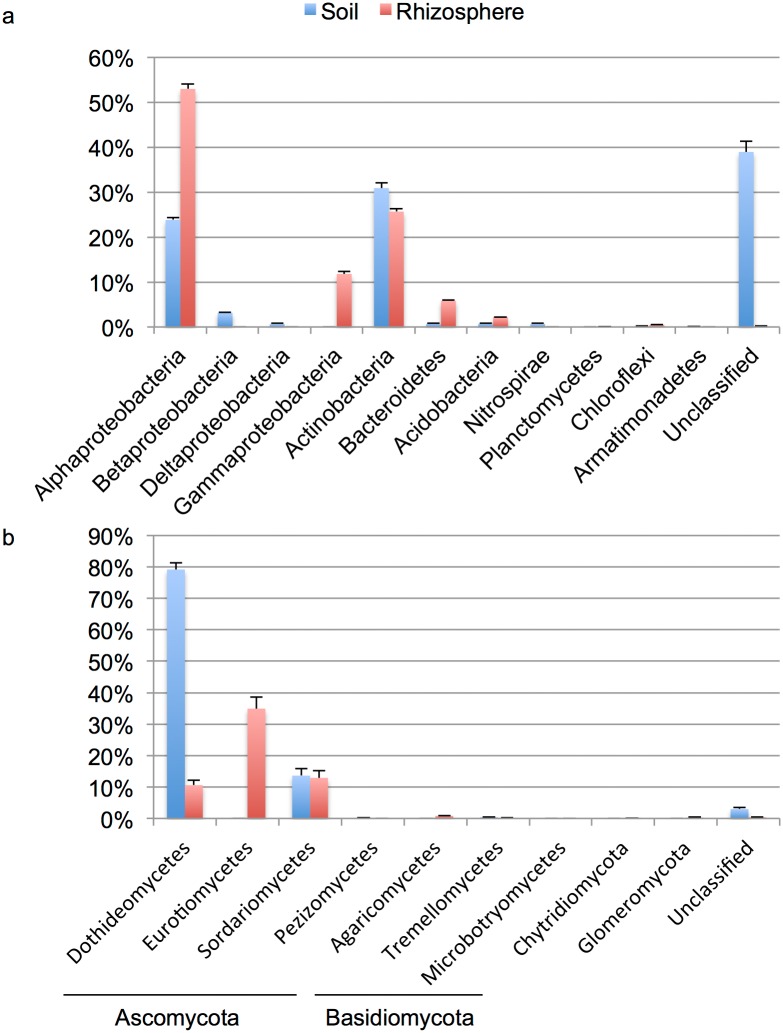
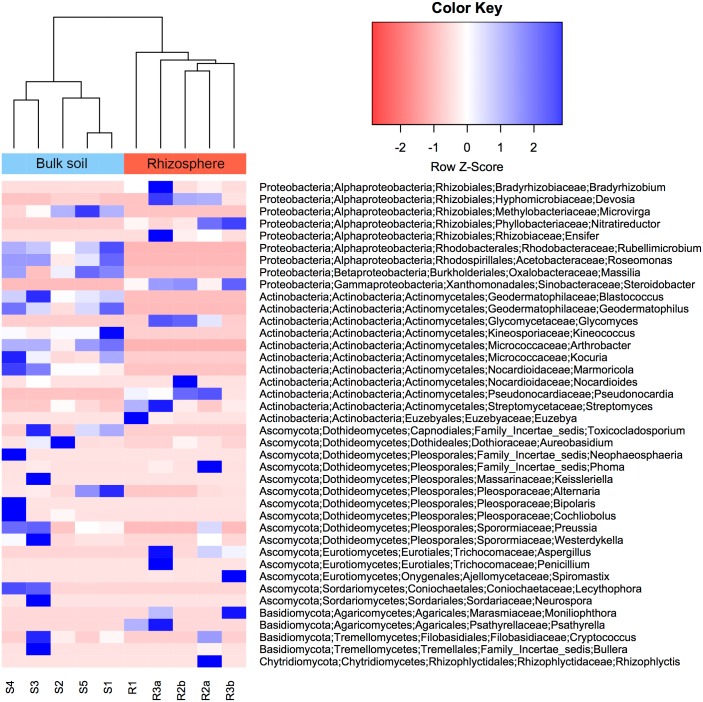
The rhizosphere bacterial communities of W. mirabilis predominantly consisted of sequences from the Proteobacteria (66%, mostly Alphaproteobacteria (53%) and Gammaproteobacteria (12%)), Actinobacteria (26%), Bacteroidetes (6%) and Acidobacteria (2%) (Figs 1a and S3a); these numbers represent the average percentage of sequences across the five samples. Bulk soil bacterial communities were jointly dominated by sequences from the Actinobacteria (31%) and Proteobacteria (28%, Alphaproteobacteria (24%), Betaproteobacteria (3%), Deltaproteobacteria (1%)). Bacteroidetes, Acidobacteria and Nitrospirae each contributed 1%. These phyla have been shown to be widely represented in rhizosphere and soil bacterial communities [7, 22–25], although their relative abundance vary in the different studies. At the genus level, Nitroreductor (20%), Steroidobacter (12%), Pseudonocardia (9%), Devosia (4%) and Glycomyces (2%) were prevalent in the rhizosphere (Fig 2); whereas Rubellimicrobium (9%), Kocuria (5%), Geodermatophilus (5%) and Microvirga (4%) were the genera most frequently found in the bulk soil. Interestingly most genera found in the rhizosphere contain isolates with plant growth-promoting activities (discussed below).
