Abstract
From early childhood to adulthood, synaptogenesis and synaptic pruning continuously reshape the structural architecture and neural connection in developmental human brains. Disturbance of the precisely balanced strengthening of certain axons and pruning of others may cause mental disorders such as autism and schizophrenia. To characterize this balance, we proposed a novel measurement based on cortical parcellation and diffusion MRI (dMRI) tractography, a cortical connectivity maturation index (CCMI). To evaluate the spatiotemporal sensitivity of CCMI as a potential biomarker, dMRI and T1 weighted datasets of 21 healthy subjects 2–25 years were acquired. Brain cortex was parcellated into 68 gyral labels using T1 weighted images, then transformed into dMRI space to serve as the seed region of interest for dMRI-based tractography. Cortico-cortical association fibers initiated from each gyrus were categorized into long- and short-range ones, based on the other end of fiber terminating in non-adjacent or adjacent gyri of the seed gyrus, respectively. The regional CCMI was defined as the ratio between number of short-range association tracts and that of all association tracts traced from one of 68 parcellated gyri. The developmental trajectory of the whole brain CCMI follows a quadratic model with initial decreases from 2 to 16 years followed by later increases after 16 years. Regional CCMI is heterogeneous among different cortical gyri with CCMI dropping to the lowest value earlier in primary somatosensory cortex and visual cortex while later in the prefrontal cortex. The proposed CCMI may serve as sensitive biomarker for brain development under normal or pathological conditions.
Keywords: maturation index, short-range association tracts, cortical connectivity, development, diffusion MRI
1. INTRODUCTION
From early childhood to adulthood, the structural architecture, neural connection and functional organization of the human brain are continuously shaped by a sequence of events, including synaptogenesis e.g. 1, 2, dendritic arborization, myelination3 and synaptic pruning4, 5. The dynamic balance of both strengthening (including myelination) of some fibers and pruning of others plays a key role in brain development. Disturbances of this balance is thought to occur in pediatric mental disorders such as autism spectrum disorder (ASD) and schizophrenia e.g. 6–8, which are two common developmental brain disorders associated with abnormal synaptic pruning. Literature has reported that the brains of individuals with ASD are characterized by hyperconnectivity in local circuits and hyperconnectivity between brain regions e.g. 9, 10, while excess synaptic pruning and functional hypoactivity were found in the brains of individuals with schizophrenia e.g. 11, 12.
Diffusion magnetic resonance imaging (dMRI), a type of MRI technique, is capable of delineating in vivo microstructural changes of white matter (WM) tracts noninvasively by measuring the water diffusion in these tracts13. With dMRI-based tractography e.g. 14, 15, WM tracts can be traced to infer structural connectivity in the human brain. Numerous studies have been conducted previously to characterize developmental human brain connectivity e.g.16–18 and network e.g. 19–22 based on dMRI tractography.
Recent studies on structural network of developing brains e.g. 19–22 suggest that emergence of the maturing brain networks are associated with both enhancement of some WM tracts and synaptic pruning of other tracts. In addition, network-based measurements representing local connectivity efficiencies were found to decrease significantly possibly due to synaptic pruning19. However, the developmental brain connectome is incomplete without an understanding of short-range association tracts. Further insights into the underlying mechanism of growing human brain networks requires information on not only microstructural enhancement of long-range association tracts, but also pruning of short-range association tracts. Delineation and quantification of short-range association tracts, combined with the existing knowledge of long-range association tracts, will provide a more complete characterization of the structural basis of observed mesoscale network changes during development. Direct measurement of the elimination of short fibers holds the key to filling an existing knowledge gap of developmental brain connectome under normal and pathological circumstances.
In this study, we defined and measured a cortical connectivity maturation index (CCMI) to characterize the balance of both strengthening of some fibers and elimination of others in the developmental human brain. Both regional and global CCMI was established with quantification of short-range association tracts reconstructed with dMRI-based tractography. dMRI and T1 weighted datasets of 21 healthy subjects 2–25 years were acquired to evaluate the spatiotemporal sensitivity of CCMI.
