Abstract
Cancer is a leading cause of death worldwide, and treating advanced stages of cancer remains clinically challenging. Epidemiological studies have shown that oxidants and free radicals induced DNA damage is one of the predominant causative factors for cancer pathogenesis. Hence, oxidants are attractive targets for chemoprevention as well as therapy. Dietary agents are known to exert an anti-oxidant property which is one of the most efficient preventive strategy in cancer progression. In this article, we highlight dietary agents can potentially target oxidative stress, in turn delaying, preventing, or treating cancer development. Some of these agents are currently in use in basic research, while some have been launched successfully into clinical trials.
Introduction
Despite great advancements in understanding the etiology–the molecular mechanisms underlying disease progression, cancer remains a leading cause of death worldwide and in the United States. Oxidative stress is a predominant causative factor in cancer development. Both reactive oxygen species (ROS) and reactive nitrogen species (RNS), referred to as oxidants, are generated as byproducts of oxygen and nitrogen metabolism, respectively, in various normal metabolic pathways (Wolfle et al. 2014). Production of oxidants in normal cells are tightly regulated by enzymes in a controlled manner regulating several signaling pathways and functions including cell division, inflammation, immune autophagy, and stress response (Finkel 2011). Any imbalance between free radicals (ROS, RNS) and antioxidants is the underlying basis of oxidative stress.
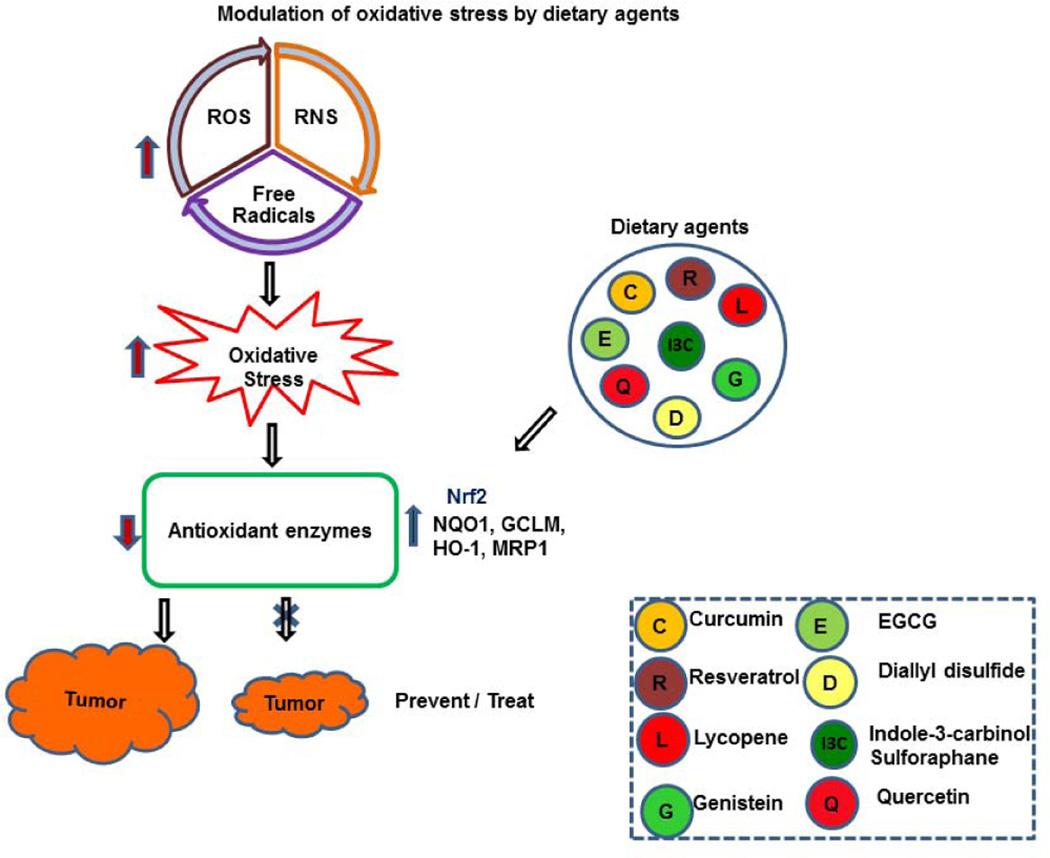
Dietary agents and supplements are major sources of anti-oxidants and are solely aimed at protecting aerobic organisms from the toxic effects of free radicals and oxidants. Anti-oxidants neutralize oxidative stress, either enzymatically (vitamins C or E or β-carotene) or non-enzymatically (superoxide dismutase [SOD], catalase [CAT], or glutathione peroxidase) to protect the organelles. Several epidemiological studies have demonstrated that changes in lifestyle and dietary habits could prevent or reduce cancer incidence (Anand et al. 2008). Therefore, this review article aims to highlight the potential roles of dietary agents exerting antioxidant properties that may impede cancer progression (Fig. 1).
Fig. 1. Modulation of oxidative stress by dietary agents.
Oxidative stress induced by increased ROS, RNS, or free radicals reduces antioxidant production, thus triggering tumor growth. Activation of antioxidants (Nrf2, NQO1, GCLM, HO-1, and MRP1) by dietary agents inhibits oxidative stress, impeding cancer progression.
Molecular function of oxidative stress
Carcinogenesis is a process that includes transforming a normal cell into a cancerous cell. It is a multistep process broadly involving either an aberrant expression of proto-oncogenes or down-regulation of tumor suppressor genes. Over decades, studies have shown that cancer cells produce elevated levels of superoxide or H2O2, leading to oxidative stress that significantly aids in the transformation of healthy cells to tumor cells. The molecular pathways involved in an oxidative response include PI3K/AKT, PKC, STATs, AP-1, Ras/Raf/MAP kinase, ERK, NF-κB, Nrf2, VEGF, and JNK signaling by regulating cell-cycle progression, survival, invasion, and metastasis of cancer cells (Table-1). These studies not only highlight the significance of ROS and free radicals in regulating cancer, but also delineate how ROS is targeted through various dietary agents.
Table 1.
Dietary compounds and major molecular targets
| Dietary Compound(s) | Molecular Targets |
|---|---|
| Curcumin | NO, COX-2, NK-kB, MAPK, PI3K/ AKT/Fox01 / BRCA-1, H2AFX and PARP-1, hsp |
| Resveratrol | NF-κB / Wnt/β-catenin |
| Lycopene | IGBPs/PDGF/VEGF PI3K/AKT/PKB, Ras/Raf/MAP, cyclin D, c-myc Bcl-2, Bcl-XL, ERK/AKT, NF-κB |
| Epigallocatechin-3-gallate | Caspase activation, Bcl-2; NF-κB MAPK/TGF-B/ EGFR / JAK/STAT; PI3K/AKT, Wnt/Notch |
| Quercetin | p53 / ras protein/ estrogen receptor/ NF-κB, EGFR/ PI3K/AKT/ MAPKs;TRPM7 |
| Diallyl disulfide | MAPKs and NF-κB/ PI3K/AKT; p53/p21 and MEK-ERK |
| Genistein | NF-κB, VEGF, PDGF, EGF, IGF, JNK1, ERK/PI3K/AKT |
| Indole-3-carbinol and Sulforaphane | Nrf2/ARE/ Wnt/β-Catenin |
Dietary agents and anticancer effects
It has evidently been proven beyond doubt that foods and dietary supplements are powerful sources of antioxidants and that regular consumption of both fruits and vegetables can either prevent or reduce cancer risk. Over decades, research has highlighted a promising approach in cancer research by tapping the enormous potential of many herbal sources of anti-oxidants in combating oxidative stress, thus targeting cancer.
