Abstract
Background
In chronic obstructive pulmonary disease (COPD), the blood vitamin D3 level is generally low, and genetic polymorphisms of vitamin D-binding protein encoded by the GC gene are associated with COPD development. In this study, we examined the relationship between GC polymorphisms and plasma vitamin D3 level in Korean patients with COPD.
Methods
The study included 175 COPD patients from the Korean Obstructive Lung Disease Cohort. Multivariate analysis was conducted with adjustment for age, body mass index (BMI), lung function, smoking status, smoking amount, and seasonal variation in blood vitamin D level. Vitamin D deficiency was defined as a plasma 25-hydroxyvitamin D3 level lower than 20 ng/mL.
Results
The mean plasma vitamin D3 level was 17.5 ng/mL. The GC1F variant (44.3%) and genotype 1F-2 (27.4%) were the most common. The plasma vitamin D3 level was lower in patients with the GC2 variant (estimated =−3.73 ng/mL) and higher in those with genotype 1F-1S (estimated =4.08 ng/mL). The GC2 variant was a significant risk factor for vitamin D deficiency (odds ratio =2.41). Among COPD clinical parameters, vitamin D deficiency was associated with a lower ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) regardless of GC polymorphisms. FEV1/FVC was higher in patients with genotype 1F-1F (estimated =3.61%) and lower in those with genotype 1F-2 (estimated =−3.31%). The 6-minute walking distance was shorter for patients with the GC1F variant (estimated =−38.91 m) and longer for those with the GC2 variant (estimated =26.98 m). The emphysema index was higher for patients with the GC1S variant (estimated =6.56%) and genotype 1F-1S (estimated =9.86%), regardless of the vitamin D level.
Conclusion
The GC2 variant is a risk factor for vitamin D deficiency, and genotype 1F-1S is a protective factor against vitamin D deficiency. GC polymorphisms and vitamin D deficiency correlate with clinical outcomes for Korean patients with COPD.
Keywords: vitamin D-binding protein, polymorphism, vitamin D, chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) has been recognized as a systemic disease associated with various comorbidities.1 Accumulating evidences demonstrate high prevalence of low vitamin D status in chronic illnesses, such as cancers, autoimmune diseases, infectious and cardiovascular diseases, and also in COPD.2–4 Although previous studies have examined the effects of vitamin D3 level or vitamin D deficiency on various clinical characteristics of COPD, the results are conflicting.5–8
Vitamin D-binding protein (VDBP) is a serum protein encoded by the GC gene located on chromosome 4q13. It was first described by J Hirschfeld in 1959 as a marker for gamma globulin in human serum.9 VDBP is the main carrier protein of 25-hydroxyvitamin D, the major circulating form of vitamin D, and 1,25-dihydroxyvitamin D, the most active vitamin D metabolite. Neutrophil-expressed VDBP activates macrophages and augments monocyte and neutrophil chemotaxis,10–13 which may contribute to chronic inflammatory response observed in COPD. Among more than 120 types of GC genetic variants, single-nucleotide polymorphisms rs4588 and rs7041 at codons 416 and 420 in exon 11 are the most common, resulting in three functional variants: GC1F(A/G), GC1S(A/T), and GC2(C/G), which give rise to six genotypes (GC1F-1F, GC1F-1S, GC1F-2, GC1S-1S, GC1S-2, and GC2-2).14 The association of GC polymorphisms with susceptibility of COPD has been explored in several studies.15–18
Although vitamin D status and GC polymorphisms are closely related, their association with clinical outcomes in COPD has not been yet investigated, since most of the previous studies on COPD have examined the individual contribution of each factor.6,16,17 Therefore, the purpose of this study was to determine the relationship between common GC polymorphisms and vitamin D3 level in Korean patients with COPD. We assumed that certain GC variants would be associated with vitamin D3 level, indicating susceptibility to vitamin D deficiency. Moreover, we aimed to evaluate the relative contribution of GC polymorphisms and vitamin D status to various clinical outcomes in patients with COPD.
Materials and methods
Study subjects
The study population consisted of 175 patients from the Korean Obstructive Lung Disease (KOLD) Cohort, which comprises patients with COPD or asthma treated in pulmonary clinics of 17 hospitals in South Korea from June 2005 to December 2011. The inclusion criteria were as follows: post-bronchodilator ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) <0.7, age over 40 years, smoking history of ten or more pack-years, and no or minimal abnormality detected by chest radiography. The study protocol was approved by the institutional review boards of the 17 hospitals included in the KOLD Cohort (Asan Medical Center, Hanyang University Guri Hospital, Inje University Ilsan Paik Hospital, Bundangcha Hospital, Kangbook Samsung Medical Center, Ewha Womans University Mokdong Hospital, Kangwon National University Hospital, Korea University Anam Hospital, Seoul National University Hospital, Seoul National University Bundang Hospital, Hallym University Medical Center, Konkuk University Medical Center, Ajou University Hospital, National Medical Center, The Catholic University of Korea Seoul St Mary’s Hospital, The Catholic University of Korea Yeouido St Mary’s Hospital, Severance Hospital), and informed written consent was obtained from all the patients.
