Abstract
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder characterized by deposits of aggregated amyloid-β (Aβ) peptide and neurofibrillary tangles in the brain parenchyma. Despite considerable research to elucidate the pathological mechanisms and identify therapeutic strategies for AD, effective treatments are still lacking. In the present study, we found that salidroside (Sal), a phenylpropanoid glycoside isolated from Rhodiola rosea L., can protect against Aβ-induced neurotoxicity in four transgenic Drosophila AD models. Both longevity and locomotor activity were improved in Sal-fed Drosophila. Sal also decreased Aβ levels and Aβ deposition in brain and ameliorated toxicity in Aβ-treated primary neuronal culture. The neuroprotective effect of Sal was associated with upregulated phosphatidylinositide 3-kinase (PI3K)/Akt signaling. Our findings identify a compound that may possess potential therapeutic benefits for AD and other forms of neurodegeneration.
Keywords: Alzheimer’s disease, amyloid-β, salidroside, Drosophila, neuroprotective effect
Introduction
Alzheimer’s disease (AD) is a progressive and fatal brain disorder that affects ~36 million people worldwide, a number that is expected to double over the next 20 years.1 Neuropathologically, it is characterized by the accumulation of extracellular senile plaques and intracellular neurofibrillary tangles, ultimately leading to neuron loss and brain atrophy.2 Amyloid-β (Aβ) is generated through a successive cleavage of the amyloid precursor protein (APP) by beta-secretase 1 (BACE-1) and gamma-secretase in brain tissues; according to the amyloid hypothesis of AD, Aβ overproduction occurs when the homeostatic processes that govern these processes are disrupted.3 The Aβ peptide can form insoluble oligomers and protofibrils, which are deposited into senile and neuritic plaques. These processes activate neurotoxic cascades that ultimately lead to neuronal dysfunction and cellular death.3,4 Thus, an important strategy for AD is to prevent the accumulation of these toxic Aβ assemblies. Although extensive research on AD onset and progression has been performed, there are currently no effective treatments to prevent or ameliorate this debilitating neurodegenerative disease.
Drug treatments for AD are primarily designed to slow down cognitive decline and ameliorate behavioral symptoms, but these drugs provide limited therapeutic effects. Traditional Chinese medicines are considered new and promising candidates for the treatment of AD. Many novel compounds isolated from Chinese herbal medicines, some of which improve dementia with fewer side effects, may be useful for the prevention and treatment of AD.5 Salidroside (Sal) is an active ingredient extracted from the root of Rhodiola rosea L., which is extensively used in traditional folk medicine in Asian and European countries and has been reported to exhibit various strong pharmacological activities.6 Its main effects are described as antioxidative, antiapoptosis, antitumor, antifatigue, antidepressive, adaptogenic, cardioprotective, and hepatoprotective.6 Sal also exerts neuroprotective effects in the setting of brain injury; it protects neurons from glutamate-induced oxidative stress and apoptosis.7,8 Although Sal has been shown to be therapeutically effective against cognitive decline during aging,9 it remains unknown whether Sal is able to antagonize Aβ-induced toxicity in AD.
In the present study, we investigated the therapeutic potential of Sal in transgenic Drosophila AD models and further explored the underlying mechanisms of Aβ-induced neuronal degeneration. We show that Sal can significantly improve locomotor functions and prolong fly life span. It also protects cultured mouse neurons against Aβ-induced toxicity. Furthermore, we provide evidence that these effects are induced by changes to the phosphatidylinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway.
Materials and methods
Animals and drugs
All Drosophila stocks were raised in 50 mL plastic vials and maintained at 25°C under a 12:12-hour light:dark cycle at constant 65% humidity as previously described.10 We used the pan-neuronal elav-GAL4 to express transgenes.11 The upstream activating sequence (UAS) transgenes used in the experiments were APP/BACE (UAS-APP/BACE), single insert Aβ42 (UAS-Aβ42), double insert Aβ42 (UAS-Aβ42; UAS-Aβ42), and Aβ42 E22G (UAS-Aβ42 E22G), all of which have been described previously.12 Canton-S (CS) flies were used as wild-type controls.