Curcumin
The well-known pleiotropic effect of curcumin with curcuminoid, the yellow pigment (turmeric), has been well documented for its traditional medicinal properties against various human ailments in both Chinese and Indian systems of medicine since ancient times. As a naturally occurring polyphenol isolated from the rhizome of Curcuma longa, curcumin (turmeric) has gained popularity because of its safety, low cost, and abundance. Approved by the U.S. Food and Drug Administration (FDA), studies have shown that consumption of curcumin is safe, even at higher doses, with no toxicity in animals (Sridhar et al. 2014) or humans (Lao et al. 2006). The exceptional therapeutic potential of curcumin as an anti-inflammatory, anti-oxidant, hypoglycemic, anti-angiogenic, pro-apoptotic, and anti-cancer agent has been extensively studied. As an anti-inflammatory agent, it inhibits production of NO and COX-2 as well as NF-kB activation (Bhaumik et al. 2000; Surh et al. 2001). It also scavenges ROS and decreases specificity protein transcription factors by targeting microRNAs (Gandhy et al. 2012). The possible therapeutic effects of curcumin on several diseases are mainly associated with inhibition of oxidative stress and its downstream mediators. Several studies have evidently shown that curcumin inhibits free radical formation (Jain et al. 2015), lipid peroxidation (Al-Rubaei et al. 2014), DNA damage (Tokac et al. 2013), and damage to cytochrome p450, but it induces glutathione-S-transferase (Koe et al. 2014).
It is well documented that curcumin’s anti-cancer properties make it feasible in preclinical trials to modulate multiple cell-signaling pathways through mitigation or prevention of many different types of cancers, including multiple myeloma and colorectal, pancreatic, breast, prostate, lung, head, and neck, in both animal models and humans (Devassy et al. 2015). In lung cancer, curcumin induces apoptosis accompanied by changes in intracellular oxidative stress-related enzymes and also by phosphorylation and activation of the mitogen-activated protein kinase signaling pathway factors c-Jun N-terminal kinase, p38, and extracellular signal-regulated kinase (Yao et al. 2015). In pancreatic B cells, curcumin attenuates palmitate-induced apoptosis through PI3K/AKT/Fox01 and mitochondrial survival pathways (Hao et al. 2015). A decrease in ROS with a concomitant increase in various antioxidant and DNA repair genes, such as BRCA-1, H2AFX, and PARP-1, were observed after curcumin treatment (Jain et al. 2015). Studies have also shown that curcumin down-regulates heat shock proteins and histone deacetylation in tumor cells under oxidative stress. It also induces apoptosis by modulating apoptosis-related proteins and also arrests the cell cycle by inhibiting tumor markers (Sarkar et al. 2014).
An approximate daily intake of 60–100 mg of curcumin in adults has been reported in the Indian diet (Tayyem et al. 2006). Based on dietary intake, dosages as high as 12 g/day during the first 3 months of treatment are in Phase I, II, and III clinical trials (Dhillon et al. 2008). In several studies using curcumin on multiple molecular targets, it has been demonstrated that cancer therapy is limited because of curcumin’s poor stability and bio-availability resulting from its increased oxidative degradation. However, a recent study has clearly shown that an encapsulation of curcumin as a redox nanoparticle rapidly scavenges the free radicals and ROS by overcoming the oxidative degradation, thus making it more potential than curcumin alone in cancer therapy (Thangavel et al. 2015).
Resveratrol
Phytoestrogens are phenolic compounds that are naturally occurring bioactive food components with diverse chemopreventive properties including antioxidant and angiogenic activities (Cao et al. 2005; Siddiqui et al. 2015). Of the various classes of phytoestrogens, resveratrol (trans-3,4′,5-trihydroxystilbene), found in varying concentrations in many plants such as grapes, berries and nuts, has evidently showed that it interferes with all three stages of carcinogenesis (initiation, promotion, and progression). As a potent anti-oxidant, it plays a crucial role by regulating several antioxidant enzymes, including glutathione peroxidase, glutathione–S-transferase, and glutathione reductase (Yen et al. 2003). It also prevents low density lipoprotein oxidation (Frankel et al. 1993) and inhibits platelet aggregation (Olas et al. 2001).
While the effects of resveratrol on cancer are at this time uncertain, numerous studies have clearly highlighted its beneficial effects, such as cardiovascular and cancer preventive properties. In vitro findings in several labs have shown its anti-cancer effects in breast, skin, gastric, colon, esophageal, prostate, and pancreatic cancer as well as leukemia (Gali-Muhtasib et al. 2015). Activation of NF-κB due to changes in cytokine and ROS levels in various diseases, including cancer, has been decreased by resveratrol (Feitelson et al. 2015). The anti-cancer properties of resveratrol include apoptotic induction by modulating the levels of Fas and FasL (Gu et al. 2015), reducing surviving expressions of both of these and Wnt/β-catenin signaling pathways (Fukada et al. 2007), and inhibiting angiogenesis (Garvin et al. 2006). It’s antioxidant activity prevents tumor formation resulting from DNA damage (Kalra et al. 2008).
As a chemopreventive agent, resveratrol has a dual role; it is anti-apoptotic and pro-apoptotic, and these diverse health benefits are enabled in a dose-dependent manner. Low doses maintain protection from various types of diseases; however, higher doses have been shown to inhibit tumor growth (Mukherjee et al. 2010). It is rapidly absorbed and metabolized and is safe in long-term administration against several pathological conditions. Although many studies have highlighted the potential therapeutic effects of resveratrol on various diseases, including cancer, whether it targets both survival and apoptotic signaling pathways needs to be ascertained through clinical trials.
Lycopene
Lycopene is a naturally occurring carotenoid that gives red color to fruits and vegetables. Predominantly rich in tomatoes (0.9 to 92.7 mg/100g) and tomato-based products (51 to 59.7 mg/100g), more than 80% of total lycopene intake in the Western diet is through these sources.
Lycopene possesses significant antioxidant activity by quenching and inactivating the ROS (Guder et al. 2014). Studies have shown that it prevents and reduces the risk of heart disease and several cancers including lung and prostate cancer (Arab, Steck 2000; Kim, Kim 2015). Both lycopene and its indirect effects play beneficial roles in preventing oxidative reactions that arise from the formation of nitrosamines (Yegin et al. 2015). It potentially targets the mechanisms involved in cell cycle arrest and apoptotic induction by abolishing growth factor receptor signaling pathways (Rao et al. 2006). Studies have shown that it inhibits cancer cell growth by inhibiting ROS and by decreasing ERK expression (Palozza et al. 2010).
