Abstract
This investigation reports a rapid and simple screening technique for the quantification of titanium and zinc in commercial sunscreens using portable X-ray fluorescence spectroscopy (pXRF). A highly evolved technique, inductively coupled plasma-mass spectroscopy (ICP-MS) was chosen as a comparative technique to pXRF, and a good correlation (r2 > 0.995) with acceptable variations (≤25%) in results between both techniques was observed. Analytical figures of merit such as detection limit, quantitation limit, and linear range of the method are reported for the pXRF technique. This method has a good linearity (r2 > 0.995) for the analysis of titanium (Ti) in the range of 0.4–14.23 wt%, and zinc (Zn) in the range of 1.0–23.90 wt%. However, most commercial sunscreens contain organic ingredients, and these ingredients are known to cause matrix effects. The development of appropriate matrix matched working standards to obtain the calibration curve was found to be a major challenge for the pXRF measurements. In this study, we have overcome the matrix effect by using metal-free commercial sunscreens as a dispersing media for the preparation of working standards. An easy extension of this unique methodology for preparing working standards in different matrices was also reported. This method is simple, rapid, and cost-effective and, in comparison to conventional techniques (e.g., ICP-MS), did not generate toxic wastes during sample analysis.
Keywords: Sunscreens, Metals, Quantitation, Portable x-ray fluorescence spectroscopy analyzer, Inductively coupled plasma-mass spectrometry
1. Introduction
Overexposure to solar radiation can lead to skin problems such as sunburns, premature aging, inflammation of the skin, DNA damage, cellular damage, and increased risk of melanoma [1–5]. From the complete spectrum of solar radiation reaching the Earth’s surface, UV-A (320–400 nm) and UV-B (280–320 nm) radiation is harmful to humans [6,7]. Sunscreens protect from solar radiation based on the UV-filters employed in their preparation. UV-filters are classified into two types: chemical and physical. Chemical UV-filters (organics) are only effective against some parts of the solar spectrum (wavelengths above 320 nm), whereas physical UV-filters (inorganics) are helpful in the protection against both UV-A and UV-B radiation. The usage of physical UV-filters is more pronounced in commercial sunscreens that are marketed to provide complete protection against harmful UV radiation [5–8]. The most prevalent physical UV-filters employed in sunscreens are metal oxides (e.g., TiO2 and ZnO) [9,10]. ZnO can provide efficient protection against UV-A and UV-B radiation, whereas TiO2 is only effective against UV-B. In 2007, the U.S. Food and Drug Administration (FDA) included in the Federal Register that commercial sunscreens must be labeled as containing UV-A and UV-B protection ingredients [11]; however, in 2011, the inclusion of the UV-A and UV-B statements was removed, as it was not directly conclusive to users [11].
In the last few years, the usage of nanoscale metal oxides in sunscreens has increased, as this provides a more esthetic nature to sunscreens upon application onto skin and an increased sun protection action due to the higher surface area attributed to their particle size [9]. Nonetheless, the toxicity of these metal oxides to humans is still not completely established, and there are no regulations on the amounts and sizes of metal oxides that can be employed to formulate sunscreen products. Different researchers have expressed their concern with the toxicity issues associated with metal oxides used in sunscreens as they might generate reactive oxygen radicals when exposed to UV radiation, and these radicals can cause DNA and cell damage [2,5,12].
Currently, different techniques have been employed to detect and characterize metal oxide nanoparticles in sunscreen products; however, none of them reports the quantification of metal oxides on an as-is basis (raw sunscreens without processing). Contado et al. [13] developed an interesting technique by combining the flow field flow fractionation with inductively coupled plasma-atomic emission spectroscopy (ICP-AES) to determine size distribution and quantification of TiO2 in sunscreens. In order to perform these studies, sunscreens were first acid digested using a microwave digestion system. Zachariadis et al. [14] used ICP-AES and atomic absorption (AA) spectrometry to determine Ti contents in sunscreens. Studies were performed on both acid digested and direct slurries of sunscreens, and it was concluded that acid digestion is necessary for the accurate and precise quantitation of metals. Salvador et al. [15] developed methods for quantification of Ti, Zn, and Fe in commercial sunscreens. They utilized ICP-AES and microwave digestion for Ti analysis and flame atomic absorption spectrometry and emulsification for Zn and Fe analysis. Other techniques such as inductively coupled plasma-mass spectrometry (ICP-MS), X-ray photoelectron spectroscopy (XPS), and energy dispersive X-ray spectroscopy (EDS) have been employed as elemental analysis techniques. ICP-MS, ICP-AES, and AA techniques are destructive in nature and requires tedious sample preparation (microwave digestion, drying of samples, filtration, etc.), while spectroscopic techniques (e.g., XPS and EDS) demand high vacuum conditions and can analyze only dry samples.
Since the invention of handheld portable X-ray fluorescence spectroscopy (pXRF) spectrometers, elemental analysis has become much simpler and user-friendly, as it does not demand highly qualified personnel for operation, does not require elaborate sample preparation protocols, and it can be utilized as a field analytical tool. The use of pXRF has been explored rigorously in the mining industry [16,17]. The usage of pXRF in environmental studies and some pharmaceutical applications is also growing due to the minimal sample preparation time and non-destructive nature of the technique [16–21]. pXRF analyzers have also been successfully employed in the detection of toxic elements such as Pb, As, Cd, and Ag in FDA-regulated products [22–24]. With the development of techniques like pXRF spectroscopy which can rapidly detect metals in the parts per million (ppm) ranges without sample preparation, this technique can be used as an approach to saving time and money. A recent study from our group [22] has demonstrated the quantification of Ag in dietary supplements by using pXRF, with limits of quantification as low as 10 ppm. Melquiades et al. [25] developed an analytical method using a pXRF analyzer for detection of Ti in sunscreens which was further used for SPF determination. The final Ti concentration was determined by employing correction factors developed by using a series of equations. Developing pXRF methods for the quantification of metals in commercial products could be beneficial for industries and regulatory agencies as this is a rapid, non-destructive technique which is both cost-effective and time efficient.
Different approaches have been made to quantify metals by using XRF spectroscopy. There are several different factors such as absorption effects [26,27], enhancement effects [26,28], Compton normalization [26], matrix matching [27,28], grain size, and particle size influences [29], involved in the accurate quantification of results. These effects need to be considered in determining metal concentrations by XRF depending on the individual circumstance of the final sample of interest. The determination of absorption coefficients, enhancement coefficients, and interference coefficients is tedious as the calculations vary by sample composition. In this particular study, there is minimal or no Rayleigh scattering [30], the main effects that could contribute to the quantification include the physical matrix effects [31] and Compton effects [26]. To eliminate the physical matrix effects and Compton effects, one can use the matrix matched standards to develop calibration curves that in return can be used to generate the empirical influence coefficients which play a major role in the quantification studies [28]. The calibration studies must be performed in a similar matrix to that of the sample of interest. Using this matrix matching, the absorption or enhancement effects are accounted for in both the standards and samples of interest.
