Abstract
Acute myocardial infarction, the clinical manifestation of ischemia-reperfusion (IR) injury, is a leading cause of death worldwide. Like ischemic preconditioning (IPC) induced by brief episodes of ischemia and reperfusion, ouabain preconditioning (OPC) mediated by Na/K-ATPase signaling protects the heart against IR injury. Class I PI3K activation is required for IPC, but its role in OPC has not been investigated. While PI3K-IB is critical to IPC, studies have suggested that ouabain signaling is PI3K-IA-specific. Hence, a pharmacological approach was used to test the hypothesis that OPC and IPC rely on distinct PI3K-I isoforms. In Langendorff-perfused mouse hearts, OPC was initiated by 4 min of ouabain 10 μM and IPC was triggered by 4 cycles of 5 min ischemia and reperfusion prior to 40 min of global ischemia and 30 min of reperfusion. Without affecting PI3K-IB, ouabain doubled PI3K-IA activity and Akt phosphorylation at Ser473. IPC and OPC significantly preserved cardiac contractile function and tissue viability as evidenced by left ventricular developed pressure and end-diastolic pressure recovery, reduced lactate dehydrogenase release, and decreased infarct size. OPC protection was blunted by the PI3K-IA inhibitor PI-103, but not by the PI3K-IB inhibitor AS-604850. In contrast, IPC-mediated protection was not affected by PI-103 but was blocked by AS-604850, suggesting that PI3K-IA activation is required for OPC while PI3K-IB activation is needed for IPC. Mechanistically, PI3K-IA activity is required for ouabain-induced Akt activation but not PKCε translocation. However, in contrast to PKCε translocation which is critical to protection, Akt activity was not required for OPC. Further studies shall reveal the identity of the downstream targets of this new PI3K IA-dependent branch of OPC. These findings may be of clinical relevance in patients at risk for myocardial infarction with underlying diseases and/or medication that could differentially affect the integrity of cardiac PI3K-IA and IB pathways.
Keywords: preconditioning, PI3K, ischemia-reperfusion injury, Na/K-ATPase
1. Introduction
Cardiac glycosides such as ouabain or digoxin are classically known as specific inhibitors of the Na/K-ATPase ion-pumping function. This inhibition, which results in subsequent increase in intracellular Na+ and increased Na+/Ca2+ exchange, is required for their well-known cardiac positive inotropic action [1, 2]. Binding of ouabain to the catalytic α-subunit of the Na/KATPase protein complex also initiates a cascade of intracellular signaling events that trigger protection against ischemia-reperfusion (IR) injury [3-9]. By analogy to ischemic preconditioning (IPC) induced by several brief episodes of ischemia before a prolonged ischemia, this protection against IR induced by ouabain is referred to as ouabain preconditioning (OPC). Known signaling events downstream from Na/K-ATPase that are critical to OPC include the activation of Src kinase and PKCε, opening of mitoK-ATP channels, and production of ROS [3, 4]. However, a possible role of Class I phosphoinositide 3-kinases (PI3K) in OPC has not been investigated. Emerging concepts in preconditioning and interventions against IR, as well as recent key findings in cardiac ouabain signaling centered on PI3K prompted us to address this question. Indeed, shortly after we reported OPC in the rat heart [3, 4], Liu et al observed a ouabain induced activation of the class I PI3K/Akt pathway in rat neonatal cardiac myocytes [10]. From a mechanistic stand-point, a main finding of the Liu paper was that ouabain-induced PI3K activation is class IA-specific. Class IA and IB PI3Ks are both important regulators of metabolism, survival, differentiation and growth in numerous cell types, including cardiac myocytes [11-14]. PI3K IA and IB also differ in key functional and structural characteristics. Class IA catalytic subunits (p110α, β and δ) form a complex with the regulatory subunit p85 and are activated through tyrosine kinase signaling. Class IB catalytic subunits (p110γ) associate with the regulatory subunit p101 and are typically activated by G-protein-coupled receptors [15]. In the heart, PI3K IA and IB have been associated with distinct processes. Activation of PI3K IA in response to stimuli such as insulin, IGF or exercise is associated with physiological hypertrophy [16, 17]. In contrast, PI3K IB activation by isoproterenol, endothelin, or cardiac overload leads to pathological hypertrophy [18, 19]. Both canonical PI3K IA- (insulin, insulin-like growth factor, epidermal growth factor) and IB- (acetylcholine, opioid, bradykinin) activators can trigger preconditioning, but the respective roles of PI3K IA and IB in different forms of preconditioning have not been specifically evaluated in most instances [6, 18, 20-24]. Exceptions include IPC, in which PI3K IB requirement has been established [25, 26], as well as adenosine (PI3K-IB) [25], and epoxyeicosatrienoic acid (PI3K-IA) [27]. At least theoretically, distinct PI3K Class I requirements could influence the efficiency of preconditioning treatments in subsets of patients at risk for myocardial infarction and presenting with medication and comorbidities that differentially affect Class IA and IB PI3K [28-30]. Likewise, selective PI3K isoform inhibitors used in the treatment of inflammatory diseases and cancer [31-33] may affect the efficacy of preconditioning treatments in the heart of these patients.
Taken together, these more recently recognized aspects of Na/K-ATPase and PI3K-I signaling in cardiac IR prompted us to evaluate the role of PI3K IA vs. IB in OPC-induced protection against IR. Specifically, we used a pharmacological approach to test the hypothesis that OPC and IPC rely on distinct PI3K-I isoforms.
2. Material and Methods
2.1 Langendorff perfusion
Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85-23, revised 1996). All mice were housed in pathogen-free conditions and maintained with 12 h dark/light cycle with free access to food and water. C57BL/6J male mice from Jackson Laboratories (Bar Harbor, ME) were 10–14 week old at the time of experimentation. Mice were injected intraperitoneally with pentobarbital (70mg/kg) and heparin (1000 IU/kg). Hearts were rapidly removed, placed into ice cold (4 °C) Krebs–Henseleit (KH) buffer and mounted on a non-recirculating Langendorff apparatus. Retrograde perfusion was performed using oxygenated KH buffer containing (in mM) NaCl (118.0), KCl (4.0), CaCl2 (1.65), KH2PO4 (1.3), MgSO4 (1.2), Ethylene glycol bis (2-aminoethylether)-N, N, N′, N′-tetraacetic acid (0.3), NaHCO3 (25), D-glucose (11.0), with a pH of 7.4. The perfusion flow rate was kept constant at about 2.5 mL/min, initially set to obtain a coronary perfusion pressure of about 70 mmHg as described [1, 34]. Hearts were paced at 9.7 Hz (4 V) throughout the experimental protocol. Isovolumic left ventricular developed pressure (LVDP) and end diastolic pressure (EDP) were recorded throughout the protocol [34]. After 10 to 15 min of equilibration, one of the protocols detailed below was initiated.