Fig 1. Relative proportions of the (a) bacteria and (b) fungi associated with the Welwitschia rhizosphere and bulk soil.
Error bars indicate mean ± SE.
Fig 2. Heatmap displaying the most abundant genera for rhizosphere and bulk soil samples.
Samples are clustered based on the percent relative abundance of the forty dominant genera (twenty bacteria and twenty fungal genera) shown as rows in this figure. Taxonomy for each genus is presented in the order: phylum, class, order, family, genus. Sample nomenclature indicates the sample type (S = bulk soil; R = rhizosphere), replicate (S = 1 to 5, R = 1 to 3) and pseudoreplicate (a, b).
Rhizosphere fungal communities consisted mainly of Ascomycota (98%, Eurotiomycetes (35%), Sordariomycetes (13%), Dothideomycetes (11%)) (Figs 1b and S3b). Basidiomycota, Glomeromycota and Chytridiomycota contributed 1%, 0.3% and 0.1%, respectively. Bulk soil fungi were also dominated by sequences from the Ascomycota (96.6%), but primarily composed by Dothideomycetes (79%) and Sordariomycetes (14%). Basidiomycota represented a discrete 0.4%. This is in contrast to what has been reported in a global study, where Basidiomycota encompassed the largest proportion of sequences [21]. However, to the best of our knowledge, desert soils were not sampled in the later study. At the genus level, Aspergillus (19%), Spiromastix (16%), Phoma (6%) and Aternaria (3%) dominated rhizosphere fungal communities (Fig 2); whereas Alternaria (28%) and Lecythophora (4%) were prevalent in soil fungal communities. Alternaria and Phoma are plant pathogens, while Aspergillus, Spiromastix and Lecythophora can be classified as saprotrophs (Data file S2 in [21]).
Strikingly, an average of 7% and 39% of the bacterial sequences, for rhizosphere and bulk soil respectively, remained unclassified at the phylum level with the RDP classifier tool (Figs 1a and S3a). For fungi, using the UNITE database, unclassified sequences represented only 0.4% and 3% of the total, for rhizosphere and bulk soil samples respectively (Figs 1b and S3b). The fact that most unclassified sequences were retrieved from bulk soil samples suggests that this ecosystem remains substantially understudied.
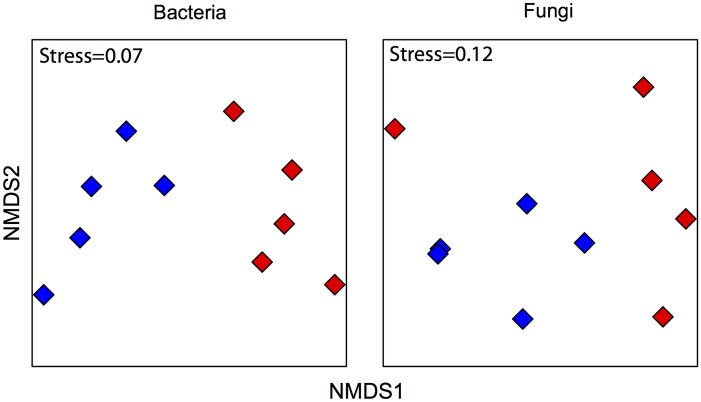
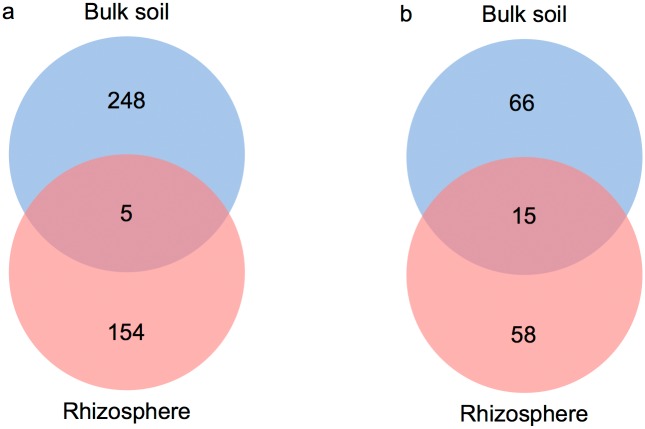
An nMDS plot showed that bacterial and fungal communities were significantly different between bulk and rhizosphere soil samples (ANOSIM: Rbacteria = 0.8, Rfungi = 0.4; both P < 0.05), as measured by weighted UniFrac dissimilarities (Fig 3). This was supported using a Venn diagram (Fig 4a and 4b), as only 1% of the bacterial OTUs and 11% of the fungal OTUs, respectively, were shared between the bulk and the rhizosphere soil. On the basis of previous studies [7, 25–27] and the data presented above this is not an unexpected result. Differences in community composition are probably due to chemical differences between the two environments. Bulk soil has relatively low nutrient concentrations, whereas roots exude organic carbon and other nutrients [28]. This could explain, for example, why Protobacteria are more abundant in the rhizosphere, as many members of this phylum tend to dominate in environments where organic resources are more available [29]. Overall, these results suggest that Welwitschia strongly selects its rhizosphere microbiota.
Fig 3. nMDS ordination plot (UniFrac dissimilarity matrix).
Each point represents the bacterial or fungal community of an individual sample. Rhizosphere communities are indicated by red diamonds, while bulk soil communities are denoted by blue diamonds.
