Abstract
Approximately 75% of adults over the age of 65 years are affected by two or more chronic medical conditions. We provide a conceptual justification for individualized absolute risk calculators for competing patient-centered outcomes (PCO) (i.e. outcomes deemed important by patients) and patient reported outcomes (PRO) (i.e. outcomes patients report instead of physiologic test results). The absolute risk of an outcome is the probability that a person receiving a given treatment will experience that outcome within a pre-defined interval of time, during which they are simultaneously at risk for other competing outcomes. This allows for determination of the likelihood of a given outcome with and without a treatment. We posit that there are heterogeneity of treatment effects among patients with multiple chronic conditions (MCC) largely depends on those coexisting conditions.
We outline the development of an individualized absolute risk calculator for competing outcomes using propensity score methods that strengthen causal inference for specific treatments. Innovations include the key concept that any given outcome may or may not concur with any other outcome and that these competing outcomes do not necessarily preclude other outcomes. Patient characteristics and MCC will be the primary explanatory factors used in estimating the heterogeneity of treatment effects on PCO and PRO. This innovative method may have wide-spread application for determining individualized absolute risk calculations for competing outcomes. Knowing the probabilities of outcomes in absolute terms may help the burgeoning population of patients with MCC who face complex treatment decisions.
Keywords: Multiple chronic disease, heterogeneity, propensity scores, longitudinal study, absolute risk, competing outcomes, decision tools
INTRODUCTION
A growing proportion of the US population has multiple chronic conditions (MCC) [1]. Approximately 75% of adults over the age of 65 years are affected by two or more chronic medical conditions [2]. As the prevalence of older adults with chronic conditions increases, so do health care utilization, treatment burden, and the frequency of adverse drug events. Considering its sizeable impact on the US population, the Department of Health and Human Services published “Multiple Chronic Conditions: A Strategic Framework” and outlined strategies for addressing the health needs of affected patients [1]. National surveys have identified prevalent combinations of chronic conditions among persons who are hospitalized or visit ambulatory care settings [3]. Methods for readily identifying chronic disease clusters and developing coordinated care management strategies are among the goals outlined in the framework. The individualized absolute risk (AR) calculator for competing patient-centered outcomes (PCO) and patient reported outcomes (PRO) described here specifically addresses the heterogeneity of treatment effects (HTE) among individuals with different profiles of comorbidity. The AR of an outcome refers to the probability that a person receiving a given treatment will experience that outcome within a pre-defined interval of time, during which they are simultaneously at risk for other outcomes of interest, i.e., competing outcomes. AR has been found to be widely applicable across health conditions, datasets and outcomes and much more interpretable by patients and healthcare providers than traditional measures of relative risk, such as odds ratios and hazard ratios.
Multiple Chronic Conditions: A Primary Source of Heterogeneity of Treatment Effects
It is helpful to place the role of HTE within the broader issue of MCC. Multiple chronic conditions increase health care utilization, impose burdens on both the individual and the health care system, and compromise adherence to medications and other therapeutic regimens [4–9]. For these reasons, the identification of models of health care that improve outcomes of persons with multiple diseases is a vital area of medical research [10–13]. Further attesting to the relevance of HTE induced by MCC, it has been reported that MCC affect the prognoses of heart disease, diabetes, and cancer [13, 14]. Compelling research documents the adverse effect of MCC on three of the most important PCO/PRO, namely, survival, functional ability and quality of life [15–21]. Certain combinations of conditions occur at higher rates than others and some of these exhibit supra-additive effects on health outcomes [22–29]. There is a critical need for the development of methods that enable the increasing number of older patients with MCC [30], with the advisement of their medical providers, to navigate complex treatment decisions.
Clinical Relevance
To date the goal of health care, namely “the maximization of benefit and minimization of harm,” has largely focused on single diseases. However, it is not uncommon for older persons to have between 5 and 10 medications for a comparable number of indicated conditions. No one has yet undertaken an in depth examination of how the treatment of these multiple conditions affects PCO and PROs, such as disability and self-reported health. Shared clinical decision-making must eventually be predicated on the explicit goal of maximizing benefit and minimizing harm to overall health, rather than disease specific outcomes. The potential harms inherent in disease-specific treatments in patients with multiple diseases have been previously documented [7, 8, 31–33]. Clinicians, policy makers, and investigators have called for innovative and feasible methods for enhancing shared clinical decision-making with patients having multiple diseases [34–37]. We posit that a quantitative understanding how patient characteristics and MCC contribute to HTE is particularly important because unexamined treatment tradeoffs hold unknown potential for harm. It is likely that unintentional adverse effects are at once widespread and frequently not documented. Determining how specific treatments for a primary disease are influenced by patient characteristics and co-existing diseases will heighten awareness of this issue. Increased awareness can potentially lead to constructive changes in clinical practice, including development of treatment plans that account for the HTE induced by MCC.
