Abstract
The diversity of chronic pain syndromes and the methods employed to study them make integrating experimental findings challenging. This study performed coordinate-based meta-analyses using voxel-based morphometry imaging results to examine gray matter volume (GMV) differences between chronic pain patients and healthy controls. There were 12 clusters where GMV was decreased in patients compared with controls, including many regions thought to be part of the “pain matrix” of regions involved in pain perception, but also including many other regions that are not commonly regarded as pain-processing areas. The right hippocampus and parahippocampal gyrus were the only regions noted to have increased GMV in patients. Functional characterizations were implemented using the BrainMap database to determine which behavioral domains were significantly represented in these regions. The most common behavioral domains associated with these regions were cognitive, affective, and perceptual domains. Because many of these regions are not classically connected with pain and because there was such significance in functionality outside of perception, it is proposed that many of these regions are related to the constellation of comorbidities of chronic pain, such as fatigue and cognitive and emotional impairments. Further research into the mechanisms of GMV changes could provide a perspective on these findings.
Perspective
Quantitative meta-analyses revealed structural differences between brains of individuals with chronic pain and healthy controls. These differences may be related to comorbidities of chronic pain.
Keywords: Chronic pain, voxel-based morphometry, meta-analysis, gray matter volume
Understanding the neurobiologic basis of chronic pain is important because it affects 100 million Americans and annually costs more than $500 billion.18,95 Chronic pain syndromes represent debilitating conditions that negatively impact quality of life and productivity. The International Association for the Study of Pain (IASP) defines chronic pain as “pain without apparently biological value that has persisted beyond the normal tissue healing time.”14 It is also associated with increased fatigue and changes in cognitive ability and emotional states.18,40,103 Treatments are often derived from experimental acute pain models,55 but chronic pain states, unlike acute pain states, are often associated with alterations in centrally mediated pain processing.1,30,106 To develop better treatments, greater understanding of central factors associated with pain is needed.
Because pain processing is facilitated by complex neural networks involving perception, cognition, and emotion, understanding chronic pain's neurobiology is challenging. Voxel-based morphometry (VBM) has revealed differences in gray matter volume (GMV) of specific brain regions in chronic pain patients compared with healthy controls,59,80,81 commonly found in the anterior cingulate cortex (ACC), thalamus, basal ganglia, and insula.58,59 Although the most commonly reported finding is regional GMV decrease associated with persistent pain,59 increased regional GMV is also found.37,38,83,85,87,96,104,107 Disparate findings across studies make integrative conclusions on the basis of qualitative assessments difficult. Discrepancies between studies likely reflect differences in samples where pain etiology could be a substantial factor. However, the experience of chronic pain could have implications for brain morphology, irrespective of etiology. Only meta-analysis methods are likely to identify trends among heterogeneous chronic pain populations.
Although the above refers to structural investigations of chronic pain versus healthy subjects, there are also functional imaging studies of experimentally induced pain. The relationship between brain function and structural changes is not well elucidated. However, structural and functional changes being somehow related makes sense.10,45,46,77 The functional pain network includes somatosensory cortices, ACC, thalamus, insula, basal ganglia, hippocampus, and temporal and parietal cortices.16,59,86,93 Given the diverse pain literature, unanimous pain network definitions are difficult. Hence, integration of experimental findings26 and comparisons between studies is imminent.
Coordinate-based meta-analysis21,48,49 is commonly used for aggregating imaging results across studies. Statistical pooling with activation likelihood estimation (ALE) integrates existing data while avoiding some limitations of single studies, allowing dominant trends to emerge. Here, we performed meta-analyses of VBM imaging results26,31,62,63 to investigate GMV differences between brains of healthy controls and patients with chronic pain. We included 23 studies3,4,12,28,29,37,38,47,74,75,78,81-85,87,88,96,99-101,107 with different attributes to identify changes associated with chronic pain that commonly occur, irrespective of etiology, age, sex, etc. We hypothesized that GMV differences in individuals with chronic pain would manifest in regions that are typically associated with pain, such as the ACC, somatosensory cortices, insula, and thalamus. We utilized functional characterization (FC), where we seeded significant clusters as regions of interest and determined the functions that were significantly associated with them in the BrainMap database.22 We hypothesized that we would identify the strongest function–location correspondences between regions with altered GMV and perceptual processes. We also hypothesized that we would observe correspondences associated with affective and cognitive processing.
Methods
VBM Literature Search
We conducted a comprehensive PubMed search for structural magnetic resonance images and chronic pain, in addition to examining review papers and tracing references from retrieved papers. Studies were captured up to early July 2012. The employed search terms were “chronic pain and voxel-based morphometry” and “chronic pain and VBM.” Because of the statistical methods employed, coordinate-based meta-analyses have certain requirements for studies to be included. Only studies that analyzed local changes in GMV based on structural magnetic resonance images using VBM were included in our meta-analysis; the reported changes included both increases and decreases in patient GMV relative to control subjects. Of note, VBM preprocessing yields either modulated or unmodulated images. When performing image normalization (warping), gray matter values (within a voxel) can be adjusted (modulated) to the amount of local displacement, such that areas that are expanded/shrunk during the normalization step undergo reduction/amplification in intensity proportional to the alteration in volume; alternatively, gray matter values can be preserved within the normalization step, in which case normalized images are considered “unmodulated.” Modulated images refer to local brain volume, whereas unmodulated images refer to local brain density or concentration. Both analyses based on modulated and those based on unmodulated images were included in this study. As most of the analyses included in this meta-analysis are based on modulated images, we generally refer to GMV.
White matter volume analysis and analyses executed by methods other than VBM were excluded. Furthermore, only whole-brain results reported in stereotactic space (eg, Talairach or Montreal Neurological Institute [MNI]) as standard coordinates (eg, x, y, z) were selected for inclusion. Only between-group comparisons between chronic pain patients and healthy controls were analyzed. Studies were excluded if they 1) did not report stereotactic coordinates of maximal brain structure changes, 2) did not report any comparisons between healthy subjects and chronic pain patients, 3) only reported coordinates as results of a region-of-interest analysis, or 4) did not report results in English. Using our inclusion criteria, we identified 23 peer-reviewed articles, jointly reporting on 490 patients and 509 healthy controls and 235 foci (see Table 1 for study data and included diagnoses).