Herein we report the development of a versatile method using a pXRF analyzer which can be used for detection and quantitation of both Ti and Zn concentrations in commercial sunscreens without any sample preparation. The concentration of Ti and Zn obtained using pXRF was confirmed and validated by ICP-MS. ICP-MS samples were prepared by microwave acid digestion prior to analysis. Contents of metal oxides employed in preparation of commercial sunscreens are usually more than ≥0.40 wt% for TiO2 and ≥1.0 wt% for ZnO (FDA allowed concentrations as per 21 CFR 352.10: up to 25% of TiO2 and up to 25% of ZnO). Therefore, there is no need to use a technique like ICP-MS, which can detect elements in parts per billion (ppb) ranges. Our results show that pXRF is a powerful tool for rapid screening and quantification of metal oxides in sunscreen products, and the methodology can be extended to detection of other metals apart from the Ti and Zn in complex matrices.
2. Experimental setup
2.1. Reagents and materials
Ti (1000 and 10,000 mg kg−1), Zn (1000 and 10,000 mg kg−1), and Sc (1000 mg kg−1) single-element ICP-MS standard solutions were acquired from Ultra Scientific (Metuchen, NJ, USA), Ricca Chemical Co. (Arlington, TX, USA), and Spex CertiPrep Group (Metuchen, NJ, USA). TiO2 powder (NIST 1898) was purchased from the National Institute of Standards and Technology (Gaithersburg, MD, USA) and used as a standard reference material. Formulated TiO2 products were obtained from (JO40S4 dispersion, 30% TiO2 by weight) Kobo Products Inc. (South Plainfield, NJ, USA) and (Eusolex T-S, 75.0% Ti by weight) EMD (Philadelphia, PA, USA), and utilized as certified reference materials. ZnO powders (99.999%) were purchased from Sigma Aldrich (St. Louis, MO, USA) and used as a standard reference material. ZnO reference materials with 5.88, 12.36, and 17.59 wt% Zn were purchased from African Mineral Standards (AMIS 0144, AMIS 0145, and AMIS 0152) (Johannesburg, South Africa) and used as certified reference materials. 32 mm XRF sample cups and polypropylene X-ray film (12.0 μm thick) were obtained from Premier Lab Supply (St. Lucie, FL, USA). Nitric acid (HNO3, Optima 67–70%) and hydrofluoric acid (HF, Optima 41–51%) were purchased from Fisher Scientific (Houston, TX, USA). Type I ultra-pure water (18 MΩ · cm) was available through an EMD Millipore water purification system (Model No: Direct-Q 3UV, Billerica, MA, USA).
2.2. Sunscreen products
In our market survey, the majority of the commercially available sunscreens were ZnO-based products, rather than TiO2. The reason is likely due to the broad UV-filtering nature of ZnO (filters both UV-A and UV-B radiation), as compared to TiO2 (filters only UV-A radiation). Many of the manufacturers use a mixture of both metal oxides to address the complete UV-filtration problem. Thirty-eight commercial sunscreen products that claim to contain one element (i.e., Ti or Zn) or both were randomly obtained from various retailers. Each of these products was given a unique identification name such as SUNsnXX. Where SUN-refers to sunscreen, sn-refers to arbitrary serial number (from 01 to 38), XX-refers to sunscreens without any metals, TX-refers to sunscreens containing Ti only, TZ-refers to sunscreens containing both Ti and Zn, and XZ-refers to sunscreens containing Zn only. The total list of the sunscreens along with metal concentration from the label claims is included in Table 1. The sample names are consistent in all of the characterization studies. SUN36XX (matrix A), SUN37XX (matrix B), and SUN38XX (matrix C) are the sunscreens that have been utilized in the preparation of their metal oxides and this value is used to determine the Ti and Zn concentration from label claims of sunscreen products. These Ti and Zn concentrations were further used for comparison against the pXRF and ICP-MS determined values, because these techniques can only detect metals but not their metal oxides.
Table 1.
List of sunscreens investigated along with their metal content determined from their label claims.
| No. | Sample ID | [Ti] wt% | [Zn] wt% |
|---|---|---|---|
| 1 | SUN01XZ | – | 6.43 |
| 2 | SUN02XZ | – | 4.82 |
| 3 | SUN03XZ | – | 6.43 |
| 4 | SUN04TX | 1.20 | – |
| 5 | SUN05TZ | 1.80 | 3.21 |
| 6 | SUN06XZ | – | 16.06 |
| 7 | SUN07XZ | – | 16.06 |
| 8 | SUN08TZ | 0.60 | 1.00 |
| 9 | SUN09XZ | – | 11.24 |
| 10 | SUN10XZ | – | 16.06 |
| 11 | SUN11XZ | – | 16.06 |
| 12 | SUN12XZ | – | 16.06 |
| 13 | SUN13TZ | 4.80 | 3.05 |
| 14 | SUN14TZ | 3.84 | 4.82 |
| 15 | SUN15TX | 1.20 | – |
| 16 | SUN16XZ | – | 14.94 |
| 17 | SUN17XZ | – | 20.08 |
| 18 | SUN18XZ | – | 5.62 |
| 19 | SUN19XZ | – | 18.07 |
| 20 | SUN20XZ | – | 7.31 |
| 21 | SUN21XZ | – | 20.08 |
| 22 | SUN22XZ | – | 5.54 |
| 23 | SUN23XZ | – | 14.94 |
| 24 | SUN24XZ | – | 9.40 |
| 25 | SUN25XZ | – | 15.06 |
| 26 | SUN26XZ | – | 16.06 |
| 27 | SUN27TZ | 5.40 | 2.41 |
| 28 | SUN28TZ | 3.60 | 4.82 |
| 29 | SUN29XZ | – | 19.84 |
| 30 | SUN30XZ | – | 7.23 |
| 31 | SUN31XZ | – | 12.85 |
| 32 | SUN32XZ | – | 16.06 |
| 33 | SUN33TZ | 4.50 | 4.02 |
| 34 | SUN34XZ | – | 5.54 |
| 35 | SUN35TZ | 0.40 | 9.32 |
| 36 | SUN36XX | – | – |
| 37 | SUN37XX | – | – |
| 38 | SUN38XX | – | – |
2.3. Sample preparation and pXRF analysis
2.3.1. Sunscreen samples
A specified amount (~4 g) of commercial sunscreen product was weighed into XRF sample cups using an analytical balance (the amount chosen was enough to cover half of the sample cup). Then, the sample cup was immediately covered with a Mylar film and secured with the snap-on ring supplied along with sample cup. After the XRF sample cup was secured with product, the cup was tapped to ensure uniform spreading of the sunscreens along the cup. Samples were analyzed by placing the film side of sample cup on pXRF sample window. An Olympus Innov-X X-5000 pXRF analyzer (Waltham, Massachusetts, USA) equipped with a tantalum (Ta) anode X-ray source was used for analysis. All the samples were analyzed in seven replicates using mining mode with beam energy of 1–35 kV (9 mm spot size and 200 μA current) and measurement time of 120 seconds. The pXRF instrument directly reports the weight percent of metals detected along with the standard deviation for each measurement, and this number was used as is for further comparison against ICP-MS results and reported label claims of sunscreens. Response factor and offset were generated from calibration data, details on how these factors were determined are mentioned below in the XRF working standards analysis section (2.5). Sunscreen samples were tested in user-defined mode settings where the response factor and offset are inputted into the pXRF instrument manually.