Decision Aids
Section 3506 of The Affordable Care Act encourages shared decision making in health care between patients and their providers using decision aids to better align care with patient preferences. These decision aids are intended to be evidence-based and inform patients of the risks and benefits of tests and treatments, as well as their relative effectiveness. Individualized AR tools addresses this call, as well as the Institute of Medicine’s 2001 report on Crossing the Quality Chasm by addressing 3 of the top 10 rules to redesign care [34]. Specifically, we address the following: 1) care is customized according to patient needs and values; 2) the patient is the source of control; and 3) decision-making is evidence-based.
MATERIALS AND METHODS
Individualized Absolute Risk for Competing Outcomes
We propose an innovative methodology to calculate individualized AR for competing outcomes that acknowledges patients’ health outcome preferences. The proposed methodology includes several conceptual innovations. First, we are dealing with outcomes whose does not preclude the patient being at risk for other outcomes. This differs from the typical statistical assumption that the competing outcome (e.g., death) precludes the possibility of the patient experiencing another outcome. The proposed technique involves the development of separate logistic regression models that estimate individual probabilities for each outcome. The AR method then estimates the probability of one outcome occurring before the other. For example, going to the hospital does not preclude disability or loss of mobility and these events can occur in many different orderings [38–40].
Lower Bias and Variance of Estimated Treatment Effects with Propensity Score Matching
Randomized controlled trials (RCT) are powered to examine treatment effect on a primary endpoint, but often exclude those with MCC. Even with more inclusive RCTs, the number of possible treatments and condition combinations make it prohibitive to address all treatment questions. Real time treatment studies using registries are beginning to be used and our proposed methods would enhance their application. For these reasons detailed calculations of individualized AR for persons with MCC are often best performed from analyses of observational data that may have multiple PCO and or PRO. Because observational studies typically have unbalanced patient characteristics with respect to treatment including MCC, we purpose propensity score (PS) matching to construct a reference group (those not taking a specific treatment) that is well-balanced with the treatment group regarding important covariates. We incorporate recent simulation-based findings regarding optimal selection of the variables included in the PS models [41]. These practices are intended not simply to balance the covariates, but to also minimize the bias and variance of the estimated treatment effects, the primary motivation for employing PS.
Propensity score matching, first introduced by Rosenbaum and Rubin in 1983, has been used and validated in hundreds of clinical and epidemiological studies over the last 30 years [42]. We use a SAS software macro that was first introduced in 2005 that has been externally reviewed and used in a large number of studies to conduct the analyses [43].
Competing Outcomes
Using competing PCO and PROs, we will produce an array of AR calculations that account for a wide range of personal characteristics and comorbid conditions among persons receiving a given treatment for their medical condition. This implies that when a multi-provider-patient team considers the effect of specific treatments they must take into account the multiple outcomes important to the patient. While we will illustrate our method with pairs of competing outcomes, the AR approach is not inherently limited to just pairs of outcomes. Thus, this demonstration shows how an AR calculator can be used to calculate the probabilities of occurrence of multiple outcomes within the same one year timeframe. A one year follow-up interval for outcomes is reasonable for patients with MCC, particularly those who experience episodic exacerbations, as they are simultaneously at risk of separate-causes of hospitalization or disability. These severely ill patients are also likely to have their treatment plans changed over the course of a year, as their primary or comorbid diseases progress. A one year interval, therefore, represents a practical sampling of the natural course of MCC on patient centered outcomes, and has been chosen for demonstrating our proposed methodology.