Embryonic day 18 mouse pups for use in primary neuronal culture were taken from pregnant C57BL mice that were purchased directly from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, People’s Republic of China). Animal experiments were performed according to the National Institutes of Health (Bethesda, MD, USA) Guide for the Care and Use of Laboratory Animals and approved by the Shanghai Jiao Tong University School of Medicine Animal Care and Use Committee.
Sal (purity >99.7%) was obtained from the Green Valley Pharmaceutical Corporation (Shanghai, People’s Republic of China). It was dissolved in phosphate-buffered saline (PBS) to a stock concentration of 100 mM and stored at −20°C. Aricept (donepezil hydrochloride) was supplied by Eisai Pharmaceutical Co., Ltd. (Tokyo, Japan).
Longevity assay
Newly emerged flies were collected within 24 hours after eclosion for the experiment. At least 100 flies of each genotype were collected and divided into fresh food vials (20 per vial). Food vials were changed every 2–3 days, and the number of dead flies was counted at that time. Survival curves were analyzed using Kaplan–Meier estimation and log-rank statistical analysis.
Climbing assay
Drosophila locomotor function was measured according to the climbing assay as previously reported.13 Thirty male flies for each group were tested with the same protocol. The experiment was performed three times. The results were calculated using the following formula:
| (1) |
Immunofluorescence confocal microscopy
The heads of 15 flies in each group were randomly selected and dissected in cold PBS. The brains were fixed in 4% paraformaldehyde at room temperature, washed three times with PBS, and then incubated with 0.2% Triton X-100 for 30 minutes. The brains were dissected after 70% formic acid treatment. Brains were incubated with 6E10 (1:200; Covance, Princeton, NJ, USA) at 4°C overnight. These brains were then incubated with the Alexa Fluor 488 secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) at room temperature for 1 hour and analyzed by confocal microscopy (Leica SP5, Wetzlar, Germany). The total numbers of 6E10 punctae in the whole brains were counted and analyzed.
Aβ measurement
The levels of Aβ40 and Aβ42 were measured using commercially available human Aβ enzyme-linked immunosorbent assay kits (ExCell Bio, Shanghai, People’s Republic of China) according to the manufacturer’s protocol. At least 50 fly heads were dissected and immediately homogenized in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 8.0, 0.5% sodium deoxycholate, 1% Triton X-100, and 150 mM NaCl) containing 1% sodium dodecyl sulfate (SDS). The absorbances of the supernatants and Aβ peptide standards were read at 450 nm. The Aβ concentrations were calculated according to the standard curve.
Primary cortical neuron culture and treatment
The cortical neurons of embryonic day 18 C57BL mice were plated as previously described.14 The cultured neurons were transfected with APPsw and tdTomato plasmids or pretreated with lenti-APP at day in vitro 2, and then the neurons were exposed to the test compounds (Sal at 50 μM, 100 μM, or 200 μM; Aricept 10 μM) at day in vitro 4 for 24 hours. The neurons were visualized using a confocal microscope (Leica SP5) or collected for Western blot analyses. Axonal lengths were measured with ImageJ software (National Institutes of Health, Bethesda, MD, USA). In brief, we performed “freehand” measurements to identify the longest axon for each neuron from the soma to terminal. For statistical purposes, at least 30 neurons per culture were quantified.
Western blot analysis
After treatment, primary cortical neurons were washed with PBS and extracted with lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing protease inhibitor cocktail (Hoffman-La Roche Ltd., Basel, Switzerland) and 1 mM phenylmethylsulfonyl fluoride for 30 minutes on ice. The total protein lysates were separated by SDS-polyacrymlamide gel electrophoresis and analyzed by Western blotting with primary antibodies (Akt, phosphorylated Akt [p-Akt], mTOR, phosphorylated mTOR [p-mTOR], ribosomal protein S6 kinase [p70S6K], phosphorylated p70S6K [p-p70S6K; 1:1,000] and β-actin [1:5,000]) from Cell Signaling (Danvers, MA, USA) overnight at 4°C, followed by horseradish peroxidase-conjugated secondary antibody (1:10,000) for 2 hours at room temperature. The results were analyzed by using ImageJ software (National Institutes of Health). The mean density of each band was normalized to the β-actin signal in the same sample and averaged.
