Abstract
Purpose
Long noncoding RNAs have been proved to play important roles in the tumorigenesis and development of human gastric cancer (GC). Our study aims to investigate the expression and function of Homeobox A transcript at the distal tip (HOTTIP) in GC.
Methods
HOTTIP expression was detected in GC tissues and cell lines by using quantitative reverse transcription polymerase chain reaction. Association between HOTTIP levels and clinicopathological factors and patient prognosis was also analyzed. MTT, flow cytometry, and transwell invasion and migration assays were used to investigate the role of HOTTIP in the regulation of biological behaviors of GC cells.
Results
HOTTIP expression was remarkably increased in GC tissues and cell lines compared with that in the normal control. Clinicopathologic analysis revealed that high HOTTIP expression correlated with larger tumor size, deeper invasion depth, positive lymph node metastasis, advanced TNM stage, and shorter overall survival. Multivariate regression analysis identified HOTTIP overexpression as an independent unfavorable prognostic factor in GC patients. Moreover, HOTTIP downregulation by si-HOTTIP transfection impaired GC cell proliferation, promoted cell apoptosis, and reduced cell invasion and migration.
Conclusion
These findings suggested that HOTTIP may contribute to GC initiation and progression, and would be not only a novel prognostic marker but also a potential therapeutic target for this disease.
Keywords: long noncoding RNA, HOTTIP, gastric cancer, prognosis
Introduction
Gastric cancer (GC) is the fourth most prevalent human malignancy and the second leading cause of cancer deaths worldwide.1 The majority of GC patients are diagnosed at advanced stage due to vague initial symptoms.2 Despite recent advances in surgical techniques, new chemotherapy regimens, radiotherapy, and molecular-targeted therapy, the clinical outcome of GC patients remains dismal, with a 5-year survival rate of 25% or less.3 Previous studies have reported many oncogenes and tumor suppressor genes closely associated with GC,4–6 but the highly complex molecular mechanisms underlying its carcinogenesis and progression are still obscure. Therefore, it is urgent to identify reliable biomarkers of GC for its early diagnosis, effective therapy, and prognosis evaluation.
Long noncoding RNA (lncRNA), >200 nucleotides in length, is a type of noncoding RNA molecule that can regulate gene expression in transcriptional or post-transcriptional level.7,8 Recent research has shown that lncRNAs participate in a large number of cellular processes, such as cell proliferation, differentiation, apoptosis, and cell cycle progression.9 Emerging evidence indicates that lncRNAs play important roles in the biology of human cancers, which may provide a new but promising way to deal with cancer.10 Functional lncRNAs may be applied for cancer diagnosis and prognosis, and also act as potential novel therapeutic targets. For example, increased expression of lncRNA BRAF activated non-coding RNA (BANCR) confers poor prognosis in patients suffering from malignant melanoma and retinoblastoma.11,12 lncRNA Hox transcript antisense intergenic RNA (HOTAIR) is a negative prognostic factor for osteosarcoma, lung cancer, and colorectal cancer.13–15 lncRNA very-low-density lipoprotein receptor (VLDLR), Plasmacytoma variant translocation 1 (PVT1) and growth arrest-specific transcript 5 (GAS5) could regulate tumor cell responses to chemotherapy.16–18 However, the understanding of the expression and function of lncRNAs in GC is still in the early stage.
Homeobox A (HOXA) transcript at the distal tip (HOTTIP) is a recently functionally characterized lncRNA located at the 5′ end of the HOXA cluster.19 Increased HOTTIP expression has been reported in tongue squamous cell carcinoma,20 lung cancer,21 pancreatic cancer,22 and hepatocellular carcinoma.23 In these tumors, HOTTIP may serve as a potential oncogene, and HOTTIP overexpression was associated with enhanced cell proliferation, reduced apoptosis, and increased cell migration. However, no report of HOTTIP in GC has been found. In the present study, we examined HOTTIP expression in GC tissues and cell lines. We also investigated the correlation between HOTTIP levels and clinicopathological characteristics and overall survival of GC patients. Moreover, we explored the role of HOTTIP in the regulation of biological behaviors of GC cells.