Applying Propensity Scoring Methods to a Confirmatory Study of Heterogeneity of Treatment Effects to Calculate Absolute Risks of Competing Outcomes
As patients with chronic obstructive pulmonary disease (COPD) nearly always have MCC, and because COPD has several recommended treatments, COPD is an excellent example for illustrating the advantages of the AR approach. Our method will combine PS methods and a confirmatory study of HTE to calculate ARs (probabilities) of competing outcomes (i.e., whichever occurs first) within a year’s time to illustrate the effect of a specific treatment for COPD. As we expect the HTE to be driven largely by patient characteristics and multi-morbid conditions, inclusion of these factors will enable more precise calculation of the probabilities of the competing outcomes. For COPD patients for whom treatment is being decided, we will provide probabilities of pairs of competing outcomes conditional on receipt of each specific treatment. The heterogeneity of these probabilities, as determined by the collection of subgroups with specific characteristics being modeled, will be chosen from frequently occurring combinations of chronic conditions among the patient sample [44]. We will do so by developing an analytic methodology that provides subgroup specific information based on patient-specific characteristics.
Data Used To Illustrate the Individualized Absolute Risk Methodology
We reviewed several cohort studies of older adults for use in the development of methodologies. We selected the Medical Expenditures Panel Survey (MEPS) for the 2008 to 2012 panels to demonstrate the study methods. MEPS is a panel study of a nationally representative sample of the U.S. civilian non-institutionalized population [45]. Participants are chosen to represent the country as a whole and include individuals and families from a wide variety of ages, incomes, geographic settings, and racial/ethnic backgrounds (Table 1). MEPS is therefore representative of racial and ethnic minorities, individuals with disabilities, and individuals with multiple chronic diseases (Table 2). A panel is a sample of households selected to participate in the study over a period of time. Each year a new panel or sample of households throughout the country is introduced into the study. The new sample is made up of households that participated in the National Health Interview Survey, conducted by the National Center for Health Statistics, the previous year [46]. Information is collected about each household member’s health and health care utilization over a two-year period beginning on January 1 of the year the household enters the study and ending December 31 the following year. Five rounds of interviews are conducted to provide annual data for two calendar years.
Table 1.
MEPS 2008–2012: N=2,587 Participants Aged 40 and over with Chronic Bronchitis, Emphysema or COPD 2009 Panel Data Only
| Demographic: | (%) |
|---|---|
| Age med., Range | 63.0 40–85 |
| Female | 348 (61.5) |
| White | 433 (76.5) |
| Black | 109 (19.3) |
| Hispanic | 60 (10.6) |
| <High school | 190 (33.6) |
| High school/vocational | 182 (32.2) |
| ≥ Some college | 190 (33.6) |
| Income < $15,000 | 145 (25.6) |
| $15,001–35,000 | 182 (32.2) |
| $35,000 | 239 (42.2) |
| Below/Near Poverty Line | 172 (30.4) |
| Married | 280 (49.5) |
| Widowed | 106 (18.7) |
| Never/divorced/separated | 171 (30.2) |
Table 2.
Chronic Conditions and Medications 2009 Panel Data Only
| Co-existing Chronic Conditions | (%) |
|---|---|
| Cardiovascular: | |
| Hypertension | 401 (70.9) |
| Coronary Heart Disease | 136 (24.0) |
| Angina | 71 (12.5) |
| Myocardial Infarction | 98 (17.3) |
| Dysrhthmias | 24 (4.2) |
| Heart Failure | 27 (4.8) |
| Other | 170 (30.0) |
| Diabetes | 137 (24.2) |
| Visual Impairment | 106 (18.7) |
| Cognitive Impairment | 99 (17.5) |
| Stroke | 91 (16.1) |
| Depressive Symptoms | 84 (14.8) |
| Bone and Cartilage Disorders | 35 (6.2) |
| Anemia | 20 (3.5) |
| Lung Cancer | 14 (2.5) |
| Medications: | |
| Beta-Agonist-Inhaled | 202 (35.7) |
| Inhaled Anticholinergic | 81 (14.3) |
| Steroids-Inhaled | 133 (23.5) |
| Steroids-Oral | 74 (13.1) |
| Smoking Cessation | 21 (12.9) |
Balancing Patient Characteristics to Reduce Bias
As we are using an observational study, we will verify the balance in patient characteristics and MCC between those receiving a specific COPD treatment and those defining the reference group (persons with COPD that are not receiving that treatment). We calculate PS for COPD treatment for all participants and use a matching algorithm, in concert with appropriate calipers, to match one to two reference patients to each patient receiving a specific treatment for COPD [47]. PS allow for the assessment of whether the characteristics of those receiving a specific COPD treatment overlap enough with those not being treated, thereby yielding an unbiased estimate of the treatment effect from the data. By combining PS with longitudinal individualized AR calculation, the association of treatment will be estimated in a way that more closely approximates causal inference. Through the development of this methodology and its protocol, future researchers will be able to apply individualized AR calculators to a broad array of treatment decisions.