Blood collection and measurement of plasma vitamin D3 level
Plasma samples were assayed for 25-hydroxyvitamin D3 using a radioimmunoassay kit (DiaSorin, Stillwater, MN, USA), and vitamin D deficiency was defined as plasma levels of 25-hydroxyvitamin D3 lower than 20 ng/mL.19
COPD clinical parameters
COPD status was assessed according to four parameters: pulmonary function, 6-minute walking (6MW) distance, quality of life evaluated by St George’s Respiratory Questionnaire (SGRQ), and emphysema index.
Genotyping
DNA was extracted from blood for GC genotyping. The region that included two-point mutation at codons 416 and 420 in exon 11 (causing Glu416/Asp and Thr420/Lys substitutions) was amplified by polymerase chain reaction (PCR) using the following primers: upstream, 5′-TAATGAGCAAATGAAAGAAG-3′ and downstream, 5′-TGAGTAGATTGGAGTGCATAC-3′ to obtain a 462 bp product. PCR was performed in a DNA Thermal Cycler (PerkinElmer Inc., Waltham, MA, USA) in a reaction volume of 40 μL containing 100 ng DNA, 1.5 mM MgCl2, 10 mM Tris Cl (pH 8.3), 40 mM KCl, 4% dimethyl sulfoxide, 0.2 mL of each deoxynucleoside triphosphate (dNTP) (Amersham Biosciences KK, Tokyo, Japan), 0.5 μM of each primer, and 3.75 units of Taq DNA polymerase (Bioneer, Daejeon, Korea). After amplification, restriction fragment-length polymorphism analysis was performed by digesting PCR products with HaeIII (Toyobo, Osaka, Japan) or EcoT14 I (Takara Bio, Otsu, Japan) at 37°C overnight. GC1S was cut by HaeIII into 295 and 167 bp fragments and by EcoT14 I into 302 and 156 bp fragments, while GC1F was not cut by either enzyme.
Computed tomography data acquisition and analysis
Volumetric computed tomography (CT) scans were performed at full inspiration and expiration using a 16-multiple detector CT scanner (Somatom Sensation; Siemens Medical Systems, Erlangen, Germany). The images were reconstructed using the soft kernel B30f (Siemens Medical Systems) from the thoracic inlet to the lung base. Images of the entire lungs were automatically extracted using the in-house software, and the attenuation coefficient of each pixel was measured and calculated. The cutoff level between normal lung density and low-attenuation area was defined as −950 HU. Quantitative assessment of emphysema was expressed as the percent of low attenuation.20
Statistical analysis
Categorical variables were analyzed using chi-square test or Fisher’s exact test, and continuous variables were analyzed using Student’s t-test or the Mann–Whitney U-test. Normally distributed variables were presented as mean ± standard deviation, and non-normally distributed variables were presented as median values and interquartile range. Multiple linear regression analysis was conducted to investigate the effects of GC variants/genotypes on vitamin D3 level, and to evaluate the relationship of GC variant/genotypes and vitamin D deficiency with various clinical parameters in COPD. Statistical adjustment was performed for lung function, age, body mass index (BMI), smoking status (current/former), smoking amount (pack-years), and seasonal variation in plasma vitamin D3 level. Logistic linear regression analysis was conducted to determine significant GC variants/genotypes for vitamin D deficiency. In all analyses, a P-value <0.05 was considered to be statistically significant.
Results
Patients’ characteristics
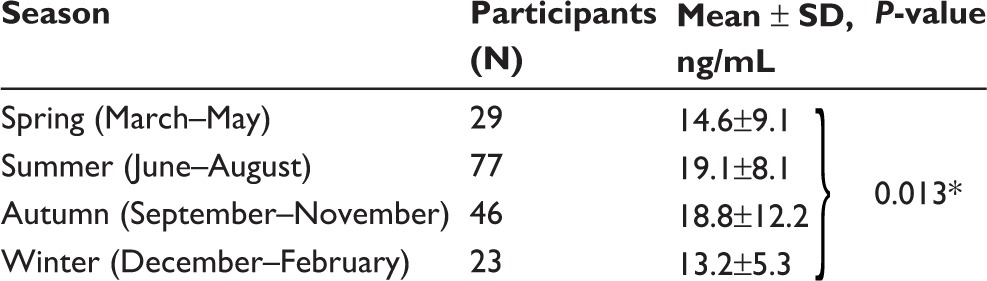
Baseline characteristics of the study population are summarized in Table 1. Mean age was 66.4 years with male dominance (97.7%). Disease staging was performed according to the previous Global Initiative for Chronic Obstructive Lung Disease (GOLD): 12 (6.9%), 87 (49.7%), 64 (36.6%), and 12 (6.9%) patients were categorized into stages I, II, III, and IV, respectively. The GC1F variant (44.3%) and genotype 1F-2 (27.4%) were the most common. The mean plasma level of vitamin D3 was 17.5 ng/mL with seasonal variations; it was higher in summer (June–August) and fall (September–November) than in spring (March–May) and winter (December–February) (Table 2).