Materials and methods
Patients and clinical specimens
Fresh primary GC tumor tissues and matched NATs (≥3 cm away from tumor margin) were collected from 98 pathologically confirmed GC patients in Changzhou No 2 Hospital between January 2009 and May 2010. All samples were frozen immediately in liquid nitrogen and stored at −80°C until analysis. Patients with two or more different malignancies were excluded. None of the patients had received preoperative radiotherapy or chemotherapy. Patient characteristics are shown in Table 1. Follow-up data were available for all patients. Overall survival was defined as the amount of time from the day of primary surgery to the date of death or the end of follow-up (for living patients). The ethical committees of Changzhou No. 2 Hospital affiliated to Nanjing Medical University approved this study, and written informed consent was obtained from all patients.
Table 1.
Correlation between HOTTIP expression and different clinicopathological features in patients with gastric cancer
| Characteristics | High HOTTIP expression (%) | Low HOTTIP expression (%) | P-value |
|---|---|---|---|
| Age (years) | |||
| ≥60 | 34 (47.9) | 37 (52.1) | 0.652 |
| <60 | 15 (55.6) | 12 (44.4) | |
| Sex | |||
| Male | 26 (46.4) | 30 (53.6) | 0.541 |
| Female | 23 (54.8) | 19 (45.2) | |
| Differentiation | |||
| Well-moderate | 12 (40.0) | 18 (60.0) | 0.273 |
| Poor | 37 (54.4) | 31 (45.6) | |
| Lauren type | |||
| Intestinal | 27 (43.5) | 35 (56.5) | 0.142 |
| Diffuse and mixed | 22 (61.1) | 14 (38.9) | |
| Tumor size | |||
| ≥5 cm | 36 (61.0) | 23 (39.0) | 0.006 |
| <5 cm | 13 (33.3) | 26 (66.7) | |
| Invasion depth | |||
| T1, T2 | 14 (36.8) | 24 (63.2) | 0.031 |
| T3, T4 | 35 (58.3) | 25 (41.7) | |
| TNM stage | |||
| I/II | 11 (33.3) | 22 (66.7) | 0.013 |
| III | 38 (58.5) | 27 (41.5) | |
| Lymphatic metastasis | |||
| Negative | 10 (33.3) | 20 (66.7) | 0.028 |
| Positive | 39 (57.4) | 29 (42.6) |
Abbreviation: HOTTIP, HOXA transcript at the distal tip.
Cell culture and RNA interference
Human GC cell lines (AGS, SGC-7901, BGC-823, and MKN-28) and human normal gastric epithelial cell line GES-1 were obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). The cells were maintained in Roswell Park Memorial Institute 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL streptomycin sulfate. Cultures were incubated in a humidified atmosphere of 5% CO2 at 37°C.
lncRNA HOTTIP small interfering RNA (si-HOTTIP) and nontargeting siRNA (si-NC) were purchased from Sigma-Aldrich (St Louis, MO, USA). GC cells were transfected with siRNA by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The cells were harvested for further assays 48 hours after transfection.
RNA extraction, reverse transcription, and quantitative reverse transcription polymerase chain reaction
Total RNA was extracted using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. RNA was reverse transcribed into cDNA using a Reverse Transcription Kit (Takara, Dalian, People’s Republic of China). HOTTIP expression levels were measured with quantitative reverse transcription polymerase chain reaction (qRT-PCR) using an ABI7500 system and the SYBR Green PCR Master Mix (Takara). GAPDH was used as an internal control. The primer sequences for HOTTIP were 5′-GTGGGGCCCAGACCCGC-3′ (forward) and 5′-AATGATAGGGACACATCGGGGAACT-3′ (reverse). Each assay was performed in triplicate, and relative HOTTIP expression was normalized to GAPDH using the 2−ΔCt method. The fold change of HOTTIP in GC relative to the matched NAT was determined by the 2−ΔΔCt method, where ΔΔcycle threshold (CT) = (CTHOTTIP − CTGAPDH) (in GC samples) − (CTHOTTIP − CTGAPDH) (in NATs).
