Abstract
Microfold or membranous (M) cells are specialized intestinal epithelial cells responsible for host immunity. The speG mutant of Salmonella Typhimurium (S. Typhimurium) is a nonreplicating strain within human cells to be a candidate vaccine vector for interacting with M cells. We conducted this study to identify the genes are differently expressed between in vitro M cells and Caco-2 cells, and to determine whether S. Typhimurium and speG affect the transcriptomes of both cell types. In vitro M cells and Caco-2 cells were infected with wild-type (WT) S. Typhimurium, its ΔspeG mutant, or none for 1 h for RNA microarrays; the transcriptomes among the 6 pools were pairwisely compared. Genetic loci encoding scaffold (e.g., HSCHR7_CTG4_4, HSCHR9_CTG9_35), long noncoding RNA, membrane-associated protein (PITPNB), neuron-related proteins (OR8D1, OR10G9, and NTNG2), and transporter proteins (MICU2 and SLC28A1) were significantly upregulated in uninfected M cells compared with uninfected Caco-2 cells; and their encoding proteins are promising M-cell markers. Significantly upregulated HSCHR7_CTG4_4 of uninfected in vitro M cells were speG-independently downregulated by S. Typhimurium infection that is a remarkable change representing an important but unreported characteristic of M cells. The immune responses of in vitro M cells and Caco-2 cells can differ and reply on speG or not, with speG-dependent regulation of KYL4, SCTR, IL6, TNF, and CELF4 in Caco-2 cells, JUN, KLF6, and KCTD11 in M cells, or speG-independent modulation of ZFP36 in both cells. This study facilitates understanding of the immune responses of in vitro M cells after administering the S. Typhimurium ΔspeG mutant as a future vaccine vector.
Introduction
Microfold or membranous (M) cells, are specialized intestinal epithelial cells that are involved in gut immunity that relies on collaboration between antigen-sampling of M cells and lymphoid or dendritic cells; therefore, M cells could a good target for delivery of mucosal vaccines into hosts for inducing cellular and humoral immunity [1]. M cells reside in 10% of epithelial cells within the follicle-associated epithelium (FAE) overlaid on the lymphoid follicles of gut-associated lymphoid tissue, including Peyer’s patches, and isolated lymphoid follicles or solitary intestinal lymphoid tissue as non-FAE intestinal villous M cells [2]. M cells are crucial for gut immunity because pathogens and macromolecules within the intestinal lumen can transcytose across M cells into the submucosa of Peyer’s patches to interact with antigen presenting cells and activate subsequent immune responses [2]. The intestinal epithelium consists of 6 major cell types: absorptive columnar epithelial cells, mucin-secreting goblet cells, enteroendocrine cells, antimicrobial peptide-secreting Paneth cells, undifferentiated cells, and M cells [3]. The intestinal epithelial cells constitute a host defense barrier against pathogens during enteric infection. Tight junctions among intestinal epithelial cells, the unique organelles localized to the apical-lateral region of the intestinal epithelium, can block the movement of macromolecules and pathogens across the intestinal epithelium to its basolateral side [4]. However, M cells have sparse irregular microvilli apically, and pocket-like cytoplasmic invagination harboring immune cells basolaterally. These distinctive morphological features enable M cells to uptake and transcytose intestinal antigens to underlying lymphoid tissues where antigen-presenting cells can present the internalized antigens to T cells for initiating protective immune responses [2].
Information on the mechanisms of M cell differentiation remains scant. A few studies have indicated that the epithelial–mesenchymal transition (EMT)-regulating transcription factor Slug, receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL), and SpiB might mediate M-cell development [5, 6]. Primary epithelial cells cultured from FAE isolated from bovine terminal rectum and intestinal epithelial cells in the murine ligated ileal loops containing Peyer’s pactches can be transformed into M cells by S. Typhimurium that SopB-dependently activates Wnt/β-catenin signaling leading to induction of both RANKL and its receptor RANK [6]. The expression of the M cell-specific marker vimentin expression can be controlled via the Wnt/β-catenin signaling pathway following an association with Slug [7]. In the mice studies, M cells located over Peyer’s patches might be derived from Lgr5 stem-cell-derived epithelial cells, and the development of M cells requires the RANKL-induced expression of SpiB [8] or the SpiB-independent pathway after S. Typhimurium infection [9]. However, the detailed mechanism underlying M-cell development requires further clarification.
Because human M cells are typically unapproachable, and in vivo M cells in animal studies cannot be used to answer all questions regarding human M cells, a convenient in vitro human M-cell model is required. Therefore in vitro M cells were firstly established by coculturing the human colon carcinoma cells line, Caco-2, with lymphocytes isolated from Peyer’s patches of BALB/c mice, and the increased internalization of bacteria was demonstrated in this model [10]. Thereafter, a modified in vitro M-cell model was established by coculturing Caco-2 cells with human Raji B cells [11], thus providing a simple method for investigating human M cells. However, little is known regarding the mechanisms underlying this in vitro model.
Salmonella is one of the enteropathogenic bacteria that can penetrate the intestinal epithelial barrier through M cells from the intestinal lumen into the lamina propria. However, whether Salmonella preferentially invades M cells rather than enterocytes in humans remains obscure because so far no human study in this issue has been conducted. Animal studies have been used to demonstrate that Salmonella enterica serovar Typhimurium (S. Typhimurium) preferably invades M cells located in the FAE of Peyer’s patches in mice [12, 13]. These M cells are a critical entry site for the colonization and internalization of Salmonella in Salmonella pathogenesis. However, the cellular responses of M cells after Salmonella infection remain unclear.
To date, the phenotypic characterization of speG has not been investigated in Salmonella within human intestinal epithelial cells although the expression of speG has been annotated in the previous studies using RNA-sequencing. The expression of speG in S. Typhimurium was downregulated in some of the 22 in vitro infection-relevant environmental conditions, particularly late stationary phase and pH3 shock [14]. The RNA transcriptomic expression of speG was non-significantly expressed at early stationary phase of S. Typhimurium [15]. However, the small regulatory RNAs of speG were non-significantly expressed in S. Typhimurium after invasion into murine macrophages for 18 h [16]. In all Shigella species, sepG encodes spermidine N1-acetyltransferase mediating intracellular spermidine accumulation [17]. Another study using human non-intestinal epithelial cells, Caenorhabditis elegans, and C57/BL6 mice (Nramp-) demonstrated that polyamines are required for virulence in S. Typhimurium, which is especially controlled by putrescine and spermidine through the stimulation of expression of their biosynthetic genes at 5 distinct genetic loci: speA, speB, speC, speDE, and speF, and these polyamines function as a signal priming S. Typhimurium for intracellular survival [18]. Our preliminary results revealed that deletion of speG can attenuate intracellular replication but not colonization and invasion of S. Typhimurium in human epithelial cells [19]. Therefore, this characteristic enables the S. Typhimurium speG mutant to function as a potential oral vaccine vector.
For this study, we used the well established in vitro M-cell model as previously described [20]. The in vitro M cells and Caco-2 cells were infected with wild-type (WT) S. Typhimurium and speG-deleted strains. The transcriptomes of in vitro M cells and Caco-2 cells before and after S. Typhimurium infection were obtained using RNA microarrays, after which we conducted a pairwise comparison. We identified significantly regulated genes of the in vitro M cells compared with Caco-2 cells, those of both cells after S. Typhimurium infection relative to non-infected cells, and those of both the cells after the depletion of speG compared with the WT strain. These results were then confirmed by conducting a quantitative real-time polymerase chain reaction (qRT-PCR) of the selected identified genes. Overall, this microarray study enables a thorough investigation of the genes involved in the differentiation of in vitro M cells, and furthers our understanding regarding the gene expression of in vitro M cells and Caco-2 cells that can be postinfectiously regulated by S. Typhimurium and its speG gene.
Materials and Methods
Bacterial strains and culture
The S. Typhimurium WT strain SL1344, Salmonella pathogenicity island-1 (SPI-1) mutant strain ΔspaS, and speG-deleted strain ΔspeG were used in this study. The SPI-1 mutant strain ΔspaS was provided by Professor Duncan Maskell. The speG gene in S. Typhimurium SL1344 was deleted using the lambda Red recombinase-mediated integration of linear PCR amplicons as previously described [21], with the gene replaced by a kanamycin-resistance gene. Before each experiments, these strains were recovered from GermBank (CMPTM Culture Media, Taiwan) stocks maintained at −80°C onto Luria–Bertani (LB) agar plates, which were subsequently incubated at 37°C for growing colonies. For cell infections, the single colonies of each strains were aerobically grown in 3 mL of Luria–Bertani (LB) broth (Difco) at 37°C for 18 h to reach a bacterial density of 1 × 109 CFU/mL as overnight cultures. If necessary, kanamycin (50 μg/mL) was used for maintaining ΔspaS and ΔspeG.