2.3.2. XRF working standards for Ti and Zn
More than 70 wt% of the ingredients used in cream type commercial sunscreens are organic materials that serve as a matrix for dispersing the physical UV-filters such as TiO2 and ZnO. All commercial sunscreens used in this study were in cream form; hence, the final appearance of the working standard was similar to a cream and the matrix composition of working standards was similar to commercial sunscreens. The chemical composition of the matrix sunscreens is described in the supporting information (SI-Section 1). Three sunscreens were employed to prepare working standards which did not contain any Ti or Zn in their formula: SUN36XX (matrix A), SUN37XX (matrix B), and SUN38XX (matrix C). To eliminate matrix effects, working standards were matrix matched by dispersing a known amount of standard metal oxide powder in three different matrices: matrix A, B, or C. The final metal concentration in sunscreen must fall within the limits of standard calibration data. The concentration of Ti standards was 0, 2.02, 4.89, 7.39, and 14.24 wt%. To prepare Zn standards, the same sunscreen matrices (matrix A, B, or C) were employed and the final concentration of Zn was 0, 1.94, 3.95, 8.06, 15.94, and 23.90 wt%. Both Ti and Zn standards were tested by pXRF analyzer in mining mode using factory default settings, where the response factor was 1 and offset was 0 for all elements. A standard calibration curve was developed by plotting the nominal concentrations of working standards on X-axis, and pXRF determined values on Y-axis. OriginPro 9.0 software was utilized for performing linear regression data analysis.
2.4. ICP-MS sample preparation
To determine the total Ti and Zn concentration, sunscreen products were first digested in an acid matrix using a CEM (Matthews, NC, USA) MARS-Xpress microwave digester (maximum power: 1600 W). Approximately 100 mg of sunscreen, 3 mL of HNO3, and 1 mL of HF were transferred into a digestion vessel. Samples were then digested at 210 °C for 20 minutes. To determine Zn concentration, 4 mL of HNO3 was used. After microwave digestion, the sample solutions were diluted to 50 mL with ultra-pure water. A second dilution step was performed by diluting 50–100 μL of diluent to 50 mL with ultra-pure water and internal standard (Sc). The samples were analyzed using an Agilent (Santa Clara, CA, USA) 7700X ICP-MS with a PFA inert kit and nebulizer in the case of Ti, whereas for Zn analysis, the inert kit was not necessary. The concentrations of Ti (mass: 47) and Zn (mass: 66) were analyzed in triplicate using He gas mode. Reagent blanks containing 3 mL HNO3 and 1 mL HF for Ti, and 4 mL HNO3 used for Zn analysis, were prepared simultaneously and analyzed along with the samples.
2.5. Statistical analysis
Six independent empirical calibration curves were developed separately for Ti and Zn working standards in different matrices (A, B, or C). The pXRF correction factors are determined by performing linear regression on the raw data. Equation of the linear regression is as follows:
| (1) |
where x is the nominal concentration of working standard and y is the wt% of metal from pXRF analyzer. Slope of the line (m) and value of y-intercept (b) are determined using Origin Pro software and are further used for the calculation of the response factor (1/m) and offset (−b/m). Individual response factors and offsets are determined for Ti and Zn metals and are inputted manually into the pXRF analyzer software. The pXRF analyzer’s software automatically uses these correction factor values while computing the metal content, hence the final readout of pXRF analyzer is considered as a true metal concentration of any measured sample.
Accuracy of the pXRF method was determined by computing the recovery value (%RV1) of commercial samples by using the following equation. Where [Ti]XRF or [Zn]XRF is the Ti or Zn wt% determined by the pXRF analyzer and [Ti] nominal or [Zn]nominal is the Ti or Zn reported on the label of commercial sunscreens.
| (2) |
Accuracy of the ICP-MS method was determined by computing the recovery value (%RV2) of commercial samples by using the following equation. Where [Ti]ICP-MS or [Zn]ICP-MS was the Ti or Zn wt% determined by the ICP-MS analyzer.
| (3) |
Correlation between the ICP-MS and XRF techniques was determined by calculating the difference value (DV) according to the following Eq. (4).
| (4) |
2.6. Quality control parameters
Each batch consisted of 10 commercial sunscreens. The batch testing was carried out in the following order: ECV (energy calibration verification), IB (instrument blank), ICV (initial calibration verification), CCV (continuous calibration verification), samples 1–10, CRM (certified reference material), CCV, ICV, IB, and ECV. Method validity was verified by ≤ 10.00% deviation in the CCV, ICV, CRM, and IB values measured before and after an analytical batch. The stainless steel standard coupon supplied by Innov-X Company was utilized for ECV. IB was always the same matrix, which was utilized for making working standards. Ti-ICV was performed using a standard developed from mixing standard TiO2 powder purchased from a different manufacturer in the same matrix as a working standard. CCV was verified by using 7.95 wt% Ti working standard. Ti-CRM which was obtained from a different vendor and used as received. Commercial sunscreens containing Zn were also tested in a similar way as mentioned above. Zn-ICV was developed by mixing Zn powder from AMIS with the same matrix used for preparation of working standard. Zn-CRM was a certified reference material obtained from a different vendor. A 8.06 wt% Zn working standard was chosen as CCV.