Table 1.
Baseline characteristics of the study population
| Characteristics | Total participants (N=175)* |
|---|---|
| Sex, male | 171 (97.7) |
| Age, years | 66.4±6.8 |
| Height, cm | 165.4±6.2 |
| BMI, kg/m2 | 22.9±3.4 |
| Smoking status | |
| Current | 45 (25.7) |
| Former | 130 (74.3) |
| Pack-years | 45.6±23.1 |
| Underlying diseases | |
| Coronary vascular disease | 18 (10.3) |
| Cerebrovascular disease | 7 (4.0) |
| Hypertension | 55 (31.4) |
| Diabetes mellitus | 19 (10.9) |
| Chronic kidney disease | 3 (1.7) |
| Liver disease | 7 (4.0) |
| Asthma | 50 (28.6) |
| Malignancy | 6 (5.1) |
| FEV1, L | 1.51±0.51 |
| FEV1, % predicted | 57.5±18.0 |
| FVC, L | 3.16±0.84 |
| FVC, % predicted | 84.1±20.9 |
| FEV1/FVC, % | 48.0±10.6 |
| GOLD classification | |
| I | 12 (6.9) |
| II | 87 (49.7) |
| III | 64 (36.6) |
| IV | 12 (6.9) |
| Measured with emphysema index, % (N=131) | 21.9±16.5 |
| Measured with 6MW distance, meters (m) (N=129) | 438.7±77.5 |
| Plasma 25-hydroxyvitamin D3 level, ng/mL | 17.5±9.5 |
| VDBP variants (N=350)# | |
| GC1F | 155 (44.3) |
| GC1S | 83 (23.7) |
| GC2 | 112 (32.0) |
| Genotypes (N=175) | |
| 1F-1F | 35 (20.0) |
| 1F-1S | 37 (21.1) |
| 1F-2 | 48 (27.4) |
| 1S-1S | 8 (4.6) |
| 1S-2 | 30 (17.1) |
| 2-2 | 17 (9.7) |
Note:
Data are presented as the number of patients (percentage) or mean ± standard deviation.
Number of total variants; because there are two variants in each individual, double the number (350) of 175 is included in the analysis.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; 6MW, 6-minute walking; VDBP, vitamin D-binding protein.
Table 2.
Seasonal variation in baseline plasma vitamin D level
|
Note: *One-way analysis of variance was used.
Abbreviation: SD, standard deviation.
Association of vitamin D3 level with GC variants and genotypes
Vitamin D3 plasma levels and vitamin D deficiency distribution were compared between different GC variants and genotypes (Table 3). Patients with the GC2 variant had lower plasma vitamin D3 levels than non-GC2 patients (15.5 vs 19.9 ng/mL, P=0.002) and higher proportion of vitamin D deficiency (78.9% vs 58.8%, P=0.004). Patients with genotype 1F-1S had higher plasma levels of vitamin D3 than non-1F-1S individuals (20.7 vs 16.7 ng/mL, P=0.021), while those with genotype 1S-2 had lower plasma levels of vitamin D3 than non-1S-2 patients (14.2 vs 18.2 ng/mL, P=0.034). There was no difference in the distribution of vitamin D deficiency among different genotypes.
Table 3.