In vitro M-cell culture model
The in vitro M-cell culture model was established as previously described, with minor modifications [20]. The Raji B cells, a cell line of human Burkitt’s lymphoma purchased from the Bioresource Collection and Research Center, Taiwan (BCRC No. 60116, originally from ATCC No. CCL-86), were grown in 90% RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco), and 1% L-glutamine (Gibco), 1.5 g/L of sodium bicarbonate (Sigma), 4.5 g/L of glucose (Sigma), 10 mM of HEPES (Sigma), and 1.0 mM of sodium pyruvate (Sigma) at 37°C in 5% CO2. The Caco-2 cells, a C2BBe1 clone of a Caucasian human’s colon adenocarcinoma, purchased from the Bioresource Collection and Research Center, Taiwan (BCRC No. 60182, originally from ATCC No. CRL-2102), were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% FBS (Sigma), 100 μM of nonessential amino acids (Sigma), 1% L-glutamine (Gibco), and 0.01 mg/mL of transferrin (Sigma) at 37°C in 5% CO2. After trypsinization, 5 × 105 Caco-2 cells (3–6 passages since obtainment from BCRC) were seeded onto the 9.5-mm-diameter collagen (10 μg/cm2)-precoated polycarbonate Millipore membrane inserts with 3-μm pores in Millicell 24-well plates, and were incubated at 37°C in 5% CO2 for 14 d. At this stage, the transepithelial electrical resistance (TEER) values across the Caco-2 cell monolayers were measured using a voltage meter EVOM (World Precision Instruments) in accordance with manufacturer instructions. After the polarization of Caco-2 cells with adequate epithelial barrier integrity was achieved and validated using TEER values higher than 300 Ω·cm2, Raji B cells at a density of 5 × 105 cells/well were added into the basolateral chamber, which contained one-third of the complete RPMI medium and two-thirds of the complete DMEM. The co-cultures were continued for an additional 6 d to induce M-cell-like monolayer formation. During the 20-d cultures, 100 units/mL of penicillin and 0.1 mg/mL of streptomycin (Sigma) were administered in the culture media and removed 2 h before the experiments. The 20-d-old polarized Caco-2 cells that were not cocultured with Raji B cells were used as the controls.
Electron micrographs of in vitro M cells and Caco-2 cells
In vitro M cells and Caco-2 cells were morphologically evaluated using electron microscopy after 20 d of culturing. During harvesting, each well of the cell monolayers was washed with PBS twice and fixed with 2.5% glutaraldehyde in a 0.1-M phosphate buffer for further processing before analysis through scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
For SEM, the glutaraldehyde-fixed samples were washed with a 0.1-M phosphate buffer supplemented with 3% sucrose (Sigma) for 60 min. The samples were subsequently treated with 1% aqueous osmium tetroxide (Sigma) for 15 min. After treatment, the samples were dehydrated in acidified 2,2-dimethoxypropoane (Sigma) for 5 min, and transferred to 100% ethanol (Sigma) for 2 min. All of the samples were then critically point-dried in liquid CO2 by using a K850 critical point dryer (Quorum Technologies), mounted on aluminum stubs, and putter-coated with a mixture of gold and palladium by using an Eiko IB-2 ion coater (Eiko). Finally, these prepared samples were observed using a Hitachi SU3500 scanning electron microscope.
For TEM, the glutaraldehyde-fixed samples were post-fixed in 1% osmium tetroxide for 2 h, washed with PBS, dehydrated in a graded series of ethanol, and finally rinsed with propylene oxide (Sigma). The samples were then embedded in Epon (Serva), and incubated at 65°C for 48 h. The thick sections were cut using an ultra-microtome (0.05 μm) and mounted on mesh grids. Finally, the sections were observed under the Hitachi H-600 or HT-7700 transmission electron microscope.
Transcytosis assay
The in vitro M cells were functionally assessed using a transcytosis assay. In brief, the overnight cultures of WT S. Typhimurium and ΔspaS were diluted 1:100 in fresh LB broth and incubated with shaking for 3.5 h at 37°C until the mid-log phase of growth was reached. After replacement with the serum-free media, the mid-log cultures of the bacterial strains were coincubated with in vitro M cells or Caco-2 cells at a multiplicity of infection (MOI) of 25 for 1 h in three independent experiments. After 1-h bacterial infection, the integrity of the infected cell monolayers were validated by their TEER values of >250 Ω·cm2. The mid-log cultures and media from the basolateral chambers were then serially diluted and plated out on LB agar plates prior to overnight incubation at 37°C. The bacterial colony-forming units (CFUs) were counted, and the number of the transcytosed bacteria was calculated compared with the initial inoculums, expressed as CFU per initial inoculum of 108 CFU.
Treatment of in vitro M cells and Caco-2 cells for RNA isolation
Using the same protocol as that used for the transcytosis assay, the in vitro M cells and Caco-2 cells were treated with WT S. Typhimurium and ΔspeG at an MOI of 25, or with the LB broth as the noninfection control, for 1 h in two independent experiments. The total RNAs of both the cells from these 3 conditions were isolated using TRIzol reagent (Invitrogen) in accordance with the manufacturer instructions, and the preformed RNA was purified using an RNeasy Mini Kit (Qiagen). The RNA concentration was determined using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific), and the integrity of the RNA samples was validated using the ratio of absorbance at 260 nm and 280 nm, as well as the RNA integrity number determined using Bioanalyzer 2100 (Agilent Technology) with an RNA 6000 Nano LabChip kit (Agilent).
RNA microarrays
The total RNA samples were reverse-transcribed to cDNAs and subsequent cRNA, which were subsequently labeled with Cy3-CTP (CyDye, Agilent) by using an Agilent Low Input Quick-Amp Labeling kit (Agilent) in accordance with manufacturer instructions. Thus, the Cy3-labled cRNAs were fragmented into 50–100 nucleotides through incubation with a fragmentation buffer at 60°C for 30 min. All of the fragmented labeled cRNA were pooled and hybridized to Agilent SurePrint G3 human V2 GE 8 × 60K arrays that had been tiled with 50 599 human gene probes (Agilent) at 65°C for 17 h. The array chips were washed, dried, and then scanned using an Agilent microarray scanner at 535 nm for Cy3-CTP. The scanned images were quantified and analyzed using Feature Extraction 10.5.1.1 software (Agilent). The background values were corrected using the spatial detrend surface value, and were normalized by quantile. Finally, the gene expression levels in each array group were analyzed using the DAVID database (http://david.ncifcrf.gov). The relative gene expression levels were compared to the mean of each array and their fold-change values were calculated. The heap map with genes in each group that upregulated or downregulated more than 2-fold was constructed based on their normalized values by using GeneSpring multiomic analysis software (Agilent). The microarray data has been deposited in GEO (http://www.ncbi.nlm.nih.gov/geo/) and is accessible via GEO Accession Number GSE73880.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The primers of the selected genes identified in the RNA microarrays were designed using Primer3 and BLAST online (http://www.ncbi.nlm.nih.gov/tools/primer-blast/), and are listed in S1 Table. The total RNAs from the in vitro M cells and Caco-2 cells were isolated using the TRIzol reagent (Invitrogen) in accordance with manufacturer instructions. Each microgram of isolated total RNA was treated with one units of DNase I (New England Biolabs) at 37°C for 10 min in order to remove any residual genomic DNA, and the mixtures were heat-inactivated at 75°C for 10 min. The purified RNA samples were then reverse-transcribed into cDNAs by using an iScript cDNA synthesis kit (Bio-Rad). The mixtures were incubated at 25°C for 5 min and at 42°C for 30 min before heating at 85°C for 5 min. Finally, qRT-PCR was conducted in triplicate using 50 ng of cDNA and specific primers in each reaction by using an iQ SyBr green supermix kit (Bio-Rad) on a CFX96 real time PCR system (Rio-Rad). Each reaction involved incubation at 95°C for 3 min for denaturation, followed by 40 cycles of DNA amplifications were amplified at 95°C for 15 s, at 55°C for 30 s, and at 72°C for 30 s. Three housekeeping genes, GAPDH, RPLP, and HPRT, were used as internal controls. The expression level of each gene was calculated using the ΔΔCt method. To ensure speG can be expressed after invasion of S. Typhimurium into host cells, we infected Caco-2 cells with S. Typhimurium SL1344 at a MOI of 25 for 6 h and extracted the RNAs from the mid-log cultures and the intracellular bacteria after lysing the infected Caco-2 cells using 10% Triton X-100. Then, the RNAs were processed as above and qRT-PCR was performed using the above protocol and the designed primers (S1 Table) for quantifying the expression levels of speG in S. Typhimurium SL1344 isolated from mid-log cultures and bacterial infected Caco-2 cells, both of which were normalized against mRNA levels of 16s as internal controls. All the values of mRNA expression levels were compared to the mean of those in mid-log cultured S. Typhimurium SL1344 and the data were expressed as mean ± standard deviation.
Statistical analysis
A Student’s t-test was performed for a between-group comparison involving the transcytosis assay and qRT-PCR in this study. A p value < 0.05 was considered as significant difference. Transcriptomes among the 6 pools (uninfected, SL1344-infected or ΔspeG mutant-infected in vitro M cells and Caco-2 cells) were subjected to a pairwise comparison after correction through two-way ANOVA to determinate the p values. A p value < 0.01 with > 2- or < –2-fold change was considered statistically significant.