3. Results and discussion
3.1. Calibration curves
An empirical calibration curve of Ti working standards in matrix A is shown in Fig. 1(a). XRF emission lines in mining mode for Ti were observed at 4.49 and 4.91 KeV, which corresponds to Kα1 and Kβ1 lines of Ti, respectively [18,32]. During pXRF analysis of the working standards, no other spectral emission lines from different elements were observed, which also confirms the purity of the standard materials. The coefficient of determination (r2) for the calibration curve was found to be 0.9979. From the equation of linear regression, the response factor and offset were determined and corresponding data are listed in Table 2. From data in Table 2, one can identify that the response factors and offsets are similar for all three matrices (i.e., A, B, C) used for making the working standards.
Fig. 1.
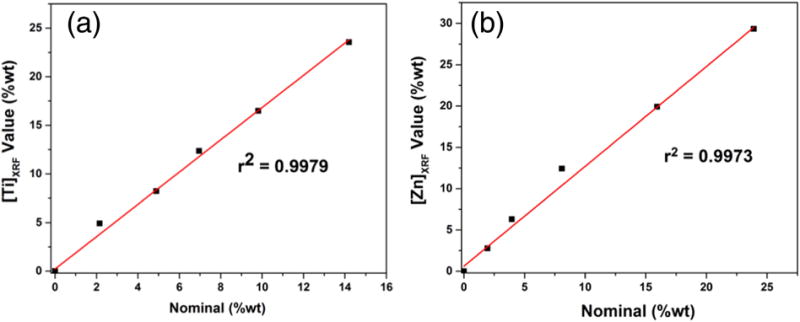
(a) Calibration curve of Ti working standards prepared by using matrix A. (b) Calibration curve of Zn working standards prepared by using matrix A.
Table 2.
Linear regression equation, coefficients of determination, response factors, and offset values of working standards developed using different sunscreen matrices.
| Matrix | Working standard | Linear regression equation | Coefficient (r2) | Response factor | Offset |
|---|---|---|---|---|---|
| Matrix A | Ti | y = 1.66x + 0.22 | 0.9979 | 0.60 | −0.14 |
| Matrix B | Ti | y = 1.59x + 0.30 | 0.9999 | 0.64 | −0.19 |
| Matrix C | Ti | y = 1.59x + 0.35 | 0.9986 | 0.63 | −0.22 |
| Matrix A | Zn | y = 1.21x + 0.61 | 0.9973 | 0.83 | −0.51 |
| Matrix B | Zn | y = 1.22x + 0.68 | 0.9965 | 0.82 | −0.56 |
| Matrix C | Zn | y = 1.24x + 0.65 | 0.9967 | 0.81 | −0.52 |
An empirical calibration curve of Zn working standards in matrix A is shown in Fig. 1(b). The linear regression equation, coefficient of determination, response factor, and offset are listed in Table 2. The XRF Kα1 and Kβ1 emission lines of Zn are identified at 8.61 and 9.55 KeV, respectively [33]. Empirical calibration curves developed by using working standards in matrix B and matrix C are shown in the supporting information (Figures S1 and S2) and their corresponding linear regression equation, coefficient, response factor, and offset are presented in Table 2. The developed method can be versatile considering the fact that a similar kind of commercial metal-free sunscreen can be used for matrix matching of working standards. The curves exhibit good linearity over a wide range; 0–14.23 wt% in case of Ti, and 0–23.94 wt% in case of Zn.
3.2. Analysis of commercial sunscreens and method validation
3.2.1. Powder form working standards
Initial studies were made by developing Ti and Zn working standards in powder form and empirical calibration curves were developed using the data from pXRF. Ti and Zn powder standards were prepared by using boric acid as a diluent. The response factor and offset values generated from empirical calibration curves of powder working standards are inputted manually into the pXRF analyzer software, and commercial sunscreens were then analyzed using these factors. Although the ICV, CCV, and CRM produced with powder standard reference materials for the quality control purpose were found to be within the limits of deviation (≤ ± 10.0%), commercial sunscreens investigated show more than 30.0% of deviation when compared to the reported values by the vendors (i.e., label claims) and to ICP-MS results. These studies proved that the calibration curves prepared with powder standards were not appropriate to apply for the measurement of commercial sunscreens. The test results summarized in Table 3 show metal concentrations of Ti and Zn in each sample. Considering the data from the method validation tools, it is conclusive that powder standards are ideal for only powder samples and there is significant contribution from the matrix in commercial sunscreens. Absorption or enhancements in the XRF signals due to the surrounding elements play an important role in the quantitation of metals of interest.
Table 3.
Recovery values and difference values of Ti- and Zn-based commercial sunscreens tested using the correction factors from powder working standards.
| Sample ID | Metal | Reported | XRF (wt%) | RV1 | ICP-MS (wt%) | RV2 | DV |
|---|---|---|---|---|---|---|---|
| SUN01XZ | Zn | 6.43 | 10.1 ± 0.01 | 157.19 | 7.13 ± 0.02 | 110.94 | 34.52 |
| SUN02XZ | Zn | 4.82 | 7.85 ± 0.03 | 163.54 | 4.70 ± 0.01 | 97.92 | 50.26 |
| SUN05TZ | Zn | 3.21 | 4.78 ± 0.00 | 149.38 | 3.11 ± 0.03 | 96.89 | 42.64 |
| SUN06XZ | Zn | 16.06 | 22.5 ± 0.01 | 140.00 | 17.14 ± 0.15 | 106.21 | 27.58 |
| SUN09XZ | Zn | 11.24 | 15.6 ± 0.02 | 139.64 | 11.20 ± 0.10 | 100.18 | 32.91 |
| SUN04TX | Ti | 1.20 | 0.54 ± 0.01 | 45.00 | 1.25 ± 0.01 | 104.17 | −82.1 |
| SUN08TZ | Ti | 0.60 | 0 | 0 | 0.55 ± 0.0 | 91.67 | −200.00 |
| SUN28TZ | Ti | 3.60 | 6.66 ± 0.01 | 185.00 | 4.38 ± 0.08 | 121.67 | 40.94 |
| SUN27TZ | Ti | 5.40 | 8.94 ± 0.02 | 165.56 | 5.82 ± 0.12 | 107.78 | 42.64 |
| SUN33TZ | Ti | 4.50 | 6.01 ± 0.02 | 133.56 | 4.52 ± 0.03 | 100.44 | 28.78 |
3.2.2. Matrix matched working standards
Commercial sunscreens that are available from different manufacturers vary a lot in composition and contain approximately 70% of organics, which are mainly light elements (atomic number <16). Quantitative XRF analysis is complicated as the measured intensities are not only related to the analytes of interest but also depend on physical matrix effects including interferences, homogeneity of the samples, particle grain sizes, sample thickness, state of sample (solid, liquid, and semi-solid), and so on [26–29]. In addition, when there is excess of lighter elements in the samples, this leads to an effect called Compton scattering, which limits the accurate quantification due to the inelastic scattering of incident X-rays [26,28,30]. To eliminate the physical matrix effects and Compton effects, matrix matched standards are required. Standard calibration data generated from the matrix matched standards is used in generating empirical influence coefficients (response factors and offset); these are directly used by instrument software to quantify the results.
