Abstract
Allodiploidization is a fundamental yet evolutionarily poorly characterized event, which impacts genome evolution and heredity, controlling organismal development and polyploid cell-types. In this study, we investigated the sex determination system in the allodiploid and sterile ATCC 42981 yeast, a member of the Zygosaccharomyces rouxii species complex, and used it to study how a chimeric mating-type gene repertoire contributes to hybrid reproductive isolation. We found that ATCC 42981 has 7 MAT-like (MTL) loci, 3 of which encode α-idiomorph and 4 encode a-idiomorph. Two phylogenetically divergent MAT expression loci were identified on different chromosomes, accounting for a hybrid a/α genotype. Furthermore, extra a-idimorph-encoding loci (termed MTLa copies 1 to 3) were recognized, which shared the same MATa1 ORFs but diverged for MATa2 genes. Each MAT expression locus was linked to a HML silent cassette, while the corresponding HMR loci were located on another chromosome. Two putative parental sex chromosome pairs contributed to this unusual genomic architecture: one came from an as-yet-undescribed taxon, which has the NCYC 3042 strain as a unique representative, while the other did not match any MAT-HML and HMR organizations previously described in Z. rouxii species. This chimeric rearrangement produces two copies of the HO gene, which encode for putatively functional endonucleases essential for mating-type switching. Although both a and α coding sequences, which are required to obtain a functional cell-type a1-α2 regulator, were present in the allodiploid ATCC 42981 genome, the transcriptional circuit, which regulates entry into meiosis in response to meiosis-inducing salt stress, appeared to be turned off. Furthermore, haploid and α-specific genes, such as MATα1 and HO, were observed to be actively transcribed and up-regulated under hypersaline stress. Overall, these evidences demonstrate that ATCC 42981 is unable to repress haploid α-specific genes and to activate meiosis in response to stress. We argue that sequence divergence within the chimeric a1-α2 heterodimer could be involved in the generation of negative epistasis, contributing to the allodiploid sterility and the dysregulation of cell identity.
Introduction
Ploidy variation has played a major role in the evolution of many extant eukaryotic lineages [1]. In yeasts, numerous studies have demonstrated how variations in the ploidy state took place frequently during evolutionary history [2] [3]. Allodiploid offspring often have strong selective disadvantages due to their sterility [4]. However, in some instances, the increased genome size and complexity of allodiploids may enhance heterosis and/or adaptive flexibility [5], particularly at the edges of the ancestral species' range, where they are more likely to encounter stress [6] [7]; [8]. Life history models note that there is an intricate interplay between ploidy variation and alterations of mating, meiosis and sporulation patterns [9]. Consequently, the ploidy state affects the genetic composition at sex-determining loci, giving rise to the ploidy-dependent initiation of dedicated transcriptional programs [10].
In the well-studied model organism Saccharomyces cerevisiae, the meiosis of diploid cells is triggered by environmental cues, such as nutrient depletion, and gives rise to four haploid meiotic spores with two distinct mating-types (MATa and MATα). Haploid spores eventually re-establish diploid MATa/MATα lines by one of three processes: (1) by mating with their own mitotic daughter cells after switching their mating-type in a process catalysed by the endonuclease HO (HOmothallism); (2) by mating with another spore created by the same meiotic event (intratetrad mating); or, more rarely, (3) by mating with an unrelated individual (outcrossing) [11]. The a1-α2 protein heterodimer is one of the master regulators of cell identity and sexual development (as reviewed in [12] and [13], respectively). The a1 and α2 proteins are homeodomain transcriptional factors encoded by the MATa1 and MATα2 genes at the active MATa and MATα loci, respectively. In MATa/MATα diploid cells, α2 interacts with a1 to bind DNA as a heterodimer and transcriptionally repress mating genes, preventing polyploidy and aneuploidy. Furthermore, the a1-α2 heterodimer inhibits the expression of haploid-specific genes (h-sgs) and activates the developmental switch from mitosis to meiosis under appropriate stress stimuli [14] [15]. Two h-sg targets of negative regulation by the a1-α2 heterodimer are MATα1 (encoding the transcription factor that positively regulates the α-specific genes) and HO genes (encoding an endonuclease, which cleaves a specific DNA sequence at the MAT locus during the first step of mating-type switching) [16]. Furthermore, a1-α2 heterodimer controls the sexual development by inhibiting the RME1 (Repressor of Meiosis 1) gene. In haploid cells, the RME1 transcriptional factor prevents the expression of the IME1 (Inducer of Meiosis 1) gene [17] [18] and positively regulates the transcription of adhesion-specific genes, such as the FLO11 gene, in response to nutrient depletion [19]. In diploid cells, the a1-α2 heterodimer binds to the RME1 gene promoter, repressing its transcription and thereby relieving IME1 gene repression. The a1-α2 repressor complex directly controls entry into meiosis through the regulation of the IME4 gene, which is required for the full expression of IME1 [20]. The a1-α2 heterodimer negatively regulates the antisense (AS) long non-coding RNA (lncRNA) [21] [22]. This lncRNA (also termed as Regulator of Meiosis 2 or RME2) is transcribed in haploid cells and blocks the expression of the IME4 gene. In diploid cells, the a1-α2 complex represses RME2 expression, allowing sense (S)-IME4 to be transcribed under starvation conditions.
Yeasts of the non-whole genome duplication (non-WGD) Zygosaccharomyces rouxii species complex are adapted to grow in food with high solute concentrations and frequently experience variations in ploidy, resulting in different modes of reproduction [23]. This complex includes the haploid heterothallic or homothallic (self-fertile) Z. rouxii species, which undergoes mating and subsequent meiosis under salt stimuli [23] [24]; the diploid Zygosaccharomyces sapae species, which reproduces mainly by clonality and ascospores are rarely observed [25]; and a group of anueploid/allodiploid strains of unclear taxonomical position (termed mosaic lineage) [23] [26]. Within the latter, the allodiploid ATCC 42981 strain has been extensively studied for its ability to withstand high concentrations of alkali metal cations and for its capability to produce glycerol under salt stress [27] [28]. From a molecular point of view, the ATCC 42981 genome displays rDNA heterogeneity [26] [29], additional chromosomes compared to Z. rouxii [28], and diploid DNA [26]. Gordon and Wolfe [29] showed that the ATCC 42981 genome contains two partially divergent complements, namely the T- and P- subgenomes, which could arise from a recent allodiploidization event between Z. rouxii carrying the T- subgenome and another not-yet-recognized species harbouring the P- subgenome (so far represented by the unique strain NCYC 3042 and referred to as Zygosaccharomyces pseudorouxii nom. inval. by James et al. [30]). Despite having a DNA diploid content, the ATCC 42981 strain has not been observed to undergo meiosis under different standard and stress growth conditions [25].
Because gene information retained at the MAT expression loci is essential to ensure appropriate haploid/diploid cell-type identity and functional cell development, several efforts have recently been made to characterize MAT loci in haploid Z. rouxii [31] and diploid Z. sapae [32], but not in the allodiploid ATCC 42981 strain. Like S. cerevisiae, haploid Z. rouxii wild strains possess a three-cassette system consisting of MATa or MATα expression loci and two silent cassettes of both idiomorphs, HMR and HML, respectively, which act as donor sequences during the mating-type switching presumably catalysed by HO endonuclease [33] [34]. S. cerevisiae maintains both HML and HMR cassettes at different locations on the same chromosome harbouring the MAT expression locus, whereas Z. rouxii only conserves the MAT/HML linkage on chromosome C and the HMR locus on chromosome F [34]. This structural organization implies that, differently from S. cerevisiae, Z. rouxii exploits the ectopic recombination between non-homologous chromosomes to switch the mating-type. Congruently, Watanabe et al. [31] found that sex chromosomes represent hyper-mutational hotspots, with flanking regions of MAT, HML and HMR cassettes remaining highly variable among Z. rouxii haploid strains. Compared to Z. rouxii, the Z. sapae diploid species has one more copy of the HO gene and displays an unusual aααα genotype, with a redundant number of divergent MATα loci but without any HMR silent locus. This complex architecture of sex-determining chromosomal regions prevents mating-type switching due to the lack of the HMR cassette and hampers sexual development due to the imbalance in divergent mating-type genes.
Here, we determined the structure and functions of the three-locus sex-determining system in the allodiploid ATCC 42981 strain. The transcriptional profiling of these genes under meiotic-inducing salt stress revealed how the hybrid genetic configuration at the MAT loci contributes to allodiploid sterility. To the best of our knowledge, our report presents the first evidence that a chimeric sex-determination system accounts for the incomplete silencing of the h-sg program and contributes to the prevention of switching from mitosis to meiosis in allodiploid yeasts.
Materials and Methods
Strains, culture conditions and mating test
The Zygosaccharomyces strains used in this work are listed in Table 1. Strains were routinely cultured at 28°C in YPD (1% w/v yeast extract, 1% w/v peptone, 2% w/v dextrose) medium with or without 15% (w/v) agar and stored at 4°C. For long-term preservation, strains were stored at -80°C in YPD medium containing 25% glycerol (v/v) as a cryopreservative. To increase the probability of observing conjugated or un-conjugated spore-containing ascii, sporulation was tested by inoculating the early stationary phase culture of ATCC 42981 on five different media [YPD, YPDA, malt extract agar (MEA; Difco), MEA supplemented with 6% (w/v) NaCl (6%NaCl-MEA), and YNB5%GNaCl (1% w/v yeast extract, 5% w/v dextrose, 6.7 g/l yeast nitrogen base, 2.0 M NaCl)] for 3 weeks.
Table 1. Details of strains used in the present study.
Ploidy data were obtained from Solieri et al. [23] [2,6], while the genotype of strain ABT301T at the active MAT loci was obtained from Solieri et al. [32].
| Strains | Other collections | Source | Current taxonomical positions | Mating-type/thallism | Spore | Ploidy ratio |
|---|---|---|---|---|---|---|
| CBS 732T | NCYC 568, NRRL Y-229 | Grape must | Z. rouxii | MATα/homothallic | - | 1.3 |
| CBS 4837 | NYC 1682, NRRL Y2547 | Miso | mosaic lineage | MATa/heterothallic | + | 1.96 |
| CBS 4838 | NRRL Y2584 | Miso | mosaic lineage | MATα/heterothallic | + | 1.90 |
| ATCC42981 | - | Miso | mosaic lineage | nd | nd | 2.1 |
| ABT301T | CBS 12607, MUCL54092 | TBV | Z. sapae | aαααMATα/MATα/heterothallic | + | 2.0 |
| NCYC 3042 | CBS 9951 | Soft drink | Z. pseudorouxiinom. inval. | nd | nd |
nd, not determined; TBV, Traditional Balsamic Vinegar.