Table 1. Studies Used for VBM Chronic Pain Meta-Analysis.
| Author | NSubjects | NCoordinates | Pain Type | Patient Demographics | Pain Duration | Significance Level (P <) | Modulated/Unmodulated | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| C | P | C >P | P >C | ||||||
| Apkarian 2004 | 26 | 26 | 4 | 0 | Chronic back pain | 38% male; 43.7 years | >1 year | .01* | Unmodulated |
| As-Sanie 2012 | 6 | 12 | 1 | 0 | Chronic pelvic pain | 0% male; 24.2 years; 83% white | >6 months | .05* | Modulated |
| Buckalew 2008 | 8 | 8 | 2 | 0 | Chronic low back pain | 50% male; 74.5 years; 75% PHS education | >3 months | .001 | Modulated |
| Geha 2008 | 28 | 26 | 1 | - | Complex regional pain syndrome | 14% male; 40.7 years | >3 months | .05* | Modulated |
| Gerstner 2011 | 9 | 9 | 7 | - | Temporomandibular disorder | 0% male; 25.4 years | >3 months | .001 | Modulated |
| Gustin 2011 | 36 | 21 | 6 | 1 | Trigeminal neuropathic pain | 19% male; 55 years | >1 year | .01* | Modulated |
| Gwilym 2010 | 16 | 16 | 2 | 12 | Osteoarthritis | 50% male; 68 years | Unspecified | .001 | Modulated |
| Kuchinad 2007 | 10 | 10 | 5 | 0 | Fibromyalgia | 0% male; 52 years | >3 months | .05* | Modulated |
| Rocca 2006 | 15 | 16 | 21 | 0 | Migraine | 6% male; 42.7 years | >2 years | .001 | Unmodulated |
| Rodriguez-Raecke 2009 | 32 | 32 | 16 | 0 | Primary hip osteoarthritis | 41% male; 66.8 years | >12 months | .001 | Modulated |
| Ruscheweyh 2011 | 31 | 45 | 26 | 0 | Low back, headache, joint pain | 36% male; 66 years | >12 months | .05* | Modulated |
| Schmidt-Wilcke 2005 | 20 | 20 | 16 | 0 | Chronic tension-type headache | 50% male; 33.9 years | Unspecified | .05* | Unmodulated |
| Schmidt-Wilcke 2006 | 18 | 18 | 5 | 3 | Chronic back pain | 50% male; 50.4 years | >6 months | .001 | Unmodulated |
| Schmidt-Wilcke 2007 | 22 | 20 | 2 | 4 | Fibromyalgia | 5% male; 53.6 years | >3 months | .001 | Unmodulated |
| Schmidt-Wilcke 2008 | 31 | 35 | 4 | 0 | Migraine | 9% male; 32.4 years | Unspecified | .05* | Unmodulated |
| Schmidt-Wilcke 2010 | 11 | 11 | 9 | 0 | Chronic facial pain | 18% male; 52.2 years | >3 months | .001 | Modulated |
| Schweinhardt 2008 | 14 | 14 | 0 | 4 | Chronic vulvar pain | 0% male; 25.7 years | >6 months | .05* | Unmodulated |
| Seminowicz 2010 | 49 | 56 | 18 | 7 | Irritable bowel syndrome | 0% male; 32.2 years | >1 year | .1* | Modulated |
| Tu 2010 | 32 | 32 | 9 | 7 | Primary dysmenorrhea | 0% male; 23.8 years | >6 months | .005 | Modulated |
| Valet 2009 | 25 | 14 | 13 | 0 | Pain disorder | 0% male; 51.1 years | >2 years | .05* | Unmodulated |
| Valfre 2008 | 27 | 27 | 11 | 0 | Migraine | 22% male; 34.9 years | 20.6 ± 8.9 years† | .05* | Modulated |
| Vartiainen 2009 | 28 | 8 | 7 | - | Chronic widespread pain | 13% male; 47 years | >3 years | .001 | Unmodulated |
| Younger 2010 | 15 | 14 | 1 | 11 | Temporomandibular disorder | 0% male; 38 years | >1 year | .05* | Modulated |
Abbreviations: C, number of control subjects; P, number of patients; C >P, number of foci where GMV was significantly greater in controls than patients; P >C, number of foci where GMV was significantly greater in patients than controls.
NOTE. A 0-count in P >C coordinates indicates that patient increases were tested for, but none were found; a dash indicates that it was unspecified whether patient increases were tested for.
Study was corrected for multiple comparisons.
Average duration.
The diagnostic criteria for chronic pain syndromes have been diverse, evolving over time and varying between the communities included in the studies, although standardized methods and criteria (eg, IASP criteria, Liverpool criteria, International Headache Society criteria, and American College of Rheumatology guidelines) have recently aided in patient classification. Most diagnoses were determined by clinicians and/or made using previously established, medically accepted diagnostic criteria; the common denominators for inclusion in a chronic pain population were pain duration greater than a specified amount of time (minimum range: 3 months to 1 year) and pain present immediately prior to testing. Because it was our aim to give a quantitative overview of the chronic pain literature, we chose to include all studies that were published in peer-reviewed journals and investigated gray matter differences in patients with chronic pain, regardless of the mode of diagnosis. That is, although the diagnostic criteria are heterogeneous across the included studies, all were accepted by the scientific community as contributing to the knowledge of the neurobiologic substrates of chronic pain and were therefore deemed eligible for inclusion in our meta-analysis.