In this study, working standards were prepared with organic matrices which were similar to the commercial sunscreens. We employed commercial sunscreens which were free from any metal oxides such as TiO2 and ZnO as matrix materials. With this approach, one can not only eliminate the complex matrix compounding process but also save time and be economical. Calibration curves developed from working standards in organic matrices exhibited good linearity and coefficients of determination (Fig. 1 and Table 2). Correction factors developed using these new working standards were proven to be ideal for analyzing commercial samples. The robust nature of the developed method had been investigated by analyzing different commercial samples containing varying amounts of metal oxides. In the case of Ti-based sunscreens, sunscreens were found to contain ~0.40–6.00 wt% of Ti. Whereas for Zn-based sunscreens, sunscreens were found to contain ~1.00–20.00 wt% of Zn. Ti was analyzed in nine commercial sunscreens, and Zn was analyzed in twenty-six commercial sunscreens using both ICP-MS and pXRF techniques. Commercial sunscreens were analyzed by calculating recovery values (RV1 and RV2) and difference values (DV1). Method validity is continuously verified by checking CCV, ICV, and CRM, twice for every analytical batch tested. Method validation results for both, Ti and Zn, are presented in Table 4. Deviations for all the method validation tools were always found to be ≤10.0%. Commercial sunscreen samples were measured in seven replicates; the results presented here are an average of seven replicates. RV1, RV2, and DV1 are listed in Table 5 and Table 6 for Ti- and Zn-based sunscreens, respectively. The efficiency of the pXRF method can be validated by comparing the results against ICP-MS values. Difference values between ICP-MS and pXRF were computed using the DV1 equation. The DV1 values can be acceptable if the range of deviation is between ±25.0%; all sunscreens tested were found to be within these limits. Considering the DV1 values, the results from our current studies indicate that the pXRF can be utilized as a field technique where rapid screening of metals in the sunscreens can be achieved. Method detection limit (MDL) and method quantification limit (MQL) were determined from the standard deviation value of the lowest concentration working standard. MDL was 3 times the standard deviation and MQL was 10 times the standard deviation. The MDL and MQL values for both the Ti and Zn elements are listed in Table 9.
Table 4.
Method validation results of Ti and Zn from the analysis of matrix A working standards and commercial sunscreens.
| Product Name | Nominal (wt%) | XRF (wt%) | RV1 |
|---|---|---|---|
| [Ti]-ICV | 13.73 | 14.88 ± 0.04 | 108.43 |
| [Ti]-CCV | 8.16 | 7.83 ± 0.04 | 95.96 |
| [Ti]-CRM | 19.30 | 17.60 ± 0.04 | 91.39 |
| [Zn]-ICV | 7.55 | 7.61 ± 0.01 | 100.91 |
| [Zn]-CCV | 6.31 | 6.67 ± 0.01 | 105.72 |
| [Zn]-CRM | 12.38 | 13.47 ± 0.01 | 108.81 |
Table 5.
Recovery values and difference values of Ti-based commercial sunscreens tested using the correction factors from matrix A working standards.
| Sample ID | Label claim Ti (wt%) | [Ti]XRF (wt%) | %RV1 | [Ti]ICP-MS (wt%) | %RV2 | % DV |
|---|---|---|---|---|---|---|
| SUN04TX | 1.20 | 1.38 ± 0.01 | 114.92 | 1.25 ± 0.01 | 104.17 | 9.81 |
| SUN05TZ | 1.80 | 2.16 ± 0.01 | 116.06 | 1.95 ± 0.02 | 108.33 | 10.04 |
| SUN08TZ | 0.60 | 0.64 ± 0.01 | 107.51 | 0.55 ± 0.04 | 91.67 | 15.79 |
| SUN14TZ | 3.84 | 3.57 ± 0.02 | 93.08 | 3.31 ± 0.03 | 86.20 | 7.55 |
| SUN15TX | 1.20 | 1.51 ± 0.01 | 125.77 | 1.42 ± 0.02 | 118.33 | 5.98 |
| SUN27TZ | 5.40 | 6.85 ± 0.02 | 126.92 | 5.82 ± 0.12 | 107.78 | 16.20 |
| SUN28TZ | 3.60 | 4.89 ± 0.02 | 136.11 | 4.38 ± 0.08 | 121.67 | 11.09 |
| SUN33TZ | 4.50 | 4.54 ± 0.02 | 101.01 | 4.52 ± 0.03 | 100.44 | 0.45 |
| SUN35TZ | 0.40 | 0.40 ± 0.01 | 99.43 | 0.37 ± 0.00 | 92.5 | 7.59 |
Table 6.
Recovery values and difference values of Zn-based commercial sunscreens tested using the correction factors from Matrix A working standards.