A clinical trial involving a large population of prostate cancer patients undergoing radical prostatectomy with 4.2 years follow-up with lycopene supplementation have shown smaller tumors (Kirsh et al. 2006). Antioxidant properties and other signaling mechanisms of lycopene are primarily responsible for its therapeutic effects, targeting growth factors, signaling pathways, antioxidant response element (ARE) regulation, cell cycles, apoptosis, cell invasion, and metastasis (Trejo-Solis et al. 2013). Findings on ethanolic extract of lycopene have shown that it triggers induction of Phase II detoxification enzymes by activating ARE and its transcription factor nuclear factor E2-related factor 2, thus enhancing its anti-tumorigenic effect (Ben-Dor et al. 2005).
Lycopene exerts its effects by regulating several growth factors and signaling pathways, including reduction of IGF-1 levels with enhanced IGBPs (breast, lung, colorectal, and prostate cancers) (Giovannucci et al. 1995), PDGF (Chan et al. 2009), and VEGF (Chen et al. 2012). At attainable concentrations, it modulates cancer progression by down-regulating PI3K/AKT, and PKB and the kinase metabolic pathways of Ras, Raf, and MAP. It also targets the subsequent expression of genes involved in cell proliferation (cyclin D, Bcl-2, and Bcl-XL), cell cycles, apoptosis (Kolberg et al. 2015; Rotelli et al. 2015), inflammation, angiogenesis, invasion, and metastasis (Trejo-Solis et al. 2013). Cell cycle progression is arrested by lycopene at phases G0/G1 and S by decreasing the expression of cyclin D and c-myc (Ono et al. 2015).
Many preclinical epidemiological studies have shown that lycopene at physiological concentrations arrests cell proliferation in gastric (ERK pathway), colon (AKT signaling), breast, and prostate (NF-κB) cancers (Palozza et al. 2010), but further clinical trials are needed to explore all its potential therapeutic roles. Because of its free radical scavenging property, lycopene has been proven beyond doubt to be a natural potential anti-oxidant compound. Moreover, its multi-targeting effects allow it to remain a promising dietary agent for cancer therapy, attracting scientists for extensive research.
Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG), a polyphenol in green tea (Camellia sinensis) popularly consumed as a health-promoting beverage around the world, is another significant phytochemical. As a powerful anti-oxidant, anti-inflammatory, and anti-proliferative compound, it is the most potent of all catechins.
In recent years, the potential effects of green tea have been intensively studied in animal models, in vitro, and in human studies against many pathological conditions. Induction of antioxidant enzymes and Phase II metabolism by ECGC has been highlighted in both animal and human models (Lambert, Elias 2010). To a greater extent, it can inhibit tumorigenesis in the stomach, lungs, liver, breast, and colon during all three stages of cancer through multiple signaling pathways. The compound ginseng, found in green tea, has a synergistic effect with anti-cancer drugs in arresting colon cancer cell proliferation. These polyphenols inhibits cancer cell survival via several growth factor receptors. It has been shown that ECGC induces apoptosis via an ROS-dependent mechanism (Lao et al. 2006) and caspase activation with altered Bcl-2 family member expression, decreasing NF-κB kinase activity and therefore reducing nitric oxide production along with up- or down-regulation of a number of enzymes involved in MAPK, oncogenes, and tumor suppressor genes (Beltz et al. 2006). Enhanced expression of the genes responsible for TGF-B signaling, mediated by ROS, was evident following EGCG treatment (Lambert, Elias 2010). Furthermore, several studies have proven that it also inhibits tumor growth mediated by multi-signaling pathways - EGFR, JNK, STAT, PI3K/AKT, Wnt, or Notch (Ma et al. 2014).
Studies emanating from various groups have highlighted that to a greater extent, ECGC prevents metastatic properties in various cancers, such as skin, prostate, liver, lung, breast, pancreatic, and other cancers; however, further studies will elucidate the protective effects of green tea against cancer metastasis (Khan, Mukhtar 2010). A few clinical trials have also clearly demonstrated the anti-tumorigenic property of ECGC, showing delayed cancer onset followed by lower recurrence rates in breast cancer patients (Stages I and II) (Fujiki et al. 1999). The efficacy of green tea extracts, both in ointment and capsule forms, has further confirmed that ECGC is effective in treating cervical lesions, a beneficial therapy for HPV patients (Erdogan 1972). These results clearly highlight that ECGC, used either alone or in synergistic treatment with other chemotherapy drugs, has great potential in cancer prevention and therapy, and it also explains its demanding roles in both Phase II and III clinical trials.
Quercetin
Of the known bioflavonoids, quercetin (3,3′,4′,5,7-pentahydroxyflavone) has gained interest in research because of its several potential health effects as an anti-inflammatory, immunemodulator, anti-atherogenic, anti-oxidant, anti-hypertensive, and anti-toxic (Boersma et al. 2002; Rani et al. 2015). Major plant sources of the flavonoid, a brilliant citron yellow pigment, include red onions, tomatoes (organic), honey, fruits, and leafy vegetables (Rani et al. 2015). Average daily intake is 30 mg in Western countries (Noroozi et al. 2000). Various effects of quercetin in the presence of low and high levels of reduced GSH has been clearly demonstrated to possess both anti-oxidant (low) and pro-oxidant (high) properties (Robaszkiewicz et al. 2007).
Quercetin, either alone or in combination, has significant anti-cancer effects by inducing cell death through apoptosis in leukemia, lung, hepatoma, oral, and colon cancer cell lines (Atashpour et al. 2015; Lee et al. 2015; Liu et al. 2015; Maurya, Vinayak 2015; Yuan et al. 2015; Refolo et al. 2015), inducing the apoptotic pathway, down-regulating mutant p53, inhibiting the ras protein, and targeting estrogen receptor binding capacity (Rani et al. 2015). The molecular mechanisms by which it exerts its therapeutic effects include inhibition of NF-κB (Zheng et al. 2014) and EGFR (Fridrich et al. 2008), thus modulating the downstream PI3K/AKT pathway. Apoptotic induction is also evinced by the action of quercetin inhibiting MAPKs and transient receptor potential melastatin 7 (TRPM7) channels in gastric tumors (Kim et al. 2014) and regulating Bcl-2 and Bax in breast cancers (Duo et al. 2012). A recent study has revealed that it also reverses tamoxifen resistance in breast cancer cells (Wang et al. 2015). Another important mechanism is its ability to modulate estrogen receptors, thus inducing apoptosis, which is ERα-dependent. Furthermore, it renders a protective dual role against E2-related cancers, especially in ERα- and ERβ-expressing organs (Bulzomi et al. 2012).
A well-known therapeutic dietary agent, quercetin also regulates cell cycle progression, targeting several factors, including p21, cyclin B, p27, CDK, and topoisomerase II, thus inducing cell cycle arrest either at the G2/M or G1/S stages or a location specific to tumor origin (Gibellini et al. 2011). Recent studies have evidently shown that it reduces the production of hyperalgesic cytokines and oxidative stress and also activates the opioid-dependent analgesic pathway, thus making it a most relevant therapeutic option in treating cancer-associated pain (Calixto-Campos et al. 2015). However, further investigation is recommended. Its poor solubility in water is a hurdle that is improvised by combining it with either (polyethylene glycol) (Yuan et al. 2006) or sulfobutyl ether-7beta-cyclodextrin, augmenting its anti-cancer effects. These beneficial effects of quercetin make it a potential cancer therapy.