To study sexual compatibility, 2-to-4-day-old cultures of the ATCC 42981 strain were incubated alone or in a mixture with mating-tester strains Z. rouxii CBS 4837 (mating-type a) or CBS 4838 (mating-type α) in both MEA and 6%NaCl-MEA media at 27°C for 3 weeks. Samples were examined microscopically every week using phase-contrast optics to detect conjugation.
The YNB5%G [1% w/v yeast extract, 5% w/v dextrose, 6.7 g/l yeast nitrogen base (Difco)] and the YNB5%GNaCl (1% w/v yeast extract, 5% w/v dextrose, 6.7 g/l yeast nitrogen base, 2.0 M NaCl) media were used for RNA extraction from cells grown under unstressed and salt-stressed conditions, respectively.
PCR conditions and sequencing
Genomic DNA (gDNA) extraction was performed by a phenol-based method from stationary grown cells after mechanical lysis according to Hoffman and Winston [35]. The quantity of DNA was evaluated spectrophotometrically using a NanoDrop ND-1000 device (Thermo Scientific). All PCR reactions were performed on a T100 Thermalcycler (Bio-Rad) in a 25-μl reaction volume containing 200 ng of template gDNA. For PCR amplification < 2 kb rTAQ DNA polymerase (Takara, Japan) and for PCR amplification ≥ 2 kb Phusion Hot Start II Polymerase (Thermo Scientific, Waltham, MA) along with buffer HF (5x) or LA Taq DNA polymerase (Takara, Japan) with GC buffer I (2x), were used according to the manufacturer’s instructions. All primers used in this study are listed in S1 Table; primers were designed with Primer 3 software (http://primer3.sourceforge.net/) and provided by either MWG (Heidelgerg, Germany) or BMR Genomics (Padova, Italy). The PCR products were resolved on 1.2% (w/v) agarose gels stained with ethidium bromide and their size was estimated by comparison with 100 bp or 1 kb DNA Ladder Plus (Fermentas) as molecular size markers. PCR products were purified using the DNA Clean & Concentrator™-5 (DCC™-5) Kit (Zymo Research) according to the manufacturer’s instructions. Moreover, when required, DNA fragments were purified from 1% agarose gels using the Gene JET Gel Extraction Kit (Thermo Scientific, Waltham, MA). Finally, all PCR products were sequenced on both strands through a DNA Sanger sequencing process performed by either MWG (Heidelgerg, Germany) or BMR Genomics (Padova, Italy).
Genomic walking procedure
The overall strategy for determining mating-type-like (MTL) loci is depicted in S1 Fig (panel A). Briefly, three MATα copy-specific primer pairs were designed on the ZsMTLα copies 1, 2 and 3 cassettes previously cloned in Z. sapae and used to clone the corresponding MATα1 (partial) and MATα2 (full-length) coding regions in the ATCC 42981 genome (S1 Fig and S1 Table). Complete MATα1 loci were obtained in a second round of PCR walking using reverse primers (A, B, C and A_D) built on the 3’-end regions potentially flanking the MAT and HML/HMR cassettes [31] [32] (S1 Fig). Similarly, three primer pairs were used to amplify putative MTLa loci in the ATCC 42981 strain (S1 Fig, panel B). Two primer pairs were designed to specifically amplify the ZsMATa1 and ZsMATa2 genes, respectively, whereas a third primer pair was built to completely include MATa1 ORF and partially MATa2 ORFs, respectively. In order to further extend the MATa2 coding DNA sequence, a second round of PCR walking was performed by combining a MATa2-specific reverse primer and all the available forward primers (1, 2 and 3) built on the 5’-end regions flanking the MAT and HML/HMR cassettes [31] (S1 Fig). The DNA regions flanking MTLa and MTLα loci were characterized through the semi-nested and direct PCR approaches as previously reported [32] (S2 Fig).
The overview of the strategy employed for ATCC 42981 HO gene characterization is summarized in S3 Fig, and a detailed primer list is given in S1 Table.
Bioinformatics analyses
Sequences were assembled and edited using DNAStar Software (DNASTAR, Inc. Madison, Wisconsin USA). Multiple nucleotide and amino acid sequence alignments were performed using Clustal W2 [36]. Searches for nucleotide and protein sequence homologs were carried out in the GenBank database with Blastn and Blastp algorithms, respectively [37]. Phylogenetic analysis was conducted on aa sequences using MEGA6 [38]. The phylogenetic relationship was inferred using the neighbour-joining (NJ) method. Support percentages for the nodes of NJ-trees were computed using bootstrapping analysis with 1,000 replications and were shown next to the branches when ≥ 60%. For domain identification, Pfam-searches [39] were run on http://pfam.sanger.ac.uk/. Structure predictions were obtained with Jpred3 [40] and validated according to Martin et al. [41]. Sequences were submitted to the EMBL/GenBank databases under the accession numbers from KT598024 to KT598027 and from KT694298 to KT6942302.
PFGE-Southern blotting assays
Chromosomal DNA preparation in plug, pulsed-field gel electrophoresis (PFGE), and Southern blotting assays were performed as previously reported [26] [32]. Primers engaged in probe synthesis for Southern blot analysis are listed in S1 Table.
Subgenome assignment of mating-type and HO gene copies
PCR subgenome genotyping was performed using gDNA extracted from Z. pseudorouxii NCYC 3042 as a T- subgenome template. PCR amplification of all MATα1, MATα2, MATa1, MATa2, and HO gene copies present in ATCC 42981 genome was carried out with the primer pairs listed in S1 Table. To avoid false negative results, we tested two alternative primer pairs for each target gene. ATCC 42981 gDNA was also included as a positive control. Z. rouxii CBS 732T gDNA was not included in PCR reactions as one of the two putative parental genetic complements because its genome project was available.
RNA extraction and RT-PCR
Zygosaccharomyces cells were pre-cultured in YPD medium for 24 h at 28°C under shaking conditions (150 rpm), washed in physiological solution (9 g/l NaCl), and used to inoculate two sets of baffled Erlenmeyer flasks (E-flasks) containing 70 ml of YNB5%G (standard growth condition) and YNB5%GNaCl (hyperosmotic growth condition) media, respectively (initial OD600nm 0.02–0.04). Inoculated E-flasks were incubated at 28°C under shaking conditions (150 rpm) and yeast growth was spectrophotometrically monitored at 600 nm two times a day. Cells at the stationary phase (no change in OD measurement detected in at least three consecutive readings) were harvested and frozen at -80°C. RNA extractions were carried out using the ZR Fungal/Bacterial RNA MicroPrep™ Kit (ZymoReasearch, Irvine, California) according to the manufacturer’s instructions. Purified RNAs were submitted to additional DNase digestion in solution and cleaned up with the RNA Clean & Concentrator™-5 Kit (ZymoReasearch, Irvine, California).
Total RNAs were reverse transcribed using 0.5 μM oligo (dT) and RevertAid H Minus Reverse Transcriptase (Thermo Scientific, Waltham, USA) according to the manufacturer's instructions. In the case of IME4 transcript analyses, instead of oligo (dT) the forward and reverse primers ZrIME4F1 and ZrIME4R1 were used for the cDNA synthesis of AS-IME4 lncRNA and S-IME4 mRNA, respectively (S1 Table). Then a common pair of internal primers (ZrIME4_F2/ZrIME4_R2; S1 Table) was used to amplify the same cDNA fragment from each template, if any. Experiments were carried out with three biological replicates and non-reverse transcribed (NRT) controls were performed for each biological replicate. cDNA template (25 ng) was PCR-amplified with primers listed in S1 Table and successful amplification was checked by electrophoresis in 2% agarose gel in 0.5X TBE Buffer.
Quantitative PCR (qPCR) assays
For relative expression level analysis, qPCR reactions were performed using 1 ng/μl of cDNA, 0.3 μM of each primer, and the Maxima™ SYBR Green/ROX qPCR Master Mix (Fermentas, USA) according to the manufacturer’s instructions. Primers were designed to selectively amplify MATa1 exons as well as different copies of MATα1, MATα2 and HO genes (S1 Table). PCR efficiency was in silico predicted for each primer set using the open source tool Primer Efficiency (http://srvgen.upct.es/index.html; [42]). Preliminary, the presence and the estimated size of amplicons were checked by RT-PCR on cDNA from stationary-phase cells (three biological replicates) under standard conditions, whereas unspecific amplifications and primer-dimer formation were checked by melting curve analysis after RT-qPCR assay. All qPCR reactions were run in the Applied Biosystems 7300 Real-Time PCR instrument (Applied Biosystem, Foster City, CA, USA). The Z. rouxii housekeeping gene ACT1 (ZrACT1; XM002497273) was used as a reference gene, according to Leandro et al. [43]. The relative expression of different gene transcripts was calculated by the ΔΔCt method and converted to the relative expression ratio (2−ΔΔCt) for statistical analysis [44]. For this purpose, a dilution series of standard points from pooled control cDNAs was exploited (concentration range 10–0.02 ng/μl) in technical triplicates. Amplification profiles, baselines and thresholds were analysed with 7300 SDS 1.4. PCR-Miner [45] [46] was applied as an alternative method when the low gene expression level did not provide enough dynamic range to build a reliable standard curve. In this case, reaction efficiency and the fractional cycle number at threshold (Ct) were estimated by relying on the kinetics of individual PCR reactions containing 5 ng of cDNA template from three biological replicates (salt-treated and controls), including six technical replicates each.
Results
Morphological characterization and mating test
We first assessed the mating behaviour of the ATCC 42981 strain in pure and mixed cultures with the Z. rouxii mating partners CBS 4837 (mating-type a) and CBS 4838 (mating-type α), respectively. Examination under the microscope did not show any evidence of mating reaction of ATCC 42981 cells neither with Z. rouxii CBS 4837 or CBS 4838 tester strains, even after 3 weeks of incubation both on MEA and 6% NaCl-MEA media (data not shown). Even if rare mating events cannot be ruled out, this result suggests that the ATCC 42981 strain did not respond to Z. rouxii pheromone signalling or that there was an imbalanced or deficient organization in MTL loci. Based on our previous observations [25], ATCC 42981 cells grown on MEA medium displayed an adhesive phenotype with clamps on mother and daughter cells that remained attached to each other, but they were not able to form ascii in pure culture (data not shown). In contrast, Z. sapae ABT 301T rarely forms ascii at the same conditions [25]. As salt has been reported to induce meiosis in Zygosaccharomyces yeasts [24], we tested the capability of the ATCC 42981 strain to form ascospores in YNB5%G NaCl and 6%NaCl-MEA media. No conjugated ascii were observed over time in any media tested. Furthermore, an increase in the adhesive phenotype was detected in cells grown under salt stress (data not shown).