Plasma vitamin D level according to GC variants and genotypes
| Variables | Participants (N) | Vitamin D level, ng/mL* | Vitamin D deficiency# |
|---|---|---|---|
| Variant | |||
| GC1F | 120 | 18.3±9.6 | 79 (65.8) |
| Non-GC1F | 55 | 15.7±9.0 | 43 (78.2) |
| P-value | 0.094 | 0.099 | |
| GC1S | 75 | 18.3±10.7 | 52 (69.3) |
| Non-GC1S | 100 | 16.9±8.5 | 70 (70.0) |
| P-value | 0.328 | 0.924 | |
| GC2 | 95 | 15.5±8.8 | 75 (78.9) |
| Non-GC2 | 80 | 19.9±9.7 | 47 (58.8) |
| P-value | 0.002 | 0.004 | |
| Genotype | |||
| 1F-1F | 35 | 18.5±7.0 | 20 (57.1) |
| Non-1F-1F | 140 | 17.3±10.0 | 102 (72.9) |
| P-value | 0.500 | 0.070 | |
| 1F-1S | 37 | 20.7±11.4 | 23 (62.2) |
| Non-1F-1S | 138 | 16.7±8.8 | 99 (71.7) |
| P-value | 0.021 | 0.260 | |
| 1F-2 | 48 | 16.4±9.5 | 36 (75.0) |
| Non-1F-2 | 127 | 17.9±9.5 | 86 (67.7) |
| P-value | 0.336 | 0.350 | |
| 1S-1S | 8 | 22.2 (11.7–33.6) | 4 (50.0) |
| Non-1S-1S | 167 | 15.5 (10.9–22.0) | 118 (70.7) |
| P-value | 0.189 | 0.246 | |
| 1S-2 | 30 | 14.2±8.1 | 25 (83.3) |
| Non-1S-2 | 145 | 18.2±9.6 | 97 (66.9) |
| P-value | 0.034 | 0.075 | |
| 2-2 | 17 | 15.0 (8.1–19.9) | 14 (82.4) |
| Non-2-2 | 158 | 15.8 (11.3–22.8) | 108 (68.4) |
| P-value | 0.164 | 0.233 |
Notes:
Data are presented as mean ± standard deviation, median (interquartile range) or P-value.
Data are presented as the number of patients (percentage) or P-value. Vitamin D deficiency was defined as plasma level of 25-hydroxyvitamin D3 lower than 20 ng/mL.
Effects of GC variants/genotypes on plasma vitamin D3 level
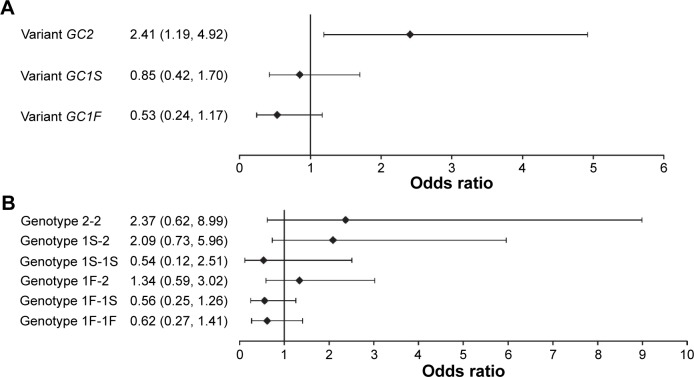
Multiple linear regression analysis of the association between GC variants/genotypes and plasma vitamin D3 levels indicated that plasma vitamin D3 levels were lower in patients carrying the GC2 variant (estimated =−3.73 ng/mL, P=0.010) and higher in those with genotype 1F-1S (estimated =4.08 ng/mL, P=0.020) (Table 4). Logistic regression analysis indicated that the GC2 variant was a significant risk factor for vitamin D deficiency (odds ratio =2.41, 95% confidence interval: 1.19–4.92, P=0.015) (Figure 1).
Table 4.
Adjusted estimates of various factors for plasma vitamin D level
| Model 1: variant GC2
|
Model 2: genotype 1F-1S
|
|||||
|---|---|---|---|---|---|---|
| Estimated | Standard error | P-value | Estimated | Standard error | P-value | |
| Variant/genotype | −3.73 | 1.44 | 0.010 | 4.08 | 1.73 | 0.020 |
| Age, years | 0.06 | 0.11 | 0.589 | 0.07 | 0.11 | 0.525 |
| BMI, kg/m2 | −0.07 | 0.23 | 0.781 | −0.09 | 0.23 | 0.687 |
| FEV1, % predicted | −0.04 | 0.05 | 0.463 | −0.04 | 0.05 | 0.403 |
| FEV1/FVC | 0.20 | 0.09 | 0.029 | 0.25 | 0.09 | 0.005 |
| Smoking status | −2.71 | 1.64 | 0.099 | −2.39 | 1.65 | 0.149 |
| Smoking, pack-years | 0.05 | 0.03 | 0.096 | 0.06 | 0.03 | 0.061 |
| Seasonal variation in plasma vitamin D level | 0.10 | 0.78 | 0.901 | 0.04 | 0.79 | 0.957 |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Figure 1.
Risk of vitamin D deficiency in COPD patients with various GC polymorphisms.
Notes: (A) Three different variants; (B) six different genotypes. Data are presented as odds ratio and 95% confidence interval for each GC variant and genotype.