Results
Electron microscopy shows morphological transformation of Caco-2 cells into in vitro M cells
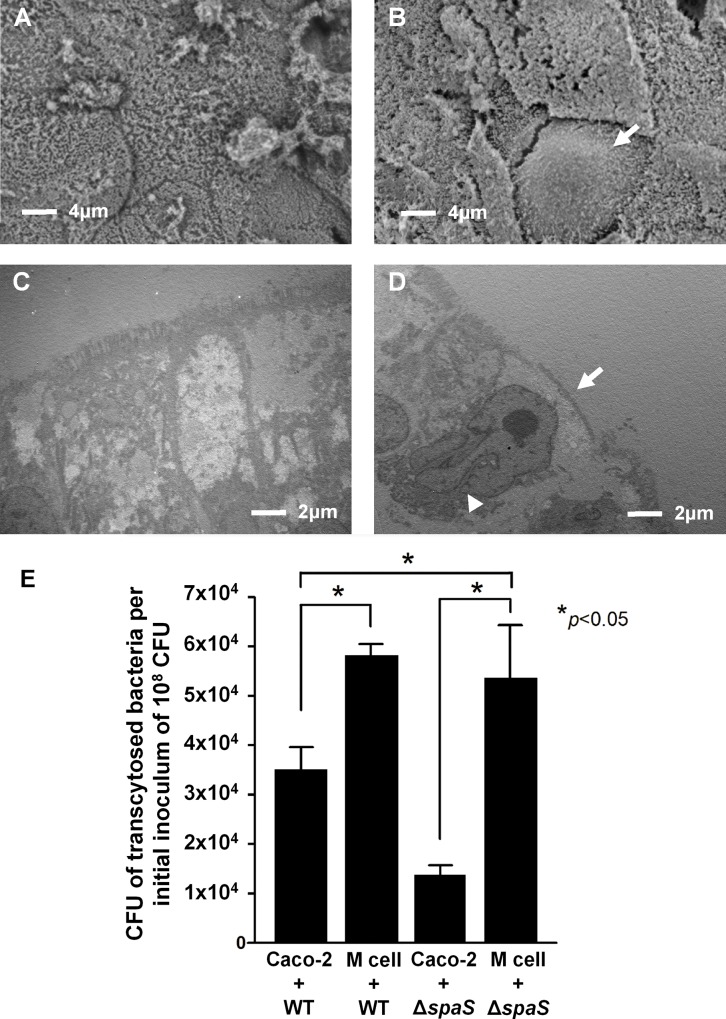
For confirming whether the 20-d-old polarized Caco-2 cells had been transformed into in vitro M cells after coculturing with Raji-B cells 6 d prior, we performed SEM and TEM to assess the morphological changes between in vitro M cells and Caco-2 cells. The SEM images showed that intact normal microvilli were expressed on the apical sides of the polarized Caco-2 cells (Fig 1A), and the number of irregular microvilli diminished on the cell surfaces of the in vitro M cells (Fig 1B). The cross-sectional TEM images showed that the lengths of microvilli and cell shape were normal in the Caco-2 cells (Fig 1C); however, the in vitro M cells had sparse shortened irregular microvilli on the apical surface, with basolateral invaginations harboring lymphocytes (Fig 1D). Under electron microscopy, these M-cell-like cells were not found in the Caco-2 cells as the controls, but were identified in approximately 5–10% of the polarized Caco-2 cells cocultured with Raji B cells, which is close to the previous report [2]. Therefore, these morphological characteristics confirmed that the Caco-2 cell monolayers cocultured with Raji B cells transformed into a cell population of an epithelium containing M-cell-like cells.
Fig 1. Morphological and functional assessment of polarized Caco-2 cells and in vitro M cells.
Scanning electron micrographs (SEM) (2700×) of (A) 20-day-old polarized Caco-2 cells and (B) in vitro M cells. (C) Ultrastructural features of the polarized Caco-2 cell monolayer in the transmission electron micrographs (TEM). (D) Characteristic features of in vitro M cells in the TEM, showing irregular shortened microvilli on the apical surface (arrow) and a basolateral pocket harboring lymphocytes (arrow head). (E) Bacterial translocation rates after wild-type (WT) S. Typhimurium and ΔspaS infections in Caco-2 cell and in vitro M cells. In vitro M cells and Caco-2 cells were individually infected with WT S. Typhimurium and ΔspaS (multiplicity of infection = 25) for 1 h. The translocation rates of each group were calculated using the bacterial numbers in the media from the basolateral chambers of Transwells compared with the initial inoculums of 108 colony-forming unit (CFU). *p < 0.05 was considered as statistically significant.
Transcytosis assay proves functional translocation of noninvasive S. Typhimurium across in vitro M cells
In addition to the morphological observation of the in vitro M cells, we conducted a transcytosis assay to assess a critical function of M cells (i.e., to translocate particles from their apical to basolateral sides). The SPI-1 genes, such as spaS, of S. Typhimurium encode the apparatus of the type III secretion system (T3SS) which mediates bacterial colonization in the gastrointestinal tract [13, 22]. In our preliminary study, we found that S. Typhimurium ΔspaS is a noninvasive mutant strain compared with WT S. Typhimurium in human epithelial cells [19]. Therefore, we used this mutant strain as a noninvasive bioparticle in the transcytosis assay to determine whether it could be translocated by in vitro M cells. First, we observed that the transcytosis rate of S. Typhimurium ΔspaS was significantly lower than that of WT S. Typhimurium in the Caco-2 cells (gray versus white bar in Fig 1E). This result is consistent with our previous finding on noninvasiveness of S. Typhimurium ΔspaS [19]. In addition, our data revealed that the transcytosis rate of S. Typhimurium ΔspaS in M cells was significantly higher than that in Caco-2 cells (black versus gray bar in Fig 1E), and similar to that of WT S. Typhimurium in M cells (striated bar in Fig 1E). These data indicated that in vitro M cells can translocate this attenuated noninvasive bacteria from the apical to basolateral side, which is a major characteristic of M cells. In a similar manner, WT S. Typhimurium can be translocated across M cells more easily than across Caco-2 cells (striated versus white bar in Fig 1E), which could be a result of M-cell transcytosis as well as possibly a phenomenon of cell tropism for S. Typhimurium. Therefore, our in vitro M-cell culture model showed that both invasive and noninvasive S. Typhimurium strains could translocate across in vitro M-cell monolayers, which were successfully formed by transforming polarized Caco-2 cells into M-like cells with a capacity of particle transcytosis.
Microarray analysis identifies 20 significantly upregulated genes of in vitro M cells compared with Caco-2 cells
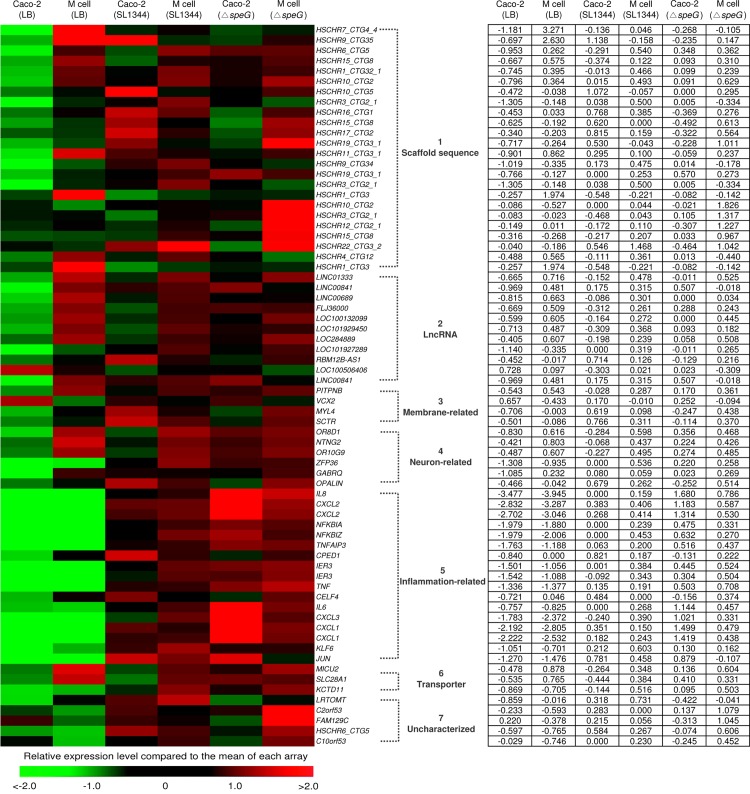
Prior to micoarray analysis, we first confirmed that the expression level of speG in S. Typhimurium within the infected Caco-2 cells was 1.6-fold higher than in the extracellular S. Typhimurium from mid-log culture before invasion (S1 Fig). After we morphologically and functionally confirmed our in vitro M-cell culture model, we further compared the global transcriptomes between in vitro M cells and Caco-2 cells, and investigated the influence of S. Typhimurium and its speG gene in the host cells by using RNA microarrays. Among 50,599 genes, we identified 70 genes that were significantly upregulated or downregulated, and they were classified into 8 groups based on their relative or potential functions (Fig 2). Microarray analysis revealed that 20 genes of the uninfected in vitro M cells were significantly upregulated compared with the uninfected polarized Caco-2 cells, and the functions of these 20 genes were grouped into 6 different categories: 6 scaffold sequences, 7 long noncoding RNA (lncRNA) genes, one membrane-associated gene, 3 neuron-related protein-encoding genes, 2 transporter-related genes, and one uncharacterized X-linked member gene (Table 1). Among the 6 scaffold sequences belonging to the uncharacterized portion of the bacterial genome, the most significantly upregulated gene of the in vitro M cells was HSCHR7_CTG4_4 (17.637-fold change, Table 1), which encodes a hypothetical protein and is located between HTR5A antisense RNA 1 and PAXIP1 antisense RNA 1 on chromosome 7. Another 2 markedly upregulated scaffold sequences with limited information are HSCHR9_CTG35 which is close to PTPN3 (protein tyrosine phosphatase nonreceptor type 3) on chromosome 9, and HSCHR10_CTG2, which is located between PITRM1 (pitrilysin metallopeptidase 1) and LOC101927880 (lncRNA) on chromosome 10. The other 3 scaffold sequences, HSCHR6_CTG5, HSCHR15_CTG8, and HSCHR1_CTG32_1, encode hypothetical products with unknown functions. In addition, 7 lncRNA genetic loci were significantly upregulated in the in vitro M cells, with a change in RNA expression levels ranging from 2.038- to 2.622-fold and with unknown functions (Table 1). The highly expressed PITPNB gene (2.086-fold change) of the in vitro M cells encodes phosphatidylinositol transfer protein β, which catalyzes the transfer of phosphatidylinositol and phosphatidylcholine, and is a cytoplasmic protein associated with the cell membrane. Compared with the Caco-2 cells, the in vitro M cells had 3 significantly upregulated genes, OR8D1, OR10G9, and NTNG2, which encode neuron-related proteins. The OR8D1 (2.47-fold change) and OR10G9 (2.07-fold change) genes encode olfactory receptors 8D1 and 10G9, respectively, whereas NTNG2 (2.11-fold change) encodes the netrin-G2 precursor. Moreover, 2 transporter-related genes of the in vitro M cells were remarkably upregulated. The MICU2 (2.36-fold change) gene encodes calcium uptake protein 2, and SLC28A1 (2.32-fold change) encodes a nucleoside transporter. The only significantly downregulated gene, VCX2, encodes variable charge X-linked protein 2 (−2.463-fold change, Table 1).