Diallyl disulfide
Diallyl disulfide (DADS; 4,5-dithia-1,7-octadiene), is a major organosulphur compound present in garlic and few allium plants. Recent studies have shown that the natural compound, allicin, has significant anti-mitotic effects both in vivo (Milner 2006) and in vitro (Huang et al. 2007). It has been shown that DADS scavenges free radicals and oxidants by stimulating the activities of GPX, glutathione reductase, and SOD (Yin et al. 2014).
Diallyl disulfide prevents hemorrhagic cystitis by inhibiting oxidative damage and the MAPKs and NF-κB pathways (Kim et al. 2015). Possible protective anti-oxidant mechanisms are explained by various authors and include activation of Nrf2 pathways by inhibition of NF-κB activation, thus preventing hepatotoxicity (Lee et al. 2014). Koh et al. reported the dose-dependent role of DADS on PC-12 cells, resulting in a neuroprotective effect at lower concentrations by activating PI3K and AKT and inhibiting GSK-3 activation, cytochrome-c release, caspase 3 activities, and PARP cleavage while it is cytotoxic at higher concentrations (Koh et al. 2005).
As an effective anti-cancer agent, DADS inhibits growth and the metastatic potentials of various cancers, such as breast, gastric, leukemia, esophageal, squamous cell carcinoma, prostate, colorectal adenoma, uterine, lung, and skin cancers (Lin et al. 2008; Nagaraj et al. 2010; Lu et al. 2004). Epidemiologic studies have undoubtedly shown that the frequency of contracting gastric cancer is significantly reduced with increased consumption of garlic (Yi, Su 2013). Studies have also shown that it reduces PSA, a well-known prostate cancer biomarker (Gunadharini et al. 2006). Induction of apoptosis is related to the anti-proliferative effect of DADS mediated through the signaling pathways of EGFR, ERK, and PKM2 (Ma et al. 2014), p53, p21, and MEK-ERK (Yuan et al. 2015), and also by inducing cell cycle arrest.
These studies clearly show the multi-tasking effect of these organosulfur compounds. Modulating the cellular redox state, detoxify carcinogens, induce cell cycle arrest and apoptosis, and inhibit angiogenesis and cell invasion, underline DADS as a potent therapeutic agent because it has few toxic effects on healthy cells. Most in vivo and in vitro studies used this compound at higher concentrations; therefore, the feasibility of using such high concentrations in human clinical trials needs to be reviewed before it can be used for prevention or treatment of cancer.
Genistein
The predominant food sources of the phytoestrogens genistein and daidzein are soybeans and their by-products, lupin, chick peas, and other legumes. Present as β-glucoside, genistein is an isoflavone that exhibits anti-oxidant, anti-proliferative, and anti-carcinogenic properties, and it is more potent than daidzein. The anti-oxidant and anti-cancer effects as well as other pharmacological properties of genistein are brought about by free genistein aglycone, found after digestion of the glycosylated form of genistein in the small intestine (Polkowski et al. 2004). A potent anti-oxidant, genistein results in elevated levels of anti-oxidant enzymes such as glutathione peroxidase, SOD, and glutathione reductase, increasing the scavenging of free radicals and reducing lipid peroxidation (Wei et al. 2015).
Several studies have clearly proven the differential effect of genistein as an estrogenic and anti-estrogenic, attributing to the treatment of hormone-related cancers (Banerjee et al. 2008). The average daily dietary intake of isoflavones is comparatively low in Western countries (2 mg) than in Asian countries (25–50 mg) (van Erp-Baart et al. 2003; Messina et al. 2006), a causative factor of disparity observed in the frequency of breast and prostate cancers. Soybeans modulate carcinogenesis by targeting tumor initiation, proliferation, and progression. Genistein augments its anti-cancer properties through downregulating several molecular pathways – NF-kB, VEGF, PDGF, EGF, IGF, JNK1, ERK/PI3K/AKT (Varinska et al. 2015). In vivo and in vitro studies have proven the efficacy and synergistic effects of genistein in inhibiting cell proliferation and inducing apoptosis in liver, lung, colon, and prostate cancers (Kim et al. 2005; Zhang et al. 2013; Wang et al. 2014; Ito et al. 2014). Based on epidemiological data, the role of isoflavone in gastric cancer is debatable because schools of thought differ (Hara et al. 2013; Koh et al. 2005). The paradoxical effect of genistein is that it has an anti-tumorigenic effect at higher concentrations (>10 uM) in both estrogen receptor-positive and -negative breast cancer cells; however, at lower concentrations, it stimulates the proliferation of ER-positive breast cancer cell lines (Seo et al. 2006). Besides concentration, soy intake also plays a crucial role in breast cancer because it is shown to be protective prior to puberty, reducing during adolescence, and tumorigenic pre-menopausal.
As a FDA-approved drug and in Phase I and II trials for the treatment of metastatic colorectal cancer, genistein has gained more importance (Spagnuolo et al. 2015). Although variations occur between the epidemiologic in vitro and in vivo studies, more research and additional clinical trials are required to validate genistein as a potent anti-cancer drug. Nonetheless, it has thus far been shown to exert chemo-preventive effects and therefore is a promising anti-cancer agent.
Indole-3-carbinol and Sulforaphane
Another natural compound, predominantly found in members of the Brassica genus (cabbage, radishes, cauliflower, broccoli, sprouts, and daikon), is glucosinolate. After consumption of glucosinolate, the active organosulfur compounds indole-3-carbinol (I3C) and sulforaphane, which possess anti-cancer properties, are formed. Both the phytochemicals have potent antioxidant, anti-carcinogenic, and anti-atherogenic properties; however they increased the expression of genes encoding antioxidant enzymes (CAT, SOD, GR, and GPX) in hepatoma cells through Nrf2 and ARE signaling pathways (Krajka-Kuzniak et al. 2015). Findings have also shown that the anti-tumorigenic effect of I3C is partly achieved by one of its major byproducts, diindolylmethane, which acts as an anti-angiogenic, inducing cell death.
Highlighting the anti-tumorigenic properties, both in vivo and in vitro studies have showed that I3C arrests the G1 cell cycle and inhibits the growth of breast cancer cells through degradation of Cdc25A (Wu et al. 2010). Numerous in vitro, in vivo, and human studies have shown its targeted ability to suppress cell proliferation in various cancer models [breast (Ebert et al. 2007), prostate (Traka et al. 2008), colon (Pappa et al. 2006), lung (Higdon et al. 2007), and leukemia (Ho et al. 2011)]. However, most therapeutic studies reveal that I3C is more potent for hormonal-dependent cancers such as breast and cervical cancer under in vivo conditions. Further studies have shown sulforaphane as a potent inducer of Phase II detoxication enzymes (Fahey, Talalay 1999), and I3C reversed the cytotoxic effect of dexamethasone by blocking ROS overproduction and Nrf2 expression enhancement (Lin et al. 2015). Sulforaphane also has a therapeutic role in neurodegenerative disorders other than cancer (Wnt and β-catenin).