Fig 4. Venn diagram showing the number of shared phylotypes of (a) bacteria and (b) fungi between the rhizosphere and bulk soil communities.
Closer analysis of the rhizosphere samples showed that an average of 16.8% and 2.5% of the bacterial and fungal OTUs, respectively, were shared between any two of these communities. Furthermore, no fungal and only 6 bacterial OTUs (representing 38% of the reads) were consistently present across the samples (S4 Fig). These included Nitratireductor, Steroidobacter, Pseudonocardia and three Phylobacteriaceae OTUs. Overall, this indicates a larger degree of variability between the Welwitschia rhizosphere communities. This appears to be a common feature of host-associated microbial communities (see [30] and references therein) and appears to contradict the evidence for selectivity presented above. However, this apparent contradiction can be resolved assuming a competitive lottery model, applied to explain the variability of epiphytic bacterial community of the green alga Ulva australis [30, 31]. In this model microbial communities are hypothesized to occupy a certain niche (e.g. the rhizsosphere) based on the functions they perform; that is, microbial assemblages are based on functional guilds rather than species. Consequently, the set of species present in any rhizosphere community would be determined by which members of the guild, available in the surrounding soil, colonise the space available first. Future work with a larger number of samples, deeper sequencing and including functional attributes (genes) is needed to resolve whether microorganisms present in the Welwitschia root microbiome are recruited stochastically from the local soil community or actively based on the functions they perform.
It has been postulated that the ability of some desert plant species to survive under extreme conditions is linked to the fact that they associate with plant growth-promoting microbes [32, 33]. Several bacterial and fungal OTUs classified as belonging to plant-beneficial microbes were unique to or overrepresented in the rhizosphere of Welwitschia. These included, for instance, three different genera (i.e. Bradyrhizobium, Ensifer and Mesorhizobium) and three OTUs (family Phylobacteriaceae) of rhizobia, which have the ability to fix atmospheric nitrogen. Acinetobacter and Sphingomonas, known to solubilize soil-insoluble phosphate [34]. Nitratireductor, commonly reported as able to reduce nitrate to nitrite, that could be involved in nitrogen metabolism. Steroidobacter, recognised to produce brassinosteroids, which have been reported to control seed germination, stem and root elongation, vascular differentiation, leaf expansion and stress protection in plants [35]. Pseudonocardia as well as Rhizophagus were also found in the rhizosphere of Welwitschia plants. Pseudonocardia is well-known for producing antibiotic compounds [36], which can theoretically counteract some phytophatogenic microbes, whereas Rhizophagus, an arbuscular mycorrhizal fungus (AMF), could potentially supply phosphorus and other nutrients to Welwitschia in exchange for plant carbon [37]. It is noteworthy that Nitratireductor, Steroidobacter, Pseudonocardia and the three Phylobacteriaceae OTUs were core members of the rhizosphere community (see above). However, more research is needed to elucidate the role of the members of these microbial communities, as it is well known that plant growth promoting characteristics are strain dependent.