Greater Interpretability of Absolute Risk
One of the reasons the proposed method of integrating PS into the creation of individualized AR for competing outcomes methods is needed is because results in the medical literature are typically presented in relative terms, such as odds ratios, risk ratios or hazard ratios, which are not easily interpreted by patients or their physicians. The longitudinal individualized AR for competing outcomes is the gross probability of an outcome within a specific period of time in the context of a competing outcome. It is increasingly common in the medical literature for measures of relative risk to be partnered with a presentation of AR to bring clearer meaning and interpretation of research results.
Methodologies recommended by experts in medication-related research include precise definition of medications used, establishment of temporal precedence, proper evaluation of clinical indication and contraindication bias, and adjustment for confounding or for the propensity to receive the medication of interest [48–52]. Similarly, there are several prerequisite elements to justify causality in chronic disease, such as strength (graded association between cumulative dose or duration of use and outcome), biological gradient, consistency, biological plausibility (coherence), and the establishment of temporal precedence. Quantifying the ARs of competing clinical outcomes and PCO and PROs, for persons receiving medication for a primary condition in the presence of multiple diseases and including patient characteristics, is one of the most pressing areas in patient-centered decision-making. With careful attention to design and analytical issues, we are developing methodology that has potentially wide-spread use.
The challenges of assessing treatment effects in observational studies have been well chronicled [42, 53–56]. Treatments are non-random factors often intricately linked to the diseases and their severity and to other predisposing or prognostic factors. Furthermore, within a medication class, different agents may have different effects. Our study initially will consider the following common treatments for the primary condition of COPD: beta agonists, inhaled anticholinergics, and steroids. Our methods for calculating AR have the additional advantage of allowing adjustment for competing outcomes [57].
Illustration of Absolute Risk Methods with Chronic Obstructive Pulmonary Disease (COPD)
We emphasize that AR, when appropriately calculated, is a very sophisticated statistical measure [57]. It is not a simple probability of occurrence taken from contingency tables. Rather, it is a well-developed approach that calculates the gross probability of an event within a specific interval of time in the context of competing outcomes. For the purposes of the AR calculations proposed here, our definition of competing outcomes is that of two outcomes for which a patient is simultaneously at risk, where the patient is interested in knowing the probability of each occurring first given concurrent risk for the second. A methodologically correct AR, therefore, requires longitudinal modeling of both primary and competing outcomes. Only a sophisticated, model-based form of this methodology allows for adjustment of both fixed and time-varying patient characteristics when estimating these risks.
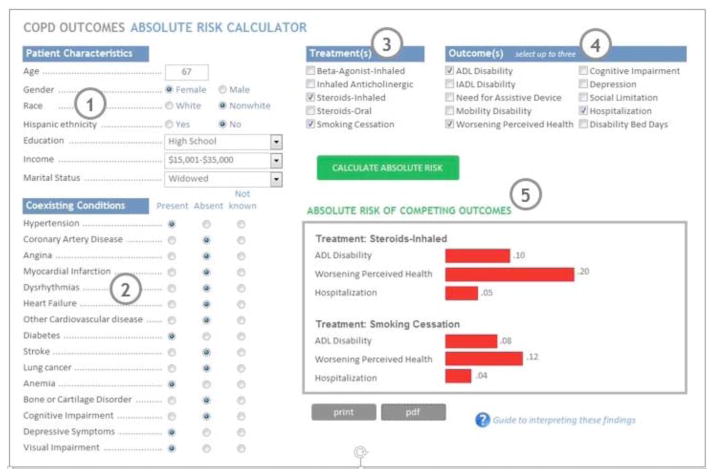
For a person receiving a specific treatment for COPD relative to those not receiving the treatment, the AR is calculated for “outcome 1” occurring before the competing “outcomes 2”. Conversely, the AR for “outcome 2” occurring before the competing “outcomes 1” is calculated (Figure 1 circle 5). We expect the AR values to be strongly conditional on patient characteristics and the specific MCC of those receiving the treatment. The method will be illustrated by characterizing the effects of common treatments for COPD medications and will demonstrate how much the efficacy of these treatments, as measured by their effect on AR, is modified by the presence of specific chronic conditions.