2.2 Experimental protocol
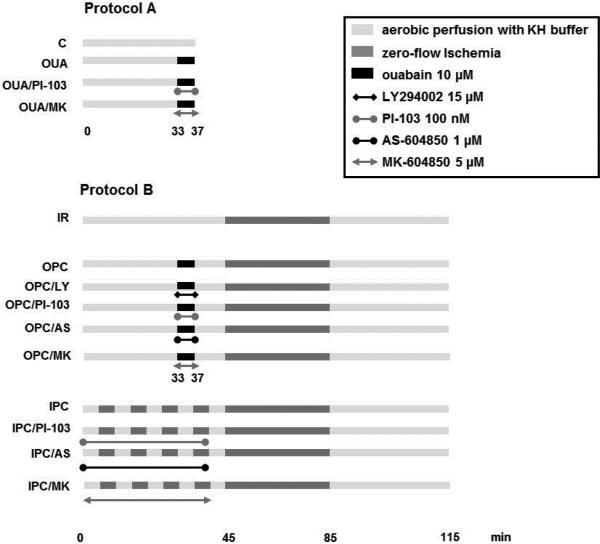
(Figure 1). Protocol A was applied to study signaling events initiated by ouabain. Protocol B was applied to assess IR injury. The IR group was perfused with KH buffer for 45 min, subjected to 40-min zero-flow ischemia, and reperfused for 30 min with KH. IPC was induced by 4 cycles of 5 min ischemia followed by 5 min of reperfusion before the onset of ischemia. OPC was induced by 4 min of perfusion with ouabain (Sigma, Saint-Louis, MO, USA) 10 μM that ended 8 min before ischemia. For infarct size measurement, 90 min of reperfusion were added to protocol B.
Figure 1. Perfusion protocols.
Hearts from 10-14 week old C57/Bl6 male mouse were studied. Protocol A was used to assess trigger phase signaling (PI3K, Akt and PKCε). Protocol B was used to evaluate ischemia/reperfusion (IR) injury. After equilibration, IR was triggered by 40 min of zero-flow ischemia followed by 30 min of reperfusion, unless otherwise stated. Ischemic preconditioning (IPC) was induced by 4 cycles of 5min zero-flow ischemia and 5 min reperfusion. Ouabain preconditioning (OPC) was induced by 4min of exposure to ouabain 10μM followed by 8min washout before ischemia. KH: Krebs Henseleit buffer.
2.3 Lactate dehydrogenase (LDH) activity measurement
Coronary effluent was collected for 30 s at 0, 1, 2, 3, 4, 5, 10, 15, 20, and 30 min of reperfusion. LDH activity was determined colorimetrically using a Cytotoxicity Detection Kit (Roche Applied Science, Indianapolis, IN, USA) as described [3].
2.4 Tissue Preparation
Hearts were quick-frozen in liquid nitrogen at the end of protocol A (Fig. 1). The left ventricle was powdered and ~100 mg of tissue were added to 1 ml of ice-cold radioimmunoprecipitation assay buffer (RIPA- 50mM Tris-base, 150 mM NaCl, 1% IGEPAL, 0.25% sodium deoxycholate, 1 mM EDTA, pH 7.4) containing 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 mM NaF, 10 nM okadaic acid, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and homogenized in a 10 ml homogenizer by 15 up-and-down strokes. The resulting lysates were centrifuged at 14,000×g for 15 min [9].
2.5 PI3 Kinase activity assay
One mg of heart lysate proteins were incubated with anti PI3K p85α Antibody B-9 (Santa cruz, CA, USA) to assess PI3K IA activity, or with anti PI3K p110γ Antibody H-199 (Santa Cruz, CA, USA) to assess PI3K IB activity. Following overnight incubation at 4°C, prewashed Protein A Agarose beads (Millipore, MA, USA) were added and incubated for 3h at 4°C. The immune complex was then washed four times with buffer A (100 mM NaCl, 1 mM Na3VO4, and 20 mM HEPES, pH 7.5) and resuspended in buffer B (180 mM NaCl-20 mM HEPES, pH 7.5). PI3K activity in immunoprecipitates was assayed directly on the beads by a standard procedure as previously described [10], using phosphatidylinositol (Avanti Polar Lipids, Alabaster, AL, USA) and [γ -32P]ATP as substrates. All reactions were performed at room temperature and stopped after 10 min by addition of 1 M HCl. The lipids were extracted with chloroform-methanol (1:1), spotted on a thin-layer chromatography plate, and separated with chloroform-acetone-methanol-glacial acetic acid-H2O (40:15:13:12:8). The radioactivity of the phosphorylated lipid products was quantified by a PhosphorImager (Molecular Dynamics, CA, USA).
2.6 Protein Electrophoresis and Immunoblotting
Protein in tissue lysates were added to Laemmli sample buffer, boiled for 5 min, separated on 10% SDS-PAGE, transferred onto a nitrocellulose membrane and probed with anti phospho-Akt (Ser473, Cell Signaling Technology, MA, USA). The blots were then stripped and probed with anti total Akt (Cell Signaling Technology, MA, USA), as described previously [1]. Immunoblots were then quantified, and the ratios of p-Akt to Akt were calculated. The ratio of band density of each group was then divided by untreated controls to calculate the -fold increase/decrease from multiple independent experiments.
2.7 PKCε translocation from cytosolic to particulate fraction
After perfusion according to Protocol A (Figure 1), hearts were snap frozen in liquid nitrogen and homogenized in buffer 1 containing (in mM) EGTA(10), EDTA(1), DTT(0.5), PMSF(1), proteinase inhibitor cocktail, Tris-HCl(20), pH 7.5. Homogenates were then centrifuged at 100,000 ×g for 1h at 4°C. The supernatants designated as the cytosolic fractions were removed and saved. The pellets were sonicated and centrifuged at 25,000×g in buffer 1 containing 1% Triton. The supernatants were collected as the particulate fractions. Cytosolic and particulate fractions were used in SDS-PAGE and immunoblotting using the anti-PKCε antibody C-15 (Santa Cruz, CA, USA) as we have previously described [3].
2.8 Statistical Analysis
Statistical analysis was performed using one-way ANOVA followed by Tukey's multiple comparison post hoc test, or by Student t-test. P<0.05 was considered statistically significant.