Cell proliferation assay
Cell proliferation was analyzed using MTT assay. Briefly, ~1×103 cells were seeded into a 96-well plate and incubated for 1, 2, 3, and 4 days. At the indicated time point, 20 μL of MTT (5 mg/mL) (Sigma-Aldrich) was added into each well and incubated for another 4 hours. Then the supernatants were removed and 150 μL of DMSO (Sigma-Aldrich) was added to terminate the reaction. The absorbance value (optical density [OD]) was measured at 490 nm on a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Detection of apoptosis by flow cytometry
Forty-eight hours after transfection, the GC cells were harvested, washed, and resuspended in ice-cold phosphate-buffered saline. The cells were then treated with propidium iodide (10 μg/mL; Sigma-Aldrich) and Annexin V-FITC (50 μg/mL, BD Biosciences, San Jose, CA, USA) in the dark for 15 minutes at room temperature, and examined by flow cytometry (FACScan; BD Biosciences).
Cell invasion and migration assays
Cell migration and invasion assays were performed using transwell chambers (8 μm pore size; BD Biosciences). For the migration assay, approximately 1×105 GC cells in serum-free media were seeded into the upper chambers after siRNA transfection. The lower chamber contained medium with 20% fetal bovine serum as a chemoattractant. Following a 48-hour incubation, the cells located on the lower surface of the chamber were stained and counted using a microscope (Olympus Corp., Tokyo, Japan). The invasion assay protocol was similar to the migration assay except that the upper chambers were first covered with Matrigel.
Statistics
All statistical analyses were performed using the SPSS 17.0 software package (SPSS, Chicago, IL, USA). The significance of differences between groups was estimated by Student’s t-test and chi-square test. Survival curves were constructed with the Kaplan–Meier method and compared by log-rank test. The significance of survival variables was evaluated using a multivariate Cox proportional hazards regression analysis. P<0.05 was considered statistically significant.
Results
Increased HOTTIP expression in GC tissues and cell lines
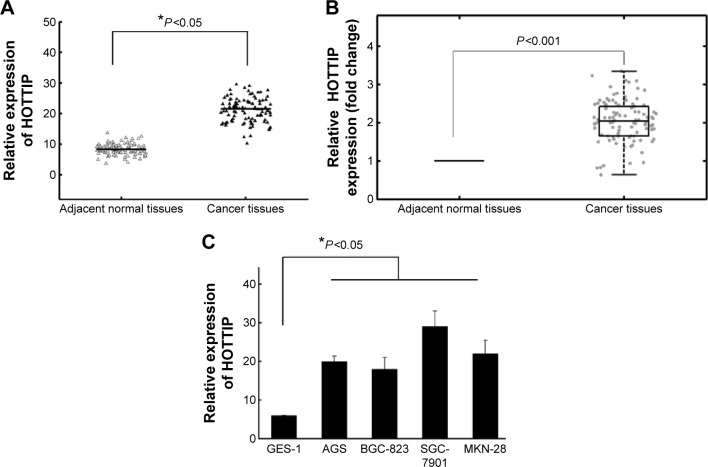
HOTTIP expression in GC tissues and cell lines was measured by qRT-PCR. Figure 1A and B showed a significant high expression of HOTTIP in GC tissues compared with NATs (P<0.05). HOTTIP expression was also significantly increased in four GC cell lines compared to normal gastric epithelial cell GES-1 (Figure 1C, P<0.05). Since SGC-7901 and MKN-28 exhibited relative high HOTTIP expression among all tested cell lines, these two cell lines were chosen for the subsequent in vitro experiments.
Figure 1.
Relative HOTTIP expression levels in gastric cancer tissues and cell lines.