2. METHODS AND MATERIALS
2.1 Participants
A total of 21 healthy children, adolescents and young adults between the ages of 2 and 25 years (16 Male and 5 Female; mean age = 13.03 years, standard deviation = 8.32 years) were recruited and scanned at the Beijing Children’s Hospital of Capital Medical University in Beijing, China. The study was approved by the Institutional Review Board (IRB) at Beijing Children’s Hospital. Subjects or their guardians (if subjects are under 18 years old) gave written informed consents for all study procedures. All participants were medically healthy and had no known neurological or psychiatric disorders. They were not under any intervention or medication known to affect the central nervous system.
2.2 Acquisition of dMRI and T1 weighted image
All MR scans were performed on a Philips 3T Achieva Magnetic Resonance System (Philips Healthcare, Best, The Netherlands). dMRI were acquired using single-shot, echo-planar imaging (EPI) sequence with Sensitivity Encoding parallel imaging scheme (SENSE, reduction factor = 2.3). Diffusion parameters were as follows: TR/TE=7960/83ms, field of view (FOV) = 224×224mm2, in-plane imaging resolution= 2×2mm2, axial slice thickness=2mm, slice number=65 covering the entire brain without a slice gap, 30 independent diffusion-weighted directions23, uniformly distributed in space, with b-value of 0 and 1000 sec/mm2. T1 weighted images were acquired using magnetization-prepared rapid gradient-echo (MPRAGE) sequence with FOV = 200×180mm2, in-plane imaging resolution= 1×1mm2, slice thickness=1mm, slice number=160 covering the entire brain. The T1 weighted images provided superior gray and white matter contrast and were used for parcellation of the cerebral cortex. dMRI and T1 weighted images were acquired within the same session.
2.3 Fiber tracing from a parcellated cortical gyrus
The pipeline for WM fiber tracing from a certain cortical gyrus (inferior parietal gyrus or IPG was used as an example), namely cortical parcellation determining the tractography seed region of interest (ROI), is demonstrated in Figure 1a–1h. Based on the T1 weighted image of each subject (Figure 1a) the brain cortical surface of each hemisphere was rendered and parcellated into 34 gyral labels24 (Figure 1b) using FreeSurfer (http://surfer.nmr.mgh.harvard.edu, Version 5.0.1), a semi-automated software suit. Fiber assignment of continuous tractography (FACT)15 was used to trace the whole brain fibers (Figure 1f) for all subjects in Diffusion Toolkit (http://www.trackvis.org/dtk/). After tensor fitting, fractional anisotropy (FA) threshold of 0.2 and a principal eigenvector turning angle threshold of 50 degrees were used for FACT tractography. Linear affine transformation was applied to reorient and transform the parcellated cortical labels into dMRI space with skull-stripped b0 image (Figure 1e) and skull-stripped T1 weighted images as the transformation target and subject, respectively with Diffeomap (http://www.mristudio.org). The same linear transformation re-sliced the gyral labeled image using nearest neighbor interpolation. Due to the dense WM zones just beneath the infragranular layers of the cortex impeding tracking25, the parcellated cortical ribbon in dMRI space (Figure 1c) was then dilated by 8mm (dilated IPG shown in green overlaid on skull-stripped b0 image in Figure 1g) using a custom program written in IDL (Interactive Data Language 8.2.3, http://www.exelisvis.com) to get through the dense WM zone for initiating fiber tracking. The depth of dilation has been evaluated in our previous study26. Using the whole brain dilated cortical ribbon as a binary mask, cortico-cortical association fibers with both terminations within the mask were retained (cortex-spinal cord, cortex-brainstem, and cortex-thalamus fibers were excluded in this study). Cortico-cortical association fibers initiated from IPG as a seed ROI are shown in Figure 1h.
Figure 1.
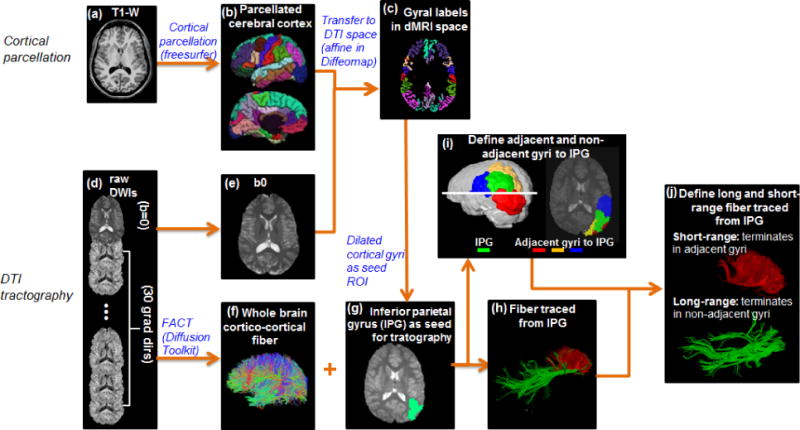
The schematic pipeline of the cortical parcellation (a–c), fiber tracing (d–h) and categorization of long- and short-range fibers (i–j) from a certain cortical gyrus with T1-weighted (a) and dMRI data (d).