Anatomic Likelihood Estimation
The meta-analyses were performed using the revised21 ALE approach for coordinate-based meta-analysis of neuroimaging results.48,97 This procedure identifies areas showing a convergence of findings across different experiments whether they are activations in functional studies or morphometric changes in anatomic studies. We use the term anatomic likelihood estimation (ALE) when applying this method to anatomic studies.31 The studies' reported coordinates were compiled; those coordinates that were reported in Talairach space were converted to MNI space using the Lancaster transform,50 and the analysis was performed in MNI space. The ALE analysis treats each focus as the center of a 3D Gaussian probability distribution so as to model the spatial uncertainty associated with the foci. This technique uses the number of subjects to weight the size of the distribution around each point, assuming that a larger sample size should have less associated spatial uncertainty because of the reduction in contributions of interindividual differences.21
For each structural magnetic resonance study, this probabilistic approach will yield a modeled anatomic effects map (analogous to a modeled activation map in functional coordinate-based meta-analyses) where each voxel has an associated probability of being a true observed effect.98 In an ALE meta-analysis, the union of the modeled activation maps is computed at each voxel to provide an unbiased estimate of spatial convergence across experiments. A random effects inference model is used that compares the ALE scores resulting from the union of spatially contingent modeled activation values with the ALE scores derived from a null-distribution permutation procedure reflecting a random spatial association between findings. This comparison filters the “true” ALE scores from those obtained by chance.20,62 We performed separate ALE meta-analyses for reported coordinates associated with increases and decreases in patients' GMV relative to controls, with inference performed at a cluster-level corrected P < .05 with a cluster-forming voxel-level threshold of P < .01 (uncorrected for family-wise error). Cluster localization and identification was executed using Talairach Daemon51,52 via GingerALE cluster analysis.20,21,98
Functional Characterization
Interpretation and contextualization of regions found to have structural differences can be aided by determining the functional roles those regions play. The ALE analysis yielded a set of regions where structural differences were consistently reported between controls and patients. Using a neuroinformatics approach, we explored the range of functions that have been associated with these regions across the literature using prior results archived in the BrainMap database.22 When studies are entered into the database, large amounts of descriptor information related to the experiment are also recorded. One of these pieces of data is the behavioral domain that the experiment targets. There are 5 primary behavioral domain categories: cognition, action, perception, emotion, and interoception. Many of these domains are further divided into subdomains. Paradigm classes categorize the specific task employed (see http://brainmap.org/scribe/ for the complete BrainMap taxonomy and domain and subdomain definitions). In particular, forward inference is the probability of observing activity in a brain region given knowledge of the psychological process, whereas reverse inference is the probability of a psychological process being present given knowledge of activation in a particular brain region. In the forward inference approach, a cluster's functional profile was determined by identifying taxonomic labels, for which the probability of finding activation in the respective cluster was significantly higher than the overall chance (across the entire database) of finding activation in that particular cluster. Significance was established using a binomial test (P < .05, corrected for multiple comparisons using Bonferroni's method62). That is, we tested whether the conditional probability of activation given a particular label (P[Activationj|Domain]) was higher than the baseline probability of activating the region in question per se (P[Activation]). Significance was then assessed by means of a chi-square test. An association of task X to brain region Y obtained in these analyses does not necessarily imply that neural activity in region Yis limited to task X.
Results
The largest, most significant region of reduced GMV in patients was observed spanning several structures in the right hemisphere. Cluster 1 began inferiorly in the putamen and claustrum around z = −14. It then continued from the claustrum to the insula (Brodmann area [BA] 13), moving superiorly and finally extended into the posterior inferior frontal gyrus (IFG; BA 44) on the superior end. The next largest and most significant cluster, cluster 2, began inferiorly in the left ACC (BA 24) and extended into BA 32 in the ACC. It continued superiorly into the left cingulate gyrus (BA 32) and began to cross into the right cingulate gyrus (BA 32). It also extended bilaterally into the medial frontal gyri (BA 9). Cluster 3 was found primarily in the left insula (BA 13) and extended slightly into the superior temporal gyrus (STG; BA 38). Cluster 4 was located in the left thalamus, including a submaximum in the medial dorsal nucleus. Cluster 5 contained a submaximum in subgyral gray matter (BA 21), but also included the right posterior STG (BA 22) and the right insula (BA 13). Cluster 6 was located slightly left of midline between z = 43 and z = 53 and included the medial frontal gyrus (MeFG; BA 6) and the cingulate gyrus (BA 31). Cluster 7 spanned the left IFG with BA 9 superiorly and BA 44 inferiorly. Cluster 8 was positioned in the right MeFG (BA 6) with a submaximum in the right paracentral lobule (BA 31); it also continued into the posterior midcingulate cortex. Cluster 9's submaximum was situated in the right superior frontal gyrus (SFG, BA 10), and the cluster descended into the right MeFG (BA 10). Cluster 10 was located primarily in the right anterior middle frontal gyrus (BA 6) with a small extension into the superior frontal gyrus (SFG, BA 6) at z = 54. Cluster 11 was found in the left posterior middle frontal gyrus. Cluster 12 included a small portion of the left IFG (BA 9), superior to cluster 7, and extended into the insula. Figure 1 and Table 2 display the clusters and their volumes and coordinates.
Figure 1.
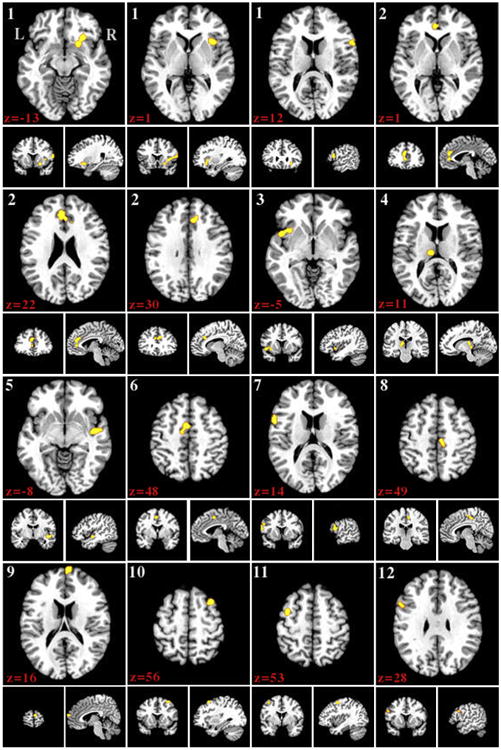
Regions where gray matter volume was greater in control subjects than patients displayed on the Colin27 template brain. Numbers indicate z slice and are displayed in MNI coordinates. Results were taken at cluster level P < .05, with a cluster forming threshold of P < .01 uncorrected.
Table 2. Control Subjects GMV > Chronic Pain Patients GMV ALE Clusters.