Notes: (A) HOTTIP expression was significantly higher in gastric cancer tissues than in the corresponding NATs. HOTTIP levels were calculated by the 2−ΔCt method and normalized to GAPDH. (B) The fold change of HOTTIP in GC relative to the matched NAT in 98 GC patients. (C) Increased HOTTIP expression in four GC cell lines compared to normal gastric epithelial cell line GES-1. *P<0.05.
Abbreviations: GC, gastric cancer; HOTTIP, HOXA transcript at the distal tip; NAT, normal adjacent tissue.
Correlation between HOTTIP expression and clinical features
We further analyzed the association between HOTTIP expression levels and clinicopathological characteristics of GC. GC samples were classified into HOTTIP low expression group (n=49) and HOTTIP high expression group (n=49) according to the median HOTTIP expression level of all GC samples. The association between clinicopathological characteristics and HOTTIP expression is summarized in Table 1. We found that HOTTIP level was associated with tumor size, tumor depth, lymph node metastasis, and clinical stage. However, we did not find any significant correlation between HOTTIP levels and other clinicopathological features, such as patient’s sex, age, Lauren type, and cancer differentiation.
Prognostic values of HOTTIP expression in GC
We further evaluated the associations of HOTTIP expression level with survival of GC patients. Survival analysis indicated that patients in low HOTTIP expression group had better 5-year overall survival than those in high HOTTIP expression group (P<0.001, Figure 2). Univariate analysis revealed that HOTTIP expression, tumor size, tumor depth, lymphatic invasion, and TNM stage were prognostic factors for patient’s prognosis (Table 2). Multivariate analysis confirmed high HOTTIP expression (P=0.015, relative risk [RR] =2.54) as an unfavorable prognostic factor for GC patients independent of other clinicopathological factors, including depth of infiltration (P=0.003, hazard ratio [HR] =3.15), lymph node status (P=0.012, HR =2.76), and TNM stage (P=0.008, HR =2.92; Table 2).
Figure 2.
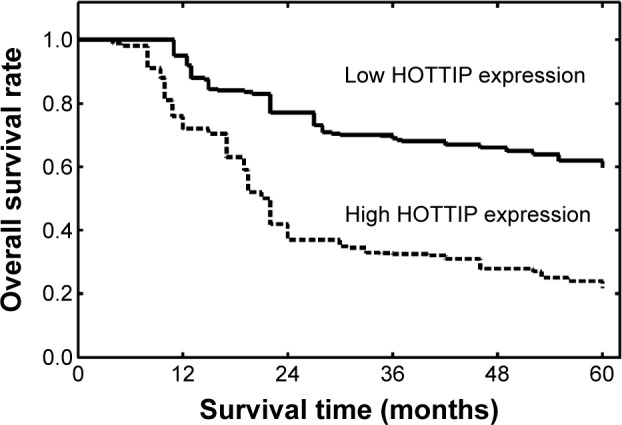
Kaplan–Meier survival curves of gastric cancer patients based on HOTTIP expression status.
Note: Patients in the high HOTTIP expression group had significantly poorer prognosis than those in low HOTTIP expression group (P<0.001, log-rank test).
Abbreviation: HOTTIP, HOXA transcript at the distal tip.
Table 2.
Univariate and multivariate analysis of overall survival in 98 gastric cancer patients
| Variables | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| RR | P-value | RR | P-value | |
| Age (years) | 0.94 | 0.225 | 1.44 | 0.095 |
| Sex | 1.41 | 0.136 | 0.98 | 0.272 |
| Differentiation | 1.55 | 0.094 | 1.58 | 0.074 |
| Lauren type | 1.33 | 0.142 | 1.16 | 0.214 |
| Tumor size | 3.09 | 0.006 | 1.53 | 0.082 |
| Invasion depth | 2.85 | 0.015 | 3.15 | 0.003 |
| Lymphatic metastasis | 2.57 | 0.028 | 2.76 | 0.012 |
| TNM stage | 3.94 | <0.001 | 2.92 | 0.008 |
| HOTTIP expression | 3.78 | <0.001 | 2.54 | 0.015 |
Abbreviations: HOTTIP, HOXA transcript at the distal tip; RR, relative risk.