Figure 1.
Simulated COPD outcomes AR calculator. Key: (1) Patient characteristics are entered; (2) Comobid conditions are selected; (3) Treatments are selected; (4) Competing outcomes are selected; (5) Absolute Risk estimates for competing outcomes are displayed, for each selected treatment.
Demonstration of Individualized Absolute Risk Calculator
Future individualized AR Calculators will be web-based. A mock screen shot combining the input and output screens of a hypothetical AR Calculator for our illustrative COPD example is shown in the Figure 1. First, the patient’s MCC and characteristics are entered; for this example we display a 67 year old, non-white woman with a high school education, low income, with hypertension, diabetes, anemia, depressive symptoms and visual impairments (Figure 1 circles 1 & 2). Possible treatments are selected; for this example inhaled steroids and smoking cessation (circle 3). Then the patient’s outcome priorities over the next year are selected from the list; for this example having disabilities in activities of daily living (ADL), worsening perceived health, and hospitalization (circle 4). Next, the calculator would graphically display the individualized AR for that patient for their priorities (circle 5).
DISCUSSION
Our proposed methodology will fill critical gaps in the estimation of treatment effect for persons with MCC and address several methodologic challenges including: HTE, causal modeling, interpretable estimates (e.g. AR) for patients and healthcare providers, and a shared decision-making approach for PCO and PRO that are competing outcomes over a planning horizon. We develop this methodology so that it can be applied widely for treatments of different index conditions, although it will be illustrated with a nationally-representative cohort of U.S. adults with COPD which often co-occurs with other chronic conditions.
Future decision tools for MCC reflecting the outcome priorities of these complex patients may better address their quality of life and reduce their burden of medications. We present our methodology development in the context of competing dichotomous outcomes that do not necessarily preclude one another. A limitation of the proposed approach is that non-dichotomous outcomes, such as count of disabilities, days in skilled nursing facilities, or cost of care, do not fit this context and additional methodology will be needed to address them. Additional limitations include that longitudinal weights for complex sampling designs are not incorporated and it does not apply to recurrent events. Future modelling approaches should consider modifications for these, as well as allowing for a choice of causal modeling technique, and hierarchical models to capture healthcare system factors or other clustering. Methodological development of ways to rigorously account for MCC is just beginning and the aforementioned limitations and modifications will be resolved by researchers committed to address this growing public health need.
SUMMARY
We are proposing a simple method of calculating the absolute risk of important patient centered outcomes among persons suffering from COPD. The methodology we are presenting depends on the use of observational data which is typically much more comprehensive and representative of a broader population than RCTs. We will account for the imbalance of covariates with propensity score matching which moves us closer to causal inference. Because we are covering outcomes that do not preclude one another, the proposed methodology will be very helpful to persons with MCC who face complex treatment decisions. Because quality of life is of such high priority to such patients, and because it is highly personal, this methodology may serve as an aid in helping patients decide on treatments based on the gross probabilities of differing outcomes and their personal set of values. Although it has been proposed that the development of methods for facilitating the treatment of those with MCC has been presented as a national priority, the method proposed here is one of the first to answer that call.
Acknowledgments
This work was supported by the National Institute of Aging at the National Institutes of Health (R01 AG047891, R21 AG045148, P30 AG021342). Dr. Vaz Fragoso is a recipient of a Merit Award from the Department of Veterans Affairs.