| Cluster No. | Volume (mm3) | x | y | z | ALE Value × 109 | xS | yS | zS | Label | BA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3752 | 38.06 | 17.21 | −1.71 | 2.15 | 28 | 22 | −10 | R subgyral gray matter | 47 |
| 2.09 | 22 | 12 | −12 | R putamen | ||||||
| 1.94 | 62 | 16 | 12 | R inferior frontal gyrus | 44 | |||||
| 1.76 | 36 | 16 | 0 | R insula | 13 | |||||
| 1.53 | 50 | 18 | 8 | R precentral gyrus | 44 | |||||
| 1.42 | 62 | 16 | 20 | R inferior frontal gyrus | 45 | |||||
| 2 | 3464 | −.63 | 35.71 | 19.29 | 2.01 | −6 | 40 | 18 | L medial frontal gyrus | 9 |
| 1.91 | −4 | 40 | 2 | L anterior cingulate cortex | 32 | |||||
| 1.72 | 6 | 30 | 27 | R anterior cingulate cortex | 32 | |||||
| 1.57 | 2 | 30 | 24 | R cingulate gyrus | 32 | |||||
| 1.55 | 14 | 34 | 30 | R medial frontal gyrus | 9 | |||||
| 3 | 1552 | −42.22 | 10.6 | −6.49 | 1.87 | −48 | 8 | −8 | L superior temporal gyrus | 38 |
| 1.66 | −36 | 14 | −4 | L insula | 13 | |||||
| 4 | 1280 | −11.09 | −22.72 | 6.6 | 1.81 | −8 | −21 | 11 | L medial dorsal nucleus | |
| 1.58 | −12 | −28 | −4 | L thalamus | ||||||
| 5 | 1104 | 47.75 | −7.61 | −9.36 | 1.92 | 46 | −8 | −10 | R subgyral gray matter | 21 |
| 6 | 960 | −5.12 | −2.67 | 47.88 | 1.99 | −4 | 0 | 48 | L medial frontal gyrus | 6 |
| 1.51 | −10 | −10 | 46 | L cingulate gyrus | 31 | |||||
| 7 | 952 | −59.16 | 9.8 | 15.23 | 2.05 | −60 | 12 | 13 | L inferior frontal gyrus | 44 |
| 1.46 | −60 | 12 | 24 | L inferior frontal gyrus | 9 | |||||
| 8 | 952 | 8.33 | −25.39 | 47.67 | 1.81 | 10 | −29 | 46 | R paracentral lobule | 31 |
| 1.56 | 6 | −22 | 50 | R medial frontal gyrus | 6 | |||||
| 1.48 | 6 | −16 | 54 | R medial frontal gyrus | 6 | |||||
| 9 | 736 | 6.04 | 65.93 | 14.53 | 1.99 | 6 | 68 | 14 | R superior frontal gyrus | 10 |
| 10 | 640 | 33.13 | 15.58 | 54.93 | 1.97 | 34 | 16 | 56 | R middle frontal gyrus | 6 |
| 1.26 | 24 | 12 | 54 | R superior frontal gyrus | 6 | |||||
| 11 | 592 | −38.42 | 1.31 | 53.58 | 1.60 | −40 | −1 | 52 | L middle frontal gyrus | 6 |
| 12 | 368 | −54.8 | 11.87 | 28.21 | 1.56 | −58 | 14 | 30 | L inferior frontal gyrus | 9 |
NOTE. The x, y, and z coordinates define the weighted center of cluster. xS, yS, and zS define the local submaxima. All foci are listed in MNI coordinates. Results were taken at cluster level P < .05, with a cluster forming threshold of P < .01 uncorrected.
Figure 2 displays the region in which significantly increased GMV was identified in patients relative to controls. There was only 1 cluster in this comparison (Table 3). It was located in the hippocampus and the parahippocampal gyrus (BA 36).
Figure 2.
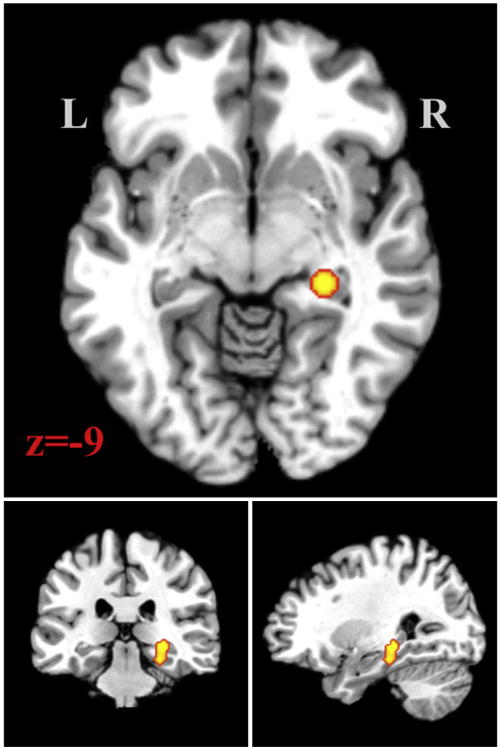
Region where gray matter volume was greater in patients than control subjects displayed on the Colin27 template brain. Number indicates z slice and is displayed in MNI coordinates. Results were taken at cluster level P < .05, with a cluster forming threshold of P < .01 uncorrected.
Table 3. Chronic Pain Patients GMV > Control Subjects GMV ALE Clusters.
| Cluster No. | Volume (MM3) | x | y | z | ALE Value × 109 | xS | yS | zS | Label | BA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1096 | 27.1 | −29.14 | −13.06 | 2.15 | 28 | −30 | −8 | Hippocampus | |
| 1.87 | 26 | −28 | −18 | Parahippocampal gyrus | 36 |
NOTE. The x, y, and z coordinates define the weighted center of cluster. xS, yS, and zS define the local submaxima. All foci are listed in MNI coordinates. Results were taken at cluster level P < .05, with a cluster forming threshold of P < .01 uncorrected.
The FC results in BrainMap (Fig 3) identified a large constellation of domains associated with the above-mentioned regions. Only a few of the regions showed significance in the domain of somesthesis; rather, the majority of results were associated with cognitive and emotional domains. These function–location correspondences identified strong associations between cluster 1 and cognition, emotion, pain, and gustation within the perception domain, bladder interoception, and anxiety. Cluster 2 was involved in emotion (significantly in fear and sadness), cognition (significantly in knowledge of one's body and attention), perception (significantly in gustation, olfaction, and pain), sexuality interoception, and action inhibition. Cluster 3 showed associations with the domains of action inhibition and speech execution, speech and syntax language cognition, and music cognition. Cluster 4 showed involvement in the action domain, specifically in execution and speech execution. Cluster 5 was associated with audition perception and music cognition. Cluster 6 was shown to be involved in action domains, specifically execution, motor learning, and imagination. Cluster 7 primarily showed association with cognitive domains such as language syntax, language phonology, language orthography, time, working memory, and action imagination. Cluster 8 was involved in action execution and explicit memory cognition. Cluster 9 was associated with vision perception and explicit memory cognition. Cluster 10 showed significant involvement in cognitive processes, including explicit memory and working memory. Cluster 11 showed strong association with explicit and working memory, space, and language semantics cognition, as well as vision perception and action imagination. Cluster 12 was involved with cognition, emotion, pain perception, action inhibition, bladder interoception, gustation perception, and emotional anxiety. The cluster in which patient GMV was greater than controls showed association with emotion and cognition, specifically space and explicit memory cognition.
Figure 3.