| Sample | Label claim Zn (wt%) | [Zn]XRF (wt%) | %RV1 | [Zn]ICP-MS (wt%) | %RV2 | %DV |
|---|---|---|---|---|---|---|
| SUN01XZ | 6.43 | 7.96 ± 0.01 | 124.35 | 7.13 ± 0.02 | 111.25 | 11.12 |
| SUN02XZ | 4.82 | 6.04 ± 0.01 | 125.75 | 4.70 ± 0.01 | 97.92 | 24.92 |
| SUN03XZ | 6.43 | 8.71 ± 0.01 | 136.08 | 7.32 ± 0.62 | 114.38 | 17.35 |
| SUN05TZ | 3.21 | 3.50 ± 0.00 | 109.33 | 3.11 ± 0.03 | 96.89 | 12.16 |
| SUN06XZ | 16.06 | 17.9 ± 0.01 | 110.92 | 17.14 ± 0.15 | 106.21 | 4.10 |
| SUN07XZ | 16.06 | 18.6 ± 0.01 | 115.49 | 16.54 ± 0.17 | 102.73 | 11.78 |
| SUN09XZ | 11.24 | 12.3 ± 0.01 | 109.98 | 11.22 ± 0.50 | 100.18 | 9.33 |
| SUN10XZ | 16.06 | 17.9 ± 0.01 | 111.65 | 16.78 ± 0.24 | 104.22 | 6.88 |
| SUN11XZ | 16.06 | 18.7 ± 0.01 | 116.26 | 17.52 ± 0.18 | 108.82 | 6.61 |
| SUN12XZ | 16.06 | 18.6 ± 0.01 | 115.64 | 18.16 ± 0.17 | 112.80 | 2.49 |
| SUN14TZ | 4.82 | 5.52 ± 0.01 | 115.08 | 5.65 ± 0.28 | 117.71 | −2.26 |
| SUN16XZ | 14.94 | 14.8 ± 0.01 | 99.24 | 16.21 ± 0.20 | 108.72 | −9.12 |
| SUN17XZ | 20.08 | 19.6 ± 0.01 | 97.26 | 18.85 ± 0.14 | 93.83 | 3.59 |
| SUN18XZ | 5.62 | 7.61 ± 0.01 | 135.83 | 6.19 ± 0.15 | 110.54 | 20.55 |
| SUN19XZ | 18.07 | 11.1 ± 0.01 | 61.56 | 9.19 ± 0.06 | 50.66 | 19.46 |
| SUN20XZ | 7.31 | 9.10 ± 0.01 | 124.64 | 7.37 ± 0.84 | 102.19 | 19.84 |
| SUN21XZ | 20.08 | 19.7 ± 0.01 | 98.12 | 18.59 ± 0.21 | 92.54 | 5.86 |
| SUN22XZ | 5.54 | 6.95 ± 0.01 | 126.32 | 5.63 ± 0.03 | 102.73 | 20.62 |
| SUN23XZ | 14.94 | 15.2 ± 0.01 | 101.81 | 14.95 ± 0.05 | 100.40 | 1.40 |
| SUN24XZ | 9.40 | 11.4 ± 0.01 | 120.84 | 9.93 ± 0.03 | 105.74 | 13.30 |
| SUN25XZ | 15.06 | 18.6 ± 0.01 | 123.02 | 14.91 ± 1.10 | 98.94 | 21.72 |
| SUN26XZ | 16.06 | 17.8 ± 0.01 | 110.34 | 16.59 ± 0.04 | 104.78 | 5.17 |
| SUN28TZ | 4.82 | 5.01 ± 0.01 | 104.37 | 4.93 ± 0.01 | 102.71 | 1.54 |
| SUN30XZ | 7.23 | 8.88 ± 0.01 | 123.27 | 7.75 ± 0.42 | 107.64 | 13.52 |
| SUN32XZ | 16.06 | 18.1 ± 0.01 | 112.15 | 17.32 ± 0.01 | 107.27 | 4.45 |
| SUN35TZ | 9.32 | 11.1 ± 0.01 | 118.46 | 11.20 ± 0.31 | 120.22 | −1.47 |
Table 9.
MDL and MQL values of Ti and Zn for all three matrices.
| Matrix
|
Matrix A
|
Matrix B
|
Matrix C
|
|||
|---|---|---|---|---|---|---|
| (wt %) | Ti | Zn | Ti | Zn | Ti | Zn |
| MDL | 0.06 | 0.01 | 0.06 | 0.01 | 0.06 | 0.01 |
| MQL | 0.19 | 0.03 | 0.22 | 0.03 | 0.19 | 0.04 |
3.3. Analysis of commercial sunscreens using working standards developed from different matrices
In order to further establish the validity of making standards using commercial sunscreen matrices, two other metal-free commercial sunscreens were utilized to develop separate working standards for Ti and Zn. The respective empirical calibration curves of Ti and Zn working standards in matrix B and matrix C are shown in the supporting information (Figures S1 and S2). The linear regression equation, coefficient of determination, response factor, and offset values are presented in Table 2. These correction factors are further utilized for quantitative analysis and were automatically applied to the pXRF analyzer software during the final samples analysis. Eight commercial sunscreens were individually tested for Ti and Zn separately using the correction factors from each sunscreen matrix. These data are presented in Tables 7 and 8. In the case of Ti, the DV1 values are still within ±25.0% range, which prove that the other two sunscreen matrices are equally as good as matrix A. The MDL and MQL values for Ti and Zn from matrix B and matrix C are presented in Table 9. These studies prove that the current method can be extended by developing working standards using commercially available metal-free sunscreens as matrix materials. Matrix matching is an easy and efficient way to address the matrix problems and it does not involve any tedious mathematical calculations and assumptions.
Table 7.
Data comparison of Ti commercial samples obtained from different working standards.
| Sample ID | Label claim Ti (wt%) | ICP-MS (wt%) | Matrix A
|
Matrix B
|
Matrix C
|
|||
|---|---|---|---|---|---|---|---|---|
| [Ti]XRF | %DV | [Ti]XRF | %DV | [Ti]XRF | %DV | |||
| SUN04TX | 1.20 | 1.25 ± 0.01 | 1.38 ± 0.01 | 9.81 | 1.22 ± 0.01 | −2.35 | 1.16 ± 0.01 | −7.47 |
| SUN05TZ | 1.80 | 1.95 ± 0.02 | 2.16 ± 0.01 | 10.04 | 2.49 ± 0.01 | 24.32 | 2.42 ± 0.01 | 21.67 |
| SUN08TZ | 0.60 | 0.55 ± 0.04 | 0.64 ± 0.01 | 15.79 | 0.52 ± 0.01 | −6.57 | 0.51 ± 0.01 | −7.94 |
| SUN14TZ | 3.84 | 3.31 ± 0.03 | 3.57 ± 0.02 | 7.55 | 3.66 ± 0.02 | 9.93 | 3.55 ± 0.00 | 7.11 |
| SUN15TX | 1.20 | 1.42 ± 0.02 | 1.51 ± 0.01 | 5.98 | 1.49 ± 0.00 | 4.54 | 1.39 ± 0.01 | −7.47 |
| SUN28TZ | 3.60 | 4.38 ± 0.08 | 4.89 ± 0.02 | 11.09 | 4.86 ± 0.01 | 10.39 | 4.68 ± 0.01 | 6.52 |
| SUN33TZ | 4.50 | 4.52 ± 0.03 | 4.54 ± 0.02 | 0.45 | 5.79 ± 0.01 | 24.59 | 5.65 ± 0.02 | 22.22 |
| SUN35TZ | 0.40 | 0.37 ± 0.00 | 0.40 ± 0.01 | 7.59 | 0.37 ± 0.01 | 0.00 | 0.29 ± 0.01 | −22.89 |
Table 8.