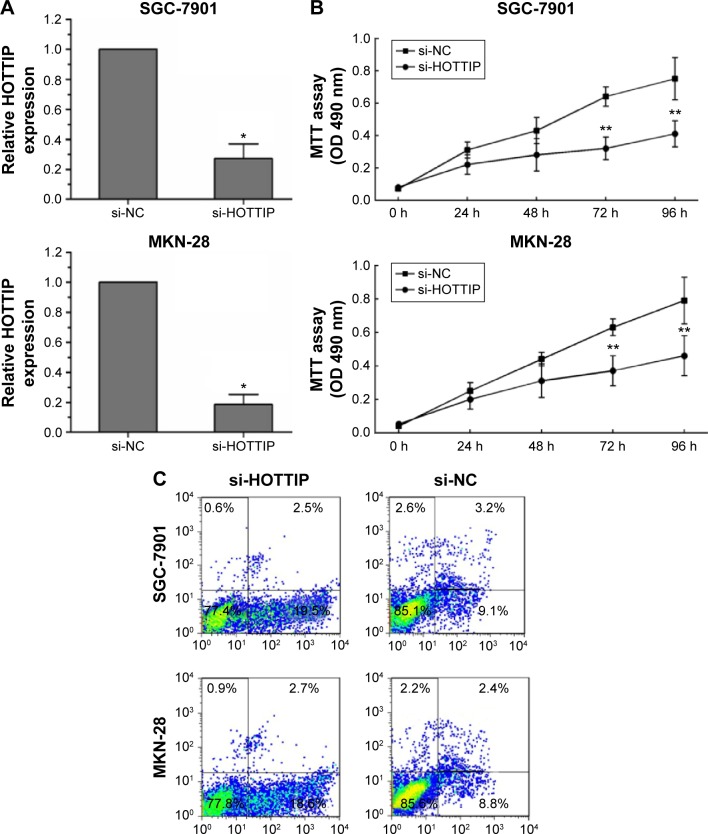
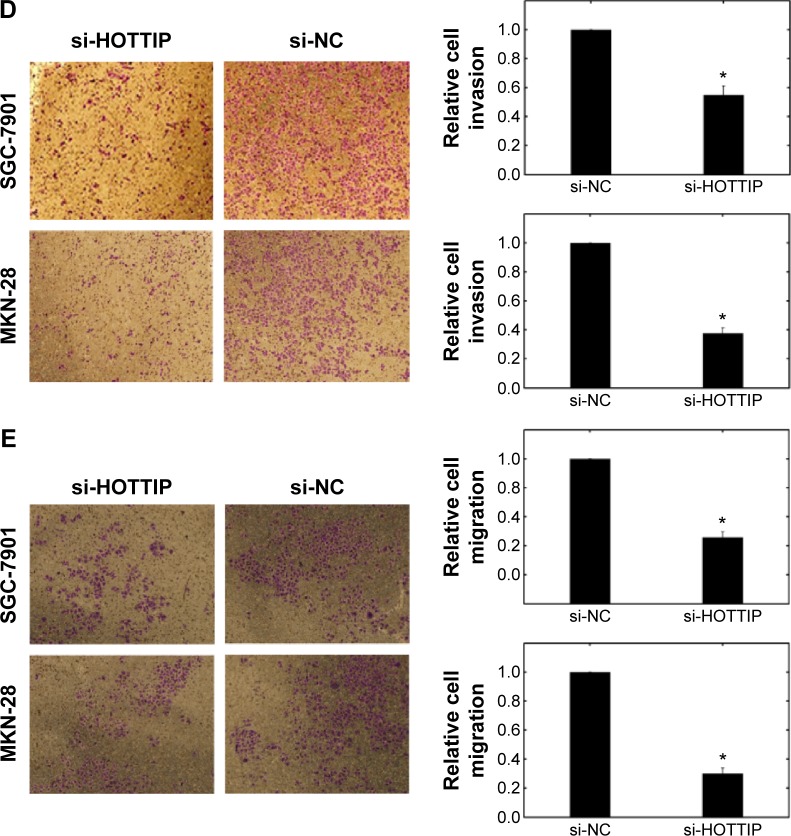
Effects of HOTTIP downregulation on the biological behaviors of SGC-7901 and MKN-28 cells
Finally, we explored the role of HOTTIP in regulating the biological behaviors of GC cells. HOTTIP expression in SGC-7901 and MKN-28 cells was evidently downregulated after si-HOTTIP transfection (Figure 3A). As shown in Figure 3B and C, HOTTIP downregulation impaired GC cell proliferation and promoted cell apoptosis compared to the si-NC group. In addition, we observed reduced cell invasion/migration in SGC-7901 and MKN-28 cells after si-HOTTIP transfection (Figure 3D and E).