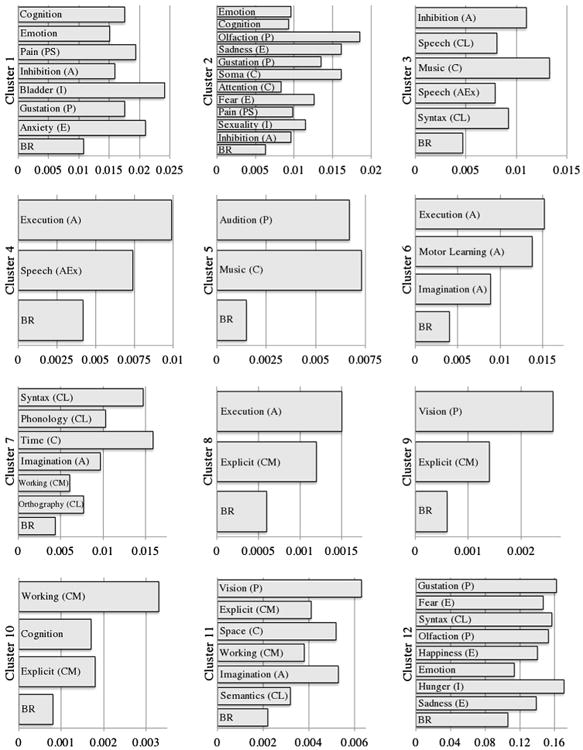
Functional characterization results. Behavioral domains in which the region of interest was significantly involved compared to baseline. Significance at P < .05. A, action; BR, base rate; C, cognition; E, emotion; Ex, execution; I, interoception; L, language; M, memory; P, perception; S, somesthesis.
Discussion
The results are discussed by GMV change and cluster groupings with shared regions below.
Decreased GMV in Chronic Pain
Clusters 1, 7, and 12
The IFG present in all 3 clusters is occasionally reported in imaging studies of pain; it shows decreased GMV in pain patients82 and correlates negatively with pain questionnaire scores.96 The IFG is active during pain catastrophizing in fibromyalgia patients.33,82,96 It is involved in language processing and working memory27 and contributes to emotional empathy.89 In an empathy study, IFG activity was increased in chronic pain patients while they rated the pain intensity of characters in pictures or cartoons.36 The IFG's involvement in pain may relate to emotional states experienced during pain.
The insula (clusters 1 and 12 here, also 3 and 5) has a significant role in pain processing. Activation in the anterior insula is associated with the affective dimension of pain processing and expectation of pain, whereas posterior activations are associated with the sensation and somatotopy of pain.65,68,79
Work to date has shown that the putamen (cluster 1) has a role in the somatotopic pain processing8 and likely modulates STG activity.53 The FC of clusters 1 and 12 confirmed this role in emotional processing. It also revealed that these clusters contain regions involved in cognition, interoception, action, and pain perception. Cluster 7's FC indicated roles in cognitive language and memory processes.
Cluster 2
Cluster 2 was found in the ACC, a prominent component activated in experimental pain and structurally altered in chronic pain.16,17,58,59,70,72,86,92-94 In most pain studies, the ACC appeared to be involved in the affective aspects of pain processing.16,17,70,72,94 However, our finding of patient GMV decrease is in an ACC subregion involved in emotion, extending into midcingulate cortex areas involved in pain modulation6 and fear and avoidance.102 Cluster 2's FC revealed multiple associations, including emotion, cognition, and pain perception. Given these findings, it appears the ACC is involved in the comorbidity of altered emotional control,2 regardless of the precise location. Some of these behavioral domains may be attributed to the MeFG in the cluster; it is part of the dorsolateral prefrontal cortex, shown to modulate pain perception.56
Clusters 3 and 5
Clusters 3 and 5 were found in the left and right STG and insula (discussed above). The STG is typically associated with auditory perception,44 speech perception and comprehension,11,90 and music processing.66 The FC showed involvement in inhibition, speech, audition, language, and music. However, STG is active in many induced pain studies.7,23-25,64,76,105 The STG has largely been ignored in pain imaging studies, likely in part because many investigators use region-of-interest analyses and also because a link between STG function and pain is not obvious.
A possible STG role in pain processing is in efference copy.53 Efference copy and corollary discharge are responsible for monitoring mismatches between predicted and actual sensation.41-43,57 STG activity is associated with efference copy and is likely mediated by the putamen,53 a region involved in pain processing. When there is an error signal because of mismatch, subjects try to correct the error and perceive increased sense of effort.5,91 Research implicates efference copy as driving sense of effort.60,61,71,73,91 Thus, we hypothesize that STG involvement in pain is due to mismatches between pain expectation and perception and that this constant mismatch leads to central fatigue.
Cluster 4
The thalamus is a pain region16,93 involved in affective and sensory processes related to pain.13 Its FC, however, showed only association with action and speech execution. This could possibly be due to a bias in the BrainMap database (discussed further below), causing certain study types to be overrepresented. Another possibility is that patients may experience difficulty completing actions and speaking because of their pain, fatigue, and an increased sense of effort.
Clusters 6 and 8
Both clusters enter the cingulate gyrus in a subregion that is associated with response selection, the posterior midcingulate cortex; this region is involved in skeletomotor orientation.102 This is compatible with cluster 6's FC results, which indicated a role in action, such as execution, motor learning, and imagination. The 2 clusters entered MeFG in BA 6 with cluster 6 in the supplementary motor area. In a functional study in fibromyalgia, the supplementary motor area showed activations in the high subjective pain condition in controls but not patients.34 The FCs revealed that cluster 8 was significantly involved in explicit memory and action execution. Cluster 6 was associated with action domains.
Cluster 9
The SFG is associated with many cognitive processes and is implicated in introspection,32 working memory,9 and spatial processing.9 Although the SFG has also been associated with pain processing,29,74,101,107 its role in chronic pain typically is not discussed. The SFG's emergence in this analysis, its FC in memory cognition, and its involvement in introspection suggest that its role is associated with coping styles. For example, some chronic pain patients are able to adopt an internal locus of control, which is associated with diminished pain perception.19,39 Thus, the SFG may mediate patients' cognitive attempts to cope with pain.
Clusters 10 and 11
The middle frontal gyrus has been implicated in working memory54 and contingency awareness.15 These 2 functions are compatible with FC results showing significant associations with cognition and working memory. These clusters were within the premotor cortex known to be positively correlated with pain intensity.69 It is likely that these differences are related to patients living with chronic pain and developing the expectation of pain. Cluster 10 also extends into the SFG.