Isolation and characterization of MTLα loci in ATCC 42981
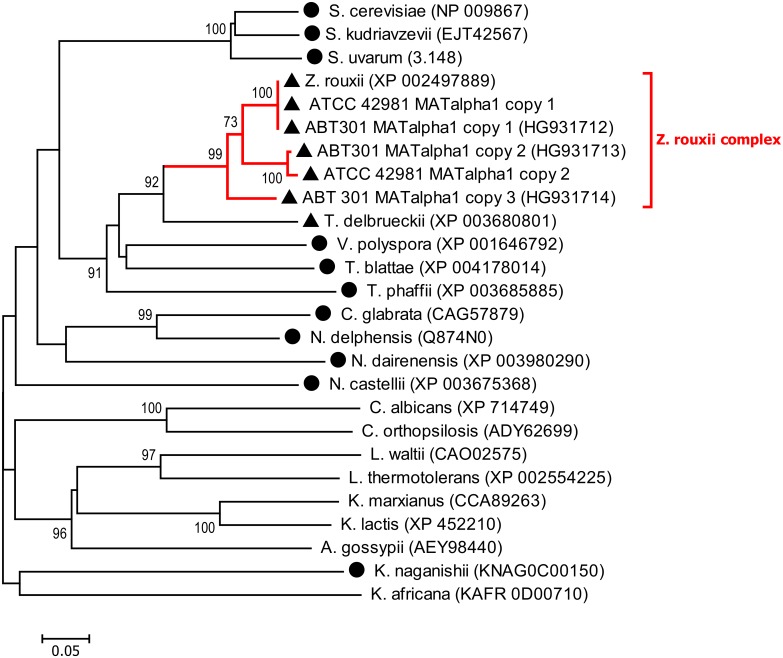
Three MTLα loci, termed ZsMTLα copies 1 to 3, have been previously described in Z. sapae as regions containing phylogenetically distinct α1 and α2 genes, respectively [32]. To establish whether strain ATCC 42981 also displays a similar genetic configuration, we exploited a PCR walking strategy. This assay relies on MATα copy-specific primers designed on the corresponding ZsMTLα cassettes [32]. PCR reactions were positive using MTLα copies 1 and 2-specific primers, whereas negative results were scored for any MTLα copy 3-specific primer set tested. We sequenced two distinct MTLα loci (hereafter referred to as MTLα copies 1 and 2) in ATCC 42981. Both loci consisted of two MAT genes encoding α1 and α2 proteins placed in opposite directions and separated by an intervening region of 343 bp. The MATα1 genes from MTLα loci 1 and 2 (termed MATα1 copies 1 and 2, respectively) were divergent from each other for 17 nt substitutions, whereas the MATα2 from MTLα loci 1 and 2 (termed MATα2 copies 1 and 2, respectively) displayed 16 nt substitutions. The deduced proteins MATα1 and MATα2 copies 1 were 87.00% and 78.67% identical to the deduced protein MATα1 and MATα2 copies 2, respectively. BLASTp search against the NCBI database showed that proteins MATα1 and MATα2 copies 1 are more similar to Z. rouxii orthologs (termed ZrMATα1 and ZrMATα2) than the paralogous proteins MATα1 and MATα2 copies 2 in the ATCC 42981 genome.
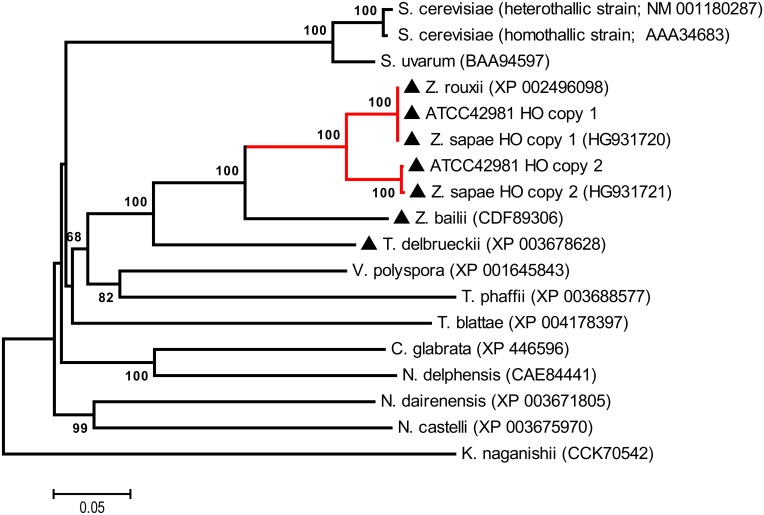
The NJ-based phylogenetic analysis was carried on MATα1 sequences from representative WGD and non-WGD species. As expected, ATCC 42981 MATα1 copy 2 protein did not group to Z. rouxii MATα1, but was instead clustered separately (bootstrapping value of 99%) together with ZsMATα1 copy 2 (Fig 1). The alignment of ATCC 42981 MATα1 copies with Z. rouxii and S. cerevisiae MATα1 proteins revealed a region of high similarity inside the MATα-HMG domain [41] with three predicted conserved α helices (data not shown).
Fig 1. Phylogenetic analysis of MATα1 proteins.
The neighbour-joining (NJ) tree shows the phylogenetic relationships between the allodiploid strain ATCC 42981 and other hemiascomycetes inferred from MATα1 proteins. Numbers on branches indicate bootstrap support percentages (1,000 pseudoreplicates) higher than 60% from NJ. The red branch indicates the Z. rouxii yeast complex, which includes Z. rouxii MATα1, ATCC 42981 MATα1 copies 1 and 2, Z. sapae MATα1 copies 1, 2, and 3 sequences. The dark dot indicates WGD species, whereas the dark triangle indicates non-WGD species with the HO gene.
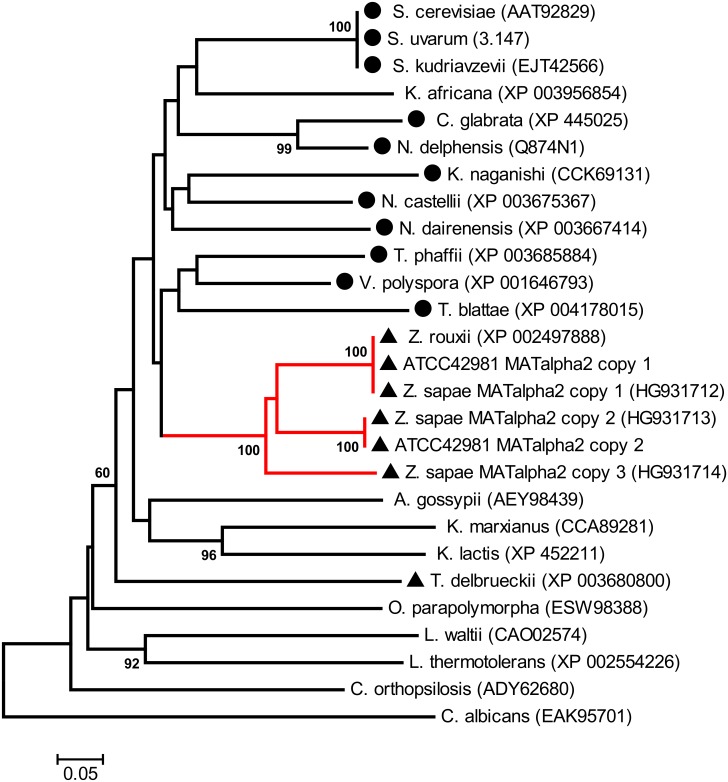
Phylogeny inferred from the MATα2 aa sequences of WGD and non-WGD species showed a tree topology congruent with the species relationships established using MATα1 sequences. The ATCC 42981 genome harbours two MATα2 variants that are related but phylogenetically distinct because of the high level of amino acid divergence (Fig 2). In particular, ATCC 42981 MATα2 copy 1 clustered with Z. rouxii MATα2 (bootstrapping 100%), whereas MATα2 copy 2 was strictly related to Z. sapae MATα2 copy 2 (bootstrapping 100%). Both copies contained a conserved HD1 class homeodomain, consisting of a three-helix globular domain which contacts both major groove bases and the DNA backbone [47] [48] (data not shown). However, portions of the protein outside the homeodomain, which mediate interactions with accessory proteins, had a different degree of conservation.
Fig 2. Phylogenetic analysis of MATα2 proteins.
The neighbour-joining (NJ) tree shows the phylogenetic relationships between the allodiploid strain ATCC 42981 and other hemiascomycetes inferred from MATα2 proteins. Numbers on branches indicate bootstrap support percentages (1,000 pseudoreplicates) higher than 60% from NJ. The red branch indicates Z. rouxii complex, which includes Z. rouxii MATα2, ATCC 42981 MATα2 copies 1 and 2, and Z. sapae MATα2 copies 1 to 3 sequences. The dark dot indicates WGD species, whereas the dark triangle indicates non-WGD species with the HO gene.
Isolation and characterization of MTLa loci in ATCC 42981
In Z. sapae the a-idiomorph encoding MTL locus harbours a MATa1-coding ORF (ZsMATa1) identical to the Z. rouxii orthologue (ZrMATa1) and a MATa2-coding ORF (ZsMATa2), which showed a 26-bp deletion compared to ZrMATa2, resulting in a 9-amino acid shorter protein [32]. To identify MTLa loci in the ATCC 42981 genome, a PCR strategy was used based on primers designed on the MATa1 and MATa2 gene sequences in Z. rouxii and Z. sapae. We found three MTLa loci, each harbouring MATa1 and MATa2 genes in opposite directions separated by a 279-bp long intergenic sequence, which differed for a single SNP (T/G) compared to the corresponding region in Z. sapae.
Sequence alignments and BLAST-type search revealed that all ATCC 42981 MTLa loci displayed identical MATa1 genes (100% identity to ZrMATa1 and ZsMATa1), whereas differed from each other in the MATa2-coding ORFs. One MATa2-coding gene (referred to as the MATa2 copy 1) was 100% identical to Z. rouxii counterpart. Another MATa2 gene (termed the MATa2 copy 2) encoded an a2 protein, which diverged from ZrMATa2 mainly at the C-terminal end (93.73% identity). A third MATa2 coding sequence, namely MATa2 copy 3, encoded a protein 94.41% and 94.24% identical to ZrMATa2 and ZsMATa2, respectively.
The NJ-tree confirmed the high degree of evolutionary conservation of MATa1 proteins within the Z. rouxii complex (bootstrapping 100%) (data not shown). Furthermore, a search with the program Pfam revealed that ATCC 42981 MATa1 proteins conserve the HD2 class homeodomain [48] [49] (data not shown).