Statistical analysis
All statistical analysis was performed using Statistical Package for the Social Sciences software 19.0 (IBM Corporation, Armonk, NY, USA). The Kaplan–Meier test was used to assess life span curve differences. Two-group comparisons were analyzed using Student’s t-tests. A comparison of three or more groups was performed using one-way analysis of variance followed by Tukey’s test. P<0.05 was considered statistically significant.
Results
Sal prolonged the life span of AD transgenic Drosophila
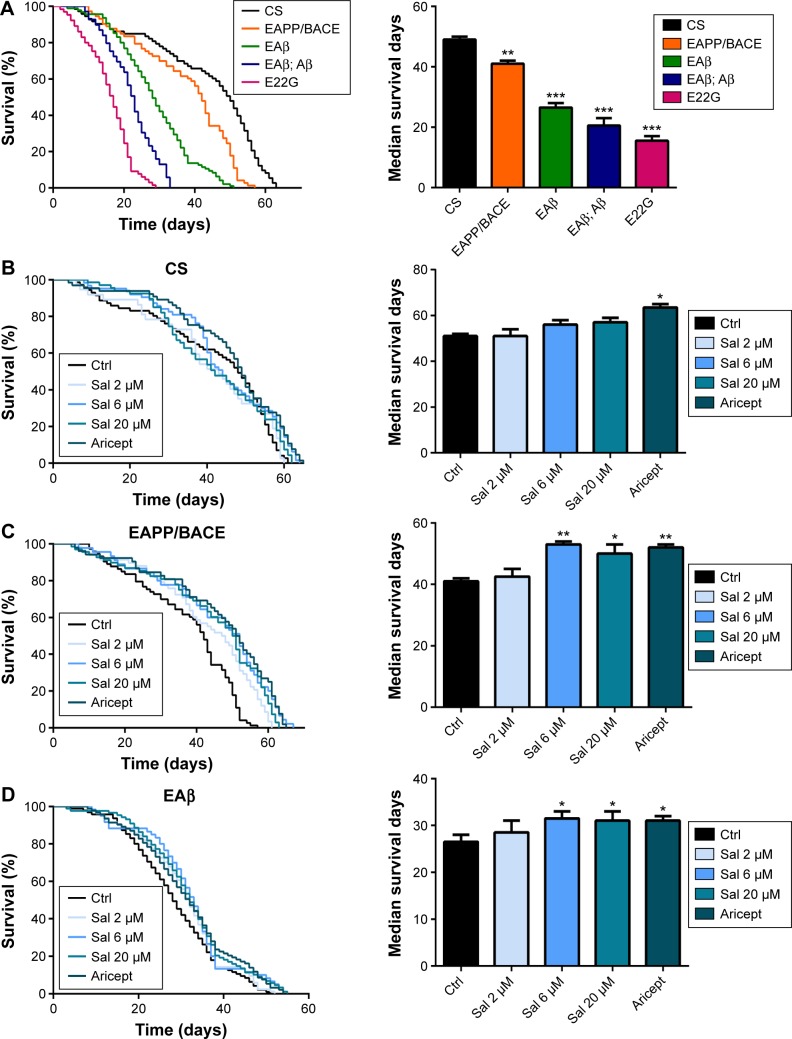
Transgenic Drosophila models expressing human Aβ or APP/BACE exhibit age-dependent AD-like pathology.15,16 These models manifest behavioral abnormalities and significantly reduce longevity,10,12 and have therefore been used to identify novel therapeutic targets.17 We assessed the life span of four AD model fly lines, such as human APP/BACE transgenic line EAPP/BACE, single and double copy Aβ lines (EAβ, EAβ; Aβ), and Aβ mutant line E22G, and observed reduced longevity in all four (Figure 1A). As the lines EAβ; Aβ and line E22G were too weak during their short life (median survival <20 days) to perform the locomotor experiment, transgenic lines EAPP/BACE and EAβ, with relatively long survival times, were utilized to assess the effect of Sal. We first examined the safety of Sal by feeding flies various Sal concentrations (2, 6, and 20 μM) or Aricept, a clinically approved medication for AD treatment. No significant changes were observed in CS flies (Figure 1B). However, in both APP/BACE and EAβ Drosophila lines, Sal treatment significantly prolonged the median survival time in a dose-dependent manner (Figure 1C and D).
Figure 1.
Sal treatment increases the life span of a transgenic AD Drosophila model.