Effects of GC variants/genotypes and vitamin D deficiency on clinical parameters of COPD
The effects of GC variants/genotypes and plasma vitamin D3 level on COPD clinical parameters (FEV1, FEV1/FVC, 6MW distance, SGRQ score, and emphysema index) were evaluated after adjusting for lung function, age, BMI, smoking status (former/current), smoking amount (pack-years), and seasonal variation in plasma vitamin D3 level (Table 5). Nine different models were analyzed with three variants and six genotypes. FEV1 and SGRQ score were not influenced by either GC variants/genotypes or vitamin D deficiency. Patients with vitamin D deficiency showed lower FEV1/FVC regardless of GC polymorphism. Patients with the GC2 variant (estimated =−2.46%, P=0.050) or genotype 1F-2 (estimated =−3.31%, P=0.015) showed lower FEV1/FVC compared to those without these polymorphisms, while those with genotype 1F-1F (estimated = 3.61%, P=0.018) showed higher FEV1/FVC. After adjustment, vitamin D deficiency was found to be associated with shorter 6MW distance for the GC1F and GC2 variants and genotypes 1F-1F, 1F-1S, and 2-2 (Table 5). The GC1F (estimated =−38.91%, P=0.009) and GC2 variants (estimated =26.98%, P=0.038) showed shorter and longer 6MW distances, respectively. For the emphysema index, the GC1S variant (estimated =6.56%, P=0.023) and genotype 1F-1S (estimated =9.86%, P=0.003) correlated with higher emphysema index values and genotype 1S-1S (estimated =−13.11%, P=0.031) with lower emphysema index compared to COPD patients without these genetic variations. However, no association was observed between the emphysema index and vitamin D deficiency.
Table 5.
Adjusted estimates of VDBP variants/genotypes and vitamin D deficiency* for FEV1/FVC, 6MW distance, and emphysema index
| Models | FEV1/FVC#
|
6MW distance†
|
Emphysema index^
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated | SE | P-value | Estimated | SE | P-value | Estimated | SE | P-value | |
| Model 1 | |||||||||
| Variant GC1F | −1.35 | 1.33 | 0.309 | −38.91 | 14.54 | 0.009 | – | – | – |
| Vitamin D deficiency | −3.39 | 1.34 | 0.013 | −33.57 | 15.17 | 0.029 | |||
| Model 2 | |||||||||
| Variant GC1S | −0.75 | 1.24 | 0.547 | – | – | – | 6.56 | 2.85 | 0.023 |
| Vitamin D deficiency | −3.26 | 1.34 | 0.016 | 1.78 | 3.37 | 0.599 | |||
| Model 3 | |||||||||
| Variant GC2 | −2.46 | 1.25 | 0.050 | 26.98 | 12.83 | 0.038 | – | – | – |
| Vitamin D deficiency | −2.67 | 1.36 | 0.051 | −35.46 | 15.60 | 0.025 | |||
| Model 4 | |||||||||
| Genotype 1F-1F | 3.61 | 1.52 | 0.018 | −19.42 | 16.57 | 0.244 | – | – | – |
| Vitamin D deficiency | −2.85 | 1.33 | 0.034 | −31.47 | 15.68 | 0.048 | |||
| Model 5 | |||||||||
| Genotype 1F-1S | −1.20 | 1.51 | 0.428 | −24.60 | 15.01 | 0.105 | 9.86 | 3.20 | 0.003 |
| Vitamin D deficiency | −3.34 | 1.34 | 0.014 | −31.05 | 15.48 | 0.048 | 2.10 | 3.31 | 0.528 |
| Model 6 | |||||||||
| Genotype 1F-2 | −3.31 | 1.35 | 0.015 | – | – | – | – | – | – |
| Vitamin D deficiency | −2.97 | 1.32 | 0.026 | ||||||
| Model 7 | |||||||||
| Genotype 1S-1S | 4.66 | 2.90 | 0.110 | – | – | – | −13.11 | 6.00 | 0.031 |
| Vitamin D deficiency | −3.05 | 1.34 | 0.023 | 0.90 | 3.38 | 0.790 | |||
| Model 8 | |||||||||
| Genotype 1S-2 | −1.35 | 1.63 | 0.408 | – | – | – | – | – | – |
| Vitamin D deficiency | −3.11 | 1.35 | 0.022 | ||||||
| Model 9 | |||||||||
| Genotype 2-2 | 3.13 | 2.06 | 0.131 | 41.84 | 24.23 | 0.088 | – | – | – |
| Vitamin D deficiency | −3.41 | 1.34 | 0.012 | −33.71 | 15.65 | 0.034 | |||
Notes:
Vitamin D deficiency was defined as plasma level of 25-hydroxyvitamin D3 lower than 20 ng/mL.