3. Results
3.1 Activation of the PI3K/Akt Pathway
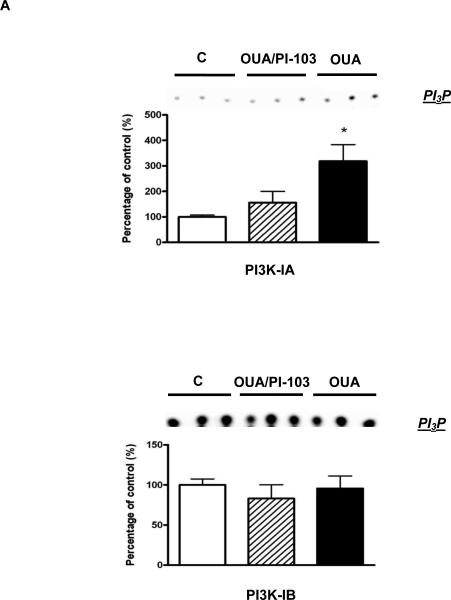
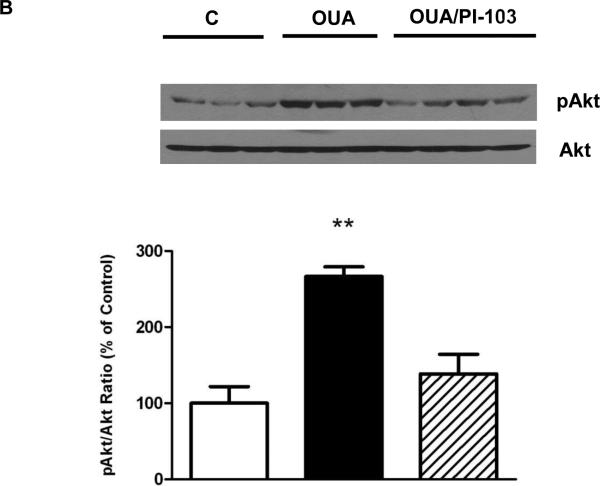
Ouabain is known to activate PI3K-IA but not PI3K-IB in rat cardiac tissue and mouse cardiac myocytes [1, 10]. However, whether such PI3K IA-specific activation occurs in the intact mouse heart exposed to ouabain at the concentration and duration of our established OPC protocol was unknown. Hence, we first tested the effect of a treatment of 4 min with 10 μM ouabain on PI3K- IA and PI3K-IB activities as well as on Akt phosphorylation. As shown in figure 2, PI3K IA but not IB activity was significantly enhanced upon ouabain treatment. This activation was blunted by co-treatment with 100 nM PI-103, a PI3K inhibitor with well described PI3K IA specificity [35]. Consistent with the above observation, the ouabain treatment also induced Akt phosphorylation at Ser 473 in a PI3K IA-dependent manner (Figure 2B).
Figure 2. OPC-induced activation of the PI3K-IA/Akt Pathway.
Mouse hearts were perfused with ouabain 10μM for 4 min in the presence or absence of PI-103 100 nM and frozen in liquid nitrogen (Protocol A in figure 1). 2A. PI3K-IA&IB Activity was measured as described in Methods. 2B. Akt. Total Akt and Ser473 phosphorylated Akt (pAkt) contents were evaluated. Values are mean ± SEM (n=3-4). * P<0.05 and ** P < 0.01 vs. control.
3.2 Characterization of OPC in the mouse heart
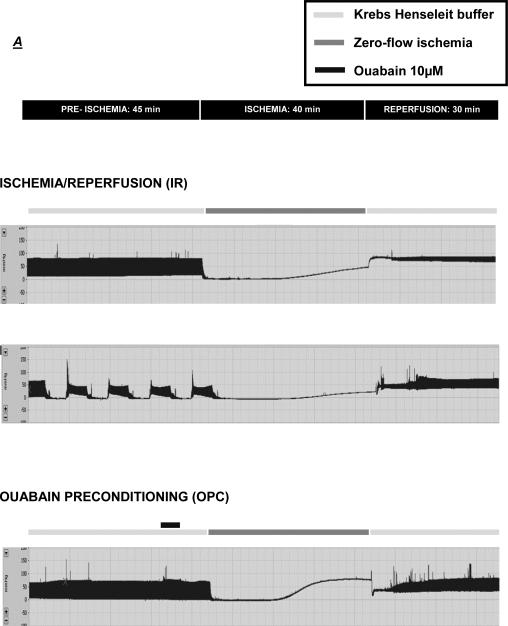
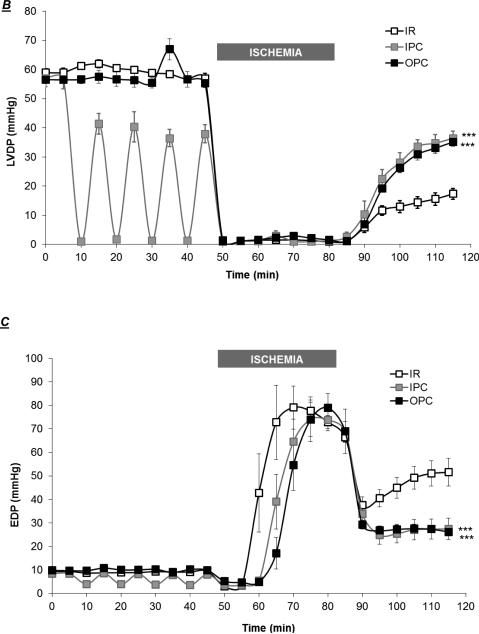
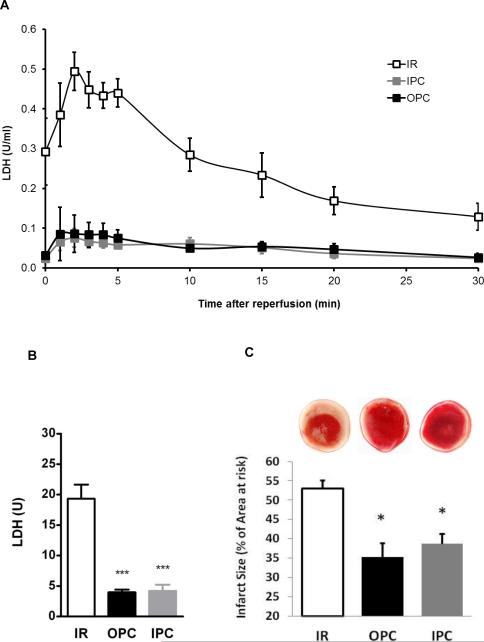
Next, we assessed the impact of OPC triggered by a protocol that we have previously used in the rat heart [3], and compared it to the protective effect of a well-established IPC protocol [36]. As shown in figure 3, baseline parameters were identical in the IR, IPC and OPC groups (t=0). Exposure to 4 min of ouabain 10 μM caused a transient modest increase in LVDP, with a peak detected at t=35 min (66.9±3.6 vs.58.4±1.1, N.S.), followed by a return to baseline value at least 5 min prior to the initiation of ischemia (Figure 3A, t=40-45 min). The LVDP trace for hearts exposed to the IPC protocol presented a typical pattern, with each 5-min ischemic episode producing a transient depression of LVDP and the subsequent 5-min reperfusion period allowing for a recovery to 70-90% of the basal value (Figure 3A). As expected, ischemia resulted in cardiac arrest in all groups. Although cardiac performance resumed within seconds of reperfusion, it was significantly compromised in all groups as shown by the significant decrease in LVDP (Figure 3A) and the increase in EDP (Figure 3B). However, significant protection by OPC and IPC was clearly detected within 10 min of reperfusion for both LVDP and EDP. After 30 min of reperfusion, LVDP recovery was 29.6±3.7% of the pre-ischemic value in untreated hearts (IR), compared to 62.1±1.4% for OPC (P<0.001 vs. IR) and 63.9 ± 3.8% for IPC (P<0.001 vs. IR). Likewise, the recovery of EDP was significantly better in OPC (26.2±1.5 mmHg) and IPC (27.4±4.5 mmHg) than IR (51.5±5.9 mmHg). To assess post-ischemic cardiac tissue damage, LDH release was evaluated in coronary effluent collected throughout the 30 min of reperfusion. Bar graphs in figure 4B show the total amount of LDH released over the 30 min of reperfusion (calculated from areas under the curves presented in Fig. 4A). The total amount of LDH released was 19.3±2.3 U in the IR group, and significantly reduced to 3.9±0.5 U (P<0.001 vs. IR) and 4.2±1.0 U (P<0.001 vs. IR) in the OPC and IPC groups, respectively. Quantitative analysis of TTC stained left ventricular cross sections revealed a significant reduction of 14-17% of the infarct size in hearts exposed to OPC and IPC (35.3±3.7 and 38.8±2.5) compared to IR (52.9±2.2, P<0.01). Taken together, these data suggest that OPC-induced protection against IR in the mouse heart is comparable to that of a standard IPC protocol.