This clearly underscores the potential use of these organosulfur compounds as therapeutic agents against cancer and other diseases mediated through suppression of free radical production, induction of apoptosis, and regulation of various signaling pathways.
Conclusion
Studies have clearly shown that both lifestyle and types of dietary intake have a significant influence on preventing the cancer incidence by activating anti-inflammatory pathways. The rapid increase in cancer research over the decades has shed some light on identifying and targeting the molecular pathways in cancer treatment. With the availability of many therapeutic methods aiming toward cancer treatment, chemoprevention by potent dietary agents is of greater significance as it targets many signaling pathways. The studies highlight the importance of anti-oxidants as one of the potential tool in cancer prevention and treatment by scavenging the effects of free radicals and oxidants. Treatment with these dietary agents rich in anti-oxidants, either alone or in combination, focuses the beneficial effects of inhibiting cell proliferation, survival, invasion, and metastasis and inducing apoptosis. It may be noteworthy, that this article emphasizes only some of the predominant dietary compounds, although there are still a larger number compounds that are being explored. However, because of the differential effects of some of these compounds, further exploration is needed. It is highly imperative to have a better understanding of the possible roles of these compounds so that they can be used in safe and effective cancer therapies. Nevertheless, consumption of dietary agents and their by products can help prevent cancer.
Acknowledgments
This work was supported by R01CA140605 and R01CA138797.
Abbreviations
- AKT/PKC
Protein kinase C
- AP-1
Activator Protein 1
- ARE
Antioxidant response element
- Bax
BCL2-associated X protein
- Bcl-2
B-cell lymphoma 2
- Bcl-xl
B-cell lymphoma-extra-large
- BRCA-1
Breast cancer 1
- CAT
Catalase
- Cdc25A
Cell Division Cycle 25A
- CDK
Cyclin-dependent kinase
- COX-2
Cyclooxygenase
- EGFR
epidermal growth factor receptor
- Fas
cell-surface Fas receptor
- FasL
Fas ligand
- Fox01
Forkhead box protein O1
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
Glutathione
- GSK-3
Glycogen Synthase Kinase 3
- H2AFX
H2A histone family
- IGBP
IGF binding protein
- IGF-1
Insulin-like Growth Factor-1
- JNK
Janus kinase
- NF-κB
Nuclear factor-kappa B
- NO
Nitric oxide
- Nrf-2
Nuclear factor erythroid 2 [NF-E2]-related factor 2
- PARP-1
(Poly (ADP-Ribose) Polymerase 1
- PDGF
Platelet-derived growth factor
- PI3K
Phosphoinositide 3-kinase
- PSA
Prostate specific antigen
- Ras/Raf/MAP
kinase
- ERK
Mitogen-activated protein kinase
- SOD
Superoxide dismutase
- STATs
Signal transducer and activator of transcription
- TGF-β
Transforming growth factor-beta
- VEGF
Vascular endothelial growth factor
- Wnt
Wingless signaling in Drosophila
References
- Al-Rubaei ZM, Mohammad TU, Ali LK. Effects of local curcumin on oxidative stress and total antioxidant capacity in vivo study. Pak J Biol Sci. 2014;17(12):1237–1241. doi: 10.3923/pjbs.2014.1237.1241. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab L, Steck S. Lycopene and cardiovascular disease. Am J Clin Nutr. 2000;71(6 Suppl):1691S–1695S. doi: 10.1093/ajcn/71.6.1691S. discussion 6S–7S. [DOI] [PubMed] [Google Scholar]
- Atashpour S, Fouladdel S, Movahhed TK, Barzegar E, Ghahremani MH, Ostad SN, et al. Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran J Basic Med Sci. 2015;18(7):635–643. [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269(2):226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem. 2006;6(5):389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, et al. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005;4(1):177–186. [PubMed] [Google Scholar]
- Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 2000;483(1):78–82. doi: 10.1016/s0014-5793(00)02089-5. [DOI] [PubMed] [Google Scholar]
- Boersma MG, van der Woude H, Bogaards J, Boeren S, Vervoort J, Cnubben NH, et al. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyl transferases. Chem Res Toxicol. 2002;15(5):662–670. doi: 10.1021/tx0101705. [DOI] [PubMed] [Google Scholar]
- Bulzomi P, Galluzzo P, Bolli A, Leone S, Acconcia F, Marino M. The pro-apoptotic effect of quercetin in cancer cell lines requires ERbeta-dependent signals. J Cell Physiol. 2012;227(5):1891–1898. doi: 10.1002/jcp.22917. [DOI] [PubMed] [Google Scholar]
- Calixto-Campos C, Correa MP, Carvalho TT, Zarpelon AC, Hohmann MS, Rossaneis AC, et al. Quercetin Reduces Ehrlich Tumor-Induced Cancer Pain in Mice. Anal Cell Pathol (Amst) 2015;2015:285708. doi: 10.1155/2015/285708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Fu ZD, Wang F, Liu HY, Han R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J Asian Nat Prod Res. 2005;7(3):205–213. doi: 10.1080/10286020410001690190. [DOI] [PubMed] [Google Scholar]
- Chan CM, Fang JY, Lin HH, Yang CY, Hung CF. Lycopene inhibits PDGF-BB-induced retinal pigment epithelial cell migration by suppression of PI3K/Akt and MAPK pathways. Biochem Biophys Res Commun. 2009;388(1):172–176. doi: 10.1016/j.bbrc.2009.07.155. [DOI] [PubMed] [Google Scholar]
- Chen ML, Lin YH, Yang CM, Hu ML. Lycopene inhibits angiogenesis both in vitro and in vivo by inhibiting MMP-2/uPA system through VEGFR2-mediated PI3K-Akt and ERK/p38 signaling pathways. Mol Nutr Food Res. 2012;56(6):889–899. doi: 10.1002/mnfr.201100683. [DOI] [PubMed] [Google Scholar]
- Devassy JG, Nwachukwu ID, Jones PJ. Curcumin and cancer: barriers to obtaining a health claim. Nutr Rev. 2015;73(3):155–165. doi: 10.1093/nutrit/nuu064. [DOI] [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Duo J, Ying GG, Wang GW, Zhang L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep. 2012;5(6):1453–1456. doi: 10.3892/mmr.2012.845. [DOI] [PubMed] [Google Scholar]
- Ebert B, Seidel A, Lampen A. Phytochemicals induce breast cancer resistance protein in Caco-2 cells and enhance the transport of benzo[a]pyrene-3-sulfate. Toxicol Sci. 2007;96(2):227–236. doi: 10.1093/toxsci/kfl147. [DOI] [PubMed] [Google Scholar]
- Erdogan E. Intraoral radiography with a new apparatus. Istanbul Univ Dishekim Fak Derg. 1972;3(3):337–357. [PubMed] [Google Scholar]
- Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem Toxicol. 1999;37(9–10):973–979. doi: 10.1016/s0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J, et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin Cancer Biol. 2015;12(35 Suppl):S25–S54. doi: 10.1016/j.semcancer.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341(8852):1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- Fridrich D, Teller N, Esselen M, Pahlke G, Marko D. Comparison of delphinidin, quercetin and (−)-epigallocatechin-3-gallate as inhibitors of the EGFR and the ErbB2 receptor phosphorylation. Mol Nutr Food Res. 2008;52(7):815–822. doi: 10.1002/mnfr.200800026. [DOI] [PubMed] [Google Scholar]
- Fujiki H, Suganuma M, Okabe S, Sueoka E, Suga K, Imai K, et al. Mechanistic findings of green tea as cancer preventive for humans. Proc Soc Exp Biol Med. 1999;220(4):225–228. doi: 10.1046/j.1525-1373.1999.d01-38.x. [DOI] [PubMed] [Google Scholar]
- Fukada K, Takahashi-Yanaga F, Sakoguchi-Okada N, Shiraishi F, Miwa Y, Morimoto S, et al. Celecoxib induces apoptosis by inhibiting the expression of survivin in HeLa cells. Biochem Biophys Res Commun. 2007;357(4):1166–1171. doi: 10.1016/j.bbrc.2007.04.077. [DOI] [PubMed] [Google Scholar]
- Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R, Darwiche N. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis. 2015;20(12):1531–1562. doi: 10.1007/s10495-015-1169-2. [DOI] [PubMed] [Google Scholar]
- Gandhy SU, Kim K, Larsen L, Rosengren RJ, Safe S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer. 2012;12:564. doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231(1):113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Gibellini L, Pinti M, Nasi M, Montagna JP, De Biasi S, Roat E, et al. Quercetin and cancer chemoprevention. Evid Based Complement Alternat Med. 2011;2011:591356. doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Alcohol, low-methionine--low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst. 1995;87(4):265–273. doi: 10.1093/jnci/87.4.265. [DOI] [PubMed] [Google Scholar]
- Gu S, Chen C, Jiang X, Zhang Z. Resveratrol synergistically triggers apoptotic cell death with arsenic trioxide via oxidative stress in human lung adenocarcinoma A549 cells. Biol Trace Elem Res. 2015;163(1–2):112–123. doi: 10.1007/s12011-014-0186-2. [DOI] [PubMed] [Google Scholar]
- Guder A, Korkmaz H, Gokce H, Alpaslan YB, Alpaslan G. Isolation, characterization, spectroscopic properties and quantum chemical computations of an important phytoalexin resveratrol as antioxidant component from Vitis labrusca L. and their chemical compositions. Spectrochim Acta A Mol Biomol Spectrosc. 2014;133:378–395. doi: 10.1016/j.saa.2014.05.056. [DOI] [PubMed] [Google Scholar]
- Gunadharini DN, Arunkumar A, Krishnamoorthy G, Muthuvel R, Vijayababu MR, Kanagaraj P, et al. Antiproliferative effect of diallyl disulfide (DADS) on prostate cancer cell line LNCaP. Cell Biochem Funct. 2006;24(5):407–412. doi: 10.1002/cbf.1262. [DOI] [PubMed] [Google Scholar]
- Hao F, Kang J, Cao Y, Fan S, Yang H, An Y, et al. Curcumin attenuates palmitate-induced apoptosis in MIN6 pancreatic beta-cells through PI3K/Akt/FoxO1 and mitochondrial survival pathways. Apoptosis. 2015 doi: 10.1007/s10495-015-1150-0. [DOI] [PubMed] [Google Scholar]
- Hara Y, Noda A, Miyata S, Minoshima M, Sugiura M, Kojima J, et al. Effects of aged garlic extract on left ventricular diastolic function and fibrosis in a rat hypertension model. Exp Anim. 2013;62(4):305–310. doi: 10.1538/expanim.62.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JN, Kang ER, Yoon HG, Jeon H, Jun W, Watson RR, et al. Inhibition of premature death by isothiocyanates through immune restoration in LP-BM5 leukemia retrovirus-infected C57BL/6 mice. Biosci Biotechnol Biochem. 2011;75(7):1234–1239. doi: 10.1271/bbb.100840. [DOI] [PubMed] [Google Scholar]
- Huang Z, Lei X, Zhong M, Zhu B, Tang S, Liao D. Bcl-2 small interfering RNA sensitizes cisplatin-resistant human lung adenocarcinoma A549/DDP cell to cisplatin and diallyl disulfide. Acta Biochim Biophys Sin (Shanghai) 2007;39(11):835–843. doi: 10.1111/j.1745-7270.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Igishi T, Takata M, Ueda Y, Matsumoto S, Kodani M, et al. Synergistic cell growth inhibition by the combination of amrubicin and Akt-suppressing agents in K-ras mutation-harboring lung adenocarcinoma cells: implication of EGFR tyrosine kinase inhibitors. Int J Oncol. 2014;44(3):685–692. doi: 10.3892/ijo.2014.2249. [DOI] [PubMed] [Google Scholar]
- Jain A, Samykutty A, Jackson C, Browning D, Bollag WB, Thangaraju M, et al. Curcumin inhibits PhIP induced cytotoxicity in breast epithelial cells through multiple molecular targets. Cancer Lett. 2015;365(1):122–131. doi: 10.1016/j.canlet.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra N, Roy P, Prasad S, Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 2008;82(7–8):348–358. doi: 10.1016/j.lfs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Khan N, Mukhtar H. Cancer and metastasis: prevention and treatment by green tea. Cancer Metastasis Rev. 2010;29(3):435–445. doi: 10.1007/s10555-010-9236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Shin HK, Park JH. Genistein inhibits insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells: a possible mechanism of the growth inhibitory effect of Genistein. J Med Food. 2005;8(4):431–438. doi: 10.1089/jmf.2005.8.431. [DOI] [PubMed] [Google Scholar]
- Kim MC, Lee HJ, Lim B, Ha KT, Kim SY, So I, et al. Quercetin induces apoptosis by inhibiting MAPKs and TRPM7 channels in AGS cells. Int J Mol Med. 2014;33(6):1657–1663. doi: 10.3892/ijmm.2014.1704. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim H. Anticancer Effect of Lycopene in Gastric Carcinogenesis. J Cancer Prev. 2015;20(2):92–96. doi: 10.15430/JCP.2015.20.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee IC, Ko JW, Moon C, Kim SH, Shin IS, et al. Diallyl Disulfide Prevents Cyclophosphamide-Induced Hemorrhagic Cystitis in Rats through the Inhibition of Oxidative Damage, MAPKs, and NF-kappaB Pathways. Biomol Ther (Seoul) 2015;23(2):180–188. doi: 10.4062/biomolther.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsh VA, Mayne ST, Peters U, Chatterjee N, Leitzmann MF, Dixon LB, et al. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(1):92–98. doi: 10.1158/1055-9965.EPI-05-0563. [DOI] [PubMed] [Google Scholar]