2.4 Categorization of long- and short-range fibers based on termination location of the other end of fibers
For all 68 pacellated cortical gyrus, the adjacent and non-adjacent gyral labels to each cortical gyral label were identified. Using IPG (shown in green in both 3D reconstructed brain and 2D slice) in Figure 1i as an example, its adjacent gyri are superior parietal gyrus(yellow), lateral occipital gyrus(red) and supra marginal gyrus(blue) and all other gyri are non-adjacent gyri to IPG. Then cortico-cortical association fibers initiated from IPG can be categorized into short- and long-range association fibers based on the other end of fibers terminating in adjacent gyri and non-adjacent gyri to IPG respectively (Figure 1j). Regional CCMI of IPG was calculated as the ratio between the number of short-range association fibers and the total number of association fibers initiated from IPG (including both short- and long-range association fibers). Global CCMI was calculated as the ratio between the total number of short-range association fibers and that of whole brain association fibers from all 68 gyral labels.
2.5 Global and regional CCMI developmental trajectory analysis
To explore the developmental change of global or regional CCMI, complex growth models (the quadratic model and the cubic model) were used. The following equation was used for fitting a quadratic or cubic model between y (global or regional CCMI) and age t, y=β0+ β1t+ β2t2+ β3t3ε. Where β0, β1, β2 and β3 were parameters to be estimated and ε was an error term. Note that β3 is for cubic fitting only. For global or regional CCMI, the age at which the lowest value of CCMI reached (the point where decrease gives ways to increase) can be determined from the first order derivative of the fitted developmental curves, which may be potentially useful index of cortical maturation. Hence, lowest CCMI of the whole brain and three representative cortical regions: primary somatosensory cortex (S1), visual cortex (V1) and prefrontal cortex were also calculated. Statistical analysis was computed using R statistical software version 3.0.2 (https://www.r-project.org/).
3. RESULTS
3.1 Long- and short-range association fibers traced from a certain cortical gyrus
Figure 2 shows the categorization and balance of long- and short-range association tracts initiated from a certain cortical gyrus of three representative subjects at 2 years (young child), 12 years (adolescent) and 22 years (adult) of age, using IPG (ROI shown in green and overlaid on the fibers) as an example. It is clear that long-range association tracts traced from IPG are strengthened and denser in older subjects (12 years and 22years) when compared to that from younger subject (2 years), while short-range association tracts look similar.
Figure 2.
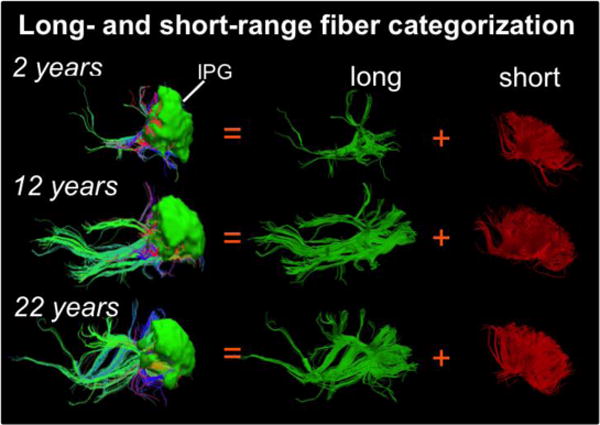
Long-, short-range association fiber categorization from a representative gyrus (inferior parietal gyrus, IPG) from 3 typical developing subjects at 2, 12 and 22 years of age.