Increased GMV in Chronic Pain
The parahippocampal gyrus is involved in pain modulation and sensitivity.35 Also, the hippocampus93 is activated during pain while the subjects experience anxiety, leading to greater pain perception.67 Accordingly, the FC shows involvement in 2 areas affected by comorbid symptoms of chronic pain, cognition, and emotion. Thus, in addition to a functional role in pain, hippocampal involvement also participates in these comorbidities.
Toward Understanding the Neurobiology of Chronic Pain
The inclusion of many types of chronic pain in this meta-analysis supports that these structural variations are associated with chronic pain in general. There were several clusters of GMV reduction in patients compared to healthy controls. It is logical to hypothesize that regional structural differences are associated with altered pain processing and sensitivity. However, the fact that many regions exhibiting GMV differences are not part of the classic “pain matrix” challenges the existence of such a well-defined matrix and suggests that altered morphology may not be completely related to altered functionality. It is likely that the comorbidities of chronic pain are associated with GMV changes.
The only cluster with increased GMV was found in the hippocampus and parahippocampal gyrus. This is a region of overlap between the functional pain network and regions that are structurally altered in chronic pain. The mechanism by which gray matter changes in either direction is unknown; further research aimed at determining why and how GMV changes could provide much more insight. Perhaps integrating findings from anatomic, functional, resting state, and connectivity analyses of chronic pain could yield a detailed model of the differences in the brains of those with chronic pain and help generate hypotheses about the origin and exacerbation of these differences.
One limitation of this meta-analysis is that the chronic pain conditions are extremely heterogeneous. For example, chronic headache disorders are often distinct from other chronic pain disorders, as are autoimmune and inflammatory disorders. It is possible that pain conditions with a neuropathic etiology are different from painful conditions without damage to the nervous system. Furthermore, many of the disorders were represented in diffuse body regions with diverse somatotopic representations. However, including as many disorders as possible was advantageous for this analysis for increased power and to attempt to identify GMV changes common to all disorder types. Most of the included studies did not control for depression, and many found differences in depression scores between patients and controls. Although this could affect the results, we believe that it supports our conclusions that many of the structural changes observed could be related to comorbidities instead of just the chronic pain. Constraints in meta-analyses are inherent to the methods used in a given set of studies. FCs are limited to those coded in the BrainMap database. Researchers interested in a particular region often use specific paradigms to study that region. Therefore, FCs could be incomplete depending on the focus of the studies. In addition, many VBM studies do not test for gray matter increases in disordered populations (3 of 23 here do not), possibly giving an inaccurate impression of the relative frequency of GMV increases and decreases. Finally, studies using different scanning parameters and scanner strengths could introduce small spatial errors into the raw data.
In summary, these meta-analyses support that chronic pain is associated with regional GMV changes. Many of the regions were not “pain matrix” regions, which implies that altered brain morphology is related not only to altered pain processing in chronic pain but also to frequent comorbidities. This is further supported by the fact that these comorbidities are present in most chronic pain disorders; therefore, disorder heterogeneity does not discount the results.
Acknowledgments
The study was funded by United States Army Medical Research Acquisition Act (USAMRAA) Grant W81XWH-11-2-0061 (D.A.R.) and National Institute of Mental Health grants R01-MH074457 (A.R.L.).
Footnotes
The authors have no conflicts of interest financial or otherwise, related to this study.
References
- 1.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, Williams D, Clauw DJ, Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: A voxel-based morphometry study. Pain. 2012;153:1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard KJ, Robin DA. Influence of continual biofeedback on jaw-pursuit tracking in healthy adults and in adults with apraxia plus aphasia. J Mot Behav. 2007;39:19–28. doi: 10.3200/JMBR.39.1.19-28. [DOI] [PubMed] [Google Scholar]
- 6.Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 7.Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: An fMRI study. Magn Reson Med. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Bingel U, Gläscher J, Weiller C, Büchel C. Somatotopic representation of nociceptive information in the putamen: An event-related fMRI study. Cereb Cortex. 2004;14:1340–1345. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]
- 9.Boisgueheneuc Fd, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B. Functions of the left superior frontal gyrus in humans: A lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- 10.Brodtmann A, Puce A, Darby D, Donnan G. Regional fMRI brain activation does correlate with global brain volume. Brain Res. 2009;1259:17–25. doi: 10.1016/j.brainres.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 11.Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cogn Sci. 2001;25:663–678. [Google Scholar]
- 12.Buckalew N, Haut MW, Morrow L, Weiner D. Chronic pain is associated with brain volume loss in older adults: Preliminary evidence. Pain Med. 2008;9:240–248. doi: 10.1111/j.1526-4637.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 13.Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: Is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78:415–418. doi: 10.1007/BF00228914. [DOI] [PubMed] [Google Scholar]
- 14.Harstall C, Ospina M. How prevalent is chronic pain. In: Carr DB, editor. IASP Pain Clinical Updates. 2. XI. Seattle, WA: International Association for the Study of Pain; 2003. [Google Scholar]
- 15.Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. NeuroImage. 2006;29:1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Chen FY, Tao W, Li YJ. Advances in brain imaging of neuropathic pain. Chin Med J (Engl) 2008;121:653–657. [PubMed] [Google Scholar]
- 17.Chiapparini L, Ferraro S, Grazzi L, Bussone G. Neuroimaging in chronic migraine. Neurol Sci. 2010;31:S19–S22. doi: 10.1007/s10072-010-0266-9. [DOI] [PubMed] [Google Scholar]
- 18.Committee on Advancing Pain Research, Care, and Education, Board on Health Sciences Policy, Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 19.Crisson JE, Keefe FJ. The relationship of locus of control to pain coping strategies and psychological distress in chronic pain patients. Pain. 1988;35:147–154. doi: 10.1016/0304-3959(88)90222-9. [DOI] [PubMed] [Google Scholar]
- 20.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eickhoff SB, Laird A, Grefkes C, Wang L, Zilles K, Fox P. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox PT, Lancaster JL. Mapping context and content: The BrainMap model. Nat Rev Neurosci. 2002;3:319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- 23.Freund W, Klug R, Weber F, Stuber G, Schmitz B, Wunderlich AP. Perception and suppression of thermally induced pain: A fMRI study. Somatosens Mot Res. 2009;26:1–10. doi: 10.1080/08990220902738243. [DOI] [PubMed] [Google Scholar]