Figure 3.
Effects of HOTTIP on the biological behaviors of SGC-7901 and MKN-28 cells.
Notes: (A) Expression of HOTTIP was significantly downregulated after si-HOTTIP transfection. *P<0.05. (B) Cell proliferation was measured by MTT assays in SGC-7901 and MKN-28 cells transfected with si-HOTTIP or si-NC. **P<0.01. (C) Flow cytometric analysis showed induced cell apoptosis after si-HOTTIP transfection. (D, E) The transwell invasion and migration assays showed that the number of invaded or migrated cells was significantly lower in the si-HOTTIP-transfected group than in the si-NC-transfected group. *P<0.05.
Abbreviations: HOTTIP, HOXA transcript at the distal tip; h, hour; si-HOTTIP, HOTTIP small interfering RNA; si-NC, nontargeting small interfering RNA; OD, optical density.
Discussion
Identifying novel molecules that take part in GC formation and progression may be helpful for improving the diagnosis, prevention, and treatment of this disease. The relationship between lncRNAs and tumors has currently become one of the focuses of cancer studies. Abnormal expressions of several lncRNAs have been reported in GC. For example, overexpression of lncRNA H19 promoted the features of GC including proliferation, migration, invasion, and metastasis.24 Plasma H19 could serve as a potential biomarker for diagnosis of GC, in particular for early tumor screening.25 Li et al26 found that high expression of lncRNA BANCR was positively associated with clinical stage, tumor depth, lymph node metastasis, and distant metastasis in GC patients. They also confirmed high expression of BANCR as an independent unfavorable prognostic factor in GC patients. Zhang et al27 demonstrated that knockdown of lncRNA PVT1 could reverse the cisplatin resistance in cisplatin-resistant GC cell lines, while upregulation of PVT1 significantly reduced GC cell apoptosis and inhibited the sensitivity of GC cells to anticancer drugs. These findings suggested that lncRNAs might play important roles in GC initiation and development, and have a great potential for clinical application.
In the present study, we first investigated HOTTIP expression in GC tissues and cell lines by RT-PCR. We observed high HOTTIP expression in GC specimens compared to NATs. Additionally, HOTTIP expression was markedly increased in GC cell lines compared with normal gastric epithelium cells. Our results provided the first evidence that high HOTTIP expression was closely associated with GC carcinogenesis. Then we correlated HOTTIP levels with different clinicopathological factors of GC tissues. We found that high HOTTIP expression was more frequently detected in GC patients with larger tumor size, deeper invasion depth, positive lymph node metastasis, and advanced TNM stage. Downregulation of HOTTIP in GC cells would reduce cell proliferation, enhance cell apoptosis, and impair cell invasion. These findings revealed that HOTTIP might be involved in GC progression and contribute to molecular-targeted therapy. Finally, our research showed that GC patients with high HOTTIP levels tended to have shorter overall survival than patients with lower levels. Multivariate Cox hazard regression analysis identified high HOTTIP expression as an independent indicator of unfavorable prognosis. To our knowledge, this is the first study to analyze the expression and clinical significance of HOTTIP in GC.