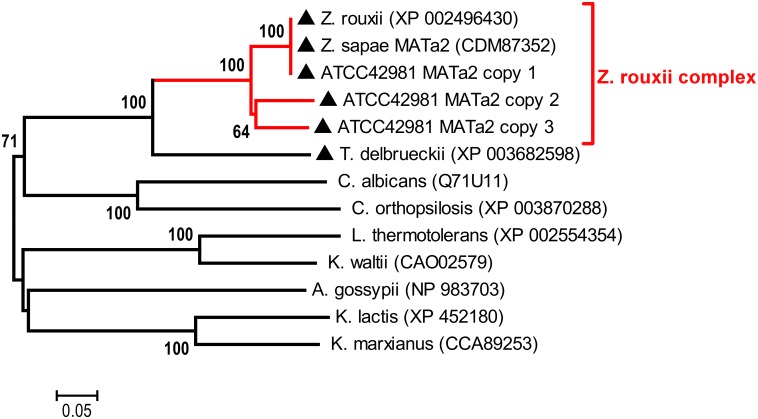
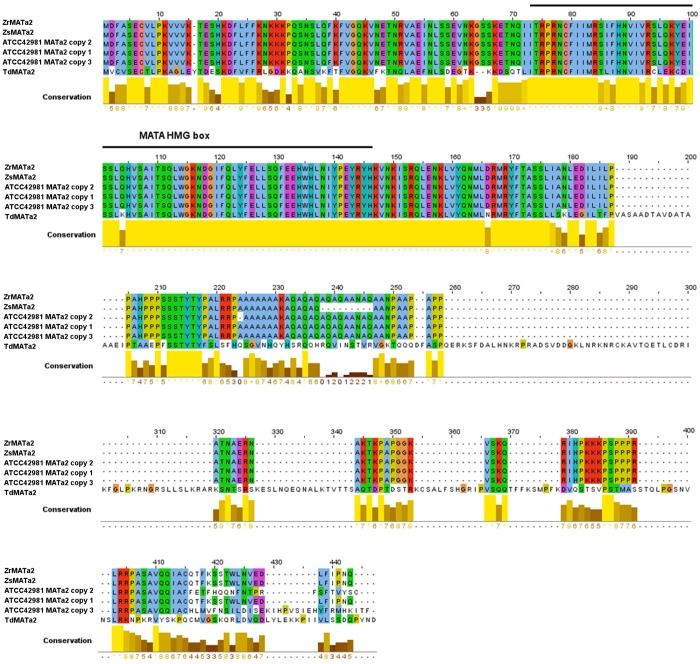
Phylogeny inferred from MATa2 amino acid sequences showed that ATCC 42981 MATa2 variants are phylogenetically distinct (Fig 3). In particular, MATa2 copy 1 clustered with Z. rouxii and Z. sapae MATa2 (bootstrapping 100%), whereas MATa2 copies 2 and 3 clustered together and were distinct from both ZrMATa2 and ZsMATa2. MATa2 proteins from ATCC 42981 conserved a single MATA-HMG box, a class I member of the HMG-box superfamily of DNA-binding proteins (Fig 4), coding a sequence spanning across the Ya and X regions.
Fig 3. Phylogenetic analysis of MATa2 proteins.
The neighbour-joining (NJ) tree shows the phylogenetic relationships between the allodiploid strain ATCC 42981 and other hemiascomycetes inferred from MATa2 proteins. Numbers on branches indicate bootstrap support percentages (1,000 pseudoreplicates) higher than 60% from NJ. The red branch indicates the Z. rouxii complex, which includes Z. rouxii MATa2, Z. sapae MATa2 and ATCC 42981 MATa2 copies 1 to 3 sequences. The dark dot indicates WGD species, whereas the dark triangle indicates non-WGD species with the HO gene.
Fig 4. Sequence comparison of MATa2 proteins.
Alignment of MATa2 from Z. rouxii (ZrMATa2, GenBank: XP002496430), Z. sapae (ZsMATa2, GenBank: CDM87352), ATCC 42981 MATa copies 1 to 3 and Torulaspora delbrueckii (TdMATa2, GenBank: XP003682598). The MATA HMG domain, which binds the minor groove of DNA, is noted (horizontal black bar). In both alignments, the amino acid identities were coloured according the Clustal X colour scheme and the conservation index at each alignment position were provided by Jalview [50].
Characterization of HO genes
Using a PCR walking approach, we identified two 1797-bp full-length ORFs in the ATCC 42981 genome, termed HO copies 1 and 2, which diverged for 177 transitional and 49 transversional mismatches. The predicted proteins HO copies 1 and 2 shared 100% and 92.47% identity with Z. rouxii HO, while they were 100% and 99.83% similar to Z. sapae HO copies 1 and 2, respectively.
NJ-based phylogeny inferred from amino acid HO sequences confirmed that HO copy 2 from ATCC 42981 branched with Z. sapae HO copy 2 with a high level of support (bootstrapping 100%), while ATCC 42981 HO copy 1, ZsHO copy 1 and ZrHO clustered together into a separate clade (Fig 5). These results showed that ATCC 42981 HO amino acid sequences are more divergent from each other than from the putative orthologs in Z. sapae. Amino acid alignment of both ATCC 42981 HOs with S. cerevisiae PI-SceI (GenBank: CAA98762), S. cerevisiae HO and Z. sapae HO copies 1 and 2 showed the highest homology in conserved motifs characteristic of intein-encoded LAGLIDADG endonucleases [51–53] (data not shown).
Fig 5. Phylogenetic analysis of HO endonucleases.
Neighbor-joining (NJ) tree shows evolutionary relationships between ATCC 42981 strain and other hemiascomycetes as inferred from HO proteins. Numbers on branches indicate bootstrap support (1,000 pseudoreplicates) from NJ. The red branch indicates Z. rouxii complex, which includes ZrHO, ZsHOs and ATCC 42981 HO sequences, whereas the dark triangle designates pre-WGD species.
PFGE-Southern blotting showed that both HO copies of ATCC 42981 strain are on chromosome F (1.6 Mbp) (S4 Fig). This chromosomal assignment resembles that of CBS 732T, but differs from that previously found in Z. sapae ABT301T [32]. We assumed that ATCC 42981 HO copies could be placed on the same chromosome or, alternatively, on two PFGE-co-migrating homeologous chromosomes.
Subgenome assignment of mating-type and HO genes
In order to infer the putative parental contributions to the ATCC 42981 MTLα, MATa and HO gene sets, we screened strain NCYC 3042 for all variants of MATa, MATα, and HO genes found in ATCC 42981. According to PCR profiles, successful amplification was obtained for copy 2 sets of MATα1, MATα2 and HO genes in the NCYC 3042 strain, while negative results were obtained for MATα and HO copy 1 genes (S2 Table). PCR results were consistent with the high degree of similarity between the copy 1 set of MATα and HO genes and Z. rouxii orthologs in the CBS 732T genome project (Figs 1, 2 and 5) and suggested that MATα and HO copies 2 originated from a Z. pseudorouxii parental genome. Interestingly, MATa2 copy-specific screening showed that the NCYC 3042 strain does not possess any MATa2 copies found in ATCC 42981 genome (S2 Table).
Reconstruction of the three-cassette system
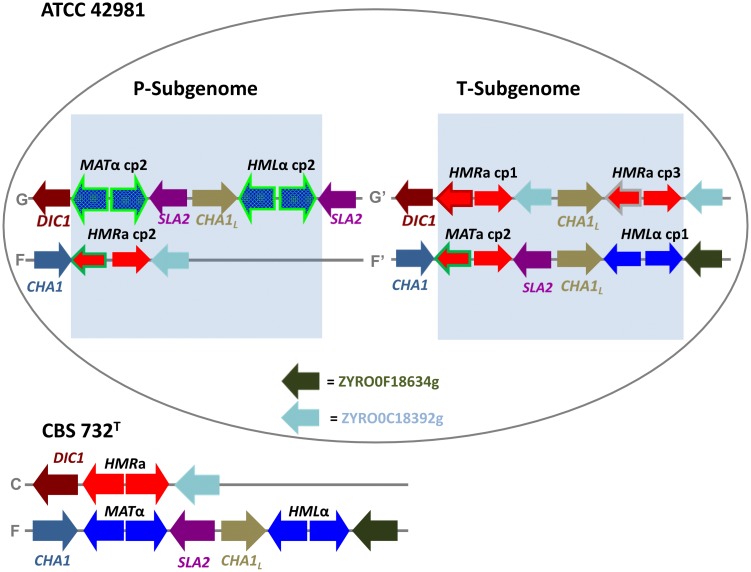
To assign the MTLa and MTLα loci as MAT expression loci, HMR or HML silent cassettes (Fig 6), we exploited direct PCR on MTL flanking regions and long-range PCRs that spanned left and right flanking regions of MTL loci (S2 Fig). Both approaches demonstrated that ZYRO0F18524g (termed CHA1L due to its similarity to CHA1 gene) and ZYRO0F18634g ORFs are located at the 5’ and 3’ ends of the MTLα copy 1 locus, respectively. Because HMLα silent cassettes are commonly downstream the CHA1L gene [31], we inferred the existence of a HMLα silent cassette containing MATα1 and MATα2 copies 1 genes (referred to as HMLα copy1) (Fig 6). Long-range PCR products obtained with the primer pairs 2/A and 3/A were positively screened through MTLα copy 2-specific primers (S2 Fig). Sequencing showed that there are two MTLα copy 2 loci flanked by the SLA2 gene at the 3’ end and by either the DIC1 gene or CHA1L at the 5’ end, respectively. It has been reported that the MATα expression loci retain the particular gene order DIC1-MATα-SLA2 in the majority of non-WGD species [54]. A similar synteny was found for MTLα copy 2, suggesting that ATCC 42981 possesses an actively transcribed MATα copy 2 locus (Fig 6). Furthermore, CHA1L upstream to the MTLα copy 2 supports the presence of an additional HMLα copy 2 cassette. Finally, we detected the following syntenic orders for MTLa loci: DIC1-MTLa copy 1-ZYRO0C18392g, CHA1-MTLa copy 2-SLA2, CHA1-MTLa copy 2-ZYRO0C18392g and CHA1L-MTLa copy 3-ZYRO0C18392g (Fig 6). The gene organization of the CHA1-MTLa copy 2-SLA2 was syntenic with that of the MATα expression locus in Z. rouxii CBS 732T, whereas the gene orders CHA1-MTLa copy 2-ZYRO0C18392g and DIC1-MTLa copy 1-ZYRO0C18392g resembled those found in HMRa silent loci of haploid Z. rouxii strains [31]. In particular, CHA1-MTLa copy2-ZYRO0C18392g resembled the HMRa cassette found in a- and α-idiomorph mixed culture of strain NBRC 1130. Although the synteny CHA1L-MTLa copy 3-ZYRO0C18392g has been never found in Z. rouxii strains, CHA1L and ZYRO0C18392g ORFs generally surround silent donor cassettes. Overall, these evidences support the notion that ATCC 42981 possesses one MATa copy 2 expression locus and three HMRa cassettes, referred to as HMRa copies 1 to 3 (Fig 6).