Notes: (A) Survival analysis of AD transgenic flies. (B) Effect of Sal on fly longevity. (C) Sal prolonged APP/BACE fly survival. (D) Sal prolonged EAβ fly survival. Kaplan–Meier cumulative survival analysis. Data are presented as mean ± SEM of three independent experiments. *P<0.05, **P<0.01, and ***P<0.001.
Abbreviations: APP, amyloid precursor protein; BACE, beta-secretase; CS, Canton-S; Ctrl, control; Sal, salidroside; AD, Alzheimer’s disease; SEM, standard error of the mean.
Sal improved locomotor activity in AD transgenic Drosophila
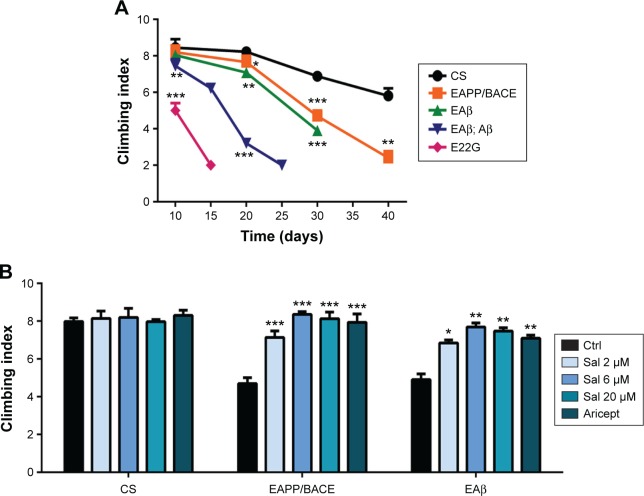
The locomotor assay is used to assess behavioral abnormalities based on negative geotaxis against gravity. We compared the locomotor activity of the four transgenic fly models to detect early Aβ-related neuronal dysfunction. Compared with control CS flies, all APP/BACE and Aβ lines exhibited significant climbing index deficits (Figure 2A). Particularly, the EAPP/BACE line and single copy Aβ42 (EAβ) line exhibited a gradual age-related decline in climbing activity. We examined the effects of Sal and Aricept in APP/BACE and Aβ42 flies and found a dose-dependent improvement in the climbing ability at 30 days comparable to the effects of Aricept (Figure 2B). These results indicate that Sal treatment reverses locomotor defects in AD flies.
Figure 2.
Salidroside increases locomotor activity.
Notes: (A) The climbing abilities of EAPP/BACE, EAβ42, EAβ42; Aβ42, and Aβ42 E22G transgenic flies were assessed after eclosion. Climbing deficits in these fly lines were compared with the control flies (CS). (B) The percentage of flies climbing up 8 cm in 10 seconds increased after salidroside or Aricept treatment in EAPP/BACE and EAβ lines at day 30. Mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001 compared to the control group with one-way ANOVA analysis followed by Tukey’s test.
Abbreviations: APP, amyloid precursor protein; BACE, beta-secretase; CS, Canton-S; ANOVA, analysis of variance; Ctrl, control; Sal, salidroside; Aβ, amyloid-β; SEM, standard error of the mean.