Data comparison of Zn commercial samples obtained from different working standards.
| Sample ID | Label claim Zn (wt%) | ICP-MS (wt%) | Matrix A
|
Matrix B
|
Matrix C
|
|||
|---|---|---|---|---|---|---|---|---|
| [Zn]XRF | %DV | [Zn]XRF | %DV | [Zn]XRF | %DV | |||
| SUN01XZ | 6.43 | 7.13 ± 0.02 | 7.96 ± 0.01 | 11.12 | 8.47 ± 0.01 | 17.63 | 8.17 ± 0.01 | 14.00 |
| SUN05TZ | 3.21 | 3.11 ± 0.03 | 3.50 ± 0.00 | 12.16 | 3.78 ± 0.01 | 19.79 | 3.56 ± 0.01 | 13.76 |
| SUN14TZ | 4.82 | 5.65 ± 0.28 | 5.52 ± 0.01 | −2.26 | 5.32 ± 0.00 | −5.17 | 5.11 ± 0.01 | −9.19 |
| SUN16XZ | 14.94 | 16.21 ± 0.20 | 14.8 ± 0.01 | −9.12 | 15.3 ± 0.02 | −5.60 | 14.8 ± 0.01 | −8.94 |
| SUN17XZ | 20.08 | 18.85 ± 0.14 | 19.6 ± 0.01 | 3.59 | 21.7 ± 0.01 | 14.41 | 21.3 ± 0.00 | 12.24 |
| SUN26XZ | 16.06 | 16.59 ± 0.04 | 17.8 ± 0.01 | 5.17 | 17.6 ± 0.01 | 5.96 | 17.3 ± 0.01 | 3.86 |
| SUN34XZ | 5.54 | 6.19 ± 0.01 | 7.05 ± 0.01 | 12.82 | 7.22 ± 0.01 | 15.13 | 6.95 ± 0.01 | 11.38 |
| SUN35TZ | 9.32 | 11.20 ± 0.31 | 11.10 ± 0.01 | −1.47 | 11.5 ± 0.01 | 5.84 | 11.4 ± 0.01 | 4.96 |
4. Conclusions
pXRF spectroscopy is demonstrated as an efficient and reliable technique to analyze Ti, and Zn in commercial sunscreens as compared to conventional techniques, such as ICP-MS and other time-consuming techniques such as ICP-AES and flame atomic absorption spectroscopy. The main problem associated with pXRF analysis of sunscreens was identified to be the matrix effects arising from organic materials present in the sunscreens. In this investigation, this problem has been efficiently addressed by matching the matrix in working standards of similar organic composition. It was determined that there is a good correlation between pXRF and ICP-MS results, within ±25% deviation. Quantification limits were as low as ~0.20 wt% in case of Ti and ~0.10 wt% in the case of Zn were observed using this technique. Wide linearity of the methods (Ti in the range of 0.30–14.23 wt% and Zn in the range of 0.7–23.90 wt%) made the analysis of commercial sunscreens much simpler. Analysis of samples by pXRF is very economical and time saving, as the method is non-destructive and sample preparation is easy. Analysis of safety-related factors such as the crystalline structure, surface coatings on metal oxide particles, and particle size distribution are envisioned as future work.
Supplementary Material
Acknowledgments
This work was performed at the Nanotechnology Core Facility (NanoCore) located in the U.S. Food and Drug Administration’s Jefferson Laboratories campus (Jefferson, Arkansas), which also houses the FDA National Center for Toxicological Research and the FDA Office of Regulatory Affairs, Arkansas Regional Laboratory. This project was graciously supported by Nanotechnology CORES (Collaborative Opportunities for Research Excellence in Science) Program administered by the FDA Office of Chief Scientist, and supported in part by an appointment to the Research Participation Program at the Office of Regulatory Affairs/Arkansas Regional Laboratory, U.S. FDA, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between U.S. Department of Energy and FDA. The views expressed in this manuscript are those of the authors and should not be interpreted as the official opinion or policy of the U.S. Food and Drug Administration, Department of Health and Human Services, or any other agency or component of the U.S. government. The mention of trades names, commercial products, or organizations is for clarification of the methods used and should not be interpreted as an endorsement of a product or manufacturer. We would like to thank Paul C. Howard, Lydia Velazquez, Germarie Sanchez-Pomales, Patrick Sisco, Haiou Qu, Yasith Nanayakkara, and Nuwan Kothalawala for their support, valuable time, and comments.
Footnotes
5. Supporting information
The composition of the matrix A, B, and C are reported. The Ti and Zn calibration curves developed from matrix B and matrix C are reported.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.sab.2015.11.008.