References
- 1.Multiple Chronic Conditions-A Strategic Framework. Washington DC: U.S. Department of Health and Human Services; 2010. cited 2013 January 29, 2013. [Google Scholar]
- 2.Anderson G. Making the Case for Ongoing Care. Robert Wood Johnson Foundation; 2010. [cited 2013]. Available from: http://www.rwjf.org/en/research-publications/find-rwjf-research/2010/02/chronic-care.html. [Google Scholar]
- 3.Wallace RB, Salive ME. The Dimensions of Multiple Chronic Conditions: Where Do We Go From Here? A Commentary on the Special Collection of Preventing. Chronic Disease Prev Chronic Dis. 2013;(10):E59. doi: 10.5888/pcd10.130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24. doi: 10.1001/jama.294.6.716. http://dx.doi.org/10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 5.Condelius A, Edberg AK, Jakobsson U, Hallberg IR. Hospital admissions among people 65+ related to multimorbidity, municipal and outpatient care. Arch Gerontol Geriatr. 2008;46(1):41–55. doi: 10.1016/j.archger.2007.02.005. http://dx.doi.org/10.1016/j.archger.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Laux G, Kuehlein T, Rosemann T, Szecsenyi J. Co- and multimorbidity patterns in primary care based on episodes of care: results from the German CONTENT project. BMC Health Serv Res. 2008;8:14. doi: 10.1186/1472-6963-8-14. http://dx.doi.org/10.1186/1472-6963-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351(27):2870–4. doi: 10.1056/NEJMsb042458. http://dx.doi.org/10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 8.Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116(3):179–85. doi: 10.1016/j.amjmed.2003.09.031. http://dx.doi.org/10.1016/j.amjmed.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Tooth L, Hockey R, Byles J, Dobson A. Weighted multimorbidity indexes predicted mortality, health service use, and health-related quality of life in older women. J Clin Epidemiol. 2008;61(2):151–9. doi: 10.1016/j.jclinepi.2007.05.015. http://dx.doi.org/10.1016/j.jclinepi.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Boyd CM, Boult C, Shadmi E, Leff B, Brager R, Dunbar L, et al. Guided care for multimorbid older adults. Gerontologist. 2007;47(5):697–704. doi: 10.1093/geront/47.5.697. http://dx.doi.org/10.1093/geront/47.5.697. [DOI] [PubMed] [Google Scholar]
- 11.McCormick WC, Boling PA. Multimorbidity and a comprehensive Medicare care-coordination benefit. J Am Geriatr Soc. 2005;53(12):2227–8. doi: 10.1111/j.1532-5415.2005.00504.x. http://dx.doi.org/10.1111/j.1532-5415.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- 12.Muller-Tasch T, Peters-Klimm F, Schellberg D, Holzapfel N, Barth A, Junger J, et al. Depression is a major determinant of quality of life in patients with chronic systolic heart failure in general practice. J Card Fail. 2007;13(10):818–24. doi: 10.1016/j.cardfail.2007.07.008. http://dx.doi.org/10.1016/j.cardfail.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Noel PH, Frueh BC, Larme AC, Pugh JA. Collaborative care needs and preferences of primary care patients with multimorbidity. Health Expect. 2005;8(1):54–63. doi: 10.1111/j.1369-7625.2004.00312.x. http://dx.doi.org/10.1111/j.1369-7625.2004.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross CP, Guo Z, McAvay GJ, Allore HG, Young M, Tinetti ME. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc. 2006;54(12):1898–904. doi: 10.1111/j.1532-5415.2006.00973.x. http://dx.doi.org/10.1111/j.1532-5415.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 15.Fortin M, Bravo G, Hudon C, Lapointe L, Dubois MF, Almirall J. Psychological distress and multimorbidity in primary care. Ann Fam Med. 2006;4(5):417–22. doi: 10.1370/afm.528. http://dx.doi.org/10.1370/afm.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL, Maltais D. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes. 2004;2:51. doi: 10.1186/1477-7525-2-51. http://dx.doi.org/10.1186/1477-7525-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279(8):585–92. doi: 10.1001/jama.279.8.585. http://dx.doi.org/10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 18.John R, Kerby DS, Hennessy CH. Patterns and impact of comorbidity and multimorbidity among community-resident American Indian elders. Gerontologist. 2003;43(5):649–60. doi: 10.1093/geront/43.5.649. http://dx.doi.org/10.1093/geront/43.5.649. [DOI] [PubMed] [Google Scholar]
- 19.Kadam UT, Croft PR. Clinical multimorbidity and physical function in older adults: a record and health status linkage study in general practice. Fam Pract. 2007;24(5):412–9. doi: 10.1093/fampra/cmm049. http://dx.doi.org/10.1093/fampra/cmm049. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295(7):801–8. doi: 10.1001/jama.295.7.801. http://dx.doi.org/10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 21.Menotti A, Mulder I, Nissinen A, Giampaoli S, Feskens EJ, Kromhout D. Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: The FINE study (Finland, Italy, Netherlands, Elderly) J Clin Epidemiol. 2001;54(7):680–6. doi: 10.1016/s0895-4356(00)00368-1. http://dx.doi.org/10.1016/S0895-4356(00)00368-1. [DOI] [PubMed] [Google Scholar]