Fig 6. Inferred genomic organization around MAT-like loci in the ATCC 42981 allodiploid genome.
Two sex homologous/homeologous chromosome pairs are depicted, namely F/F’ and G/G’. Chromosome G bears the MATα copy 2 expression locus, which is linked to the putative silent cassettes HML copy 2, whereas chromosome F’ bears the MATa copy 2 expression locus, which is linked to the putative silent cassette HML copy 1. Chromosomes F and G’ harbour three a-idiomorph HMR loci, which differ for MATa2 genes. The HMR copy 2 locus is on chromosome F, while the HMR copies 1 and 3 loci are on chromosome G’. Blue arrows represent Z. rouxii-like (blue bordered) and Z. sapae-like (light green bordered) α-idiomorph loci. Red arrows indicate a-idiomorph loci with different MATa2 genes: copy 1 (dark red surrounded), copy 2 (dark green), copy 3 (grey), respectively. Chromosomal organization of the three-cassette system in Z. rouxii haploid strain CBS 732T was reported for comparative purposes according to Souciet et al. [34]. CHA1L indicates the ZYRO0F18524g locus, while dark green and light blue arrows indicate the ZYRO0F18634g and ZYRO0C18392g loci, respectively at the right side of mating-type loci.
The chromosomal arrangement of MAT, HMR and HML cassettes was established by Southern blot analysis on PFGE-separated chromosome bands. Although PFGE-Southern blotting failed to clearly resolve the highest molecular weight chromosomes E, F and G spanning from 1.6 to 2.2 Mbp (S5 Fig), hybridization of PFGE-Southern blot with a MATα1-specific probe (suitable to recognize both MATα1 copies 1 and 2) resulted in a double band spanning from chromosome F to G for Z. rouxii CBS 732T and strain ATCC 42981 (S5 Fig). However, differently from CBS 732T, in strain ATCC 42981, the MATα1-specific probe bound less chromosome F than chromosome G, leading to two bands with different signal intensities. This result suggests that chromosome G harbours more copies of the MTLα cassette than chromosome F. When the same analysis was carried out with a MATa1-specific probe, we obtained a double band spanning from chromosome F and G of ATCC 42981 karyotype (S5 Fig). These studies suggest that MTLa loci reside on at least two of the chromosomes spanning from 1.6 and 2.2 kbp. According to PFGE-Southern blotting results, the ATCC 42981 strain arranges two expressed MAT loci (MATa and MATα copies 2), three HMRa and two HMLα silent cassettes on two homeologous chromosome pairs (G/G’ and F/F’). Taking into consideration that linked MAT and HML loci are located on a different chromosome compared to HMR [31] [54], we inferred the scenario depicted in Fig 6. Chromosome G putatively contains the expression locus MATα copy 2, which is linked to the HMLα copy 2 cassette, while chromosome F hosts the HMRa copy 2 silent cassette. The expression locus MATa copy 2 is putatively located on chromosome F’, linked with HMLα cassette copy 1. Chromosome G’ provides the two remaining silent HMRa copies 2 and 3 cassettes (Fig 6).
Gene expression analysis
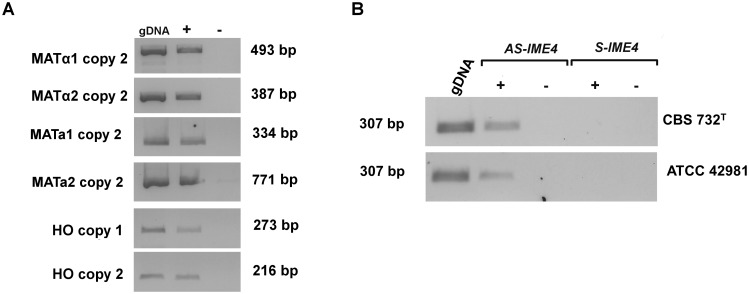
Non-quantitative RT-PCR confirmed that the expression loci MATα and MATa copies 2 are actively transcribed in ATCC 42981 cells grown in standard conditions (Fig 7). Congruently to the cassette reconstruction, the MATα1 and MATα2 genes from HMLα copy 1 were silent.
Fig 7. Expression pattern of mating-type, HO and IME4 genes.
Panel A reports positive amplified cDNAs generated with MAT and HO copy variant-specific primers from ATCC 42981 unstressed cells in stationary growth phase. Panel B depicts IME4-specific PCR products generated from CBS 732T and ATCC 42981 cDNA IME4 antisense (AS-IME4 lncRNA) and sense transcripts (S-IME4 mRNA), respectively. +/- RT indicates addition of reverse transcriptase to the cDNA synthesis reaction. For each RT-PCR reaction gDNA was used as positive control. Abbreviations: AS, anti-sense long non-coding RNA; S, sense mRNA.
According to the regulatory circuit reported in S. cerevisiae diploid cells, we expected that h-sg sets are silenced by a functional a1-α2 heterodimer. In contrast, gene expression profile analysis revealed that the MATα1 and HO genes were actively transcribed in the ATCC 42981 allodiploid strain. Similarly in haploid CBS 732T unstressed cells HO gene was actively transcribed (data not shown).
To verify whether other members of the h-sg set were expressed, we tested the presence of S-IME4 and AS-IME4 transcripts. RT-PCR analysis showed that salt-stressed ATCC 42981 diploid cells only transcribed AS-IME4, while S-IME4 was not detected (Fig 7). This pattern is similar to that displayed by haploid cells of the reference strain CBS 732T, which does not show any PCR products for S-IME4 specific RT-PCR (data not shown). The identity of AS-IME4 PCR products was confirmed by direct sequencing. We concluded that ATCC 42981 is a diploid strain that only transcribes the AS-IME4 transcript.
Transcriptional response to hyperosmotic stress
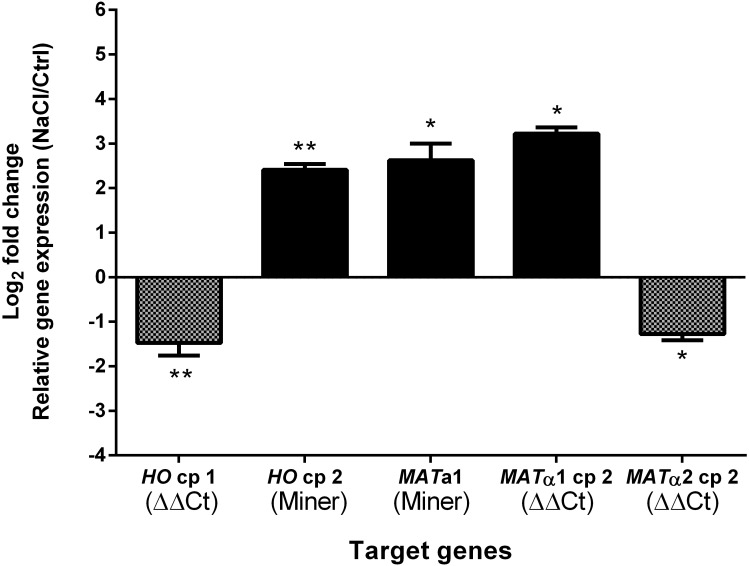
After hyperosmotic stress, ATCC 42981 cells exhibit a different expression profile for both mating-type and HO genes compared to controls. Salt stress induced higher transcript levels of MATa1 and MATα1 copy 2 (6.2- and 9.3-fold, respectively). On the contrary, MATα2 copy 2 expression was lower than that in controls (2.4-fold). HO copy 1 was slightly down-regulated (2.8-fold), whereas HO copy 2 was up-regulated (more than 5.3-fold) compared to controls (Fig 8).
Fig 8. Differential expression by quantitative real-time PCR of mating-type and HO genes in ATCC 42981 cells after hyperosmotic stress.
Expression of target genes was normalized on the reference ZrACT1 (GenBank: XM002497273). Fold change was measured by ΔΔCt or PCR Miner methods and reported as the mean (± SEM) of three biological replicates. * indicates significant difference from controls as measured by independent Student's t-tests (*P<0.05, **P<0.01).
Discussion
Previous studies demonstrated that outcrossing is a very rare event (approximately once every 50,000/110,000 generations) in yeasts as S. cerevisiae [55], Saccharomyces paradoxus [56] and Lachancea kluyveri [57]. Nevertheless, it represents a potential source of phenotypic variability (novelty) and has been extensively exploited for the genetic improvement of microbial cell factories [58]. Although many inter-mating allopatric yeasts give rise to viable hybrids, post-zygotic isolating barriers [59] prevent gene flow between species and contribute to the process of speciation [60]. Complete sets of orthologous genes are expected in yeast hybrids immediately after the merging of two parental genomes. These patterns can undergo extensive homogenization processes over evolutionary time through intragenic recombination, gene conversion and differential gene loss, shaping the offspring’s genome architectures [61] [62]. Gordon and Wolfe [29] did not find any traces of gene losses in the ATCC 42981 strain, arguing that the allopolyploidization was so recent that its genome has not had enough time to decay. These authors hypothesized that one parental subgenome resembles Z. rouxii CBS 732T and the other Z. pseudororuxii (nom. inval.) NCYC 3042. However, ATCC 42981 karyotype cannot be simply considered as an additive result between the putative parental counterparts, suggesting that some structural rearrangements have occurred in the ATCC 42981 genome compared to Z. rouxii and Z. pseudorouxii [28] [29]. Similarly, the Z. rouxii CBS 732T strain contains a SOD2-22 gene variant [63], while Z. pseudorouxii NCYC 3042 has SOD2 [30]. The detection of SOD2 and SOD22 genes, but not the SOD2-22 variant in the ATCC 42981 genome, further confirms that the subgenome complements are similar but not identical to those found in Z. rouxii CBS 732T and in Z. pseudorouxii NCYC 3042.