Figure 3. Effect of OPC and IPC on Myocardial Function during and after IR.
Representative traces of left ventricular pressures throughout the protocols (3A). Left ventricular developed pressure (LVDP, 3B) and end diastolic pressure (EDP, 3C) were continuously measured and compared in preparations exposed to a IR, IPC or OPC (figure 1). Values are means ± SEM of 5 independent experiments for each group. *** P < 0.001 vs. IR.
Figure 4. Effect of OPC and IPC on myocardial cell survival after IR.
4A. Time-dependent release of lactate dehydrogenase (LDH) in coronary effluents throughout the 30 min of reperfusion. 4B. Total LDH release calculated from the 30 min area-under-the curves. Values are means ± SEM (n=5). 4C. Infarct size as determined in hearts stained with TTC as described in Methods. Infarct size was expressed as percentage of the area risk. Values are means ± SEM (n=6). * P<0.05 and *** P<0.001 vs. IR.
3.3 Effect of PI3K activity modulation on OPC and IPC-induced protection
The above results suggested that PI3K IA but not IB is activated during the trigger phase of OPC and that OPC affords an IPC-like protection against IR. They did not reveal, however, whether PI3K IA activity plays a role in the trigger phase of OPC-induced protection. To address this question and test the hypothesis that OPC and IPC rely on distinct PI3K-I isoforms, a pharmacological approach was used.
3.3.1 Effect of pharmacological inhibition of PI3K IA and IB with LY294002 (LY)
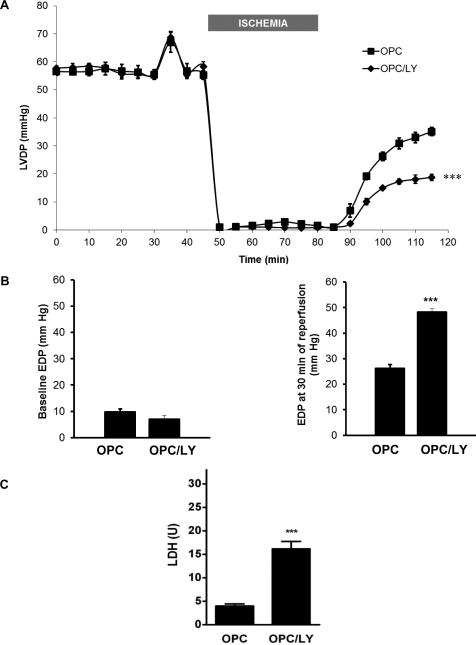
The PI3K inhibitor LY294002 (15μM) was co-perfused with ouabain as shown in figure 1 (protocol B, OPC/LY group). This LY treatment was selected because it has been previously used successfully in a Langendorff-perfused rat heart model to investigate the role of the PI3K/Akt pathway in the trigger phase of IPC [37]. Consistent with the findings reported by Yang et al. in rat hearts, we did not observe any significant change in basal cardiac function or recovery from IR with this treatment (not shown). As shown in 5A, co-administration of LY did not affect ouabain-induced increase in LVDP either (t= 33 to 37 min). However, it did significantly blunt OPC-induced post-ischemic recovery. In fact, at the end of 30 min reperfusion period, LVDP in the OPC/LY group was 32.5±2.0%, indistinguishable from that of the untreated IR group (29.3±3.7%, N.S.). Likewise, LY did not affect basal EDP (5B, left graph) but blunted OPC-induced protection against IR (5B, right graph, 48.3±1.3 mmHg vs. 26.2±1.5 mmHg, P<0.001). Consistent with a complete blockade of OPC-induced protection, the release of LDH from OPC/LY hearts was 16.2±1.6 U, significantly increased from OPC (P<0.001) and comparable to the IR level (19.3±2.3 U).
3.3.2 Effect of pharmacological inhibition of PI3K IA with PI-103
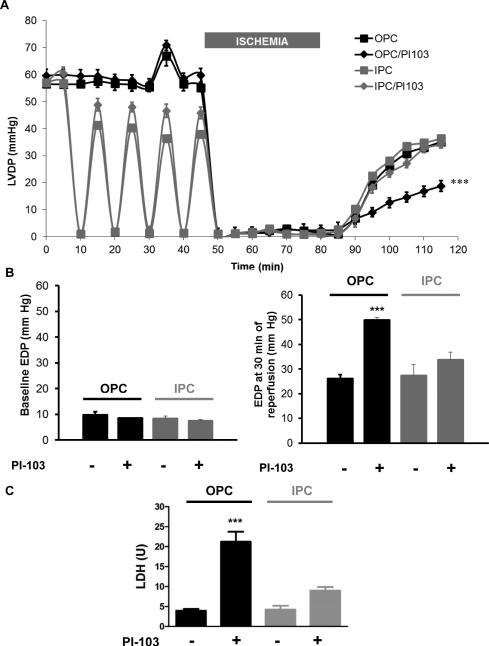
At 100 nM, addition of PI-103 did not affect basal contractility or recovery after IR (not shown), consistent with previous reports [27]. Comparable to LY, co-administration of PI-103 did not affect ouabain-induced increased in LVDP (6A, t= 33 to 37 min), but significantly blunted OPC-induced post-ischemic recovery. Consistently, this blocking effect was also clear on EDP (Figure 6B, right graph, OPC/PI group vs. OPC) and tissue injury as assessed by LDH release (Figure 6C, OPC/PI vs. OPC). In contrast, PI-103 (co-administered as shown in figure 1, IPC/PI-103 in Protocol B) did not significantly affect IPC-induced protection of LVDP (6A), EDP (6B) or tissue injury (LDH release, 6C).