Effects of Sal on Aβ aggregation and neurotoxicity
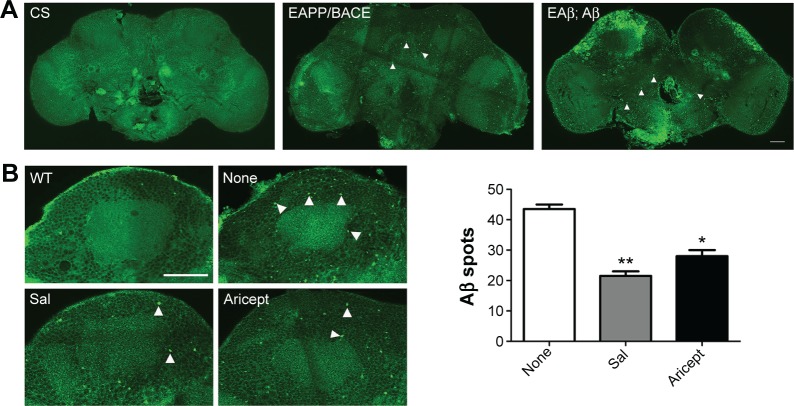
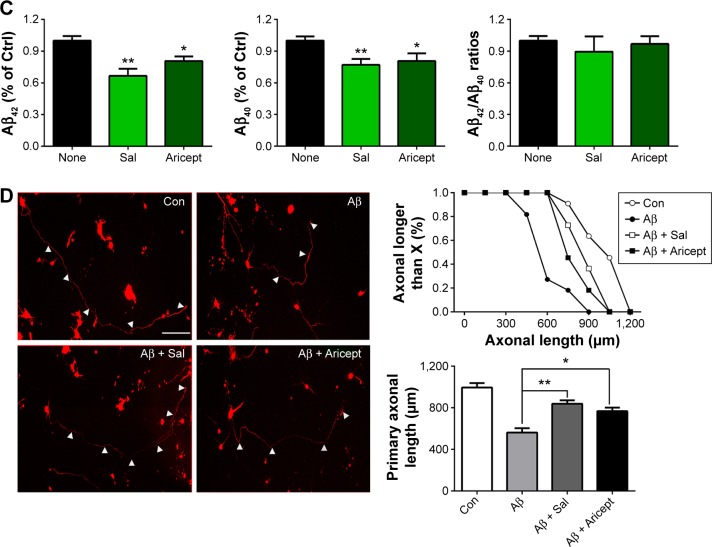
Transgenic Drosophila with nervous system expression of the human Aβ peptide fully mimics human AD pathology.15 For instance, in the EAPP/BACE and EAβ; Aβ Drosophila lines, immunofluorescence labeling with the Aβ-specific antibody revealed Aβ deposition in brains (Figure 3A). We treated the EAPP/BACE Drosophila line with Sal 6 μM or Aricept 30 μM for 30 days and found that both treatments appeared to significantly reduce amyloid plaque loads in the brain (Figure 3B), indicating that Sal treatment alleviated Aβ aggregation. In view of the finding that soluble Aβ oligomers are more neurotoxic than fibrillar Aβ and correlate strongly with AD severity,18 we further quantified soluble Aβ oligomer levels in the brain lysates and observed lower amounts of Aβ40 and Aβ42 after 30 days of either treatment, but the Aβ42/Aβ40 ratios were similar (Figure 3C). These data indicate that Sal treatment reduces Aβ levels and correlates with amyloidosis in EAPP/BACE flies. As Aβ is produced from cleaved APP that is associated with axonal abnormalities in the AD brain,19 we transfected primary cultured neurons with APP plasmids to induce neuronal degeneration and treated with Sal for 24 hours to assess its protective ability. As shown in Figure 3D, APP transfection caused neuronal abnormalies in axonal length that were reversed by Sal treatment. In these assays, >70% Sal-treated neurites were longer than 750 μM, whereas only 20% of neurites from the Aβ group were longer than 750 μM. These results indicate that Sal protects against Aβ-induced neurotoxicity.
Figure 3.
Effect of Sal on Aβ accumulation in vivo and Aβ induced neurotoxicity in vitro.
Notes: (A) Micrographs of Aβ peptide expression in intact transgenic fly brains. CS, EAPP/BACE and EAβ; Aβ flies showed spot-like staining for Aβ-antibody 6E10 staining. Scale bar in insets represent 50 µm. Arrowheads indicate 6E10-antibody positivity. (B) Immunofluorescence staining shows the level of Aβ deposition in EAPP/BACE flies after Sal or Aricept treatment for 30 days. Arrowheads indicate 6E10-antibody positivity. Scale bar, 50 μm. The number of amyloid plaques was quantified. *P<0.05, **P<0.01. (C) Aβ40 and Aβ42 levels were measured by ELISA at day 30. The values are mean ± SEM. Each value represents the mean of three experiments. *P<0.05 and **P<0.01 versus the control group. (D) Primary cultured neurons were transfected with tdTomato and APP plasmids and treated with Sal for 24 hours. The axonal lengths (arrowheads) were measured and quantified. Scale bar, 100 μm. *P<0.05 and **P<0.01 by one-way ANOVA followed by Tukey’s test.