In an allodiploid karyotype, two homeologous sex chromosomes are expected, bearing a- and α-idiomorph expression loci, respectively. Previous works demonstrated that in haploid Z. rouxii and diploid Z. sapae, HMR cassettes are located on a different chromosome compared to the MAT-HML linkage, accounting for the presence of two sex chromosomes [31] [32]. Therefore, in a recent allodiploid Zygosaccharomyces genome, we expected to identify two sex chromosome pairs. Accordingly, the ATCC 42981 strain displays an a/α genotype provided by the contribution of two sex chromosome pairs. The first sex chromosome pair consists of chromosomes G (MATα copy 2-HMLα copy 2) and F (HMRa copy 2). This subgenome complement only partially resembles that found in Z. pseudorouxii NCYC 3042 strain [30] which might have contributed to this complement with MTLα copy 2. If Z. rouxii contributed to the second chromosome set, we would expect to detect a Z. rouxii MATa2 copy 1 gene at the expression locus. In contrast, chromosome F’ (carrying MATa copy 2-HMLα copy 1) and G’ (carrying HMRa copy 1-HMRa copy 3) probably derived from a rearranged Z. rouxii genome complement [29], consistently with the mating-type loci being the hotspot of ectopic recombination in Z. rouxii strains [31]. Furthermore, an extra HMRa copy 3 was detected harbouring another partially divergent MATa2 ORF, which it might have derived from another non-Z. rouxii parental strain or resulted from an ectopic recombination event in the allodiploid ancestor. All these findings are not consistent with a simple additive set of mating-type loci recently assembled in the allodiploid genome and indicate that ATCC 42981 T- subgenome differs from its putative Z. rouxii CBS 732T counterpart. Watanabe et al. [31] found that the copy 2 variant of MATa2 gene is at the HMR silent cassette of strain NBRC1130 which undergone mating-type switching, whereas copy 1 variant occurs in MAT expression locus. This genome assortment is specular to that found in ATCC 42981 T-subgenome and hints that different MATa2 copies co-exist within Z. rouxii species, harboured by MTL loci flanked by different genes at 5’ end. We speculated that these copy variants could be relicts of past mating-type switching events. Interestingly, Z. rouxii possesses a hybrid regulation system targeting a-specific genes (a-sgs), which consists of a2-mediated activation and α2-mediated repression [64]. In haploid cells, MATa2 proteins with diverging C-terminal portions could be functionally equivalent in mediating the activation of the a-sg program.
Beyond divergent mating-type loci, the ATCC 42981 strain has also been reported to possess two divergent HO genes. Orthologous genes coming from each of the parental species should appear as paralogs in standard analyses because they are homologous genes encoded by the same genome [65]. However, the degree of divergence identified between HO gene copies 1 and 2 excludes their origin as paralogs from a recent gene duplication event and hints that HO copy 2 originated from the Z. pseudorouxii parental genome. The pattern of neutral mutations detected in the endonuclease functional domains indicates that both HO genes have been exposed to the same selective pressure.
Gene regulatory networks, such as cell-type specification circuit, have been shown to evolve significantly over time [66]. When the divergent components of these circuits are forced to interact in a hybrid background, positive or antagonistic epistatic interactions may take place [67]. Among the mechanisms cited to explain the observed loss of hybrid fertility, the Bateson-Dobzhansky-Muller (BDM) model proposes that hybrid sterility results from the lack of interaction or the malfunctioning of interacting alleles derived from divergent genomes [3] [68–70]. However, in some cases, inter-specific protein assemblies have been reported to generate novelties in protein-protein networks untested by selection in hybrid species [71] [72]. The reconstruction of the sex chromosome architecture in the ATCC 42981 genome indicates that the transcriptional regulators encoded at the MAT expression loci are phylogenetically divergent. In diploid cells, a1-α2 heterodimer acts as a master regulator of the shift from mitosis to meiotic growth under appropriate meiosis-inducing stimuli, as well as a repressor of the h-sgs under standard growth conditions. H-sgs contain the MATα1 gene, which the a1-α2 heterodimer should repress by binding the bidirectional promoter located between MATα1 and MATα2 ORFs [12]. In allodiploid ATCC 42981, the lack of MATα1 silencing could be due to: the lack of a1 and α2 proteins; the failure in the heterodimer formations; the inability of putative chimeric a1 and α2 subunits to positively interact with cis-regulatory promoter sequences. Similarly to ATCC 42981, S. cerevisiae diploids showing mutations in either a1 or α2 transcriptional factors fail to turn off MATα1 and other h-sgs [73] [74]. Additionally, Strathern et al. [75] reported that a diploid mutant with a truncated MATα2 defective allele displays a weak α phenotype and is unable to sporulate. Overall, these evidences suggest that in ATCC 42981 the cell-type specification circuit is ineffective in repressing the MATα1 gene possibly due to, among other factors, a no functional chimeric a1-α2 heterodimer.
In diploids, the entry into meiosis requires the inhibition of lncRNA AS-IME4 by a functional a1-α2 heterodimer [21]. Conversely, our results show that the ATCC 42981 strain produces anti-sense transcripts for the IME4 gene, leading to clonality as its unique mode of reproduction. The haploid-like transcriptional pattern displayed by stressed ATCC 42981 cells could also account for its increase of adhesive phenotype. In haploids grown under stress cues, a complex network of signalling modules and transcriptional factors induce clamp formation and pseudohyphal growth in order to enhance mating efficiency and chance of survival [76]. The FLO11 gene has been reported to be a key determinant of the adhesion phenotype under the positive control of the RME1 transcriptional factor. In diploids, the RME1 gene is silenced by a functional a1-α2 heterodimer, leading to the entry into meiosis in response to environmental cues. Because we observed more clamps in salt-stressed compared to unstressed ATCC 42981 cells, we argue that the chimeric a1-α2 heterodimer is also partially ineffective in repressing RME1 transcription.
In S. cerevisiae, a redundant regulatory network accounts for the three-level regulation of mating-type interconversion catalysed by the HO endonuclease, namely cell-type control (HO gene is expressed in a or α haploid cells); mother-daughter control (HO is transcribed in the mother but not in the daughter cells); and cell-cycle control (HO is expressed during the late G1 phase of the cell cycle after the point of commitment to the next cell cycle) [77]. Three transcriptional repressors are involved: the a1-α2 heterodimer, SIN3 and SIN6, respectively. These three types of negative constraints must be relieved in order for the HO gene to be transcribed. Allodiploid ATCC 42981 cells express HO mRNAs, probably due to the lack of these types of controls or to partial incompatibilities among transcriptional factors. However, the presence of HO transcripts does not imply that ATCC 42981 undergoes mating-type interconversion. Indeed, the transcriptional analysis of MAT expression loci showed that only the expected MATa copy 2 and MATα copy 2 transcripts are detected in salt-stressed cells at the stationary phase. The failure of mating-type switching induced by salt stimuli could be due to either a HO post-transcriptional control or to the lack of a fully functional network controlling the DSB-initiated gene conversion. However, other effectors could be responsible for this event, as in haploid Z. rouxii strains HO deletion determines only a slight decrease in mating-type switching frequency [31]. The Z. rouxii species complex emerged after the ancestor of hemiascomycetous yeasts had diverged from other families, such as Kluyveromyces. K. lactis has a non-functional copy of the HO gene [78] and performs mating-type switching by an alternative transposase-mediated mechanism [79]. As it is the first non-WGD clade with a functional HO gene, the Z. rouxii complex is likely to retain remnants of both mechanisms.
In haploid Z. rouxii, mating and the subsequent zygote formation occur immediately before sporulation mainly under salt stress [24] [80]. Therefore, we investigated how the ATCC 42981 cells modulate the transcription of genes coding the a1 and α2 subunits, as well as that of their downstream h-sg targets MATα1 and HO in response to long-term hypersaline stress. In diploid cells, the silencing of h-sg MATα1 and HO and the positive regulation of the MATα2 gene are controlled by a working a1-α2 heterodimer. The inability of the ATCC 42981 chimeric a1-α2 heterodimer to bind to the h-sg promoter regions may account for the observed up-regulation of MATα1 and HO genes. Functional defects of the chimeric a1-α2 heterodimer could be due to gene incompatibility between two divergent subunits and/or to the transcriptional imbalance of their encoding genes. ATCC 42981 cells could attempt to overcome these functional deficiencies by over-expressing the components of the a1-α2 transcriptional factor. Congruently, we observed an up-regulation of the a1 transcript. A similar up-regulation of the other heterodimer subunit α2 should be expected. In contrast, MATα2 gene expression appears to be down-regulated, even at a small level, suggesting an imbalance in the co-regulation of a1 and α2 subunits.
In conclusion, we demonstrated that allodiploid ATCC 42981 cells display a MATa/MATα genotype with a chimeric sex-determination system originating from the co-existence of two different parental genome complements. The protein-protein interaction incompatibility between divergent a1 and α2 subunits could switch-off the meiosis commitment genes, contributing to ATCC 42981 allodiploid sterility. The presence of a chimeric a1-α2 heterodimer promotes an unusual haploid-like transcriptional profile in cells recovered at the stationary phase and after exposure to meiosis-inducing stimuli. To the best of our knowledge, this is the first cue that the BDM interaction between the divergent a1 and α2 subunits may act as a bottleneck, preventing genetic exchanges among Zygosaccharomyces species. Recently, a novel scenario has been proposed for yeast evolution, where two ancient non-WGD ancestral species have given rise to an allodiploid cell that has doubled its genome in order to restore fertility (with a possible interval of many mitotic generations between these two events) [81]. Interestingly, one of the possible parents exhibits phylogenetic affinities with the non-WGD Z. rouxii clade. However, there are several critical open questions that still need to be answered, such as: how the genome duplication event took place and how the mechanism of restoring fertility operated [65]. Allodiploid ATCC 42981 and other strains belonging to the Zygosaccharomyces mosaic lineage [23] could serve as promising models to shed light on the transcriptional network incompatibility underlying hybrid sterility at an incipient stage of speciation, and more in general, on yeast genome evolution.
Supporting Information
Panel A shows ZsMTLα variants represented in blue and surrounded in grey (copy 1), green (copy 2) and orange (copy 3), respectively, while panel B represents MTLa loci coloured in red. Primer pairs specific for ZsMTLα copies 1 to 3 are arbitrarily referred to as αCp1-P, αCp2-P, and αCp3-P. ZsMATa1 and ZsMATa2-specific primer pairs are arbitrarily referred to as Pa1 and Pa2, while the primer pair termed Pa12 spans the complete MATa1 ORF and a portion of MATa2 gene. Solid grey arrows indicate generic flanking genes, dotted borders represent uncompleted sequences and small arrows (solid) designate gene-specific primers. Primer sequences are reported in S1 Table, according to [31] and [32]. Abbreviations: Zs, Zygosaccharomyces sapae; cp, copy; P, primer; for, forward; rev, reverse.
(TIF)
Forward and reverse MTL-specific internal primers were used to screen PCR products obtained using all possible combinations of primers spanning putative MTL-flanking genes (semi-nested PCR approach); in cases of negative results, 5' and 3' PCR walking was performed using all possible combinations of MTL-specific internal primers and MTL-flanking gene primers (direct PCR approach). Small arrows (solid) indicate gene-specific primers and degenerate primers (dotted lines). CHA1L indicates the ZYRO0F18524g locus. Primer sequences are reported in S1 Table, according to [31] and [32]. Abbreviations: cp, copy; for, forward; rev, reverse.