Figure 6. Effect of pharmacological inhibition of PI3K IA by PI-103 on OPC and IPC-induced myocardial protections.
PI-103 100 nM was co-administered with OPC or IPC treatment prior to ischemia (figure 1). Left ventricular developed pressure (LVDP, 6A), basal and final end diastolic pressures (EDP, 6B) and lactate dehydrogenase release (LDH, 6C) are shown. Values are mean ± SEM (n=5). *** P<0.001 vs. OPC.
3.3.3 Effect of pharmacological inhibition of PI3K IB with AS-604850
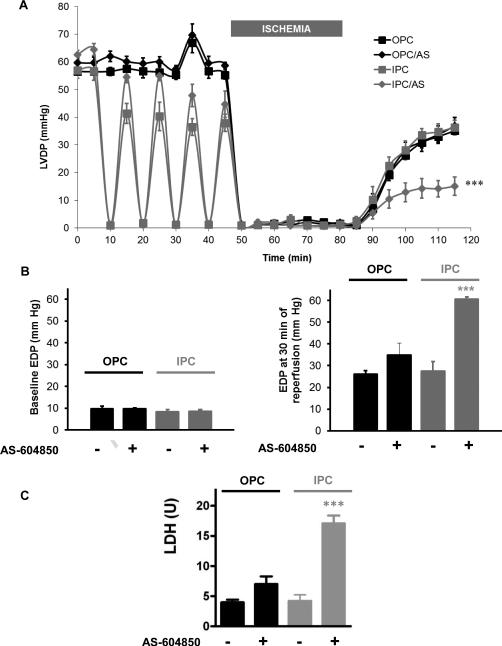
AS-604850 was used at 1 μM to specifically inhibit p110γ [38]. This AS-604850 treatment did not affect basal cardiac function or recovery after ischemia (not shown). Co-treatment with AS-604850 and ouabain did not alter LVDP (62.0±5.2% vs. 62.1±1.4% in OPC, N.S) or EDP recovery (34.9±5.4 mmHg vs. 26.2±1.5 mmHg, N.s.) after IR compared to OPC. Consistently, LDH release from AS/OPC hearts was low (7.0±1.3 U vs. 19.3±2.3 U in IR, P<0.01) and comparable to OPC (7.0±1.3 U vs. 4.0±0.5 U, N.s.). In contrast, addition of AS-604850 during IPC did blunt the protective effect on both LVDP (24.1±5.1% vs. 63.9±3.8% in IPC, P<0.001) and EDP (60.7±5.1 vs. 27.4±4.5 in IPC, P<0.001). Consistently, LDH release was significantly higher than that observed in IPC (17.1±1.3 vs. 4.2±1.0, P<0.001).
3.4 Effect of PI3K IA inhibition on ouabain-induced PKCε translocation
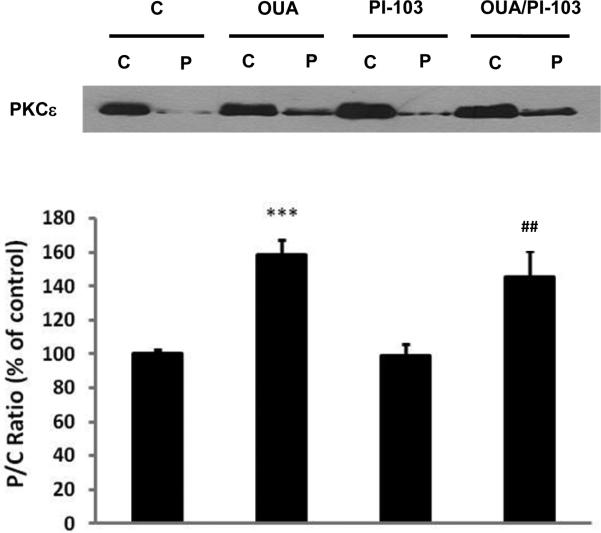
In rat cardiac tissue, 4 min of treatment with ouabain 10 μM induced PKCε activation as evidenced by translocation from the cytosolic to the particulate fraction [3]. In that model, OPC protection was blunted by a PKCε translocation inhibitor, suggesting that PKCε translocation is required for OPC [3]. To test whether PI3K IA inhibition blunted OPC by interfering with PKCε translocation, PKCε content was assayed in cytosolic (C) and particulate (P) fractions from hearts treated for 4 min with or without ouabain and/or PI-103. The results presented in figure 8 show that ouabain treatment did increase the PKCε content in the particulate fraction by about 60% (P<0.001 vs. C), comparable to our previous finding in rat hearts [3]. However, PI-103 did not affect basal P/C ratios of PKCε in the presence or absence of ouabain treatment, suggesting that PI3K IA activity was not required for PKCε translocation.
Figure 8. Effect of PI3K-IA inhibition by PI-103 on ouabain-induced PKCε activation.
Hearts were perfused for 4 min with or without ouabain and/or PI-103 according to Protocol A (Figure 1). Cytosolic (C) and particulate (P) fractions were prepared from heart lysates as described in Methods and P/C ratios of PKCε contents were compared. Upper panel: representative western blot. Lower panel: means ± SEM of 3-5 separate experiments. *** P< 0.001 vs. C, ## P < 0.01 vs. PI-103.
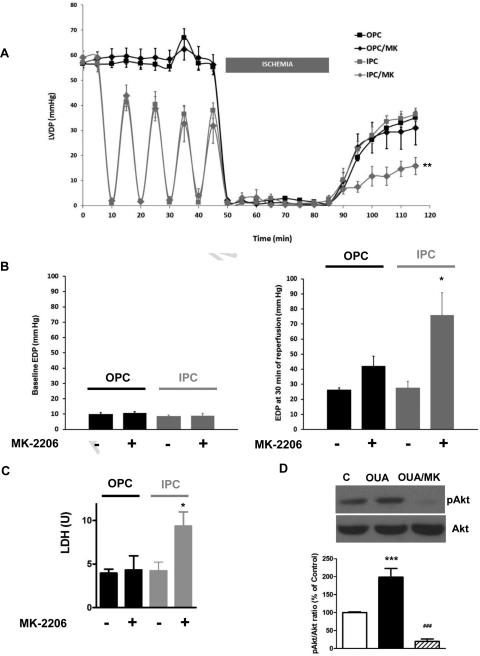
3.5 Effect of Akt inhibition on OPC and IPC-induced protection
At 5μM, addition of the pan-Akt inhibitor MK-2206 did not affect basal contractility or recovery after IR (not shown), but significantly reduced Akt phosphorylation at Ser 473 (figure 9D). Co-treatment with MK-2206 and ouabain did not alter LVDP (9A) or EDP recovery (9B) after IR compared to OPC. Consistently, LDH release from OPC/MK hearts was low and comparable to OPC (9C). In contrast, addition of MK-2206 during IPC did blunt the protective effect on both LVDP (9A) and EDP (9B, P<0.05 vs IPC). Consistently, LDH release was significantly higher than that observed in IPC (P<0.001).