Abbreviations: APP, amyloid precursor protein; BACE, beta-secretase; ELISA, enzyme-linked immunosorbent assay; h, hours; ANOVA, analysis of variance; CS, Canton-S; WT, wild type; Sal, salidroside; Ctrl, control; Aβ, amyloid-β; SEM, standard error of the mean; Con, normal control.
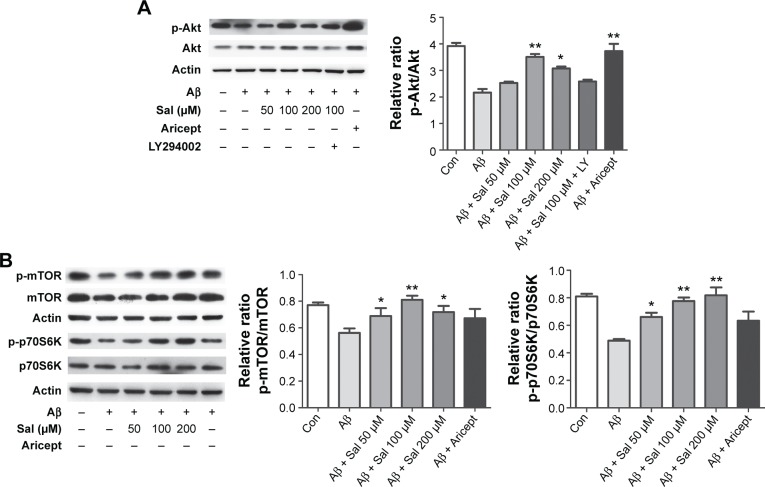
Effects of Sal on PI3K/Akt/mTOR signaling
To explore the molecular mechanism underlying Sal-induced neuroprotection, we focused on the PI3K/Akt signaling pathway that plays a crucial role in promoting neuronal survival under a variety of circumstances.20 We measured Akt protein level in primary cultured cortical neurons after treating with Sal (50, 100, or 200 μM) and Aricept (10 μM) for 24 hours. We found that APP-transfected neurons exhibited decreased p-Akt, and Sal treatment increased the level in a dose-dependent manner that is comparable to the effect of Aricept (Figure 4A). This increase was blocked by the specific PI3K inhibitor LY294002 (Figure 4A), indicating that Sal’s neuroprotective effects are at least partially mediated by PI3K/Akt signaling. As mTOR and p70S6K are downstream targets of p-Akt, we asked whether Sal altered mTOR and p70S6K activities during Aβ exposure. We found that APP-transfected neurons had decreased levels of either p-mTOR or p-p70S6K, and Sal treatment effectively restored both proteins (*P<0.05, **P<0.01; Figure 4B). Taken together, our results indicate that Sal inhibits Aβ-induced neurotoxicity by regulating PI3K/Akt signaling.
Figure 4.
Sal inhibits Aβ-induced neurotoxicity by activating the Akt/mTOR/p70S6K pathway in primary cultured cortical neurons.
Notes: (A) APP overexpressed primary cultured cortical neurons were treated with Sal or Aricept for 24 hours. Levels of Akt and phosphorylated Akt were detected and compared with the Aβ treatment group. (B) The expression levels of mTOR, p70S6K, and their phosphorylated forms were detected. All the asterisks indicate quantitatively significant differences compared with the Aβ group. All data are presented as mean ± SEM. *P<0.05 and **P<0.01 (one-way ANOVA and Tukey’s test).
Abbreviations: Sal, salidroside; mTOR, mammalian target of rapamycin; p70S6K, ribosomal protein S6 kinase; APP, amyloid precursor protein; ANOVA, analysis of variance; Aβ, amyloid-β; SEM, standard error of the mean; Con, normal control.
Discussion
AD pathogenesis is a complex process, and effective treatments remain elusive. The present study provides the first evidence that Sal treatment prolongs life span and improves locomotion in Drosophila AD models. Sal dramatically ameliorated adverse morphological changes caused by Aβ protein in the transgenic Drosophila brain. As Sal was previously reported to be nontoxic and mitigate neurotoxicity,21 our results further indicate that it is a promising drug candidate for AD. However, long-term studies are needed to assess the possibility of side effects associated with chronic administration in humans.