Fig 2. Heat map of microarray analysis and the corresponding values of fold change for the 70 significantly upregulated or downregulated genes of in vitro M cells and Caco-2 cells after infection with wild-type (WT) S. Typhimurium and ΔspeG or remaining uninfected for 1 h.
We found 70 genes of in vitro M cells and Caco-2 cells that were significantly upregulated (>2-fold change, red bricks) after treatment with WT S. Typhimurium SL1344, the speG-deleted mutant (ΔspeG), and only Luria-Bertani broth (LB). These 70 genes were classified into 8 groups according to their relative or potential functions. Red bricks represent higher gene expression levels compared with average levels (black bricks).
Table 1. Significantly upregulated or downregulated genes of in vitro M cells compared with Caco-2 cells.
| Gene | Product | Description | Fold change |
|---|---|---|---|
| Scaffold | |||
| HSCHR7_CTG4_4 | Hypothetical | Chr7:155062052–155061993 Between HTR5A-AS1 (HTR5A antisense RNA 1) and PAXIP1-AS1 (PAXIP1 antisense RNA 1) | 17.637 |
| HSCHR9_CTG35 | Hypothetical | Chr9:109401669–109435160 Close to PTPN3 (protein tyrosine phosphatase, nonreceptor type 3) | 8.860 |
| HSCHR6_CTG5 | Hypothetical | 2.179 | |
| HSCHR15_CTG8 | Hypothetical | 2.153 | |
| HSCHR1_CTG32_1 | Hypothetical | 2.082 | |
| HSCHR10_CTG2 | Hypothetical | Chr10:3309069–3309128 Between PITRM1 (pitrilysin metallopeptidase 1) and LOC101927880 (lncRNA) | 2.062 |
| Long noncoding RNA | |||
| LINC01333 | Hypothetical | Unknown | 2.622 |
| LINC00841 | Hypothetical | Unknown | 2.542 |
| LINC00689 | Hypothetical | Unknown | 2.455 |
| FLJ36000 | Hypothetical | Unknown | 2.178 |
| LOC100132099 | Hypothetical | Unknown | 2.120 |
| LOC101929450 | Hypothetical | Unknown | 2.117 |
| LOC284889 | Hypothetical | Unknown | 2.038 |
| Membrane association | |||
| PITPNB | Phosphatidylinositol transfer protein β | Catalyze the transfer of phosphatidylinositol and phosphatidylcholine | 2.086 |
| Neuron-related protein | |||
| OR8D1 | Olfactory receptor, family 8, subfamily D, member 1 | Mediate neurological system development | 2.477 |
| NTNG2 | Netrin-G2 | Maintain glutamatergic neural circuitry | 2.117 |
| OR10G9 | Olfactory receptor, family 10, subfamily G, member 9 | Mediate neurological system development | 2.075 |
| Transporter | |||
| MICU2 | Mitochondrial calcium uptake 2 | Calcium uptake protein | 2.365 |
| SLC28A1 | Solute carrier family 28 | Nucleoside transporter | 2.321 |
| Uncharacterized | |||
| VCX2 | Variable charge, X-linked 2 | Belong to X-linked members | −2.463 |
Microarray analysis identifies 25 significantly upregulated genes and 1 significantly downregulated gene of S. Typhimurium WT-infected in vitro M cells compared with Caco-2 cells
We investigated the impacts of S. Typhimurium infection on in vitro M cells and Caco-2 cells by comparing the transcriptomes of WT S. Typhimurium-infected cells with those of uninfected cells after analysis conducted using RNA microarrays. Twenty-six genes of Caco-2 cells were significantly regulated after WT S. Typhimurium infection, including 8 scaffold genes (2.646- to 2.059-fold change), 3 lncRNA genes (2.076- to −2.146-fold change), 2 membrane remodeling associated genes (2.169- to 2.379-fold change), 3 neuron-related genes (2.022- to 2.472- fold change), 9 inflammation-related genes (2.112- to 10.083-fold change), and one uncharacterized gene encoding a leucine-rich protein (S2 Table). The functions of the proteins encoded by all the identified scaffold and lncRNA genes were unknown. Regarding the 2 upregulated genes associated with membrane remodeling, MYL4 encodes myosin light chain 4, which is a hexametric ATPase cellular motor protein, whereas SCTR encodes a secretin receptor that belongs to the G protein-coupled receptor family. Moreover, the 3 neuron-related genes were upregulated, including ZFP36 encoding zinc finger protein 36, which is a myeloid cell tristetraprolin (TTP) mediating the regulation of myeloid cell differentiation, GABRQ encoding γ-aminobutyric acid (GABA) A receptor θ, which mediates neurotransmission, and OPALIN encoding oligodendrocytic myelin paranodal and inner-loop protein, which controls the development of the central nervous system. The 9 upregulated inflammatory genes are those encoding chemokines interleukin (IL)-8, which mediates the chemoattraction of neutrophils, and CXCL2, NFKBIA, NFKBIZ, and TNFFAIP3 involved in the activation of NF-κB signaling, CPED1, which mediates cell adhesion, and IER3, tumor necrosis factor (TNF), and CELF4, which mediate apoptosis.
We classified 13 significantly upregulated or downregulated genes of S. Typhimurium-infected in vitro M cells into 3 groups (S3 Table). The most highly upregulated scaffold gene HSCHR7_CTG4_4 in the uninfected in vitro M cells compared with the Caco-2 cells (Table 1) was reversely downregulated (−10.109-fold change) after WT S. Typhimurium infection (S3 Table). Similar to the upregulation in Caco-2 cells after WT S. Typhimurium infection (S2 Table), the neuron-related gene ZFP36 was significantly upregulated (2.266-fold change) in the WT S. Typhimurium-infected in vitro M cells (S3 Table). This observation indicated that ZFP36 is involved in S. Typhimurium infection. The inflammation-related genes constitute the master group of strongly regulated genes in the WT S. Typhimurium-infected in vitro M cells, with IL8 as the most highly expressed gene (14.929-fold change) and the genes encoding chemokine ligands and NF-κB activators. The JUN (3.010-fold change) and KLF6 (2.006-fold change) genes were significantly upregulated only in the WT S. Typhimurium-infected in vitro M cells (S3 Table), but neither in the WT S. Typhimurium-infected Caco-2 cells nor in the S. Typhimurium ΔspeG-infected in vitro M cells or Caco-2 cells (S2, S4 and S5 Tables), indicating that JUN and KLF6 are inflammatory factors specific to the S. Typhimurium-infected M cells and their expression requires presence of bacterial speG.
Microarray analysis identifies 22 significantly upregulated genes and 4 significantly downregulated genes of S. Typhimurium ΔspeG-infected in vitro M cells compared with Caco-2 cells
To explore the effects of the speG gene on S. Typhimurium-infected in vitro M cells and Caco-2 cells, we compared the transcriptomes of the S. Typhimurium ΔspeG-infected in vitro M cells and Caco-2 cells against those of the corresponding uninfected cells. First, we performed microarray analysis to compare the gene expression levels of S. Typhimurium ΔspeG-infected Caco-2 cells against those of uninfected Caco-2 cells (S4 Table). The scaffold genes HSCHR19_CTG3_1 and HSCHR3_CTG2_1 were significantly upregulated (2.147- to 2.11-fold change) in the S. Typhimurium ΔspeG-infected Caco-2 cells, and both of these genes were also significantly upregulated in the WT S. Typhimurium-infected Caco-2 cells (S2 Table). The LINC00841 (2.334-fold change) gene was the only upregulated lncRNA gene in ΔspeG-infected Caco-2 cells. The neuron-related gene ZFP36 was upregulated not only in the WT S. Typhimurium-infected in vitro M cells (S3 Table) and Caco-2 cells (S2 Table) but also in the S. Typhimurium ΔspeG-infected Caco-2 cells (2.423-fold change, S4 Table). The inflammatory genes also comprised the major group of considerably regulated genes in the S. Typhimurium ΔspeG-infected Caco-2 cells, with IL8 being the most significantly upregulated gene (23.865-fold change, S4 Table). This IL8 expression level of the S. Typhimurium ΔspeG-infected Caco-2 cells was higher than that of the WT S. Typhimurium-infected Caco-2 cells (10.083-fold change, S2 Table). Without speG, IL6 (3.12–fold change) and TNF (3.069-fold change) were significantly upregulated in the Caco-2 cells after S. Typhimurium infection, whereas CELF4 was not significantly upregulated (S2 and S4 Tables), suggesting that speG is involved in upregulation of IL6 and TNF and downregulation of CELF4 in the S. Typhimurium-infected Caco-2 cells.