Figure 9. Effect of Akt pharmacological inhibition by MK-2206 on OPC and IPC-induced-myocardial protections.
MK-2206 5 μM was co-administered with OPC or IPC treatment prior to ischemia (figure 1). Left ventricular developed pressure (LVDP, 9A), basal and final end diastolic pressures (EDP, 9B) and lactate dehydrogenase release (LDH, 9C) are shown. Values are mean ± SEM. * P<0.05 or ** P<0.01 vs. IPC. 9D. Akt. Hearts were perfused for 4 min with or without ouabain and/or MK-2206 according to Protocol A (Figure 1). Total Akt and Ser473 phosphorylated Akt (pAkt) contents were evaluated. Upper panel: representative western blot. Lower panel: means ± SEM of 3-5 separate experiments. *** P< 0.001 vs. C, ### P < 0.01 vs. OUA.
4. Discussion
Using a combination of highly selective PI3K isoform inhibitors in Langendorff-perfused mouse heart preparations, this study was designed to test the hypothesis that OPC and IPC are relayed by distinct PI3K isoforms. We found that, in contrast to IPC, OPC was blunted by the PI3K-IA inhibitor PI-103. Conversely, OPC was not affected by the addition of the PI3K-IB inhibitor AS-604850, a treatment that did hinder IPC. This study also revealed that PI3K-IA activation is not required for PKCε activation in the OPC signaling cascade.
Using the approach described in figure 1, we investigated the role of PI3K-IA and IB in the trigger phase (i.e. pre-ischemic period) of IPC and OPC. Because 1) all inhibitors used are known to be reversible [31, 39, 40] and 2) all protocols included a wash-out sequence at the end of the pre-ischemic period (figure 1), PI3K IA and/or IB inhibition was in all likelihood lifted before ischemia began. Consistent with this assumption, we observed that PI3K-IA-dependent Akt phosphorylation was triggered by ouabain applied after an 8 min wash-out following PI-103 treatment (not shown). Consequently, possible effects of PI3K-IA and/or IB inhibition during ischemia and/or reperfusion need not be considered here. This substantially simplifies interpretation, considering the complex and sometimes paradoxical roles of important intracellular players of preconditioning during different phases of IR (i.e. pre-ischemia vs. ischemia vs. reperfusion). Src [41], PKC [42] and ROS [43, 44] are among the best described examples of such complex patterns.
The choice of a pharmacological approach also stemmed from a need to circumvent the use of mouse models with altered expression/function of PI3K isoforms. Studies where the class IA PI3Kα and/or the class IB PI3Kγ were targeted have illustrated the prominent role of these proteins in cardiac structure and function and the complexity of the resulting phenotypes. In particular, probing preconditioning pathways has proved difficult, owing to altered basal sensitivity to IR in these models [25, 26, 45].
A few aspects of the pharmacology of the selected inhibitors should also be noted. The selectivity of ATP-competitive PI3K-IA inhibitor PI-103 towards PI3K-IA is known to be 20 times over PI3K-IB [39]. AS-604850, also an ATP-competitive PI3Kγ inhibitor, has a selectivity of 80-fold for PI3Kγ over PI3Kδ/β, and 18-fold for PI3Kγ over PI3Kα [31]. Such degrees of selectivity, combined with the design of the protocol, were critical for this study. In vitro, PI-103 was also shown to inhibit DNA-PK, the rapamycin-sensitive (mTORC1) and rapamycin-insensitive (mTORC2) complexes of the protein kinase mTOR [39]. Hence, effects of PI-103 unrelated to PI3K-IA inhibition in this study cannot be excluded. However, such effects are unlikely candidates to explain the PI-103-mediated inhibition of OPC. In fact, mTORC1 inhibition would be expected to do just the opposite, as it is typically protective against IR, as illustrated by the well-known effect of rapamycin [46]. Consistent with a minimal impact in this study, we did not observe any significant functional effect of the transient pre-ischemic exposure in the IR or the IPC group. Conversely, an inhibition of DNA-PK or mTORC2 could lead to increased damage [47, 48], but again the absence of effect in the IR (not shown) and IPC groups argue against a significant effect in the conditions of this study.
Mechanistically, we found that ouabain-induced PI3K-IA activation is required for protection by OPC. Of note, this PI3K-IA activation is not required for ouabain-induced PKCε activation (fig 8), which underscores the need for further characterization of the respective roles and interplay between PI3K-IA and PKCε, as well as other previously identified players in OPC such as Src, ROS, and the mitochondrial ATP-dependent K+ channel [3, 4, 49]. Since inhibition of either PKCε [3, 49] or PI3K-IA (present study) blunts OPC-induced protection, one wonders if and where cross talks between the two occur and/or whether they converge to the regulation of a key single effector or target multiple cellular functions critical to OPC-induced salvage. Downstream from PI3K-IA, the data in figure 2 corroborated previous findings of PI3K-IA-dependent activation of Akt by ouabain in cardiac tissue [10], but a potential role of Akt in OPC-induced protection was subsequently ruled out using pharmacological inhibition (figure 9). This was somewhat surprising, given the central role of the PI3K/Akt pathway in the regulation of cell survival and substrate utilization, which are in turn critical in IR injury and in mechanisms of protection. This new and rather unique feature of OPC warrant future investigation of the role of ouabain-induced Akt activation in OPC beyond the trigger phase.
Taken together, the results from the present study are consistent with previous reports and reveal new mechanistic distinctions between OPC and IPC pathways upstream and downstream from PI3K. Specifically, IPC is known to trigger the release of GPCR ligands (bradykinin, opioid peptides, and adenosine) [50]. The vast majority of GPCRs are associated with PI3K p110γ signaling, consistent with the PI3K-IB but not PI3K-IA requirement for IPC protection [25, 51]. In contrast, ouabain signaling is specific of PI3K-IA [10]. The proposed but not established mechanism for this effect is a ouabain-induced interaction of a proline-rich domain of the α-subunit of Na(+)/K(+)-ATPase with the SH3 domain of the p85 subunit of PI3K-IA [52]. Consistently, we observed that PI3K-IA but not IB activity is required for OPC. In IPC, early events downstream from PI3K-IB include the activation of Akt, which is in turn required for the activation of a endothelial nitric oxide synthase (eNOS)/ guanylyl cyclase (GC)/ cGMP-dependent protein kinase (PKG) sequence [25, 50, 53, 54]. In contrast, the present study revealed that Akt is not involved downstream from PI3K-IA in OPC. This finding is consistent with a previous report which excluded a major role for GC or PKG in OPC [4]. Of note, a number of similarities have also been reported between IPC and OPC intracellular pathways, which include PKCε, the mitochondrial K-ATP channel, and the generation of oxygen radicals [3, 4, 22, 55, 56].