- 22.Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Grady D, Shlipak MG. Renal insufficiency as an independent predictor of mortality among women with heart failure. J Am Coll Cardiol. 2004;44(8):1593–600. doi: 10.1016/j.jacc.2004.07.040. http://dx.doi.org/10.1016/j.jacc.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 23.Ettinger WH, Jr, Fried LP, Harris T, Shemanski L, Schulz R, Robbins J. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. CHS Collaborative Research Group. J Am Geriatr Soc. 1994;42(10):1035–44. doi: 10.1111/j.1532-5415.1994.tb06206.x. http://dx.doi.org/10.1111/j.1532-5415.1994.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 24.Felker GM, Gattis WA, Leimberger JD, Adams KF, Cuffe MS, Gheorghiade M, et al. Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol. 2003;92(5):625–8. doi: 10.1016/s0002-9149(03)00740-9. http://dx.doi.org/10.1016/S0002-9149(03)00740-9. [DOI] [PubMed] [Google Scholar]
- 25.Fortin M, Dubois MF, Hudon C, Soubhi H, Almirall J. Multimorbidity and quality of life: a closer look. Health Qual Life Outcomes. 2007;5:52. doi: 10.1186/1477-7525-5-52. http://dx.doi.org/10.1186/1477-7525-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goulet JL, Fultz SL, Rimland D, Butt A, Gibert C, Rodriguez-Barradas M, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45(12):1593–601. doi: 10.1086/523577. http://dx.doi.org/10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta RH, Dabbous OH, Granger CB, Kuznetsova P, Kline-Rogers EM, Anderson FA, Jr, et al. Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. Am J Cardiol. 2003;92(9):1031–6. doi: 10.1016/j.amjcard.2003.06.001. http://dx.doi.org/10.1016/j.amjcard.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Whitson HE, Cousins SW, Burchett BM, Hybels CF, Pieper CF, Cohen HJ. The combined effect of visual impairment and cognitive impairment on disability in older people. J Am Geriatr Soc. 2007;55(6):885–91. doi: 10.1111/j.1532-5415.2007.01093.x. http://dx.doi.org/10.1111/j.1532-5415.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 29.Whooley MA. Depression and cardiovascular disease: healing the broken-hearted. JAMA. 2006;295(24):2874–81. doi: 10.1001/jama.295.24.2874. http://dx.doi.org/10.1001/jama.295.24.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marengoni A, Winblad B, Karp A, Fratiglioni L. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am J Public Health. 2008;98(7):1198–200. doi: 10.2105/AJPH.2007.121137. http://dx.doi.org/10.2105/AJPH.2007.121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56(10):1839–44. doi: 10.1111/j.1532-5415.2008.01923.x. http://dx.doi.org/10.1111/j.1532-5415.2008.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tinetti ME, McAvay GJ, Fried TR, Allore HG, Salmon JC, Foody JM, et al. Health outcome priorities among competing cardiovascular, fall injury, and medication-related symptom outcomes. J Am Geriatr Soc. 2008;56(8):1409–16. doi: 10.1111/j.1532-5415.2008.01815.x. http://dx.doi.org/10.1111/j.1532-5415.2008.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinetti ME, McAvay GJ, Fried TR, Foody JM, Bianco L, Ginter S, et al. Development of a tool for eliciting patient priority from among competing cardiovascular disease, medication-symptoms, and fall injury outcomes. J Am Geriatr Soc. 2008;56(4):730–6. doi: 10.1111/j.1532-5415.2007.01627.x. http://dx.doi.org/10.1111/j.1532-5415.2007.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crossing the quality chasm: A new health system for the 21st century. Washington, D.C: National Academies Press; 2001. [PubMed] [Google Scholar]
- 35.Cauley JA, Ensrud KE. Considering competing risks . . Not all black and white. Arch Intern Med. 2008;168(8):793–5. doi: 10.1001/archinte.168.8.793. http://dx.doi.org/10.1001/archinte.168.8.793. [DOI] [PubMed] [Google Scholar]
- 36.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–6. doi: 10.1056/NEJMsa012528. http://dx.doi.org/10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 37.McNeil BJ, Pauker SG, Sox HC, Jr, Tversky A. On the elicitation of preferences for alternative therapies. N Engl J Med. 1982;306(21):1259–62. doi: 10.1056/NEJM198205273062103. http://dx.doi.org/10.1056/NEJM198205273062103. [DOI] [PubMed] [Google Scholar]
- 38.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919–28. doi: 10.1001/jama.2010.1568. http://dx.doi.org/10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292(17):2115–24. doi: 10.1001/jama.292.17.2115. http://dx.doi.org/10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 40.Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: a cohort study of older persons. Ann Intern Med. 2012;156(2):131–40. doi: 10.1059/0003-4819-156-2-201201170-00009. http://dx.doi.org/10.7326/0003-4819-156-2-201201170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–56. doi: 10.1093/aje/kwj149. http://dx.doi.org/10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. http://dx.doi.org/10.1093/biomet/70.1.41. [Google Scholar]
- 43.Feng WW, Jun Y, Xu R. A method/macro based on propensity score and Mahalanobis distance to reduce bias in treatment comparison in observational study. 2005 Available from: www.lexjansen.com/pharmasug/2006/publichealthre-search/pr05.pdf.