Second, we compared the transcriptome of the S. Typhimurium ΔspeG-infected in vitro M cells with that of the uninfected in vitro M cells (S5 Table). Similar to that (−10.109-fold change, S3 Table) in the WT S. Typhimurium-infected M cells, the scaffold gene HSCHR7_CTG4_4 was significantly downregulated (−12.496-fold change, S5 Table) in the S. Typhimurium ΔspeG-infected in vitro M cells. Regardless of cell types or the presence of speG in S. Typhimurium, the neuron-related gene ZFP36 was upregulated in the S. Typhimurium ΔspeG-infected in vitro M cells (2.009-fold change, S5 Table). These findings indicated that the upregulation of ZFP36 was chiefly due to S. Typhimurium, not due to speG or the cell type. The IL8 gene was the most significantly upregulated of the S. Typhimurium ΔspeG-infected in vitro M cells (22.356-fold change, S5 Table). The potassium transporter gene KCTD11 of the in vitro M cells was markedly upregulated after ΔspeG infection (S5 Table), indicating that speG plays a role in suppressing the expression of this potassium transporter of M cells during S. Typhimurium infection.
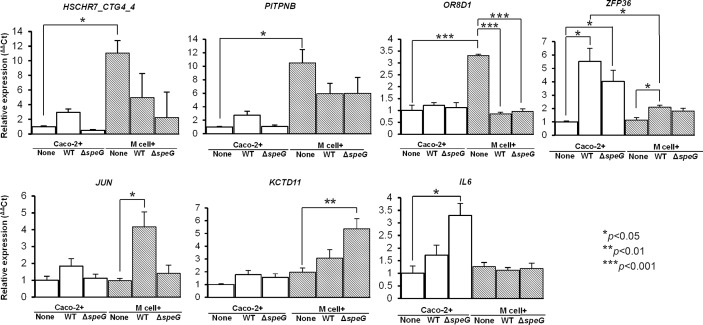
Microarray analysis is validated by qRT-PCR in the mRNA expression of HSCHR7_CTG4_4, PITPNB, OR8D1, ZFP36, JUN, KCTD11, and IL6 of in vitro M cells and Caco-2 cells after S. Typhimurium WT and ΔspeG infection
To reconfirm the microarray data, we selected 7 significantly regulated genes for qRT-PCR to quantify their mRNA expression levels in the in vitro M cells and Caco-2 cells with and without infection of S. Typhimurium WT and ΔspeG. The earlier microarray data showed that the scaffold gene HSCHR7_CTG4_4 was significantly upregulated in uninfected in vitro M cells compared with uninfected Caco-2 cells (Table 1), and remarkably downregulated in the WT S. Typhimurium- and ΔspeG-infected in vitro M cells (S3 Table). The qRT-PCR analysis results revealed reproducible data showing that HSCHR7_CTG4_4 was highly expressed in the uninfected in vitro M cells (11.5-fold higher than in the uninfected Caco-2 cells); however, the mRNA expression levels were significantly inhibited in the in vitro M cells after WT S. Typhimurium and ΔspeG infection (Fig 3). In addition, the microarray data indicated that both the PITPNB and OR8D1 genes were significantly upregulated in uninfected in vitro M cells (Table 1), which was similarly shown by a 10- and 3.3-fold change in the upregulation of PITPNB and OR8D1, respectively, in qRT-PCR analysis (Fig 3). The expression levels of OR8D1 were significantly downregulated in the in vitro M cells after S. Typhimurium WT and ΔspeG infection compared with the uninfected cells according to qRT-PCR analysis (Fig 3); however, they were nonsignificantly downregulated (−1.197- and −1.339-fold change, respectively) according to the microarray data. The gene expression of ZFP36 was significantly upregulated in the in vitro M cells and Caco-2 cells after both WT S. Typhimurium and ΔspeG infection according to both qRT-PCR (Fig 3) and microarray analyses (S2 to S5 Tables). The expression levels of ZFP36 in S. Typhimurium-infected M cells were significantly lower than those in S. Typhimurium-infected Caco-2 cells regardless of speG deletion (Fig 3), suggestive of milder ZFP36-related speG-independent inflammation in M cells than in Caco-2 cells. Moreover, the major inflammatory gene JUN was significantly upregulated only in the WT S. Typhimurium-infected in vitro M cells, but neither in the WT S. Typhimurium-infected Caco-2 cells nor in the S. Typhimurium ΔspeG-infected Caco-2 cells or in vitro M cells (Fig 3), implying that JUN is a characteristic inflammatory factor of M cells after S. Typhimurium infection and requires contribution of speG to such inflammation. The potassium transporter gene KCTD11 was significantly upregulated only in the S. Typhimurium ΔspeG-infected in vitro M cells, and IL6 was highly expressed in the S. Typhimurium ΔspeG-infected Caco-2 cells (Fig 3). The significant upregulation of these 3 genes, as determined through qRT-PCR analysis, was consistent with the microarray analysis results.
Fig 3. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of 7 selected genes, HSCHR7_CTG4_4, PITPNB, OR8D1, ZFP36, JUN, KCTD11, and IL6 in the uninfected, wild-type (WT) S. Typhimurium- and ΔspeG-infected Caco-2 cells and in vitro M cells.
The 7 significantly regulated genes indentified using RNA microarrays were further validated through qRT-PCR with their specific primers. The mRNA expression levels of each group were normalized against the geometric means of 3 house keeping genes GAPDH, RPLP and HPRT. The expression levels of individual genes were calculated using the ΔΔCt method, and expressed as the fold change compared with the geometric mean expression level of uninfected Caco-2 cells in triplicate. Data are represented as mean. ± SEM. p < 0.05 was considered to represent a significant difference.
Discussion
We successfully established an in vitro M-cell culture model to investigate the differences in the expression of several previously unreported genes in in vitro M cells when compared with Caco-2 cells; we identified a significant expression of these genes for the first time in human in vitro M cells. Researchers using rat models have indicated that Slug, RANKL, and SpiB are involved in M-cell development [5, 7]. However, none of the genes related to these 3 markers were significantly upregulated in our human in vitro M-cell model, implying the possible presence of host specificity in M cells. The phosphoinositide 3-kinase (PI3K) signaling pathway may play a potential role in M cells. Our microarray data revealed that HSCHR9_CTG35, which is located near the genes PTPN3 (Table 1), and PITPNB were significantly upregulated in our human in vitro M cells. The proteins encoded by PTPN3 and PITPNB might be involved in PI3K regulation [23, 24]. Studies have indicated that PI3K and Wnt/β-catenin signaling pathways contribute to cell differentiation. For example, the development of lymphocytes is modulated by PI3K-related signals [25]. This evidence hints that the differentiation of human in vitro M cells is also controlled via the PI3K signaling pathway. Moreover, several neuron-related genes, including NTNG2, OR8D1, and OR10G9, were strongly upregulated after the transformation of Caco-2 cells into in vitro M cells in our model (Table 1). The NTNG2 gene encodes the netrin-G2 protein, which is a potential mediator for mediating the development of the Drosophila olfactory system [26]. Moreover, the OR8D1- and OR10G9-encoded olfactory receptor proteins are potentially involved in olfactory system development. To date studies have not linked these genes with human M cells; nevertheless, MICU2, which encodes mitochondrial calcium uptake 2 protein, can regulate mitochondrial activity and cell survival [27], and SLC28A1, which encodes solute carrier family 28, a nucleoside transporter, were markedly upregulated in our in vitro M cells. The solute carrier family also potentially regulates mitochondrial activity [28]. Because mitochondrial activity is closely related to the differentiation of cell embryonal and stem cells [29], mitochondrial activity might be implicated in development of M cells. The only one significantly downregulated gene, VCX2, which encodes an uncharacterized X-linked member protein, might counteract the differentiation of human in vitro M cells. To date, X-linked members have not been characterized in M-cell development. The B cells from Peyer’s patches of X-linked immunodeficient mice distinctly interact with T cells [30]. Therefore, the X-linked members possibly regulate the microenvironment of gut immunity. These novel findings warrant further investigation for clarification.