In conclusion, we have shown that the rhizosphere of Welwitschia harbours diverse and distinct bacterial and fungal communities compared to the bulk soil. Many of the genera consistently observed in the rhizosphere samples are known to contain strains with plant-growth promoting abilities. Further investigations using culture-based approaches will help in elucidating whether or notthese microbes interact synergistically to promote Welwitschia plant health and productivity.
Supporting Information
Welwitschia plants dotted across an arid landscape (left). The exposed radial root system of a Welwitschia plant (right).
(TIF)
The taxonomic affiliation was performed using the Ribosomal Database Project Classifier (bacteria) and the UNITE database (fungi).
(PDF)
a) bacteria, b) fungi. Sample nomenclature is as in S1 Table.
(PDF)
Sample nomenclature indicates the sample type (S = bulk soil; R = rhizosphere), replicate (S = 1 to 5, R = 1 to 3) and pseudoreplicate (a, b).
(DOCX)
Acknowledgments
The authors gratefully acknowledge the Ministry of Environment and Tourism and Swakop Uranium, both in Namibia, for providing access to the plants. We thank Angie Kanandjembo, Theresa Henschel and Gert Krüger for assistance in sampling.
Data Availability
The sequence data generated in this study were deposited in the NCBI Sequence Read Archive and are available under the project number SRP061179.
Funding Statement
This work was supported by funding from the National Research Foundation, South Africa. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Swakop Uranium provided support in the form of salaries for MKL, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘author contributions’ section.
References
- 1.van der Heijden MGA, Bardgett RD, van Straalen NM. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett. 2008; 11: 296–310. [DOI] [PubMed] [Google Scholar]
- 2.Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev. 2013; 37: 634–63. 10.1111/1574-6976.12028 [DOI] [PubMed] [Google Scholar]
- 3.Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EVL, Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol. 2013; 64: 807–838. 10.1146/annurev-arplant-050312-120106 [DOI] [PubMed] [Google Scholar]
- 4.Mendes LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J. 2014; 8: 1577–87. 10.1038/ismej.2014.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013; 11: 789–99. 10.1038/nrmicro3109 [DOI] [PubMed] [Google Scholar]
- 6.Prober SM, Leff JW, Bates ST, Borer ET, Firn J, Harpole WS, et al. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol Lett. 2015; 18: 85–95. 10.1111/ele.12381 [DOI] [PubMed] [Google Scholar]
- 7.Coats VC, Pelletreau KN, Rumpho ME. Amplicon pyrosequencing reveals the soil microbial diversity associated with invasive Japanese barberry (Berberis thunbergii DC.). Molecular Ecology. 2014; 23: 1318–32. 10.1111/mec.12544 [DOI] [PubMed] [Google Scholar]
- 8.Cooper-Driver GA, Wagner C, Kolberg H. Patterns of Aspergillus niger var. phoenicis (Corda) Al-Musallam infection in Namibian populations of Welwitschia mirabilis Hook.f. J Arid Environ 2000; 46: 181–98. [Google Scholar]
- 9.Bornman C, Elsworthy J, Butler V, Botha C. Welwitschia mirabilis: Observation on general habit, seed, seedling, and leaf characteristics. Madoqua 1972; 1: 53–6. [Google Scholar]
- 10.Chaw SM, Parkinson CL, Cheng Y, Vincent TM, Palmer JD. Seed plant phylogeny inferred from all three plant genomes: Monophyly of extant gymnosperms and origin of Gnetales from conifers. Proc Natl Acad Sci USA. 2000; 97: 4086–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henschel JR, Seely MK. Long-term growth patterns of Welwitschia mirabilis, a long-lived plant of the Namib Desert (including a bibliography). Plant Ecol. 2000; 150: 7–26. [Google Scholar]
- 12.Jacobson KM, Jacobson PJ, Miller OK. The mycorrhizal status of Welwitschia mirabilis. Mycorrhiza. 1993; 3: 13–7. [Google Scholar]
- 13.Dilcher DL, Bernardes-De-Oliveira ME, Pons D, Lott TA. Welwitschiaceae from the Lower Cretaceous of Northeastern Brazil. American Journal of Botany. 2005; 92: 1294–310. 10.3732/ajb.92.8.1294 [DOI] [PubMed] [Google Scholar]
- 14.Valverde A, Makhalanyane TP, Seely M, Cowan DA. Cyanobacteria drive community composition and functionality in rock-soil interface communities. Mol Ecol. 2015; 24: 812–21. 10.1111/mec.13068 [DOI] [PubMed] [Google Scholar]