Adjusted for age, BMI, smoking status (former or current smoker), pack-years, seasonal variation in plasma vitamin D level, and FEV1 % predicted (N=174).
Adjusted for age, BMI, smoking status (former or current smoker), pack-years, seasonal variation in plasma vitamin D level, FEV1 % predicted, and FEV1/FVC (N=129).
Adjusted for age, BMI, smoking status (former or current smoker), pack-years, seasonal variation in plasma vitamin D level, FEV1 % predicted, and FEV1/FVC (N=131). ‘−’ represents data that were statistically not significant.
Abbreviations: VDBP, vitamin D-binding protein; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; 6MW, 6-minute walking; SE, standard error; BMI, body mass index.
Discussion
This study investigated the association between GC polymorphisms and vitamin D deficiency in Korean patients with COPD. Genotype 1F-1S was related with higher vitamin D levels, while the GC2 variant correlated with lower vitamin D3 levels; the latter showed a significant association with vitamin D deficiency. Moreover, in Korean patients with COPD, GC polymorphisms and vitamin D deficiency affected several clinical parameters of the disease, including FEV1/FVC, 6MW distance, and emphysema index.
Previous studies have investigated the association between GC polymorphisms and susceptibility to COPD.13,16 Thus, a meta-analysis study has revealed that in Asian population, genotype 1F-1F is a risk factor for developing COPD, while genotype 2-2 indicates protection against the disease. In Korean population, genotype 1S-1S has been identified as a risk factor for COPD.17 However, no significant association has been found between GC polymorphisms and COPD susceptibility in Caucasian population.16
Although a number of genome-wide association studies have analyzed the relationship between GC polymorphisms and vitamin D status, pathophysiology of how GC polymorphism affects vitamin D status is not clear.21–26 The GC1F and GC1S genotypes carry a single polymorphism at position 416 (Glu/Asp), while GC1F and GC2 carry a substitution at position 420 (Thr/Lys). Thus, the GC1S and GC2 genotypes encode proteins differing by two amino acids at positions 416 and 420, which accounts for the difference in isoelectric points27,28 and binding affinity to vitamin D3.29 Cohort and genome-wide association studies have revealed that GC polymorphisms are strong determinants of circulating vitamin D3 levels, which, however, may depend on ethnic background.21–26 Thus, the GC2 variant was associated with lower vitamin D3 levels in healthy young Canadian adults of East Asian and European ancestry, but not of South Asian ancestry.21 In this study, we found that the plasma vitamin D3 level and distribution of vitamin D deficiency were associated with GC polymorphisms in Korean patients with COPD.
The major function of VDBP is binding, solubilization, and transportation of vitamin D and its metabolites.27 Unlike other hydrophobic hormone carrier proteins, VDBP has high plasma concentration relative to its major ligand vitamin D3. Only less than 5% of VDBP binding sites are occupied by vitamin D sterols.30 Besides providing vitamin D bioavailability, VDBP may play a role in innate immunity (neutrophil chemotaxis and macrophage activation). Serum VDBP levels were lower in patients who developed organ dysfunction and sepsis after traumatic injury, and depended on the degree of organ dysfunction, respiratory failure, hematologic failure, and sepsis. Thus, low VDBP levels could be a significant predictor of mortality after injury.31 On the other hand, high levels of serum VDBP were associated with decreased lung function, expressed in reduced FEV1; also, high levels of airway VDBP correlated with macrophage activation.32
The structure of each GC polymorphism differs in the sugar chain.33 The oligosaccharide structure and its neighboring structure have been reported to be related with different induction of macrophage activating signal.10,33 Given the different immunomodulatory roles of VDBP according to GC polymorphism, we investigated the relationship of GC polymorphisms with COPD clinical characteristics, including vitamin D status as a covariate in this study. The results show that FEV1/FVC, 6MW distance, and emphysema index were associated with certain types of VDBP polymorphism. Although often considered as a single disease, emphysema and COPD overlap less than previously thought, and emphysema on CT shows only moderate correlation with lung function.34,35 In a previous study, we could not demonstrate the relationship between plasma vitamin D3 level and emphysema severity.6 Here, we evaluated the combined effects of GC polymorphisms and vitamin D3 level on emphysema in patients with COPD, and found that the GC1S variant and genotype 1F-1S were associated with higher emphysema index regardless of vitamin D deficiency. In contrast, genotype 1S-1S correlated with lower emphysema index, although the association was inconclusive because of a small number of genotype 1S-1S patients (N=8). In Japan, patients carrying the GC1F variant have been observed to have higher frequency of the emphysema index over 60.18 Moreover, a recent genome-wide study performed on a large cohort in the USA has revealed that certain genes previously identified as being related to pulmonary function have been found to be independently associated with emphysema rather than with pulmonary function.34
To the best of our knowledge, this is the first study to determine the combined relationship of COPD with GC polymorphisms and vitamin D deficiency in Korean population; however, it had some limitations. First, the sample size was small, especially for genotype 1S-1S; the results should be interpreted with caution. Given the diversity of GC polymorphisms, a larger number of participants are required to evaluate the role of each single GC polymorphism in COPD. Second, blood levels of VDBP, which is the key molecule to clarify the relationship between GC polymorphisms and plasma vitamin D3 level, were not measured. Finally, the information on supplementary vitamin D intake was not available. Dietary vitamin D can influence the blood level of vitamin D3; so it can affect the analysis of the association between GC polymorphism and vitamin D3 level in blood.