Because this study reveals that OPC operates through PI3K-IA rather than IB as observed for IPC, it is tempting to speculate that OPC can trigger “insulin-like” protective effects related to substrate utilization or cell survival, which may result in protection where IPC efficiency has been shown reduced or abolished [57-59]. On the other hand, the somewhat unexpected lack of requirement for Akt warrant further investigation into the mechanism involved downstream from PI3K-IA. These new mechanistic insights into two modalities of preconditioning that are rarely used or not used at all clinically suggest that novel single or combinatory approaches may be devised for patients at risk for myocardial infarction with underlying diseases and/or medication that could differentially affect the integrity of cardiac PI3K-IA and IB pathways.
Highlights.
Inhibition of PI3K-IA but not PI3K-IB blocked ouabain preconditioning (OPC).
Inhibition of PI3K-IB but not PI3K-IA blocked ischemic preconditioning (IPC).
Pre-ischemic inhibition of Akt inhibited IPC but not OPC.
Ouabain induced PKCε activation was not altered by PI3K inhibition.
PKCε translocation is necessary but not sufficient for OPC.
Figure 5. Effect of dual pharmacological inhibition of PI3K-IA and IB by LY-294002 on OPC-induced myocardial protection.
Transient exposure to ouabain was performed in the presence or absence of LY-294002 (Protocol B, figure 1). LVDP (5A), basal and final EDP (5B) and LDH release (5C) are shown. Values are mean ± SEM (n=5). *** P<0.001 vs. OPC.
Figure 7. Effect of PI3K IB pharmacological inhibition by AS-604850 on OPC and IPC-induced myocardial protections.
AS-604850 1 μM was co-administered with OPC or IPC treatment prior to ischemia (figure 1). Left ventricular developed pressure (LVDP, 7A), basal and final end diastolic pressures (EDP, 7B) and lactate dehydrogenase release (LDH, 7C) are shown. Values are mean ± SEM (n=5). *** P<0.001 vs. IPC.
Acknowledgment
This work was supported by National Heart, Lung, and Blood Institute Grant HL-36573.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors confirm that there are no conflicts of interest.
References
- 1.Bai Y, Morgan EE, Giovannucci DR, Pierre SV, Philipson KD, Askari A, et al. Different roles of the cardiac Na+/Ca2+-exchanger in ouabain-induced inotropy, cell signaling, and hypertrophy. Am J Physiol Heart Circ Physiol. 2013;304:H427–35. doi: 10.1152/ajpheart.00462.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altamirano J, Li Y, DeSantiago J, Piacentino V, 3rd, Houser SR, Bers DM. The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+-Ca2+ exchanger function. J Physiol. 2006;575:845–54. doi: 10.1113/jphysiol.2006.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierre SV, Yang C, Yuan Z, Seminerio J, Mouas C, Garlid KD, et al. Ouabain triggers preconditioning through activation of the Na+,K+-ATPase signaling cascade in rat hearts. Cardiovasc Res. 2007;73:488–96. doi: 10.1016/j.cardiores.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasdois P, Quinlan CL, Rissa A, Tariosse L, Vinassa B, Costa AD, et al. Ouabain protects rat hearts against ischemia-reperfusion injury via pathway involving src kinase, mitoKATP, and ROS. American journal of physiology Heart and circulatory physiology. 2007;292:H1470–8. doi: 10.1152/ajpheart.00877.2006. [DOI] [PubMed] [Google Scholar]
- 5.Belliard A, Sottejeau Y, Duan Q, Karabin JL, Pierre SV. Modulation of cardiac Na+,K+-ATPase cell surface abundance by simulated Ischemia/Reperfusion and Ouabain Preconditioning. Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.00374.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol. 2009;46:858–66. doi: 10.1016/j.yjmcc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa AD, Pierre SV, Cohen MV, Downey JM, Garlid KD. cGMP signalling in pre-and postconditioning: the role of mitochondria. Cardiovasc Res. 2008;77:344–52. doi: 10.1093/cvr/cvm050. [DOI] [PubMed] [Google Scholar]
- 8.D'Urso G, Frascarelli S, Zucchi R, Biver T, Montali U. Cardioprotection by ouabain and digoxin in perfused rat hearts. J Cardiovasc Pharmacol. 2008;52:333–7. doi: 10.1097/FJC.0b013e3181884448. [DOI] [PubMed] [Google Scholar]
- 9.Morgan EE, Li Z, Stebal C, Belliard A, Tennyson G, Salari B, et al. Preconditioning by subinotropic doses of ouabain in the Langendorff perfused rabbit heart. J Cardiovasc Pharmacol. 2010;55:234–9. doi: 10.1097/FJC.0b013e3181ce5e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol. 2007;293:C1489–97. doi: 10.1152/ajpcell.00158.2007. [DOI] [PubMed] [Google Scholar]
- 11.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature reviews Genetics. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 12.Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, et al. Myocardial AKT: the omnipresent nexus. Physiol Rev. 2011;91:1023–70. doi: 10.1152/physrev.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 14.Carrette F, Fabre S, Bismuth G. FOXO1, T-cell trafficking and immune responses. Adv Exp Med Biol. 2009;665:3–16. doi: 10.1007/978-1-4419-1599-3_1. [DOI] [PubMed] [Google Scholar]
- 15.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 16.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, et al. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Molecular and cellular biology. 2005;25:9491–502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–60. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 19.Oudit GY, Crackower MA, Eriksson U, Sarao R, Kozieradzki I, Sasaki T, et al. Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation. 2003;108:2147–52. doi: 10.1161/01.CIR.0000091403.62293.2B. [DOI] [PubMed] [Google Scholar]
- 20.Fuglesteg BN, Tiron C, Jonassen AK, Mjos OD, Ytrehus K. Pretreatment with insulin before ischaemia reduces infarct size in Langendorff-perfused rat hearts. Acta Physiol (Oxf) 2009;195:273–82. doi: 10.1111/j.1748-1716.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- 21.Raphael J, Rivo J, Gozal Y. Isoflurane-induced myocardial preconditioning is dependent on phosphatidylinositol-3-kinase/Akt signalling. British journal of anaesthesia. 2005;95:756–63. doi: 10.1093/bja/aei264. [DOI] [PubMed] [Google Scholar]
- 22.Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87:309–15. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Field SJ, Lee JY, Engelman JA, Cantley LC. The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signaling via the formation of a sequestration complex. The Journal of cell biology. 2005;170:455–64. doi: 10.1083/jcb.200503088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross GJ. Role of opioids in acute and delayed preconditioning. J Mol Cell Cardiol. 2003;35:709–18. doi: 10.1016/s0022-2828(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 25.Ban K, Cooper AJ, Samuel S, Bhatti A, Patel M, Izumo S, et al. Phosphatidylinositol 3-kinase gamma is a critical mediator of myocardial ischemic and adenosine-mediated preconditioning. Circulation research. 2008;103:643–53. doi: 10.1161/CIRCRESAHA.108.175018. [DOI] [PubMed] [Google Scholar]