Human M-cell makers have rarely been reported, except sialyl Lewis A antigen and galectin-9, which have been identified in M cells in human intestinal tissues [31, 32]. However, the functions of these 2 markers remain unclear. In addition, several M cell-specific proteins have been used as specific markers for identifying M cells in animal studies, with limited knowledge on their features, such as secretory granule neuroendocrine protein 1 (Sgen-1) [33], annexin A5 [34] and glycoprotein 2 (GP2) in mice [35], vimentin in rabbits [36], and cytokeratin 18 in cattle [37]. The GP2, expressed on the apical sides of murine M cells, has been well documented in previous studies. By contrast, GP2 remained undetected in a study using human in vitro M cells [38], as well as in our microarray data. Therefore, GP2 might be a poor marker for identifying human in vitro M cells, or even genuine human M cells. Our results revealed that the scaffold sequence HSCHR7_CTG4_4 exhibited the highest expression level in the transcriptome of the in vitro M cells (17.637-fold change, Table 1), which was confirmed by the similarly high mRNA expression level identified through qRT-PCR analysis (10.14-fold change, Fig 3). However, HSCHR7_CTG4_4 was significantly downregulated after S. Typhimurium infection, regardless of the presence of speG (−10.109-fold change, S3 Table; −12.496-fold change, S5 Table); that is, HSCHR7_CTG4_4 of human in vitro M cells can be speG-independently switched off by S. Typhimurium infection (green versus red bricks in Fig 2). In summary, HSCHR7_CTG4_4 can be strongly expressed noninfectiously and suppressed postinfectiously in human in vitro M cells. It is worthy of investigation whether such a drastic change from upregulation to downregulation of HSCHR7_CTG4_4 could transform more Caco-2 cells to M-like cells in our in vitro M-cell model after S. Typhimurium infection. The function of HSCHR7_CTG4_4, which encodes a hypothetical protein, is estimated to be similar to that of its neighboring genes HTR5A-AS1 and PAXIP1-AS1. HTR5A-AS1 is involved in serotonin receptor formation and PAXIP1-AS1 is involved in the expression of PAX-interacting protein 1, which encodes a nuclear protein with 6 breast cancer carboxy-terminal (BRCT) domains for maintaining genome stability, chromatin condensation, and progression through mitosis. Another gene, HSCHR9_CTG35, also had a similar gene expression to that of HSCHR7_CTG4_4 in human in vitro M cells (Table 1 and Fig 2), and this gene is located near PTPN3, which encodes a member of the protein tyrosine phosphatase family for the regulation of cellular processes and membrane-related functions. Although the phenotypes of the proteins encoded by these 2 genes remain hypothetical, the mentioned functions of their neighboring genes could possibly be involved in human M-cell development. Their encoded proteins can be potential candidate cell markers for identifying and isolating human M cells in the future. Moreover, S. Typhimurium infection can induce the transition of bovine and murine intestinal epithelial cells to M cells via activation of RANKL [6], but the expression of the RANKL genes were not significantly upregulated in the human Caco-2 cells and in vitro M cells after S. Typhimurium infection in our study. Therefore, the transformation of M cells might not be entirely controlled by the same regulatory networks in different species.
In the host, M cells provide a crucial route for bacterial internalization and transcytosis to the lamina propria. After such transcytosis across the intestinal epithelium, bacteria might encounter lymphocytes for antigen presentation to T cells and mononuclear phagocytes for the clearance of antigens [39]. Differing from the translocation of antigens across M cells into their basolateral space, intestinal epithelial cells secrete the IL-8 chemokine to attract neutrophils for migrating into the basolateral side of the infected intestinal epithelium and the subsequent clearance of bacteria [40]. IL-8 is involved in Salmonella internalization, and its transcription and secretion in the epithelium is activated via the NF-κB pathway [41]. Our data also revealed that IL8 was highly upregulated in both in vitro M cells and Caco-2 cells after S. Typhimurium infection, with the simultaneous upregulation of the genes related to the NF-κB signaling pathway for the activation of inflammatory responses. Another study also reported that IL8 transcription in in vitro M cells was upregulated after Escherichia coli and Bacteroides fragilis infections [42]; however, to date, no such report has been presented on salmonellosis. We speculated that IL-8 released from M cells might attract more gathering of immune cells beneath M cells to facilitate elimination of transcytosed bacteria. Moreover, JUN, which encodes the other major transcription factor AP-1, related to the activation of immunity, was highly expressed only in the S. Typhimurium-infected in vitro M cells according to our microarray data, implicating the significance of JUN in M cells after S. Typhimurium infection. Furthermore, a recent study using single-cell RNA-sequencing showed that the expression of type I interferon and toll-like receptor 4 related genes was upregulated within macrophages isolated from S. Typhimurium-infected mice [43]. However, the upregulated expression of these genes was not observed in our in vitro M cells and Caco-2 cells infected with S. Typhimurium. The contrasting results between phagocytic and non-phagocytic cells suggested the diversity of immune responses in different host cells after Salmonella infection.
Although in vitro M cells are derived from Caco-2 cells, our findings indicated that the immune responses of both cells can differ and involve speG. One study indicated that an SPI-1 factor of Salmonella, SipA, can induce CXC-chemokine expression in HeLa cells via the JUN pathway [44]. Salmonella can provoke membrane ruffling of host cells through its SPI- 1 factors during invasion into epithelial cells. The membrane ruffling results from cytoskeletal rearrangement, which is induced by G-coupled protein activation [45], and potentially regulated by ATPase [46]. Our microarray analysis results consistently showed that the ATPase-encoding gene MYL4 and the G-coupled protein-encoding gene SCTR were significantly upregulated in Caco-2 cells after S. Typhimurium infection but not in M cells, indication that MYL4 and SCTR are speG-dependent inflammatory factors specific to the S. Typhimurium-infected Caco-2 cells. In addition, the involvement of speG in upregulation of IL6 and TNF and downregulation of CELF4 in the S. Typhimurium-infected Caco-2 cells is also validated by our microarray analysis. By contrast, our data showed that JUN encoding transcription factor AP-1 and KLF6 encoding Kruppel-like factor 6 were significantly upregulated in M cells rather than in Caco-2 cells after S. Typhimurium infection with presence of speG. In addition, potassium transporter-encoding KCTD11 was remarkably downregulated in M cells after S. Typhimurium infection with absence of speG. Therefore, speG can modulate the expression of JUN, KLF6, and KCTD11 in the S. Typhimurium-infected M cells.
The inflammation-related gene expression of in vitro M cells and Caco-2 cells after S. Typhimurium infection was not attenuated, but augmented by the deletion of Salmonella speG, which disabled intracellular S. Typhimurium from proliferating within host cells. Our preliminary study indicated the incapability of intracellular replication, but the capability of bacterial invasion of S. Typhimurium ΔspeG in HeLa cells [18]. The expression of speG of S. Typhimurium was downregulated by the infection-relevant growth conditions such as late stationary phase, cold shock, pH shock, NaCl shock, anaerobic shock, peroxide shock, and nitric oxide shock that simulate environmental stressors during the infection of the mammalian host [14]. By contrast, the expression of speG was 1.3-fold expressed within macrophages [15], In addition, We found that the expression of speG was 1.6-fold increased in S. Typhimurium within the infected Caco-2 cells in comparison with those extracellular S. Typhimurium before invasion (S1 Fig). Therefore, we supposed that speG of Salmonella plays an influential role in immune responses after bacterial invasion into host cells, and we conducted this in vitro study utilizing two human intestinal epithelial cells. We obtained the transcriptomes of uninfected in vitro M cells and Caco-2 cells, as well as those of both cells infected with WT S. Typhimurium and ΔspeG by using RNA microarrays and performing a pairwise comparison. Most of the inflammation-related genes of S. Typhimurium ΔspeG-infected cells were more significantly upregulated compared with those of WT S. Typhimurium-infected cells, either in vitro M cells or Caco-2 cells. Evidently, IL8 of Caco-2 cells was the most strongly up-regulated among all of the genes examined after WT S. Typhimurium infection (10.083-fold change, S2 Table); however, such upregulation increased more than 2-fold after S. Typhimurium ΔspeG infection (23.865-fold change, S4 Table). Moreover, the IL8 expression of in vitro M cells was upregulated 14.929 fold after WT S. Typhimurium WT infection (S3 Table); however, this upregulation was increased approximately 1.5 times after S. Typhimurium ΔspeG infection (22.356-fold change, S5 Table). The gene expression of the other inflammatory factors such as chemokine ligand 2, NF-κB inhibitors, and tumor necrosis factor (TNF) also exhibited a similar trend, but with milder intensity.
Furthermore, the disruption of speG in S. Typhimurium significantly upregulated the IL6 expression of Caco-2 cells (3.120-fold change, S4 Table), but not upregulated in in vitro M cells (1.189-fold change, Fig 2), implying that speG can suppress IL6 upregulation in S. Typhimurium-infected Caco-2 cells. Our qRT-PCR data showed a reproducible result (Fig 3). IL-6 is an integral cytokine mediator of the acute phase response to injury and infection, and can stimulate immune responses such as the activation of T cells proliferation [47]. Whether the intestinal epithelium is an important source for IL-6 production has rarely been reported. Increased IL-6 production by macrophages, T lymphocytes, and intestinal epithelial cells is a hallmark for the pathogenesis of inflammatory bowel disease [48]. The expression of IL-6 can be enhanced at the protein or gene levels in Caco-2 cells postinfection with Salmonella [49]. In addition, IL-6 can impair tight junctions, thereby increasing the permeability of Caco-2 cells [50]. Our study was the first report in no involvement of IL6 upregulation in the inflammation of the S. Typhimurium-infected in vitro M cells that is different from the speG-involved inflammation of the S. Typhimurium-infected Caco-2 cells (Fig 3). The expression of IL6 was downregulated in uninfected Caco-2 cells, and IL6 was neutrally expressed after S. Typhimurium SL1344 infection and significantly upregulated after S. Typhimurium ΔspeG infection (Figs 2 and 3). In addition, the in vitro M cells in our study exhibited no significant upregulation of IL6 in the S. Typhimurium ΔspeG-infected M cells (Figs 2 and 3). This implied that IL6 expression was suppressed in a fully differentiated Caco-2 cells and in vitro M cells; however, S. Typhimurium initiated its expression, and the loss of speG further provoked its expression significantly in Caco-2 cells (Fig 3), and no such a phenomenon in M cells; that is, S. Typhimurium speG suppressed IL6 expression and its related inflammation only in Caco-2 cells but not M cells. Overall, speG is crucial for suppressing IL-6-involved inflammation of human intestinal epithelial cells after S. Typhimurium infection, which is not a characteristic inflammatory factor of human M cells. Disabling speG in S. Typhimurium can stimulate IL6-related responses within Caco-2 cells, which might partly explain why S. Typhimurium ΔspeG lost its capability of intracellular proliferation in our study.