(TIF)
Dotted lines represent undetermined sequences. Primer sequences are reported in S1 Table. Abbreviation: ZsHO, Zygosaccharomyces sapae HO gene.
(TIF)
Chromosomes were separated by PFGE for ATCC 42981 (1), Z. rouxii CBS 732T (2), and Z. sapae ABT301T (3). Southern blotting analysis was carried out with probe labelled to HO genes. M indicates the chromosomal size ladder (Saccharomyces cerevisiae S288C, BioRad Laboratories) in megabase pairs. ATCC 42981 chromosomes are indicated in uppercase letters (from A to G).
(TIF)
Chromosomes were separated by PFGE for Z. rouxii CBS 732T (1) and ATCC 42981 (2). Southern blotting analyses were carried out with probes labelling the α- and a-idiomorph loci. The left panel shows signals from MTLα loci Zygosaccharomyces rouxii CBS 732T (1) and the ATCC 42981 strain (2), respectively. The right panel reports separated chromosomes and signals from MTLa loci in ATCC 42981 (2). M indicates the chromosomal size ladder (Saccharomyces cerevisiae S288C, BioRad Laboratories) in megabase pairs. ATCC 42981 chromosomes are indicated in uppercase letters (from A to G).
(TIF)
(XLS)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files. All sequences are deposited in GenBank under accession numbers from KT598024 to KT598027 and from KT694298 to KT694302, and you may find the accession numbers cited in the text.
Funding Statement
The authors have no support or funding to report.
References
- 1.Van de Peer Y, Maere S, Meyer A. The evolutionary significance of ancient genome duplications. Nat Rev Genet. 2009;10(10): 725–32. 10.1038/nrg2600 [DOI] [PubMed] [Google Scholar]
- 2.Kellis M, Patterson N, Endrizzi M, Birren B, Lander ES. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature. 2003;423(6937): 241–54. [DOI] [PubMed] [Google Scholar]
- 3.Scannell DR, Butler G, Wolfe KH. Yeast genome evolution-the origin of the species. Yeast. 2007;24(11): 929–42. org/ 10.1002/yea.1515 [DOI] [PubMed] [Google Scholar]
- 4.Johnson NA. Hybrid incompatibility genes: remnants of a genomic battlefield? Trends Genet. 2010;26(7): 317–25. org/ 10.1016/j.tig.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Rieseberg LH, Raymond O, Rosenthal DM, Lai Z, Livingstone K, Nakazato T, et al. Major ecological transitions in wild sunflowers facilitated by hybridization. Science. 2003;301(5637): 1211–6. [DOI] [PubMed] [Google Scholar]
- 6.Alipaz JA, Karr TL, Wu CI. Evolution of sexual isolation in laboratory populations: fitness differences between mating-types and the associated hybrid incompatibilities. Am Nat. 2005. April 1;165(4): 429–38. [DOI] [PubMed] [Google Scholar]
- 7.Otto SP, Gerstein AC. The evolution of haploidy and diploidy. Curr Biol. 2008;18(24): 1121–4. [DOI] [PubMed] [Google Scholar]
- 8.Nolte AW, Tautz D. Understanding the onset of hybrid speciation. Trends Genet. 2010;26(2): 54–8. 10.1016/j.tig.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 9.Zörgö E, Chwialkowska K, Gjuvsland AB, Garré E, Sunnerhagen P, Liti G, et al. Ancient evolutionary trade-offs between yeast ploidy states. PLoS Genet. 2013;9(3): e1003388 10.1371/journal.pgen.1003388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heitman J. Microbial genetics: Love the one you’re with. Nature. 2009;460(7257): 807–8. 10.1038/460807a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knop M. Evolution of the hemiascomycete yeasts: On life styles and the importance of inbreeding. BioEssays. 2006;28(7): 696–708. [DOI] [PubMed] [Google Scholar]
- 12.Haber JE. Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics. 2012;191(1): 33–64. 10.1534/genetics.111.134577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granek JA., Kayıkçı Ö, Magwene PM. Pleiotropic signaling pathways orchestrate yeast development. Curr Opin Microbiol. 2011;14(6): 676–81. 10.1016/j.mib.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goutte C, Johnson AD. a1 protein alters the DNA binding of alpha2 repressor specificity. Cell. 1988; 52:875–82. [DOI] [PubMed] [Google Scholar]
- 15.Herskowitz IR. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol Rev. 1988;52(4): 536–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathias JR, Hanlon SE, O’Flanagan RA, Sengupta AM, Vershon AK. Repression of the yeast HO gene by the MATα2 and MATa1 homeodomain proteins. Nucleic Acids Res. 2004;32(22): 6469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covitz PA, Herskowitz I, Mitchell AP. The yeast RME1 gene encodes a putative zinc finger protein that is directly repressed by a1-α2. Genes Dev. 1991;5(11): 1982–9. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell AP, Herskowitz I. Activation of meiosis and sporulation by repression of the RME1 product in yeast. Nature. 1986. February 27;319(6056): 738–42. [DOI] [PubMed] [Google Scholar]
- 19.Van Dyk D, Hansson G, Pretorius IS, Bauer FF. Cellular differentiation in response to nutrient availability: the repressor of meiosis, Rme1p, positively regulates invasive growth in Saccharomyces cerevisiae. Genetics. 2003;165(3): 1045–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah JC, Clancy MJ. IME4, a gene that mediates MAT and nutritional control of meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12(3): 1078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hongay CF, Grisafi PL, Galitski T, Fink GR. Antisense transcription controls cell fate in Saccharomyces cerevisiae. Cell. 2006;127(4): 735–45. [DOI] [PubMed] [Google Scholar]
- 22.Gelfand B, Mead J, Bruning A, Apostolopoulos N, Tadigotla V, Nagaraj V, et al. Regulated antisense transcription controls expression of cell-type-specific genes in yeast. Mol Cell Biol. 2011;31(8): 1701–9. 10.1128/MCB.01071-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solieri L, Chand Dakal T, Croce MA, Giudici P. Unravelling genomic diversity of Zygosaccharomyces rouxii complex with a link to its life cycle. FEMS Yeast Res. 2013;13(3): 245–58. 10.1111/1567-1364.12027 [DOI] [PubMed] [Google Scholar]
- 24.Mori H. Life cycle in a heterothallic haploid yeast, Saccharomyces rouxii. J Ferment Technol. 1973; 51: 379–392. [Google Scholar]
- 25.Solieri L, Dakal TC, Giudici P. Zygosaccharomyces sapae sp. nov., isolated from Italian traditional balsamic vinegar. Int J Syst Evol Microbiol. 2013;63(Pt 1): 364–71. 10.1099/ijs.0.043323-0 [DOI] [PubMed] [Google Scholar]
- 26.Solieri L, Cassanelli S, Croce MA, Giudici P. Genome size and ploidy level: new insights for elucidating relationships in Zygosaccharomyces species. Fungal Genet Biol. 2008;45(12): 1582–90. 10.1016/j.fgb.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 27.Solieri L, Dakal TC, Bicciato S. Quantitative phenotypic analysis of multistress response in Zygosaccharomyces rouxii complex. FEMS Yeast Res. 2014;14(4): 586–600. 10.1111/1567-1364.12146 [DOI] [PubMed] [Google Scholar]
- 28.Pribylova L, De Montigny J, Sychrova H. Osmoresistant yeast Zygosaccharomyces rouxii: the two most studied wild-type strains (ATCC 2623 and ATCC 42981) differ in osmotolerance and glycerol metabolism. Yeast. 2007;24(3): 171–80. [DOI] [PubMed] [Google Scholar]
- 29.Gordon JL, Wolfe KH. Recent allopolyploid origin of Zygosaccharomyces rouxii strain ATCC 42981. Yeast. 2008;25(6): 449–56. 10.1002/yea.1598 [DOI] [PubMed] [Google Scholar]
- 30.James SA, Bond CJ, Stratford M, Roberts IN. Molecular evidence for the existence of natural hybrids in the genus Zygosaccharomyces. FEMS Yeast Res. 2005;5(8): 747–55. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe J, Uehara K, Mogi Y. Diversity of mating-type chromosome structures in the yeast Zygosaccharomyces rouxii caused by ectopic exchanges between MAT-like loci. PLoS One. 2013;8(4): e62121 10.1371/journal.pone.0062121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solieri L, Dakal TC, Giudici P, Cassanelli S. Sex-determination system in the diploid yeast Zygosaccharomyces sapae. G3 (Bethesda). 2014;4(6): 1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler G, Kenny C, Fagan A, Kurischko C, Gaillardin C, Wolfe KH. Evolution of the MAT locus and its Ho endonuclease in yeast species. Proc Natl Acad Sci U S A. 2004;101(6): 1632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souciet JL, Dujon B, Gaillardin C. Comparative genomics of protoploid Saccharomycetaceae. Genome Res. 2009;19(10): 1696–709. 10.1101/gr.091546.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2): 267–72. [DOI] [PubMed] [Google Scholar]
- 36.Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21): 2947–8. [DOI] [PubMed] [Google Scholar]
- 37.Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI- BLAST: a new generation of protein database search programs. Nucleic acids Res. 1997;25(17): 3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12): 2725–9. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, et al. Pfam: the protein families database. Nucleic Acids Res. 2014;42(D1): D222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 2008;36: W197–201. 10.1093/nar/gkn238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin T, Lu SW, van Tilbeurgh H, Ripoll DR, Dixelius C, Turgeon BG, et al. Tracing the origin of the fungal α1 domain places its ancestor in the HMG-box superfamily: Implication for fungal mating-type evolution. PLoS One. 2010;5(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallona I, Weiss J, Marcos E-C. pcrEfficiency: a web tool for PCR amplification efficiency prediction. BMC Bioinformatics. 2011;12(1): 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leandro MJ, Sychrová H, Prista C, Loureiro-Dias MC. ZrFsy1, a high-affinity fructose/H+ symporter from fructophilic yeast Zygosaccharomyces rouxii. PLoS One. 2013;8(7): e68165 10.1371/journal.pone.0068165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) Method. Methods. 2001;25(4): 402–8. [DOI] [PubMed] [Google Scholar]
- 45.Zhao S, Fernald RD. Comprehensive algorithm for quantitative Real-Time Polymerase Chain Reaction. J Comput Biol. 2005;12(8): 1047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, Van Den Hoff MJB, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37(6): e45 10.1093/nar/gkp045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolberger C, Vershon AK, Johnson AD, Pabo C. Crystal structure of a MATα2 homeodomain-operator model complex suggests a general for homeodomain-DNA interactions. Cell. 1991;67: 517–26. [DOI] [PubMed] [Google Scholar]