3.2 Global and regional CCMI developmental trend lines
Figure 3 demonstrates the developmental curve of whole brain or global CCMI (fitted with quadratic model) and regional CCMI (fitted with cubic model). Global CCMI decreases initially from 2 to around 16 years of age, followed by an increase from 16 to 25 years of age as seen in Figure 3a. In addition, we examined the developmental curves of regional CCMI in three representative functional regions of human brain: S1, V1 and prefrontal cortex (Figure 3b–d). Similar to global CCMI, the developmental curves of regional CCMI from all three functional regions in Figure 3b–d show an initial decrease followed by a later increase. The developmental curves also suggested spatiotemperally heterogeneous CCMI dynamics. Specifically, the CCMI reaches its lowest value at different ages across these functional regions. The prefrontal cortex drops to its lowest CCMI (~17 years) later than S1 (~9 years) and V1 (~14 years), suggesting later maturation of higher-order functional regions than primary sensorimotor regions.
Figure 3.
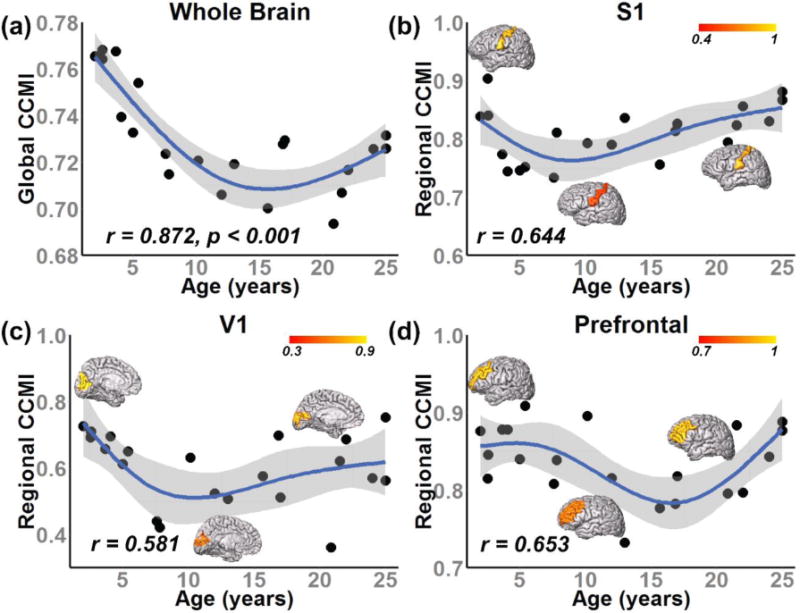
Developmental curve of global CCMI fitted with quadratic model (a) and regional CCMI fitted with cubic model from three representative regions: primary somatosensory cortex (S1) (b), visual cortex (V1) (c) and prefrontal cortex (d), respectively. Each black diamond in the scatter plot represents the CCMI from one subject. Regional CCMI displayed on a three-dimensional reconstructed brain from 3 typical developing subjects at 2, 12 and 22 years of age were shown in panels (b–d).
4. DISCUSSION
A novel maturation index characterizing the balance of long- and short-range association fibers has been proposed based on cortical parcellation from T1 weighted image and dMRI tractography. The present study explored the normative developmental patterns of both global and regional CCMI of human brain across early childhood to young adulthood (from 2 to 25 years). The CCMI values declined first, followed by an increase in both whole brain and regional levels. Heterogeneous developmental curves of regional CCMI were observed across three functional regions, including primary somatosensory cortex, visual cortex and prefrontal cortex (Figure 3b–d), by dropping to their lowest CCMI values at different time points. The spatiotemporal sensitivity of CCMI makes it a potential biomarker to characterize normal brain development and detect neuropathology.
Using histological approach, prior studies have observed synaptic overproduction in infancy, persistence of high levels of synaptic density to adolescence, followed by a decrease after adolescence e.g. 2, 5, 27. In addition, distinct regional variations in the timelines of synaptogenesis and synaptic elimination during human brain development were reported. Specifically, these progressive and regressive events have been found beginning earlier in primary sensorimotor regions and later in the prefrontal cortex e.g. 2, 5, 27. These pioneering neuropathological studies have offered unprecedented information on focal synaptogenesis, however it is extremely difficult to reveal the global pruning pattern of the entire cerebral cortex with histological approach. And these histological approaches are invasive, not practical for clinical diagnosis or evaluation. In the present study, a similar pattern has been observed in our regional CCMI, with somatosensory cortex reach its lowest value earlier than visual cortex and prefrontal cortex (Figure 3), suggesting its relevance with these cellular or molecular processing in developmental human brain. In addition, CCMI can be characterized both regionally and across the entire brain, yielding regional and global measurements. With its noninvasive nature, the CCMI can be readily derived from routine dMRI and T1 weighted images typically acquired in less than 10 minutes, making it a potential biomarker for characterizing normal brain development and detecting abnormalities related to neuropsychiatric disorders.