Our results were consistent with the previous findings in other cancers. In lung cancer A549 cells, cell proliferation and colony formation were significantly inhibited in vitro after successfully depletion of HOTTIP.21 Tumor growth in vivo was also suppressed in a mouse model. Moreover, depletion of HOTTIP caused cell cycle arrest in G0/G1 phase and induced significant cell apoptosis. In pancreatic cancer, knockdown of HOTTIP inhibited tumor cell proliferation, promoted apoptosis, and reduced migration.28 Additionally, inhibition of HOTTIP potentiated the antitumor effects of gemcitabine in vitro and in vivo.22 In tongue squamous cell carcinoma, high HOTTIP expression positively correlated with depth of infiltration (T stage), clinical stage, and distant metastasis and predicted poor survival.20 High HOTTIP expression was also associated with increased metastasis formation and decreased overall survival in patients with hepatocellular carcinoma.29 Taken together, these researches indicated that HOTTIP might serve as an oncogene in several types of human cancers. However, the complex molecular mechanisms underlying high HOTTIP expression in human cancers and its function are still incompletely known. More studies should be applied to clarify the precise mechanisms by which HOTTIP contributes to tumor formation and progression.
We are aware of some limitations in our work. First, the clinical part was a retrospective study, and the tumor sample size was relatively small. Second, we observed the effects of HOTTIP on the proliferation, apoptosis, invasion, and migration of GC cells, but its association with colony formation, cell cycle, and xenograft tumorigenesis was not involved in this study. Third, although we revealed the oncogene function of HOTTIP in GC, its probable downstream mediators are still unclear. Epithelial-to-mesenchymal transition (EMT) has been recognized as an important process that is associated with the progression and metastasis of several cancers including GC. However, the effect of HOTTIP on the EMT markers, such as E-cadherin, N-cadherin, and vimentin, has not been reported till now. Whether HOTTIP plays a role in EMT might be an interesting and important topic of future investigations.
Conclusion
In conclusion, our research confirmed elevated HOTTIP expression in GC tissues and cell lines. Our study also showed that high HOTTIP levels correlated with tumor progression and poor prognosis in GC patients. Regulation of HOTTIP expression influenced biological behaviors of GC cells. These findings suggested that HOTTIP may act as an oncogene in GC initiation and development, and would be not only a novel prognostic marker but also a potential therapeutic target for this disease.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siewert JR, Sendler A. The current management of gastric cancer. Adv Surg. 1999;33:69–93. [PubMed] [Google Scholar]
- 3.Duraes C, Almeida GM, Seruca R, Oliveira C, Carneiro F. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch. 2014;464(3):367–378. doi: 10.1007/s00428-013-1533-y. [DOI] [PubMed] [Google Scholar]
- 4.Fan H, Guo Z, Wang C. Combinations of gene ontology and pathway characterize and predict prognosis genes for recurrence of gastric cancer after surgery. DNA Cell Biol. 2015;34(9):579–587. doi: 10.1089/dna.2015.2923. [DOI] [PubMed] [Google Scholar]
- 5.Liang L, Fang JY, Xu J. Gastric cancer and gene copy number variation: emerging cancer drivers for targeted therapy. Oncogene. 2015 doi: 10.1038/onc.2015.209. [DOI] [PubMed] [Google Scholar]
- 6.Nishida Y, Kuwata T, Nitta H, et al. A novel gene-protein assay for evaluating HER2 status in gastric cancer: simultaneous analyses of HER2 protein overexpression and gene amplification reveal intratu-moral heterogeneity. Gastric Cancer. 2015;18(3):458–466. doi: 10.1007/s10120-014-0394-7. [DOI] [PubMed] [Google Scholar]