- 24.Freund W, Stuber G, Wunderlich AP, Schmitz B. Cortical correlates of perception and suppression of electrically induced pain. Somatosens Mot Res. 2007;24:203–212. doi: 10.1080/08990220701723636. [DOI] [PubMed] [Google Scholar]
- 25.Freund W, Wunderlich AP, Stuber G, Landwehrmeyer B, Klug R. Graded cutaneous electrical vs thermal stimulation in humans shows different insular and cingulate cortex activation. Somatosens Mot Res. 2010;27:15–27. doi: 10.3109/08990220903516593. [DOI] [PubMed] [Google Scholar]
- 26.Friebel U, Eickhoff SB, Lotze M. Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. NeuroImage. 2011;58:1070–1080. doi: 10.1016/j.neuroimage.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- 28.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: Abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerstner G, Ichesco E, Quintero A, Schmidt-Wilcke T. Changes in regional gray and white matter volume in patients with myofascial-type temporomandibular disorders: A voxel based morphometry study. J Orofac Pain. 2011;25:99–106. [PubMed] [Google Scholar]
- 30.Giesecke T, Gracely RH, Grant MAB, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 31.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: Application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg II, Harel M, Malach R. When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 34.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 35.Grant JA, Courtemanche J, Duerden EG, Duncan GH, Rainville P. Cortical thickness and pain sensitivity in zen meditators. Emotion. 2010;10:43–53. doi: 10.1037/a0018334. [DOI] [PubMed] [Google Scholar]
- 36.Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. NeuroImage. 2007;36:256–267. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 37.Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA. Different pain, different brain: Thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci. 2011;31:5956–5964. doi: 10.1523/JNEUROSCI.5980-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: A longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62:2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- 39.Härkäpää K, Järvikoski A, Mellin G, Hurri H, Luoma J. Health locus of control beliefs and psychological distress as predictors for treatment outcome in low-back pain patients: Results of a 3-month follow-up of a controlled intervention study. Pain. 1991;46:35–41. doi: 10.1016/0304-3959(91)90031-R. [DOI] [PubMed] [Google Scholar]
- 40.Hart R, Wade J, Martelli M. Cognitive impairment in patients with chronic pain: The significance of stress. Curr Pain Headache Rep. 2003;7:116–126. doi: 10.1007/s11916-003-0021-5. [DOI] [PubMed] [Google Scholar]
- 41.Heinks-Maldonado TH, Mathalon DH, Gray M, Ford JM. Fine-tuning of auditory cortex during speech production. Psychophysiology. 2005;42:180–190. doi: 10.1111/j.1469-8986.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 42.Hickok G, Erhard P, Kassubek J, Helms-Tillery AK, Naeve-Velguth S, Strupp JP, Strick PL, Ugurbil K. A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: Implications for the explanation of conduction aphasia. Neurosci Lett. 2000;287:156–160. doi: 10.1016/s0304-3940(00)01143-5. [DOI] [PubMed] [Google Scholar]
- 43.Houde JF, Nagarajan SS, Sekihara K, Merzenich MM. Modulation of the auditory cortex during speech: An MEG study. J Cogn Neurosci. 2002;14:1125–1138. doi: 10.1162/089892902760807140. [DOI] [PubMed] [Google Scholar]
- 44.Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, Damasio H, Steinschneider M, Reale RA, Hind JE, Brugge JF. Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol. 2000;416:79–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Ilg R, Wohlschläger AM, Gaser C, Liebau Y, Dauner R, Wöller A, Zimmer C, Zihl J, Mühlau M. Gray matter increase induced by practice correlates with task-specific activation: A combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansen-Berg H. Imaging the relationship between structure, function and behaviour in the human brain. Brain Struct Funct. 2009;213:499–500. doi: 10.1007/s00429-009-0220-x. [DOI] [PubMed] [Google Scholar]
- 47.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain? J Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laird A, Lancaster J, Fox P. BrainMap: The social evolution of a functional neuroimaging database. Neuroinformatics. 2005;3:65–78. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- 49.Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutierrez D, Moran ST, Gonzales SM, Ray KL, Franklin C, Glahn DC, Fox PT, Lancaster JL. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. NeuroImage. 2010;51:677–683. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziota JC. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mitiken SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leube DT, Knoblich G, Erb M, Grodd W, Bartels M, Kircher TTJ. The neural correlates of perceiving one's own movements. NeuroImage. 2003;20:2084–2090. doi: 10.1016/j.neuroimage.2003.07.033. [DOI] [PubMed] [Google Scholar]
- 54.Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- 55.Loeser JD, Melzack R. Pain: An overview. Lancet. 1999;353:1607–1609. doi: 10.1016/S0140-6736(99)01311-2. [DOI] [PubMed] [Google Scholar]
- 56.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 57.Martikainen MH, Kaneko KI, Hari R. Suppressed responses to self-triggered sounds in the human auditory cortex. Cereb Cortex. 2005;15:299–302. doi: 10.1093/cercor/bhh131. [DOI] [PubMed] [Google Scholar]
- 58.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 59.May A. Structural brain imaging: A window into chronic pain. Neuroscientist. 2011;17:209–220. doi: 10.1177/1073858410396220. [DOI] [PubMed] [Google Scholar]
- 60.McCloskey DI. Corollary discharges: Motor commands and perception. In: Brookes VB, editor. Handbook of Physiology: A Critical, Comprehensive Presentation of Physiological Knowledge and Concepts. Bethesda, MD: American Physiological Society; 1981. pp. 1415–1448. [Google Scholar]
- 61.McCloskey DI, Gandevia S, Potter EK, Colebatch JG. Muscle sense and effort: Motor commands and judgments about muscular contractions. In: Desmedt JE, editor. Motor Control Mechanisms in Health and Disease. New York: Raven Press; 1983. pp. 151–170. [PubMed] [Google Scholar]
- 62.Nickl-Jockschat T, Habel U, Maria Michel T, Manning J, Laird AR, Fox PT, Schneider F, Eickhoff SB. Brain structure anomalies in autism spectrum disorder—A meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp. 2011;33:1470–1489. doi: 10.1002/hbm.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nickl-Jockschat T, Schneider F, Pagel A, Laird A, Fox P, Eickhoff S. Progressive pathology is functionally linked to the domains of language and emotion: Meta-analysis of brain structure changes in schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2011;261:166–171. doi: 10.1007/s00406-011-0249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niddam DM, Yeh TC, Wu YT, Lee PL, Ho LT, Arendt-Nielsen L, Chen ACN, Hsieh JC. Event-related functional MRI study on central representation of acute muscle pain induced by electrical stimulation. NeuroImage. 2002;17:1437–1450. doi: 10.1006/nimg.2002.1270. [DOI] [PubMed] [Google Scholar]