- Koe XF, Tengku Muhammad TS, Chong AS, Wahab HA, Tan ML. Cytochrome P450 induction properties of food and herbal-derived compounds using a novel multiplex RT-qPCR in vitro assay, a drug-food interaction prediction tool. Food Sci Nutr. 2014;2(5):500–520. doi: 10.1002/fsn3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SH, Kwon H, Park KH, Ko JK, Kim JH, Hwang MS, et al. Protective effect of diallyl disulfide on oxidative stress-injured neuronally differentiated PC12 cells. Brain Res Mol Brain Res. 2005;133(2):176–186. doi: 10.1016/j.molbrainres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kolberg M, Pedersen S, Bastani NE, Carlsen H, Blomhoff R, Paur I. Tomato paste alters NF-kappaB and cancer-related mRNA expression in prostate cancer cells, xenografts, and xenograft microenvironment. Nutr Cancer. 2015;67(2):305–315. doi: 10.1080/01635581.2015.990575. [DOI] [PubMed] [Google Scholar]
- Krajka-Kuzniak V, Paluszczak J, Szaefer H, Baer-Dubowska W. The activation of the Nrf2/ARE pathway in HepG2 hepatoma cells by phytochemicals and subsequent modulation of phase II and antioxidant enzyme expression. J Physiol Biochem. 2015;71(2):227–238. doi: 10.1007/s13105-015-0401-4. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501(1):65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IC, Kim SH, Baek HS, Moon C, Kang SS, Kim SH, et al. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem Toxicol. 2014;63:174–185. doi: 10.1016/j.fct.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Hsiao M, Chang JL, Yang SF, Tseng TH, Cheng CW, et al. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch Toxicol. 2015;89(7):1103–1117. doi: 10.1007/s00204-014-1300-0. [DOI] [PubMed] [Google Scholar]
- Lin H, Gao X, Chen G, Sun J, Chu J, Jing K, et al. Indole-3-carbinol as inhibitors of glucocorticoid-induced apoptosis in osteoblastic cells through blocking ROS-mediated Nrf2 pathway. Biochem Biophys Res Commun. 2015;460(2):422–427. doi: 10.1016/j.bbrc.2015.03.049. [DOI] [PubMed] [Google Scholar]
- Lin YT, Yang JS, Lin SY, Tan TW, Ho CC, Hsia TC, et al. Diallyl disulfide (DADS) induces apoptosis in human cervical cancer Ca Ski cells via reactive oxygen species and Ca2+-dependent mitochondria-dependent pathway. Anticancer Res. 2008;28(5A):2791–2799. [PubMed] [Google Scholar]
- Liu Y, Wu YM, Zhang PY. Protective effects of curcumin and quercetin during benzo(a)pyrene induced lung carcinogenesis in mice. Eur Rev Med Pharmacol Sci. 2015;19(9):1736–1743. [PubMed] [Google Scholar]
- Lu HF, Sue CC, Yu CS, Chen SC, Chen GW, Chung JG. Diallyl disulfide (DADS) induced apoptosis undergo caspase-3 activity in human bladder cancer T24 cells. Food Chem Toxicol. 2004;42(10):1543–1552. doi: 10.1016/j.fct.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Ma YC, Li C, Gao F, Xu Y, Jiang ZB, Liu JX, et al. Epigallocatechin gallate inhibits the growth of human lung cancer by directly targeting the EGFR signaling pathway. Oncol Rep. 2014;31(3):1343–1349. doi: 10.3892/or.2013.2933. [DOI] [PubMed] [Google Scholar]
- Maurya AK, Vinayak M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol Biol Rep. 2015;42(9):1419–1429. doi: 10.1007/s11033-015-3921-7. [DOI] [PubMed] [Google Scholar]
- Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98(18):1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- Milner JA. Preclinical perspectives on garlic and cancer. J Nutr. 2006;136(3 Suppl):827S–831S. doi: 10.1093/jn/136.3.727S. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Dudley JI, Das DK. Dose-dependency of resveratrol in providing health benefits. Dose Response. 2010;8(4):478–500. doi: 10.2203/dose-response.09-015.Mukherjee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj NS, Anilakumar KR, Singh OV. Diallyl disulfide causes caspase-dependent apoptosis in human cancer cells through a Bax-triggered mitochondrial pathway. J Nutr Biochem. 2010;21(5):405–412. doi: 10.1016/j.jnutbio.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Noroozi M, Burns J, Crozier A, Kelly IE, Lean ME. Prediction of dietary flavonol consumption from fasting plasma concentration or urinary excretion. Eur J Clin Nutr. 2000;54(2):143–149. doi: 10.1038/sj.ejcn.1600908. [DOI] [PubMed] [Google Scholar]
- Olas B, Wachowicz B, Szewczuk J, Saluk-Juszczak J, Kaca W. The effect of resveratrol on the platelet secretory process induced by endotoxin and thrombin. Microbios. 2001;105(410):7–13. [PubMed] [Google Scholar]
- Ono M, Takeshima M, Nakano S. Mechanism of the Anticancer Effect of Lycopene (Tetraterpenoids) Enzymes. 2015;37:139–166. doi: 10.1016/bs.enz.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Palozza P, Colangelo M, Simone R, Catalano A, Boninsegna A, Lanza P, et al. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis. 2010;31(10):1813–1821. doi: 10.1093/carcin/bgq157. [DOI] [PubMed] [Google Scholar]
- Pappa G, Lichtenberg M, Iori R, Barillari J, Bartsch H, Gerhauser C. Comparison of growth inhibition profiles and mechanisms of apoptosis induction in human colon cancer cell lines by isothiocyanates and indoles from Brassicaceae. Mutat Res. 2006;599(1–2):76–87. doi: 10.1016/j.mrfmmm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Polkowski K, Popiolkiewicz J, Krzeczynski P, Ramza J, Pucko W, Zegrocka-Stendel O, et al. Cytostatic and cytotoxic activity of synthetic genistein glycosides against human cancer cell lines. Cancer Lett. 2004;203(1):59–69. doi: 10.1016/j.canlet.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Rani N, Velan LP, Vijaykumar S, Arunachalam A. An insight into the potentially old-wonder molecule-quercetin: the perspectives in foresee. Chin J Integr Med. 2015 doi: 10.1007/s11655-015-2073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AV, Ray MR, Rao LG. Lycopene. Adv Food Nutr Res. 2006;51:99–164. doi: 10.1016/S1043-4526(06)51002-2. [DOI] [PubMed] [Google Scholar]
- Refolo MG, D'Alessandro R, Malerba N, Laezza C, Bifulco M, Messa C, et al. Anti Proliferative and Pro Apoptotic Effects of Flavonoid Quercetin Are Mediated by CB1 Receptor in Human Colon Cancer Cell Lines. J Cell Physiol. 2015;230(12):2973–2980. doi: 10.1002/jcp.25026. [DOI] [PubMed] [Google Scholar]
- Robaszkiewicz A, Balcerczyk A, Bartosz G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int. 2007;31(10):1245–1250. doi: 10.1016/j.cellbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Rotelli MT, Bocale D, De Fazio M, Ancona P, Scalera I, Memeo R, et al. IN-VITRO evidence for the protective properties of the main components of the Mediterranean diet against colorectal cancer: A systematic review. Surg Oncol. 2015;24(3):145–152. doi: 10.1016/j.suronc.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Sarkar R, Mukherjee A, Mukherjee S, Biswas R, Biswas J, Roy M. Curcumin augments the efficacy of antitumor drugs used in leukemia by modulation of heat shock proteins via HDAC6. J Environ Pathol Toxicol Oncol. 2014;33(3):247–263. doi: 10.1615/jenvironpatholtoxicoloncol.2014010913. [DOI] [PubMed] [Google Scholar]