Recent publications suggest that intraneuronal Aβ accumulation may be an early event in AD pathogenesis, while amyloid plaques accumulate later.22,23 Inhibiting the toxicity of soluble Aβ in the brain is therefore likely to be a useful AD treatment. Our primary neuronal culture results showed that Sal effectively attenuated Aβ-induced cytotoxicity, which is consistent with previous reports about Sal’s effects in Aβ-induced cognitive deficits.24,25 We previously reported that Sal can inhibit hypoxia-induced abnormal APP processing by decreasing BACE1 expression.26 The present data suggest that Sal inhibits Aβ production.
We also provided evidence that Sal exerts its neuroprotective effects by stimulating PI3K/Akt signaling, which plays a pivotal role in neuronal survival.27 Previous studies suggested that PI3K/Akt signaling was downregulated in the AD brain,28 and activation of this pathway has been shown to prevent Aβ-induced neuronal neurotoxicity.29 We found that Sal increased the level of Akt phosphorylation, which was abolished by PI3K inhibitor LY294002. This strongly suggests that the protective effect of Sal is most likely a result of its activation of PI3K/Akt signaling. We further observed that Sal apparently upregulated mTOR signaling in primary cultured neurons. As a main downstream effector of the PI3K/Akt pathway, mTOR plays a central role in cell growth, proliferation, and cell survival. Activation of p70S6k mediated by mTOR promotes mRNA biogenesis, ribosomal protein translation, and cell growth,30 which may directly contribute to Sal’s neuroprotective effects. Importantly, mTOR signaling plays a vital role in memory formation and cognitive function that are relevant to AD.31,32 Our results reveal an intracellular signaling pathway that mediates the protective effects of Sal against Aβ-induced neurotoxicity in the AD brain.
Conclusion
In conclusion, our results demonstrate that treatment with Sal can attenuate behavioral symptoms and pathological progression in the AD models by reducing Aβ amyloidosis and activating PI3K/Akt signaling. This suggests that Sal may protect degenerated neurons and could be used to prevent and treat AD. Although Sal has been prescribed by Chinese medical physicians to patients with cardiovascular disease and was reported to exhibit various pharmacological activities, further studies are necessary to evaluate the efficacy of Sal against AD. Specifically, it is important to investigate its clinical implications and determine if it could also be useful for other neurodegenerative diseases.
Acknowledgments
The authors thank Dr Nan-Jie Xu for helpful comments on the manuscript, Dr Gang Pei for providing transgenic Drosophila, and Ilya Bezprozvanny for providing APPsw and tdTomato plasmids. This work was supported by grants from the National Natural Science Fund of China (81430022, 91332107).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wimo A, Jonsson L, Bond J, Prince M, Winblad B, Alzheimer Disease I. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1–11.e13. doi: 10.1016/j.jalz.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. Alzheimer’s disease. Subcell Biochem. 2012;65:329–352. doi: 10.1007/978-94-007-5416-4_14. [DOI] [PubMed] [Google Scholar]
- 4.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 5.Sun ZK, Yang HQ, Chen SD. Traditional Chinese medicine: a promising candidate for the treatment of Alzheimer’s disease. Transl Neurodegener. 2013;2(1):6. doi: 10.1186/2047-9158-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grech-Baran M, Syklowska-Baranek K, Pietrosiuk A. Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected spp. in vitro cultures. Phytochem Rev. 2015;14(4):657–674. doi: 10.1007/s11101-014-9368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao L, Li H, Zhang J, et al. Salidroside protects Caenorhabditis elegans neurons from polyglutamine-mediated toxicity by reducing oxidative stress. Molecules. 2014;19(6):7757–7769. doi: 10.3390/molecules19067757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xian H, Zhao J, Zheng Y, et al. MADP, a salidroside analog, protects hippocampal neurons from glutamate induced apoptosis. Life Sci. 2014;103(1):34–40. doi: 10.1016/j.lfs.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 9.Jin H, Pei L, Shu X, et al. Therapeutic intervention of learning and memory decays by salidroside stimulation of neurogenesis in aging. Mol Neurobiol. 2016;53(2):851–866. doi: 10.1007/s12035-014-9045-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Wang Y, Sui Y, et al. The combination of aricept with a traditional Chinese medicine formula, smart soup, may be a novel way to treat Alzheimer’s disease. J Alzheimer Dis. 2015;45(4):1185–1195. doi: 10.3233/JAD-143183. [DOI] [PubMed] [Google Scholar]