- 7.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482(7385):339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108(12):2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 10.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su S, Gao J, Wang T, Wang J, Li H, Wang Z. Long non-coding RNA BANCR regulates growth and metastasis and is associated with poor prognosis in retinoblastoma. Tumour Biol. 2015;36(9):7205–7211. doi: 10.1007/s13277-015-3413-3. [DOI] [PubMed] [Google Scholar]
- 12.Li R, Zhang L, Jia L, et al. Long non-coding RNA BANCR promotes proliferation in malignant melanoma by regulating MAPK pathway activation. PLoS One. 2014;9(6):e100893. doi: 10.1371/journal.pone.0100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loewen G, Jayawickramarajah J, Zhuo Y, Shan B. Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol. 2014;7(1):90. doi: 10.1186/s13045-014-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Su Y, Yang Q, et al. Overexpression of long non-coding RNA HOTAIR promotes tumor growth and metastasis in human osteosarcoma. Mol Cells. 2015;38(5):432–440. doi: 10.14348/molcells.2015.2327. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Svoboda M, Slyskova J, Schneiderova M, et al. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis. 2014;35(7):1510–1515. doi: 10.1093/carcin/bgu055. [DOI] [PubMed] [Google Scholar]
- 16.You L, Chang D, Du HZ, Zhao YP. Genome-wide screen identifies PVT1 as a regulator of Gemcitabine sensitivity in human pancreatic cancer cells. Biochem Biophys Res Commun. 2011;407(1):1–6. doi: 10.1016/j.bbrc.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Yan IK, Wood J, Haga H, Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. 2014;12(10):1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickard MR, Williams GT. Regulation of apoptosis by long non-coding RNA GAS5 in breast cancer cells: implications for chemotherapy. Breast Cancer Res Treat. 2014;145(2):359–370. doi: 10.1007/s10549-014-2974-y. [DOI] [PubMed] [Google Scholar]
- 19.Wang KC, Yang YW, Liu B, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472(7341):120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Zhao L, Wang YX, Xi M, Liu SL, Luo LL. Long non-coding RNA HOTTIP is correlated with progression and prognosis in tongue squamous cell carcinoma. Tumour Biol. 2015 Jun 10; doi: 10.1007/s13277-015-3645-2. Epub. [DOI] [PubMed] [Google Scholar]
- 21.Deng HP, Chen L, Fan T, Zhang B, Xu Y, Geng Q. Long non-coding RNA HOTTIP promotes tumor growth and inhibits cell apoptosis in lung cancer. Cell Mol Biol. 2015;61(4):34–40. [PubMed] [Google Scholar]
- 22.Li Z, Zhao X, Zhou Y, et al. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer. J Transl Med. 2015;13:84. doi: 10.1186/s12967-015-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang FH, Au SL, Wei L, et al. Long non-coding RNA HOTTIP is frequently up-regulated in hepatocellular carcinoma and is targeted by tumour suppressive miR-125b. Liv Int. 2015;35(5):1597–1606. doi: 10.1111/liv.12746. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5(8):2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Yin C, Dang Y, Ye F, Zhang G. Identification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer. Sci Rep. 2015;5:11516. doi: 10.1038/srep11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Zhang L, Zhang Y, Zhou F. Increased expression of LncRNA BANCR is associated with clinical progression and poor prognosis in gastric cancer. Biomed Pharmacother. 2015;72:109–112. doi: 10.1016/j.biopha.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XW, Bu P, Liu L, Zhang XZ, Li J. Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochem Biophys Res Commun. 2015;462(3):227–232. doi: 10.1016/j.bbrc.2015.04.121. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Jutooru I, Chadalapaka G, Corton JC, Safe S. The long non-coding RNA HOTTIP enhances pancreatic cancer cell proliferation, survival and migration. Oncotarget. 2015;6(13):10840–10852. doi: 10.18632/oncotarget.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quagliata L, Matter MS, Piscuoglio S, et al. Long noncoding RNA HOTTIP/HOXA13 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Hepatology. 2014;59(3):911–923. doi: 10.1002/hep.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]