- 48.Kües U, Casselton LA. Fungal mating type genes—regulators of sexual development. Mycol Res. 1992;96(12): 993–1006. [Google Scholar]
- 49.Anderson JS, Forman MD, Modleski S, Dahlquist FW, Baxter SM. Cooperative ordering in homeodomain-DNA recognition: solution structure and dynamics of the MATa1 homeodomain. Biochemistry. 2000;39(33): 10045–54. [DOI] [PubMed] [Google Scholar]
- 50.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9): 1189–91. 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belfort M, Roberts RJ. Homing endonucleases: keeping the house in order. Nucleic Acids Res. 1997;25(17): 3379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoddard BL. Homing endonuclease structure and function. Q Rev Biophys. 2005;38(1): 49–95. [DOI] [PubMed] [Google Scholar]
- 53.Hafez M, Hausner G, Bonen L. Homing endonucleases: DNA scissors on a mission. Genome. 2012;55(8): 553–69. 10.1139/g2012-049 [DOI] [PubMed] [Google Scholar]
- 54.Gordon JL, Armisen D, Proux-Wera E, OhEigeartaigh SS, Byrne KP, Wolfe KH. Evolutionary erosion of yeast sex chromosomes by mating-type switching accidents. Proc Natl Acad Sci. U S A. 2011;108(50): 20024–9. 10.1073/pnas.1112808108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruderfer DM, Pratt SC, Seidel HS, Kruglyak L. 2006. Population genomic analysis of outcrossing and recombination in yeast. Nat Genet. September;38(9):1077–81. Epub 2006 Aug 6 [DOI] [PubMed] [Google Scholar]
- 56.Tsai IJ, Bensasson D, Burt A, Koufopanou V. Population genomics of the wild yeast Saccharomyces paradoxus: Quantifying the life cycle. Proc Natl Acad Sci U S A. 2008. March 25;105(12):4957–62. 10.1073/pnas.0707314105 Epub 2008 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedrich A, Jung P, Reisser C, Fischer G, Schacherer J.Population genomics reveals chromosome-scale heterogeneous evolution in a protoploid yeast. Mol Biol Evol. 2015. January;32(1):184–92. 10.1093/molbev/msu295 Epub 2014 Oct 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steensels J, Snoek T, Meersman E, Nicolino MP, Voordeckers K, Verstrepen KJ. Improving industrial yeast strains: exploiting natural and artificial diversity. FEMS Microbiol Rev. 2014;38(5): 947–95. 10.1111/1574-6976.12073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunter N, Chambers SR, Louis EJ, Borts RH. The mismatch repair system contributes to meiotic sterility in an interspecific yeast hybrid. EMBO J. 1996;15(7): 1726–33. [PMC free article] [PubMed] [Google Scholar]
- 60.Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates, Inc; 2004. [Google Scholar]
- 61.Dunn B, Paulish T, Stanbery A, Piotrowski J, Koniges G, Kroll E, et al. Recurrent rearrangement during adaptive evolution in an interspecific yeast hybrid suggests a model for rapid introgression. PLoS Genet. 2013;9(3): e1003366 10.1371/journal.pgen.1003366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolfe KH. Origin of the yeast Whole-Genome Duplication. PLoS Biol. 2015;13(8): e1002221 10.1371/journal.pbio.1002221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinclová O, Potier S, Sychrová H. The Zygosaccharomyces rouxii strain CBS732 contains only one copy of the HOG1 and the SOD2 genes. J Biotechnol. 2001;88(2): 151–8. [DOI] [PubMed] [Google Scholar]
- 64.Baker CR, Booth LN, Sorrells TR, Johnson AD. Protein modularity, cooperative binding, and hybrid regulatory states underlie transcriptional network diversification. Cell. 2012;151(1): 80–95. 10.1016/j.cell.2012.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolfe KH. Yesterday’s polyploids and the mystery of diploidization. Nat Rev Genet. 2001;2(5): 333–41. [DOI] [PubMed] [Google Scholar]
- 66.Tuch BB, Galgoczy DJ, Hernday AD, Li H, Johnson AD. The evolution of combinatorial gene regulation in fungi. PLoS Biol. 2008;6(2): e38 10.1371/journal.pbio.0060038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dettman JR, Sirjusingh C, Kohn LM, Anderson JB. Incipient speciation by divergent adaptation and antagonistic epistasis in yeast. Nature. 2007;447(7144): 585–8. [DOI] [PubMed] [Google Scholar]
- 68.Brideau NJ, Flores HA, Wang J, Maheshwari S, Wang XU, Barbash DA. Two Dobzhansky-Muller genes interact to cause hybrid lethality in Drosophila. Science. 2006;314(5803): 1292–5. [DOI] [PubMed] [Google Scholar]
- 69.Lee H-Y, Chou J-Y, Cheong L, Chang N-H, Yang S-Y, Leu J-Y. Incompatibility of nuclear and mitochondrial genomes causes hybrid sterility between two yeast species. Cell. 2008;135(6): 1065–73. 10.1016/j.cell.2008.10.047 [DOI] [PubMed] [Google Scholar]
- 70.Bayes JJ, Malik HS. Altered heterochromatin binding by a hybrid sterility protein in Drosophila sibling species. Science. 2009;326(5959): 1538–41. 10.1126/science.1181756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orr HA, Turelli M. The evolution of postzygotic isolation: accumulating Dobzhansky-Muller incompatibilities. Evolution. 2001;55(6): 1085–94. [DOI] [PubMed] [Google Scholar]
- 72.Piatkowska EM, Naseeb S, Knight D, Delneri D. Chimeric protein complexes in hybrid species generate novel phenotypes. PLoS Genet. 2013;9(10): e1003836 10.1371/journal.pgen.1003836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siliciano PG, Tatchell K. Transcription and regulatory signals at the mating-type locus in yeast. Cell. 1984;37(3): 969–78. [DOI] [PubMed] [Google Scholar]
- 74.Harashima S, Miller AM, Tanaka K, Kusumoto K, Mukai Y, Nasmyth K, et al. Mating-type control in Saccharomyces cerevisiae: isolation and characterization of mutants defective in repression by a1-alpha 2. Mol Cell Biol. 1989;9(10): 4523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strathern J, Shafer B, Hicks J, McGill C. a/α-specific repression by MATα2. Genetics. 1988;120(1): 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goossens KVY, Ielasi FS, Nookaew I, Stals I, Alonso-sarduy L, Daenen L, et al. Molecular mechanism of flocculation self-recognition in yeast and its role in mating and survival. mBio 6(2): e00427–15. 10.1128/mBio.00427-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sternberg PW, Stern MJ, Clark I, Herskowitz I. Activation of the yeast HO gene by release from multiple negative controls. Cell. 1987;48(4): 567–77. [DOI] [PubMed] [Google Scholar]
- 78.Fabre E, Muller H, Therizols P, Lafontaine I, Dujon B, Fairhead C. Comparative genomics in hemiascomycete yeasts: evolution of sex, silencing, and subtelomeres. Mol Biol Evol. 2005;22(4): 856–73. [DOI] [PubMed] [Google Scholar]
- 79.Rajaei N, Chiruvella KK, Lin F, Astrom SU. Domesticated transposase Kat1 and its fossil imprints induce sexual differentiation in yeast. Proc Natl Acad Sci U S A. 2014;111(43): 15491–6. 10.1073/pnas.1406027111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mori H, Onishi H. Diploid hybridization in a heterothallic haploid yeast, Saccharomyces rouxii. Appl Microbiol. 1967;15(4): 928–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marcet-Houben M, Gabaldón T. Beyond the Whole-Genome Duplication: phylogenetic evidence for an ancient interspecies hybridization in the baker’s yeast lineage. PLoS Biol. 2015;13(8): e1002220 10.1371/journal.pbio.1002220 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panel A shows ZsMTLα variants represented in blue and surrounded in grey (copy 1), green (copy 2) and orange (copy 3), respectively, while panel B represents MTLa loci coloured in red. Primer pairs specific for ZsMTLα copies 1 to 3 are arbitrarily referred to as αCp1-P, αCp2-P, and αCp3-P. ZsMATa1 and ZsMATa2-specific primer pairs are arbitrarily referred to as Pa1 and Pa2, while the primer pair termed Pa12 spans the complete MATa1 ORF and a portion of MATa2 gene. Solid grey arrows indicate generic flanking genes, dotted borders represent uncompleted sequences and small arrows (solid) designate gene-specific primers. Primer sequences are reported in S1 Table, according to [31] and [32]. Abbreviations: Zs, Zygosaccharomyces sapae; cp, copy; P, primer; for, forward; rev, reverse.
(TIF)
Forward and reverse MTL-specific internal primers were used to screen PCR products obtained using all possible combinations of primers spanning putative MTL-flanking genes (semi-nested PCR approach); in cases of negative results, 5' and 3' PCR walking was performed using all possible combinations of MTL-specific internal primers and MTL-flanking gene primers (direct PCR approach). Small arrows (solid) indicate gene-specific primers and degenerate primers (dotted lines). CHA1L indicates the ZYRO0F18524g locus. Primer sequences are reported in S1 Table, according to [31] and [32]. Abbreviations: cp, copy; for, forward; rev, reverse.
(TIF)
Dotted lines represent undetermined sequences. Primer sequences are reported in S1 Table. Abbreviation: ZsHO, Zygosaccharomyces sapae HO gene.
(TIF)
Chromosomes were separated by PFGE for ATCC 42981 (1), Z. rouxii CBS 732T (2), and Z. sapae ABT301T (3). Southern blotting analysis was carried out with probe labelled to HO genes. M indicates the chromosomal size ladder (Saccharomyces cerevisiae S288C, BioRad Laboratories) in megabase pairs. ATCC 42981 chromosomes are indicated in uppercase letters (from A to G).
(TIF)
Chromosomes were separated by PFGE for Z. rouxii CBS 732T (1) and ATCC 42981 (2). Southern blotting analyses were carried out with probes labelling the α- and a-idiomorph loci. The left panel shows signals from MTLα loci Zygosaccharomyces rouxii CBS 732T (1) and the ATCC 42981 strain (2), respectively. The right panel reports separated chromosomes and signals from MTLa loci in ATCC 42981 (2). M indicates the chromosomal size ladder (Saccharomyces cerevisiae S288C, BioRad Laboratories) in megabase pairs. ATCC 42981 chromosomes are indicated in uppercase letters (from A to G).
(TIF)
(XLS)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. All sequences are deposited in GenBank under accession numbers from KT598024 to KT598027 and from KT694298 to KT694302, and you may find the accession numbers cited in the text.