The ZFP36 encoding zinc finger protein 36, namely TTP, was significantly upregulated in Caco-2 cells and in vitro M cells after S. Typhimurium infection, regardless of the presence of speG. TTP can regulate the function of brain-derived neurotrophic factor in the central nervous system, and iron homeostasis in fibroblasts [51]. In addition, a recent study indicated that TTP has an anti-inflammatory effect on the IL-10 production of murine macrophages [52]. IL-10 can be secreted from human colon epithelial cells, and impede pathogen clearance by inhibiting the NF-κB signaling pathway in human monocytes [53]. Therefore, we proposed that ZFP36 upregulation can suppress the IL-10 secretion of host cells, not only of phagocytic cells but possibly of intestinal epithelial cells and M cells as well after S. Typhimurium infection, and such a phenotype of ZFP6 is not be influenced by the Salmonella speG gene. Our study demonstrated that ZPF36 was significantly upregulated in the S. Typhimurium-infected Caco-2 cells and in vitro M cells with a higher intensity in the former (Fig 3), suggesting a hypothesis that milder ZPF36 expression in M cells than in Caco-2 cells contributes to the functional or morphological characteristics of M cells. The ZFP36 gene might be an important host defense factor of human intestinal epithelial cells, including M cells, during bacterial infection, and requires further study for elucidation.
Certain neuron-related genes of human intestinal epithelial cells are significantly expressed during Salmonella infection. Unlike ZFP36 which was persistently upregulated in both in vitro M cells and Caco-2 cells after WT S. Typhimurium and ΔspeG infection, the other 2 neuron-related genes, GABRQ and OPALIN, were significantly upregulated in only S. Typhimurium-infected Caco-2 cells (2.028- and 2.022-fold change, respectively; S2 Table), but not in uninfected or infected M cells (S3 and S5 Tables). The deletion of speG in S. Typhimurium normalized the upregulation of GABRQ and OPALIN in Caco-2 cells (S4 Table), implying that speG modulates the expression of these 2 genes in Caco-2 cells after S. Typhimurium infection. GABRQ encodes GABA receptor A, which is involved in synaptic transmission within the neural system [54]. Because GABA plays a role in anti-inflammation and the inhibition of autoimmune inflammation in mice [55], GABRO upregulation in S. Typhimurium-infected Caco-2 cells implied that GABRO is possibly involved in host cell defense of intestinal epithelium during salmonellosis. In addition, OPALIN mediates development of the central nervous system and its encoding protein Opalin, also known as transmembrane protein 10, is a specific marker in oligodendrocytes, which can regulate microglial activity via the TNF-α and IL-1β regulatory network in mice and rats [56]. To date, no study has reported any link between OPALIN and infection or inflammation of the intestinal epithelium. Our novel findings of these 2 neuron-related genes and the contribution of speG to their upregulation warrant further investigation for clarification into their roles in gut immunity.
We identified 11 individual lncRNA sequences in our microarray analysis, implying that the upregulation of these sequences may play a role in the regulation of in vitro M-cell development (Table 1), host responses of Caco-2 cells after S. Typhimurium infection (S2 Table), and a potential effect of speG on S. Typhimurium-infected Caco-2 cells (S4 Table). The lncRNAs have been wildly identified in the human genome. Approximately 62% of the identified genes may be transcribed into noncoding RNA, and approximately 14 000 lncRNA genes have been annotated in humans [57]. However, numerous lncRNAs have not been characterized in their functions. Several lncRNAs are involved in the regulation of immune response, including NEAT, which can mediate IL8 expression [58], and THRIL, which can regulate TNF-α expression [59]. Nevertheless, the functions of the 11 identified lncRNAs in our microarrays remain unknown.
In conclusion, we successfully utilized an in vitro M-cell model to discover a number of novel genetic loci with significantly regulated gene expression after comparing and statistically analyzing the transcriptomes of S. Typhimurium-infected and uninfected in vitro M cells and Caco-2 cells. Significantly expressed HSCHR7_CTG4_4 of uninfected in vitro M cells can be speG-independently suppressed by S. Typhimurium infection that is a remarkable change in the regulation of gene expression in M cells after S. Typhimurium infection, which represents an important but unreported cell characteristic. The immune responses of in vitro M cells and Caco-2 cells can differ and reply on speG or not, with speG-dependent regulation of KYL4, SCTR, IL6, TNF, and CELF4 of Caco-2 cells, and JUN, KLF6, and KCTD11 in M cells, or speG-independent modulation of ZFP36 in both cells. This study provides a global gene profiling of in vitro M cells before and after S. Typhimurium infection, which can be developed as a platform for examining the S. Typhimurium ΔspeG mutant as a candidate oral vaccine vector and its relevant gut immune responses.
Supporting Information
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
We thank Ms. Huei-Min Chen in the Core Facility Center at Taipei Medical University for her assistance with electron microscopy.
Abbreviations
- BCRC
Bioresource Collection and Research Center
- BRCT
breast cancer carboxy-terminal
- CFU
bacterial colony-forming unit
- DMEM
Dulbecco’s modified Eagle’s medium
- EMT
epithelial–mesenchymal transition
- FAE
follicle-associated epithelium
- FBS
fetal bovine serum
- GP2
glycoprotein 2
- IL
interleukin
- LB
Luria–Bertani
- lncRNA
long noncoding RNA
- M
microfold or memembraous
- MOI
multiplicity of infection
- NF-κB
receptor activator of nuclear factor-κB
- PI3K
phosphoinositide 3-kinase
- qRT-PCR
quantitative real-time polymerase chain reaction
- RANKL
receptor activator of nuclear factor-κB ligand
- S. Typhimurium
Salmonella enterica serovar Typhimurium
- SEM
scanning electron microscopy
- Sgen-1
secretory granule neuroendocrine protein 1
- SPI
Salmonella pathogenicity island
- T3SS
type III secretion system
- TEER
transepithelial electrical resistance
- TEM
transmission electron microscopy
- TNF
tumor necrosis factor
- TTP
tristetraprolin
- WT
wild-type
Data Availability
All relevant data are within the paper and its Supporting Information files. The microarray data has been deposited in GEO (http://www.ncbi.nlm.nih.gov/geo/) and is accessible via GEO Accession Number GSE73880.
Funding Statement
The work was supported by the following: Ministry of Science and Technology, formerly the National Science Council (NSC 102-2320-B-038-009-), https://www.most.gov.tw/en/public; National Taipei University of Technology-Taipei Medical University Joint Research Program (NTUT-TMU-101-18, NTUT-TMU-102-09), http://www-en.ntut.edu.tw/bin/home.php, http://www.tmu.edu.tw/english/main.php; and Career Development Grant, National Health Research Institutes, Taiwan (NHRI-EX103-10234SC), http://english.nhri.org.tw/NHRI_WEB/nhriw001Action.do. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yamamoto M, Pascual DW, Kiyono H. M cell-targeted mucosal vaccine strategies. Curr Top Microbiol Immunol. 2012;354:39–52. 10.1007/82_2011_134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci U S A. 2004;101(16):6110–5. 10.1073/pnas.0400969101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. 10.1146/annurev.physiol.010908.163145 [DOI] [PubMed] [Google Scholar]
- 4.Rajasekaran SA, Beyenbach KW, Rajasekaran AK. Interactions of tight junctions with membrane channels and transporters. Biochim Biophys Acta. 2008;1778(3):757–69. 10.1016/j.bbamem.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 5.Kanaya T, Hase K, Takahashi D, Fukuda S, Hoshino K, Sasaki I, et al. The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nat Immunol. 2012;13(8):729–36. 10.1038/ni.2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahoun A, Mahajan S, Paxton E, Malterer G, Donaldson DS, Wang D, et al. Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion. Cell Host Microbe. 2012;12(5):645–56. 10.1016/j.chom.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 7.Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116(Pt 3):499–511. [DOI] [PubMed] [Google Scholar]
- 8.de Lau W, Kujala P, Schneeberger K, Middendorp S, Li VS, Barker N, et al. Peyer's patch M cells derived from Lgr5(+) stem cells require SpiB and are induced by RankL in cultured "miniguts". Mol Cell Biol. 2012;32(18):3639–47. 10.1128/MCB.00434-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato S, Kaneto S, Shibata N, Takahashi Y, Okura H, Yuki Y, et al. Transcription factor Spi-B-dependent and -independent pathways for the development of Peyer's patch M cells. Mucosal Immunol. 2013;6(4):838–46. 10.1038/mi.2012.122 [DOI] [PubMed] [Google Scholar]
- 10.Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277(5328):949–52. [DOI] [PubMed] [Google Scholar]
- 11.Gullberg E, Leonard M, Karlsson J, Hopkins AM, Brayden D, Baird AW, et al. Expression of specific markers and particle transport in a new human intestinal M-cell model. Biochem Biophys Res Commun. 2000;279(3):808–13. 10.1006/bbrc.2000.4038 [DOI] [PubMed] [Google Scholar]
- 12.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994;180(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6(1):53–66. 10.1038/nrmicro1788 [DOI] [PubMed] [Google Scholar]
- 14.Kroger C, Colgan A, Srikumar S, Handler K, Sivasankaran SK, Hammarlof DL, et al. An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe. 2013;14(6):683–95. 10.1016/j.chom.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 15.Srikumar S, Kroger C, Hebrard M, Colgan A, Owen SV, Sivasankaran SK, et al. RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Salmonella Typhimurium. PLoS Pathog. 2015;11(11):e1005262 10.1371/journal.ppat.1005262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroger C, Dillon SC, Cameron AD, Papenfort K, Sivasankaran SK, Hokamp K, et al. The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci U S A. 2012;109(20):E1277–86. 10.1073/pnas.1201061109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbagallo M, Di Martino ML, Marcocci L, Pietrangeli P, De Carolis E, Casalino M, et al. A new piece of the Shigella Pathogenicity puzzle: spermidine accumulation by silencing of the speG gene [corrected]. PLoS One. 2011;6(11):e27226 10.1371/journal.pone.0027226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelsbak L, Thomsen LE, Wallrodt I, Jensen PR, Olsen JE. Polyamines are required for virulence in Salmonella enterica serovar Typhimurium. PLoS One. 2012;7(4):e36149 10.1371/journal.pone.0036149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang SB. Early interactions of non-typhoidal Salmonella with human epithelium. Doctoral thesis, UCL (University College London) 2011.