- 44.Vaz Fragoso CA, Concato J, McAvay G, Van Ness PH, Gill TM. Respiratory impairment and COPD hospitalisation in older persons: a competing risk analysis. Eur Respir J. 2012;40(1):37–44. doi: 10.1183/09031936.00128711. http://dx.doi.org/10.1183/09031936.00128711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medical Expenditure Panel Survey. cited 2013 January 28, 2013 Available from: www.meps.ahrq.gov/mepsweb.
- 46.National Health Interview Survey. 2013 Jan 28; Available from: http://www.cdc.gov/nchs/nhis.htm.
- 47.Rosenbaum PR, Rubin DB. The bias due to incomplete matching. Biometrics. 1985;41(1):103–16. http://dx.doi.org/10.2307/2530647. [PubMed] [Google Scholar]
- 48.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137(8):693–5. doi: 10.7326/0003-4819-137-8-200210150-00015. http://dx.doi.org/10.7326/0003-4819-137-8-200210150-00015. [DOI] [PubMed] [Google Scholar]
- 49.Foody JM, Cole CR, Blackstone EH, Lauer MS. A propensity analysis of cigarette smoking and mortality with consideration of the effects of alcohol. Am J Cardiol. 2001;87(6):706–11. doi: 10.1016/s0002-9149(00)01487-9. http://dx.doi.org/10.1016/S0002-9149(00)01487-9. [DOI] [PubMed] [Google Scholar]
- 50.Larson EB. Evidence, guidelines, performance incentives, complexity, and old people: a clinician’s dilemma. J Am Geriatr Soc. 2009;57(2):353–4. doi: 10.1111/j.1532-5415.2008.02125.x. http://dx.doi.org/10.1111/j.1532-5415.2008.02125.x. [DOI] [PubMed] [Google Scholar]
- 51.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. http://dx.doi.org/10.7326/0003-4819-127-8_Part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 52.Shah BR, Laupacis A, Hux JE, Austin PC. Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol. 2005;58(6):550–9. doi: 10.1016/j.jclinepi.2004.10.016. http://dx.doi.org/10.1016/j.jclinepi.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112(11):2456–66. doi: 10.1002/cncr.23452. http://dx.doi.org/10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glesby MJ, Hoover DR. Survivor treatment selection bias in observational studies: examples from the AIDS literature. Ann Intern Med. 1996;124(11):999–1005. doi: 10.7326/0003-4819-124-11-199606010-00008. http://dx.doi.org/10.7326/0003-4819-124-11-199606010-00008. [DOI] [PubMed] [Google Scholar]
- 55.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98(3):253–9. doi: 10.1111/j.1742-7843.2006.pto_293.x. http://dx.doi.org/10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laupacis A, Mamdani M. Observational studies of treatment effectiveness: some cautions. Ann Intern Med. 2004;140(11):923–4. doi: 10.7326/0003-4819-140-11-200406010-00014. http://dx.doi.org/10.7326/0003-4819-140-11-200406010-00014. [DOI] [PubMed] [Google Scholar]
- 57.Benichou J, Gail MH. Estimates of absolute cause-specific risk in cohort studies. Biometrics. 1990;46(3):813–26. http://dx.doi.org/10.2307/2532098. [PubMed] [Google Scholar]