- 15.Hartmann M, Howes CG, Vaninsberghe D, Yu H, Bachar D, Christen R, et al. Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J. 2012; 6: 2199–218 10.1038/ismej.2012.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009; 75: 7537–41. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schloss PD, Gevers D, Westcott SL. Reducing the effects of pcr amplification and sequencing artifacts on 16S rRNA-based studies. Plos One. 2011; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quince C, Lanzen A, Davenport RJ, Turnbaugh PJ. Removing Noise From Pyrosequenced Amplicons. BMC Bioinf. 2011; 12: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schloss PD, Handelsman J. Status of the microbial census. Microbiol Mol Biol Rev. 2004; 68: 686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007; 73: 5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, et al. Global diversity and geography of soil fungi. Science. 2014; 346: 1256688 10.1126/science.1256688 [DOI] [PubMed] [Google Scholar]
- 22.Bulgarelli D, Rott M, Schlaeppi K, van Themaat EVL, Ahmadinejad N, Assenza F, et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature. 2012; 488: 91–5. 10.1038/nature11336 [DOI] [PubMed] [Google Scholar]
- 23.Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012; 488: 86–90. 10.1038/nature11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fierer N, Leff JW, Adams BJ, Nielsen UN, Bates ST, Lauber CL, et al. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Natl Acad Sci USA. 2012; 109: 21390–5. 10.1073/pnas.1215210110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uroz S, Buee M, Murat C, Frey-Klett P, Martin F. Pyrosequencing reveals a contrasted bacterial diversity between oak rhizosphere and surrounding soil. Environ Microbiol Rep. 2010; 2: 281–8. 10.1111/j.1758-2229.2009.00117.x [DOI] [PubMed] [Google Scholar]
- 26.Stafford WHL, Baker GC, Brown SA, Burton SG, Cowan DA. Bacterial diversity in the rhizosphere of Proteaceae species. Environ Microbiol. 2005; 7: 1755–68. [DOI] [PubMed] [Google Scholar]
- 27.Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL, et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci USA. 2013; 110: 6548–53. 10.1073/pnas.1302837110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bever JD, Platt TG, Morton ER. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu Rev Microbiology. 2012; 66: 265–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fierer N, Bradford MA, Jackson RB. Toward an ecological classification of soil bacteria. Ecology. 2007; 88: 1354–64. [DOI] [PubMed] [Google Scholar]
- 30.Burke C, Steinberg P, Rusch D, Kjelleberg S, Thomas T. Bacterial community assembly based on functional genes rather than species. Proc Natl Acad Sci USA. 2011; 108: 14288–93. 10.1073/pnas.1101591108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S. Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J. 2011; 5: 590–600. 10.1038/ismej.2010.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jorquera MA, Shaharoona B, Nadeem SM, de la Luz Mora M, Crowley DE. Plant Growth-Promoting Rhizobacteria associated with ancient clones of creosote bush (Larrea tridentata). Microb Ecol. 2012; 64: 1008–17. 10.1007/s00248-012-0071-5 [DOI] [PubMed] [Google Scholar]
- 33.Panhwar QA, Naher UA, Jusop S, Othman R, Latif MA, Ismail MR. Biochemical and molecular characterization of potential phosphate-solubilizing bacteria in acid sulfate soils and their beneficial effects on rice growth. Plos One. 2014; 9: e116035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogut M, Er F, Kandemir N. Phosphate solubilization potentials of soil Acinetobacter strains. Biol Fert Soils. 2010; 46: 707–15. [Google Scholar]
- 35.Zarraonaindia I, Owens SM, Weisenhorn P, West K, Hampton-Marcell J, Lax S, et al. The soil microbiome influences grapevine-associated microbiota. mBio. 2015; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barke J, Seipke RF, Grüschow S, Heavens D, Drou N, Bibb MJ, et al. A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. BMC Biol. 2010; 8: 109 10.1186/1741-7007-8-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parniske M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat Rev Microbiol. 2008; 6: 763–75. 10.1038/nrmicro1987 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Welwitschia plants dotted across an arid landscape (left). The exposed radial root system of a Welwitschia plant (right).
(TIF)
The taxonomic affiliation was performed using the Ribosomal Database Project Classifier (bacteria) and the UNITE database (fungi).
(PDF)
a) bacteria, b) fungi. Sample nomenclature is as in S1 Table.
(PDF)
Sample nomenclature indicates the sample type (S = bulk soil; R = rhizosphere), replicate (S = 1 to 5, R = 1 to 3) and pseudoreplicate (a, b).
(DOCX)
Data Availability Statement
The sequence data generated in this study were deposited in the NCBI Sequence Read Archive and are available under the project number SRP061179.