- 20.Martinez-Argudo I, Jepson MA. Salmonella translocates across an in vitro M cell model independently of SPI-1 and SPI-2. Microbiology. 2008;154(Pt 12):3887–94. 10.1099/mic.0.2008/021162-0 [DOI] [PubMed] [Google Scholar]
- 21.Gust B, Challis GL, Fowler K, Kieser T, Chater KF. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A. 2003;100(4):1541–6. 10.1073/pnas.0337542100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones MA, Hulme SD, Barrow PA, Wigley P. The Salmonella pathogenicity island 1 and Salmonella pathogenicity island 2 type III secretion systems play a major role in pathogenesis of systemic disease and gastrointestinal tract colonization of Salmonella enterica serovar Typhimurium in the chicken. Avian Pathol. 2007;36(3):199–203. 10.1080/03079450701264118 [DOI] [PubMed] [Google Scholar]
- 23.Cosker KE, Shadan S, van Diepen M, Morgan C, Li M, Allen-Baume V, et al. Regulation of PI3K signalling by the phosphatidylinositol transfer protein PITPα during axonal extension in hippocampal neurons. J Cell Sci. 2008;121(Pt 6):796–803. 10.1242/jcs.019166 [DOI] [PubMed] [Google Scholar]
- 24.Venable CL, Frevert EU, Kim YB, Fischer BM, Kamatkar S, Neel BG, et al. Overexpression of protein-tyrosine phosphatase-1B in adipocytes inhibits insulin-stimulated phosphoinositide 3-kinase activity without altering glucose transport or Akt/Protein kinase B activation. J Biol Chem. 2000;275(24):18318–26. 10.1074/jbc.M908392199 [DOI] [PubMed] [Google Scholar]
- 25.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317–30. 10.1038/nri1056 [DOI] [PubMed] [Google Scholar]
- 26.Das A, Chiang A, Davla S, Priya R, Reichert H, Vijayraghavan K, et al. Identification and analysis of a glutamatergic local interneuron lineage in the adult Drosophila olfactory system. Neural Syst Circuits. 2011;1(1):4 10.1186/2042-1001-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 2012;151(3):630–44. 10.1016/j.cell.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiermonte G, Paradies E, Todisco S, Marobbio CM, Palmieri F. A novel member of solute carrier family 25 (SLC25A42) is a transporter of coenzyme A and adenosine 3',5'-diphosphate in human mitochondria. J Biol Chem. 2009;284(27):18152–9. 10.1074/jbc.M109.014118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells. 2011;29(3):486–95. 10.1002/stem.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eldridge JH, Beagley KW, McGhee JR. Immunoregulation in the Peyer's patch microenvironment. Cellular basis for the enhanced responses by the B cells of X-linked immunodeficient CBA/N mice. J Immunol. 1987;139(7):2255–62. [PubMed] [Google Scholar]
- 31.Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR. Human intestinal M cells display the sialyl Lewis A antigen. Infect Immun. 1999;67(2):946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pielage JF, Cichon C, Greune L, Hirashima M, Kucharzik T, Schmidt MA. Reversible differentiation of Caco-2 cells reveals galectin-9 as a surface marker molecule for human follicle-associated epithelia and M cell-like cells. Int J Biochem Cell Biol. 2007;39(10):1886–901. 10.1016/j.biocel.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 33.Hase K, Ohshima S, Kawano K, Hashimoto N, Matsumoto K, Saito H, et al. Distinct gene expression profiles characterize cellular phenotypes of follicle-associated epithelium and M cells. DNA Res. 2005;12(2):127–37. 10.1093/dnares/12.2.127 [DOI] [PubMed] [Google Scholar]
- 34.Verbrugghe P, Waelput W, Dieriks B, Waeytens A, Vandesompele J, Cuvelier CA. Murine M cells express annexin V specifically. J Pathol. 2006;209(2):240–9. 10.1002/path.1970 [DOI] [PubMed] [Google Scholar]
- 35.Terahara K, Yoshida M, Igarashi O, Nochi T, Pontes GS, Hase K, et al. Comprehensive gene expression profiling of Peyer's patch M cells, villous M-like cells, and intestinal epithelial cells. J Immunol. 2008;180(12):7840–6. [DOI] [PubMed] [Google Scholar]
- 36.Jepson MA, Mason CM, Bennett MK, Simmons NL, Hirst BH. Co-expression of vimentin and cytokeratins in M cells of rabbit intestinal lymphoid follicle-associated epithelium. Histochem J. 1992;24(1):33–9. [DOI] [PubMed] [Google Scholar]
- 37.Hondo T, Kanaya T, Takakura I, Watanabe H, Takahashi Y, Nagasawa Y, et al. Cytokeratin 18 is a specific marker of bovine intestinal M cell. Am J Physiol Gastrointest Liver Physiol. 2011;300(3):G442–53. 10.1152/ajpgi.00345.2010 [DOI] [PubMed] [Google Scholar]
- 38.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6(4):666–77. 10.1038/mi.2013.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–53. 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- 40.Hammond ME, Lapointe GR, Feucht PH, Hilt S, Gallegos CA, Gordon CA, et al. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J Immunol. 1995;155(3):1428–33. [PubMed] [Google Scholar]
- 41.Gewirtz AT, Rao AS, Simon PO Jr., Merlin D, Carnes D, Madara JL, et al. Salmonella typhimurium induces epithelial IL-8 expression via Ca(2+)-mediated activation of the NF-κB pathway. J Clin Invest. 2000;105(1):79–92. 10.1172/JCI8066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lapthorne S, Macsharry J, Scully P, Nally K, Shanahan F. Differential intestinal M-cell gene expression response to gut commensals. Immunology. 2012;136(3):312–24. 10.1111/j.1365-2567.2012.03581.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avraham R, Haseley N, Brown D, Penaranda C, Jijon HB, Trombetta JJ, et al. Pathogen Cell-to-Cell Variability Drives Heterogeneity in Host Immune Responses. Cell. 2015;162(6):1309–21. 10.1016/j.cell.2015.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Figueiredo JF, Lawhon SD, Gokulan K, Khare S, Raffatellu M, Tsolis RM, et al. Salmonella enterica Typhimurium SipA induces CXC-chemokine expression through p38MAPK and JUN pathways. Microbes Infect. 2009;11(2):302–10. 10.1016/j.micinf.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 45.Ma AD, Metjian A, Bagrodia S, Taylor S, Abrams CS. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase γ, a Rac guanosine exchange factor, and Rac. Mol Cell Biol. 1998;18(8):4744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma B, Qian D, Nan Q, Tan C, An L, Xiang Y. Arabidopsis vacuolar H+-ATPase (V-ATPase) B subunits are involved in actin cytoskeleton remodeling via binding to, bundling, and stabilizing F-actin. J Biol Chem. 2012;287(23):19008–17. 10.1074/jbc.M111.281873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161(9):4652–60. [PubMed] [Google Scholar]
- 48.Mitsuyama K, Sata M, Rose-John S. Interleukin-6 trans-signaling in inflammatory bowel disease. Cytokine Growth Factor Rev. 2006;17(6):451–61. 10.1016/j.cytogfr.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 49.Huang FC. Upregulation of Salmonella-induced IL-6 production in Caco-2 cells by PJ-34, PARP-1 inhibitor: involvement of PI3K, p38 MAPK, ERK, JNK, and NF-κB. Mediators Inflamm. 2009;2009:103890 10.1155/2009/103890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286(36):31263–71. 10.1074/jbc.M111.238147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bayeva M, Khechaduri A, Puig S, Chang HC, Patial S, Blackshear PJ, et al. mTOR regulates cellular iron homeostasis through tristetraprolin. Cell Metab. 2012;16(5):645–57. 10.1016/j.cmet.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaljo B, Kratochvill F, Gratz N, Sadzak I, Sauer I, Hammer M, et al. Tristetraprolin is required for full anti-inflammatory response of murine macrophages to IL-10. J Immunol. 2009;183(2):1197–206. 10.4049/jimmunol.0803883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–7. [DOI] [PubMed] [Google Scholar]
- 54.Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem. 2012;287(48):40224–31. 10.1074/jbc.R112.386664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107(6):2580–5. 10.1073/pnas.0915139107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peferoen L, Kipp M, van der Valk P, van Noort JM, Amor S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology. 2014;141(3):302–13. 10.1111/imm.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–89. 10.1101/gr.132159.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imamura K, Imamachi N, Akizuki G, Kumakura M, Kawaguchi A, Nagata K, et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell. 2014;53(3):393–406. 10.1016/j.molcel.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 59.Li Z, Chao TC, Chang KY, Lin N, Patil VS, Shimizu C, et al. The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proc Natl Acad Sci U S A. 2014;111(3):1002–7. 10.1073/pnas.1313768111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The microarray data has been deposited in GEO (http://www.ncbi.nlm.nih.gov/geo/) and is accessible via GEO Accession Number GSE73880.