Several limitations should be noted. First, relatively small sample sizes are reported. Acquisition and analysis of dMRI data from more subjects are under way. Second, all data presented are cross-sectional and further description of developmental trajectories requires a longitudinal dataset. Third, a deterministic approach of dMRI-based tractography was used to trace the whole brain association fibers. It is known the dMRI-based tractography is oversimplified compared to the underlying neuroanatomy. However, as long as it provides reproducible results, it is an important tool to delineate the macroscopic architecture of the human brain WM and to investigate its status under both normal and pathological conditions28.
5. CONCLUSION
The proposed CCMI may reflect the balance between strengthening of certain axons and pruning of others. The CCMI is spatiotemporally heterogeneous, decreasing in early childhood and increasing later with lowest CCMI reached at various ages among cortical gyri. The spatiotemporal properties of CCMI make it a potential biomarker for delineation normal brain development and diagnosis of several mental disorders such as autism spectrum disorder and schizophrenia.
Acknowledgments
This study is funded by NIH MH092535 and U54HD086984.
References
- 1.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9(2):110–22. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 2.Huttenlocher PR, De Courten C, Garey LJ, Van der Loos H. Synaptic development in human cerebral cortex. Int J Neurol. 1982;16–17:144–54. [PubMed] [Google Scholar]
- 3.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. Regional development of the brain in early life. 1967:3–70. [Google Scholar]
- 4.Huttenlocher PR. Dendritic development in neocortex of children with mental defect and infantile spasms. Neurology. 1974;24(3):203–203. doi: 10.1212/wnl.24.3.203. [DOI] [PubMed] [Google Scholar]
- 5.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–27. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 6.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Current opinion in neurobiology. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6(12):955–65. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- 8.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 10.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–11. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull. 2008;34(1):30–6. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex. 2007;17(Suppl 1):i171–81. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- 13.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrens T, Berg HJ, Jbabdi S, Rushworth M, Woolrich M. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–9. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards LJ, Yarowsky P, Donohue P, Graham E, van Zijl PC, Mori S. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33(1):27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31(30):10937–47. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Q, Peng Y, Mishra V, Ouyang A, Li H, Zhang H, Chen M, Liu S, Huang H. Microstructure, length, and connection of limbic tracts in normal human brain development. Front Aging Neurosci. 2014;6:228. doi: 10.3389/fnagi.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Shu N, Mishra V, Jeon T, Chalak L, Wang ZJ, Rollins N, Gong G, Cheng H, Peng Y, Dong Q, He Y. Development of Human Brain Structural Networks Through Infancy and Childhood. Cereb Cortex. 2015;25(5):1389–1404. doi: 10.1093/cercor/bht335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, Meuli R, Thiran JP, Grant PE. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci USA. 2010;107(44):19067–72. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yap PT, Fan Y, Chen Y, Gilmore JH, Lin W, Shen D. Development trends of white matter connectivity in the first years of life. PLoS One. 2011;6(9):e24678. doi: 10.1371/journal.pone.0024678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis EL, Jahanshad N, McMahon KL, de Zubicaray GI, Martin NG, Hickie IB, Toga AW, Wright MJ, Thompson PM. Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults. Neuroimage. 2013;64:671–684. doi: 10.1016/j.neuroimage.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42(3):515–25. [PubMed] [Google Scholar]
- 24.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, Leopold DA, Frank QY. Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc Natl Acad Sci USA. 2015;112(21):E2820–E2828. doi: 10.1073/pnas.1418198112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon T, Mishra V, Ouyang M, Chen M, Huang H. Synchronous changes of cortical thickness and corresponding white matter microstructure during brain development accessed by diffusion MRI tractography from parcellated cortex. Front Neuroanat. 2015;9 doi: 10.3389/fnana.2015.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108(32):13281–6. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Zhang J, Oishi K, Faria AV, Jiang H, Li X, Akhter K, Rosa-Neto P, Pike GB, Evans A, Toga AW, Woods R, Mazziotta JC, Miller MI, van Zijl PC, Mori S. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage. 2010;52(4):1289–301. doi: 10.1016/j.neuroimage.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]