Conclusion
This study suggests that GC polymorphisms are associated with vitamin D deficiency in Korean patients with COPD. The GC2 variant was identified as a potential risk factor for vitamin D deficiency, while genotype 1F-1S was determined as a protective factor. Vitamin D deficiency and GC polymorphisms were associated with airway obstruction and exercise capacity, and GC polymorphisms correlated with emphysema severity. The relationship between GC polymorphisms and vitamin D deficiency as well as functional differences among GC polymorphisms need to be investigated in studies on larger patient cohorts with different ethnic backgrounds.
Acknowledgments
This research was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare (HI10C2020 and A102065) and Handok Inc. (4-2013-0645).
All the members of the KOLD Study Group contributed to the recruitment of patients with COPD and collection of data and samples: Ji-Hyun Lee, Eun Kyung Kim, Tae-Hyung Kim, Tae Rim Shin, Kwang Ha Yoo, Seung Soo Sheen, Jin Hwa Lee, Seong Yong Lim, Sang Yeub Lee, Ho Il Yoon, Yong Bum Park, Yong Il Hwang, Young Sam Kim, Ji Ye Jung, Yoonki Hong, Seung Won Ra, Joon Beom Seo, Sang Min Lee, Sei Won Lee, Jae Seung Lee, Jin Won Huh, Ji Yong Moon, Hye Kyeong Park, Hye Yun Park, Jin Woo Kim, Chin Kook Rhee, Hyoung Kyu Yoon, Woo Jin Kiim, Yeon-Mok Oh, and Sang-Do Lee.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2014;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. New Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Bouillon R, Bischoff-Ferrari H, Willett W. Vitamin D and health: perspectives from mice and man. J Bone Miner Res. 2008;23(7):974–979. doi: 10.1359/jbmr.080420. [DOI] [PubMed] [Google Scholar]
- 4.Persson LJ, Aanerud M, Hiemstra PS, Hardie JA, Bakke PS, Eagan TM. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS One. 2012;7(6):e38934. doi: 10.1371/journal.pone.0038934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmgaard DB, Mygind LH, Titlestad IL, et al. Serum vitamin D in patients with chronic obstructive lung disease does not correlate with mortality – results from a 10-year prospective cohort study. PLoS One. 2013;8(1):e53670. doi: 10.1371/journal.pone.0053670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung JY, Kim YS, Kim SK, et al. Relationship of vitamin D status with lung function and exercise capacity in COPD. Respirology. 2015;20(5):782–789. doi: 10.1111/resp.12538. [DOI] [PubMed] [Google Scholar]
- 7.Kunisaki KM, Niewoehner DE, Connett JE. Vitamin D levels and risk of acute exacerbations of chronic obstructive pulmonary disease: a prospective cohort study. Am J Respir Crit Care Med. 2012;185(3):286–290. doi: 10.1164/rccm.201109-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moberg M, Ringbaek T, Roberts NB, Vestbo J. Association between vitamin D status and COPD phenotypes. Lung. 2014;192(4):493–497. doi: 10.1007/s00408-014-9582-9. [DOI] [PubMed] [Google Scholar]
- 9.Hirschfeld J. Immune-electrophoretic demonstration of qualitative differences in human sera and their relation to the haptoglobins. Acta Pathol Microbiol Scand. 1959;47:160–168. doi: 10.1111/j.1699-0463.1959.tb04844.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto N, Homma S. Vitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci U S A. 1991;88(19):8539–8543. doi: 10.1073/pnas.88.19.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piquette CA, Robinson-Hill R, Webster RO. Human monocyte chemotaxis to complement-derived chemotaxins is enhanced by Gc-globulin. J Leukoc Biol. 1994;55(3):349–354. doi: 10.1002/jlb.55.3.349. [DOI] [PubMed] [Google Scholar]
- 12.Kew RR, Sibug MA, Liuzzo JP, Webster RO. Localization and quantitation of the vitamin D binding protein (Gc-globulin) in human neutrophils. Blood. 1993;82(1):274–283. [PubMed] [Google Scholar]
- 13.Binder R, Kress A, Kan G, Herrmann K, Kirschfink M. Neutrophil priming by cytokines and vitamin D binding protein (Gc-globulin): impact on C5a-mediated chemotaxis, degranulation and respiratory burst. Mol Immunol. 1999;36(13–14):885–892. doi: 10.1016/s0161-5890(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 14.Cleve H, Constans J. The mutants of the vitamin-D-binding protein: more than 120 variants of the GC/DBP system. Vox Sang. 1988;54(4):215–225. doi: 10.1111/j.1423-0410.1988.tb03908.x. [DOI] [PubMed] [Google Scholar]