- 26.Haubner BJ, Neely GG, Voelkl JG, Damilano F, Kuba K, Imai Y, et al. PI3Kgamma protects from myocardial ischemia and reperfusion injury through a kinase-independent pathway. PloS one. 2010;5:e9350. doi: 10.1371/journal.pone.0009350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batchu SN, Chaudhary KR, El-Sikhry H, Yang W, Light PE, Oudit GY, et al. Role of PI3Kalpha and sarcolemmal ATP-sensitive potassium channels in epoxyeicosatrienoic acid mediated cardioprotection. J Mol Cell Cardiol. 2012;53:43–52. doi: 10.1016/j.yjmcc.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Varma S, Lal BK, Zheng R, Breslin JW, Saito S, Pappas PJ, et al. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H1744–51. doi: 10.1152/ajpheart.01088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, et al. PI 3-kinase p110 beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–14. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 30.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 31.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–43. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 32.Durand CA, Richer MJ, Brenker K, Graves M, Shanina I, Choi K, et al. Selective pharmacological inhibition of phosphoinositide 3-kinase p110delta opposes the progression of autoimmune diabetes in non-obese diabetic (NOD) mice. Autoimmunity. 2013;46:62–73. doi: 10.3109/08916934.2012.732130. [DOI] [PubMed] [Google Scholar]
- 33.Ni J, Liu Q, Xie S, Carlson C, Von T, Vogel K, et al. Functional characterization of an isoform-selective inhibitor of PI3K-p110beta as a potential anticancer agent. Cancer discovery. 2012;2:425–33. doi: 10.1158/2159-8290.CD-12-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutherland FJ, Shattock MJ, Baker KE, Hearse DJ. Mouse isolated perfused heart: characteristics and cautions. Clin Exp Pharmacol Physiol. 2003;30:867–78. doi: 10.1046/j.1440-1681.2003.03925.x. [DOI] [PubMed] [Google Scholar]
- 35.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer cell. 2006;9:341–9. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J, Aponte AM, Kohr MJ, Tong G, Steenbergen C, Murphy E. Essential role of nitric oxide in acute ischemic preconditioning: S-nitros(yl)ation versus sGC/cGMP/PKG signaling? Free Radic Biol Med. 2013;54:105–12. doi: 10.1016/j.freeradbiomed.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang C, Talukder MA, Varadharaj S, Velayutham M, Zweier JL. Early ischaemic preconditioning requires Akt-and PKA-mediated activation of eNOS via serine1176 phosphorylation. Cardiovasc Res. 2012 doi: 10.1093/cvr/cvs287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oaks JJ, Santhanam R, Walker CJ, Roof S, Harb JG, Ferenchak G, et al. Antagonistic activities of the immunomodulator and PP2A-activating drug FTY720 (Fingolimod, Gilenya) in Jak2-driven hematologic malignancies. Blood. 2013;122:1923–34. doi: 10.1182/blood-2013-03-492181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–47. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). The Journal of biological chemistry. 1994;269:5241–8. [PubMed] [Google Scholar]
- 41.Hattori R, Otani H, Uchiyama T, Imamura H, Cui J, Maulik N, et al. Src tyrosine kinase is the trigger but not the mediator of ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2001;281:H1066–74. doi: 10.1152/ajpheart.2001.281.3.H1066. [DOI] [PubMed] [Google Scholar]
- 42.Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacological research : the official journal of the Italian Pharmacological Society. 2007;55:523–36. doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–70. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. American journal of physiology Heart and circulatory physiology. 2003;284:H566–74. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 45.McLean BA, Kienesberger PC, Wang W, Masson G, Zhabyeyev P, Dyck JR, et al. Enhanced recovery from ischemia-reperfusion injury in PI3Kalpha dominant negative hearts: investigating the role of alternate PI3K isoforms, increased glucose oxidation and MAPK signaling. J Mol Cell Cardiol. 2013;54:9–18. doi: 10.1016/j.yjmcc.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41:256–64. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Engelman JA, Chen L, Tan XH, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volkers M, Konstandin MH, Doroudgar S, Toko H, Quijada P, Din S, et al. Mechanistic target of rapamycin complex 2 protects the heart from ischemic damage. Circulation. 2013;128:2132–44. doi: 10.1161/CIRCULATIONAHA.113.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belliard A, Sottejeau Y, Duan Q, Karabin JL, Pierre SV. Modulation of cardiac Na+,K+-ATPase cell surface abundance by simulated ischemia-reperfusion and ouabain preconditioning. Am J Physiol Heart Circ Physiol. 2013;304:H94–103. doi: 10.1152/ajpheart.00374.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–53. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–53. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, Akkuratov EE, Bai Y, Gaskill CM, Askari A, Liu L. Cell Signaling Associated with Na/K-ATPase: Activation of Phosphatidylinositide 3-Kinase IA/Akt by Ouabain Is Independent of Src. Biochemistry. 2013 doi: 10.1021/bi4011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–8. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 54.Kunuthur SP, Mocanu MM, Hemmings BA, Hausenloy DJ, Yellon DM. The Akt1 isoform is an essential mediator of ischaemic preconditioning. J Cell Mol Med. 2012;16:1739–49. doi: 10.1111/j.1582-4934.2011.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fryer RM, Hsu AK, Wang Y, Henry M, Eells J, Gross GJ. PKC-delta inhibition does not block preconditioning-induced preservation in mitochondrial ATP synthesis and infarct size reduction in rats. Basic Res Cardiol. 2002;97:47–54. doi: 10.1007/s395-002-8387-x. [DOI] [PubMed] [Google Scholar]
- 56.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–8. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 57.Sivaraman V, Hausenloy DJ, Wynne AM, Yellon DM. Preconditioning the diabetic human myocardium. Journal of cellular and molecular medicine. 2010;14:1740–6. doi: 10.1111/j.1582-4934.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juhasz B, Der P, Turoczi T, Bacskay I, Varga E, Tosaki A. Preconditioning in intact and previously diseased myocardium: Laboratory or clinical dilemma? Antioxid Redox Signal. 2004;6:325–33. doi: 10.1089/152308604322899396. [DOI] [PubMed] [Google Scholar]
- 59.Bartling B, Friedrich I, Silber RE, Simm A. Ischemic preconditioning is not cardioprotective in senescent human myocardium. Ann Thorac Surg. 2003;76:105–11. doi: 10.1016/s0003-4975(03)00186-3. [DOI] [PubMed] [Google Scholar]