- 11.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 12.Caesar I, Jonson M, Nilsson KP, Thor S, Hammarstrom P. Curcumin promotes A-beta fibrillation and reduces neurotoxicity in transgenic Drosophila. PLoS One. 2012;7(2):e31424. doi: 10.1371/journal.pone.0031424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee FK, Wong AK, Lee YW, Wan OW, Chan HY, Chung KK. The role of ubiquitin linkages on alpha-synuclein induced-toxicity in a Drosophila model of Parkinson’s disease. J Neurochem. 2009;110(1):208–219. doi: 10.1111/j.1471-4159.2009.06124.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Liu J, Chen S, et al. DJ-1 modulates the expression of Cu/Zn-superoxide dismutase-1 through the Erk1/2-Elk1 pathway in neuroprotection. Ann Neurol. 2011;70(4):591–599. doi: 10.1002/ana.22514. [DOI] [PubMed] [Google Scholar]
- 15.Greeve I, Kretzschmar D, Tschape JA, et al. Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J Neurosci. 2004;24(16):3899–3906. doi: 10.1523/JNEUROSCI.0283-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao XL, Wang WA, Tan JX, et al. Expression of beta-amyloid induced age-dependent presynaptic and axonal changes in Drosophila. J Neurosci. 2010;30(4):1512–1522. doi: 10.1523/JNEUROSCI.3699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Dam D, De Deyn PP. Animal models in the drug discovery pipeline for Alzheimer’s disease. Br J Pharmacol. 2011;164(4):1285–1300. doi: 10.1111/j.1476-5381.2011.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viola KL, Klein WL. Amyloid beta oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015;129(2):183–206. doi: 10.1007/s00401-015-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen DZ, Huettenrauch M, Mitkovski M, Pradier L, Wirths O. Axonal degeneration in an Alzheimer mouse model is PS1 gene dose dependent and linked to intraneuronal Abeta accumulation. Front Aging Neurosci. 2014;6:139. doi: 10.3389/fnagi.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11(3):297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 21.Gao J, He H, Jiang W, et al. Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer’s disease. Behav Brain Res. 2015;293:27–33. doi: 10.1016/j.bbr.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 22.Stefanova NA, Muraleva NA, Korbolina EE, Kiseleva E, Maksimova KY, Kolosova NG. Amyloid accumulation is a late event in sporadic Alzheimer’s disease-like pathology in nontransgenic rats. Oncotarget. 2015;6(3):1396–1413. doi: 10.18632/oncotarget.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ripoli C, Cocco S, Li Puma DD, et al. Intracellular accumulation of amyloid-beta (Abeta) protein plays a major role in Abeta-induced alterations of glutamatergic synaptic transmission and plasticity. J Neurosci. 2014;34(38):12893–12903. doi: 10.1523/JNEUROSCI.1201-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Zhen YF, Pu Bu Ci R, et al. Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav Brain Res. 2013;244:70–81. doi: 10.1016/j.bbr.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Yu H, Zhao X, et al. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem Int. 2010;57(5):547–555. doi: 10.1016/j.neuint.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Li QY, Wang HM, Wang ZQ, Ma JF, Ding JQ, Chen SD. Salidroside attenuates hypoxia-induced abnormal processing of amyloid precursor protein by decreasing BACE1 expression in SH-SY5Y cells. Neurosci Lett. 2010;481(3):154–158. doi: 10.1016/j.neulet.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Yang X, Lei Q, et al. PEG-PEI/siROCK2 protects against Abeta42-induced neurotoxicity in primary neuron cells for Alzheimer disease. Cell Mol Neurobiol. 2015;35(6):841–848. doi: 10.1007/s10571-015-0178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol. 2011;225(1):54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiwari SK, Seth B, Agarwal S, et al. Ethosuximide induces hippocampal neurogenesis and reverses cognitive deficits in amyloid-beta toxin induced Alzheimer’s rat model via PI3K/Akt/Wnt/beta-catenin pathway. J Biol Chem. 2015;290(47):28540–28558. doi: 10.1074/jbc.M115.652586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25(4):209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 31.Ma T, Hoeffer CA, Capetillo-Zarate E, et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(9):e12845. doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99(2):128–148. doi: 10.1016/j.pneurobio.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]