- Seo HS, DeNardo DG, Jacquot Y, Laios I, Vidal DS, Zambrana CR, et al. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat. 2006;99(2):121–134. doi: 10.1007/s10549-006-9191-2. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Sanna V, Ahmad N, Sechi M, Mukhtar H. Resveratrol nanoformulation for cancer prevention and therapy. Ann N Y Acad Sci. 2015;1348(1):20–31. doi: 10.1111/nyas.12811. [DOI] [PubMed] [Google Scholar]
- Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6(4):408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar R, Ravanan S, Venugopal JR, Sundarrajan S, Pliszka D, Sivasubramanian S, et al. Curcumin- and natural extract-loaded nanofibres for potential treatment of lung and breast cancer: in vitro efficacy evaluation. J Biomater Sci Polym Ed. 2014;25(10):985–998. doi: 10.1080/09205063.2014.917039. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Tayyem RF, Heath DD, Al-Delaimy WK, Rock CL. Curcumin content of turmeric and curry powders. Nutr Cancer. 2006;55(2):126–131. doi: 10.1207/s15327914nc5502_2. [DOI] [PubMed] [Google Scholar]
- Thangavel S, Yoshitomi T, Sakharkar MK, Nagasaki Y. Redox nanoparticles inhibit curcumin oxidative degradation and enhance its therapeutic effect on prostate cancer. J Control Release. 2015;209:110–119. doi: 10.1016/j.jconrel.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Tokac M, Taner G, Aydin S, Ozkardes AB, Dundar HZ, Taslipinar MY, et al. Protective effects of curcumin against oxidative stress parameters and DNA damage in the livers and kidneys of rats with biliary obstruction. Food Chem Toxicol. 2013;61:28–35. doi: 10.1016/j.fct.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Traka M, Gasper AV, Melchini A, Bacon JR, Needs PW, Frost V, et al. Broccoli consumption interacts with GSTM1 to perturb oncogenic signalling pathways in the prostate. PLoS One. 2008;3(7):1–14. doi: 10.1371/journal.pone.0002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo-Solis C, Pedraza-Chaverri J, Torres-Ramos M, Jimenez-Farfan D, Cruz Salgado A, Serrano-Garcia N, et al. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid Based Complement Alternat Med. 2013;2013:705121. doi: 10.1155/2013/705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp-Baart MA, Brants HA, Kiely M, Mulligan A, Turrini A, Sermoneta C, et al. Isoflavone intake in four different European countries: the VENUS approach. Br J Nutr. 2003;89(Suppl 1):S25–S30. doi: 10.1079/BJN2002793. [DOI] [PubMed] [Google Scholar]
- Varinska L, Gal P, Mojzisova G, Mirossay L, Mojzis J. Soy and breast cancer: focus on angiogenesis. Int J Mol Sci. 2015;16(5):11728–11749. doi: 10.3390/ijms160511728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tao L, Qi K, Zhang H, Feng D, Wei W, et al. Quercetin reverses tamoxifen resistance in breast cancer cells. J BUON. 2015;20(3):707–713. [PubMed] [Google Scholar]
- Wang SD, Chen BC, Kao ST, Liu CJ, Yeh CC. Genistein inhibits tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. BMC Complement Altern Med. 2014;14:26. doi: 10.1186/1472-6882-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YK, Gamra I, Davenport A, Lester R, Zhao L, Wei Y. Genistein Induces Cytochrome P450 1B1 Gene Expression and Cell Proliferation in Human Breast Cancer MCF-7 Cells. J Environ Pathol Toxicol Oncol. 2015;34(2):153–159. doi: 10.1615/jenvironpatholtoxicoloncol.2015013315. [DOI] [PubMed] [Google Scholar]
- Wolfle U, Seelinger G, Bauer G, Meinke MC, Lademann J, Schempp CM. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol Physiol. 2014;27(6):316–332. doi: 10.1159/000360092. [DOI] [PubMed] [Google Scholar]
- Wu Y, Feng X, Jin Y, Wu Z, Hankey W, Paisie C, et al. A novel mechanism of indole-3-carbinol effects on breast carcinogenesis involves induction of Cdc25A degradation. Cancer Prev Res (Phila) 2010;3(7):818–828. doi: 10.1158/1940-6207.CAPR-09-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Lin M, Wang Y, Lai Y, Hu J, Fu T, et al. Curcumin induces the apoptosis of A549 cells via oxidative stress and MAPK signaling pathways. Int J Mol Med. 2015;36(4):1118–1126. doi: 10.3892/ijmm.2015.2327. [DOI] [PubMed] [Google Scholar]
- Yegin SC, Yur F, Cetin S, Guder A. Effect of Lycopene on Serum Nitrite-Nitrate Levels in Diabetic Rats. Indian J Pharm Sci. 2015;77(3):357–360. doi: 10.4103/0250-474x.159676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen GC, Duh PD, Lin CW. Effects of resveratrol and 4-hexylresorcinol on hydrogen peroxide-induced oxidative DNA damage in human lymphocytes. Free Radic Res. 2003;37(5):509–514. doi: 10.1080/1071576031000083099. [DOI] [PubMed] [Google Scholar]
- Yi L, Su Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem Toxicol. 2013;57:362–370. doi: 10.1016/j.fct.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Yin X, Zhang R, Feng C, Zhang J, Liu D, Xu K, et al. Diallyl disulfide induces G2/M arrest and promotes apoptosis through the p53/p21 and MEK-ERK pathways in human esophageal squamous cell carcinoma. Oncol Rep. 2014;32(4):1748–1756. doi: 10.3892/or.2014.3361. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Wang H, Hu Z, Huang Y, Yao F, Sun S, et al. Quercetin inhibits proliferation and drug resistance in KB/VCR oral cancer cells and enhances its sensitivity to vincristine. Nutr Cancer. 2015;67(1):126–136. doi: 10.1080/01635581.2015.965334. [DOI] [PubMed] [Google Scholar]
- Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, et al. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin Cancer Res. 2006;12(10):3193–3199. doi: 10.1158/1078-0432.CCR-05-2365. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li Q, Zhou D, Chen H. Genistein, a soya isoflavone, prevents azoxymethane-induced up-regulation of WNT/beta-catenin signalling and reduces colon pre-neoplasia in rats. Br J Nutr. 2013;109(1):33–42. doi: 10.1017/S0007114512000876. [DOI] [PubMed] [Google Scholar]
- Zheng NG, Wang JL, Yang SL, Wu JL. Aberrant epigenetic alteration in Eca9706 cells modulated by nanoliposomal quercetin combined with butyrate mediated via epigenetic-NF-kappaB signaling. Asian Pac J Cancer Prev. 2014;15(11):4539–4543. doi: 10.7314/apjcp.2014.15.11.4539. [DOI] [PubMed] [Google Scholar]