- 65.Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguière F. Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cereb Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- 66.Platel H, Price C, Baron JC, Wise R, Lambert J, Frackowiak RS, Lechevalier B, Eustache F. The structural components of music perception. A functional anatomical study Brain. 1997;120:229–243. doi: 10.1093/brain/120.2.229. [DOI] [PubMed] [Google Scholar]
- 67.Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JNP, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JNP. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 69.Porro CA, Cettolo V, Francescato MP, Baraldi P. Temporal and intensity coding of pain in human cortex. J Neurophysiol. 1998;80:3312–3320. doi: 10.1152/jn.1998.80.6.3312. [DOI] [PubMed] [Google Scholar]
- 70.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 71.Proske U. What is the role of muscle receptors in proprioception? Muscle Nerve. 2005;31:780–787. doi: 10.1002/mus.20330. [DOI] [PubMed] [Google Scholar]
- 72.Rainville P, Duncan GH, Price DD, Carrier BÃ, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 73.Robin DA, Goel A, Somodi LB, Luschei ES. Tongue strength and endurance: Relation to highly skilled movements. J Speech Hear Res. 1992;35:1239–1245. doi: 10.1044/jshr.3506.1239. [DOI] [PubMed] [Google Scholar]
- 74.Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, Comi G, Scotti G, Filippi M. Brain gray matter changes in migraine patients with T2-visible lesions. Stroke. 2006;37:1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rottmann S, Jung K, Vohn R, Ellrich J. Long-term depression of pain-related cerebral activation in healthy man: An fMRI study. Eur J Pain. 2010;14:615–624. doi: 10.1016/j.ejpain.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 77.Rubin P, Hemmingsen R, Holm S, Møller-Madsen S, Hertel C, Povlsen UJ, Karle A. Relationship between brain structure and function in disorders of the schizophrenic spectrum: Single positron emission computerized tomography, computerized tomography and psychopathology of first episodes. Acta Psychiatr Scand. 1994;90:281–289. doi: 10.1111/j.1600-0447.1994.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 78.Ruscheweyh R, Deppe M, Lohmann H, Stehling C, Floel A, Ringelstein EB, Knecht S. Pain is associated with regional grey matter reduction in the general population. Pain. 2011;152:904–911. doi: 10.1016/j.pain.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 79.Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: An event-related functional magnetic resonance imaging study. J Neurosci. 2000;20:7438–7445. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmidt-Wilcke T. Variations in brain volume and regional morphology associated with chronic pain. Curr Rheumatol Rep. 2008;10:467–474. doi: 10.1007/s11926-008-0077-7. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt-Wilcke T, Gänssbauer S, Neuner T, Bogdahn U, May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28:1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt-Wilcke T, Hierlmeier S, Leinisch E. Altered regional brain morphology in patients with chronic facial pain. Headache. 2010;50:1278–1285. doi: 10.1111/j.1526-4610.2010.01637.x. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt-Wilcke T, Leinisch E, Gänssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Schmidt-Wilcke T, Leinisch E, Straube A, Kämpfe N, Draganski B, Diener H, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt-Wilcke T, Luerding R, Weigand T, Jürgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia: A voxel-based morphometry study. Pain. 2007;132:S109–S116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Schweinhardt P, Bushnell MC. Pain imaging in health and disease: How far have we come? J Clin Invest. 2010;120:3788–3797. doi: 10.1172/JCI43498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140:411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 88.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57.e42. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- 90.Simos PG, Breier JI, Wheless JW, Maggio WW, Fletcher JM, Castillo EM, Papanicolaou AC. Brain mechanisms for reading: The role of the superior temporal gyrus in word and pseudoword naming. Neuroreport. 2000;11:2443–2446. doi: 10.1097/00001756-200008030-00021. [DOI] [PubMed] [Google Scholar]
- 91.Solomon NP, Robin DA, Mitchinson SI, VanDaele DJ, Luschei ES. Sense of effort and the effects of fatigue in the tongue and hand. J Speech Hear Res. 1996;39:114–125. doi: 10.1044/jshr.3901.114. [DOI] [PubMed] [Google Scholar]
- 92.Tölle TR, Kaufmann T, Siessmeier T, Lautenbacher S, Berthele A, Munz F, Zieglgänsberger W, Willoch F, Schwaiger M, Conrad B, Bartenstein P. Region-specific encoding of sensory and affective components of pain in the human brain: A positron emission tomography correlation analysis. Ann Neurol. 1999;45:40–47. doi: 10.1002/1531-8249(199901)45:1<40::aid-art8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 93.Tracey I. Nociceptive processing in the human brain. Curr Opin Neurobiol. 2005;15:478–487. doi: 10.1016/j.conb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 94.Treede RD, Kenshalo DR, Gracely RH, Jones AKP. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 95.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GLG, Bromet EJ, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 96.Tu CH, Niddam DM, Chao HT, Chen LF, Chen YS, Wu YT, Yeh TC, Lirng JF, Hsieh JC. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150:462–468. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 97.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Metaanalysis of the functional neuroanatomy of single-word reading: Method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 98.Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valet M, Gundel H, Sprenger T, Sorg C, Muhlau M, Zimmer C, Henningsen P, Tolle TR. Patients with pain disorder show gray-matter loss in pain-processing structures: A voxel-based morphometric study. Psychosom Med. 2009;71:49–56. doi: 10.1097/PSY.0b013e31818d1e02. [DOI] [PubMed] [Google Scholar]
- 100.Valfre W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48:109–117. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 101.Vartiainen N, Kallio-Laine K, Hlushchuk Y, Kirveskari E, Seppanen M, Autti H, Jousmaki V, Forss N, Kalso E, Hari R. Changes in brain function and morphology in patients with recurring herpes simplex virus infections and chronic pain. Pain. 2009;144:200–208. doi: 10.1016/j.pain.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 102.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wade JB, Price DD, Hamer RM, Schwartz SM, Hart RP. An emotional component analysis of chronic pain. Pain. 1990;40:303–310. doi: 10.1016/0304-3959(90)91127-5. [DOI] [PubMed] [Google Scholar]
- 104.Wartolowska K, Hough MG, Jenkinson M, Andersson J, Wordsworth BP, Tracey I. Structural changes of the brain in rheumatoid arthritis. Arthritis Rheum. 2012;64:371–379. doi: 10.1002/art.33326. [DOI] [PubMed] [Google Scholar]
- 105.Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci. 2006;26:11501–11509. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Williams DA, Gracely RH. Biology and therapy of fibromyalgia. Functional magnetic resonance imaging findings in fibromyalgia. Arthritis Res Ther. 2006;8:224. doi: 10.1186/ar2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Younger JW, Shen YF, Goddard G, Mackey SC. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149:222–228. doi: 10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