- 15.Laufs J, Andrason H, Sigvaldason A, et al. Association of vitamin D binding protein variants with chronic mucus hypersecretion in Iceland. Am J Pharmacogenomics. 2004;4(1):63–68. doi: 10.2165/00129785-200404010-00007. [DOI] [PubMed] [Google Scholar]
- 16.Xie X, Zhang Y, Ke R, et al. Vitamin D-binding protein gene polymorphisms and chronic obstructive pulmonary disease susceptibility: a meta-analysis. Biomed Rep. 2015;3(2):183–188. doi: 10.3892/br.2014.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung JY, Choi DP, Won S, et al. Relationship of vitamin D binding protein polymorphisms and lung function in Korean chronic obstructive pulmonary disease. Yonsei Med J. 2014;55(5):1318–1325. doi: 10.3349/ymj.2014.55.5.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito I, Nagai S, Hoshino Y, et al. Risk and severity of COPD is associated with the group-specific component of serum globulin 1F allele. Chest. 2004;125(1):63–70. doi: 10.1378/chest.125.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YK, Oh YM, Lee JH, et al. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung. 2008;186(3):157–165. doi: 10.1007/s00408-008-9071-0. [DOI] [PubMed] [Google Scholar]
- 21.Engelman CD, Meyers KJ, Ziegler JT, et al. Genome-wide association study of vitamin D concentrations in Hispanic Americans: the IRAS family study. J Steroid Biochem Mol Biol. 2010;122(4):186–192. doi: 10.1016/j.jsbmb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93(9):3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perna L, Felix JF, Breitling LP, et al. Genetic variations in the vitamin D binding protein and season-specific levels of vitamin D among older adults. Epidemiology. 2013;24(1):104–109. doi: 10.1097/EDE.0b013e318276c4b0. [DOI] [PubMed] [Google Scholar]
- 26.Gozdzik A, Zhu J, Wong BY, Fu L, Cole DE, Parra EJ. Association of vitamin D binding protein (VDBP) polymorphisms and serum 25(OH)D concentrations in a sample of young Canadian adults of different ancestry. J Steroid Biochem Mol Biol. 2011;127(3–5):405–412. doi: 10.1016/j.jsbmb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006;372(1–2):33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Braun A, Bichlmaier R, Cleve H. Molecular analysis of the gene for the human vitamin-D-binding protein (group-specific component): allelic differences of the common genetic GC types. Human Genet. 1992;89(4):401–406. doi: 10.1007/BF00194311. [DOI] [PubMed] [Google Scholar]
- 29.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Human Genet. 1993;92(2):183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 30.Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin) Endocr Rev. 1989;10(3):294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- 31.Dahl B, Schiodt FV, Ott P, et al. Plasma concentration of Gc-globulin is associated with organ dysfunction and sepsis after injury. Crit Care Med. 2003;31(1):152–156. doi: 10.1097/00003246-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Wood AM, Bassford C, Webster D, et al. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax. 2011;66(3):205–210. doi: 10.1136/thx.2010.140921. [DOI] [PubMed] [Google Scholar]
- 33.Ohkura K, Nagasawa H, Uto Y, Okamura N, Murakami A, Hori H. The role of Gc protein oligosaccharide structure as a risk factor for COPD. Anticancer Res. 2006;26(6A):4073–4078. [PubMed] [Google Scholar]
- 34.Manichaikul A, Hoffman EA, Smolonska J, et al. Genome-wide study of percent emphysema on computed tomography in the general population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014;189(4):408–418. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gietema HA, Zanen P, Schilham A, et al. Distribution of emphysema in heavy smokers: impact on pulmonary function. Respir Med. 2010;104(1):76–82. doi: 10.1016/j.rmed.2009.08.004. [DOI] [PubMed] [Google Scholar]