References
- 1.Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photoimmunol Photomed. 2011;27:58–67. doi: 10.1111/j.1600-0781.2011.00557.x. [DOI] [PubMed] [Google Scholar]
- 2.Dunford R, Salinaro A, Cai L, Serpone N, Horikoshi S, Hidaka H, Knowland J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418:87–90. doi: 10.1016/s0014-5793(97)01356-2. [DOI] [PubMed] [Google Scholar]
- 3.Gamer AO, Leibold E, van Ravenzwaay B. The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicol in Vitro. 2006;20:301–307. doi: 10.1016/j.tiv.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Lin W, Xu Y, Huang CC, Ma Y, Shannon K, Chen DR, Huang YW. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J Nanoparticle Res. 2009;11:25–39. [Google Scholar]
- 5.Mancebo SE, Hu JY, Wang SQ. Sunscreens: a review of health benefits regulations, and controversies. Dermatol Clin. 2014;32:427–438. doi: 10.1016/j.det.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Serpone N, Dondi D, Albini A. Inorganic and organic UV filters: their role and effi-cacy in sunscreens and suncare products. Inorg Chim Acta. 2007;360:794–802. [Google Scholar]
- 7.Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95–112. doi: 10.2147/NSA.S19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osmond MJ, Mccall MJ. Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard. Nanotoxicology. 2010;4:15–41. doi: 10.3109/17435390903502028. [DOI] [PubMed] [Google Scholar]
- 9.Lewicka Z, Benedetto A, Benoit D, Yu W, Fortner J, Colvin V. The structure, composition, and dimensions of TiO2 and ZnO nanomaterials in commercial sunscreens. J Nanoparticle Res. 2011;13:3607–3617. [Google Scholar]
- 10.Lewicka ZA, Yu WW, Oliva BL, Contreras EQ, Colvin VL. Photochemical behavior of nanoscale TiO2 and ZnO sunscreen ingredients. J Photochem Photobiol A Chem. 2013;263:24–33. [Google Scholar]
- 11.F. Register. Sunscreen drug products for over-the-counter human use, Final rules and proposed rules. Vol. 76. F.a.D.A. Department of Health and Human Services; 2011. pp. 35620–35665. 117/Friday, June 17, 2011, Federal Register. [PubMed] [Google Scholar]
- 12.Brezová V, Gabčová S, Dvoranová D, Staško A. Reactive oxygen species produced upon photoexcitation of sunscreens containing titanium dioxide (an EPR study) J Photochem Photobiol B Biol. 2005;79:121–134. doi: 10.1016/j.jphotobiol.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Contado C, Pagnoni A. TiO2 in commercial sunscreen lotion: flow field-flow fractionation and ICP-AES together for size analysis. Anal Chem. 2008;80:7594–7608. doi: 10.1021/ac8012626. [DOI] [PubMed] [Google Scholar]
- 14.Zachariadis GA, Sahanidou E. Multi-element method for determination of trace elements in sunscreens by ICP-AES. J Pharm Biomed Anal. 2009;50:342–348. doi: 10.1016/j.jpba.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Salvador A, Pascual-Martí MC, Adell JR, Requeni A, March JG. Analytical methodologies for atomic spectrometric determination of metallic oxides in UV sunscreen creams. J Pharm Biomed Anal. 2000;22:301–306. doi: 10.1016/s0731-7085(99)00286-1. [DOI] [PubMed] [Google Scholar]
- 16.Radu T, Diamond D. Comparison of soil pollution concentrations determined using AAS and portable XRF techniques. J Hazard Mater. 2009;171:1168–1171. doi: 10.1016/j.jhazmat.2009.06.062. [DOI] [PubMed] [Google Scholar]
- 17.VanCott RJ, McDonald BJ, Seelos AG. Standard soil sample preparation error and comparison of portable XRF to laboratory AA analytical results. Nuclear Instruments and Methods in Physics Research Section A, Accelerators, Spectrometers, Detectors and Associated Equipment. 1999;422:801–804. [Google Scholar]
- 18.LeBouf RF, Miller AL, Stipe C, Brown J, Murphy N, Stefaniak AB. Comparison of field portable measurements of ultrafine TiO2: X-ray fluorescence, laser-induced breakdown spectroscopy, and Fourier-transform infrared spectroscopy. Environ Sci: Processes Impacts. 2013;15:1191–1198. doi: 10.1039/c3em00108c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horcajada P, Serre C, Vallet-Regí M, Sebban M, Taulelle F, Férey G. Metal–organic frameworks as efficient materials for drug delivery. Angew Chem. 2006;118:6120–6124. doi: 10.1002/anie.200601878. [DOI] [PubMed] [Google Scholar]
- 20.Arzhantsev S, Li X, Kauffman JF. Rapid limit tests for metal impurities in pharmaceutical materials by X-ray fluorescence spectroscopy using wavelet transform filtering. Anal Chem. 2011;83:1061–1068. doi: 10.1021/ac1028598. [DOI] [PubMed] [Google Scholar]
- 21.Mondia JP, Goh F, Bryngelson PA, MacPhee JM, Ali AS, Weiskopf A, Lanan M. Using X-ray fluorescence to measure inorganics in biopharmaceutical raw materials. Anal Methods. 2015;7:3545–3550. [Google Scholar]
- 22.Sánchez-Pomales G, Mudalige TK, Lim JH, Linder SW. Rapid determination of silver in nanobased liquid dietary supplements using a portable X-ray fluorescence analyzer. J Agric Food Chem. 2013;61:7250–7257. doi: 10.1021/jf402018t. [DOI] [PubMed] [Google Scholar]
- 23.Palmer PT, Jacobs R, Baker PE, Ferguson K, Webber S. Use of field-portable XRF analyzers for rapid screening of toxic elements in FDA-regulated products. J Agric Food Chem. 2009;57:2605–2613. doi: 10.1021/jf803285h. [DOI] [PubMed] [Google Scholar]
- 24.Anderson DL. Analysis of beverages for Hg, As, Pb, and Cd with a field portable X-ray fluorescence analyzer. J AOAC Int. 2010;93:683–693. [PubMed] [Google Scholar]
- 25.Melquiades FL, Ferreira DD, Appoloni CR, Lopes F, Lonni AG, Oliveira FM, Duarte JC. Titanium dioxide determination in sunscreen by energy dispersive X-ray fluorescence methodology. Anal Chim Acta. 2008;613:135–143. doi: 10.1016/j.aca.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 26.West M, Ellis AT, Kregsamer P, Potts PJ, Streli C, Vanhoof C, Wobrauschek P. Atomic spectrometry update. X-ray fluorescence spectrometry. J Anal At Spectrom. 2007;22:1304–1332. [Google Scholar]
- 27.Marguí E, Queralt I, Hidalgo M. Application of X-ray fluorescence spectrometry to determination and quantitation of metals in vegetal material. TrAC, Trends Anal Chem. 2009;28:362–372. [Google Scholar]
- 28.Rousseau RM. Corrections for matrix effects in X-ray fluorescence analysis—a tutorial. Spectrochim Acta B At Spectrosc. 2006;61:759–777. [Google Scholar]
- 29.Maruyama Y, Ogawa K, Okada T, Kato M. Laboratory experiments of particle size effect in X-ray fluorescence and implications to remote X-ray spectrometry of lunar regolith surface. Earth Planets Space. 2008;60:293–297. [Google Scholar]
- 30.Fernández JE. Rayleigh and compton scattering contributions to x-ray fluorescence intensity. X-Ray Spectrom. 1992;21:57–68. [Google Scholar]
- 31.Kalnicky DJ, Singhvi R. Field portable XRF analysis of environmental samples. J Hazard Mater. 2001;83:93–122. doi: 10.1016/s0304-3894(00)00330-7. [DOI] [PubMed] [Google Scholar]
- 32.Schwab NV, Da-Col JA, Terra J, Bueno MIMS. Fast direct determination of titanium dioxide in toothpastes by X-Ray fluorescence and multivariate calibration. J Braz Chem Soc. 2012;23:546–554. [Google Scholar]
- 33.Larcheri S, Armellini C, Rocca F, Kuzmin A, Kalendarev R, Dalba G, Graziola R, Purans J, Pailharey D, Jandard F. X-ray studies on optical and structural properties of ZnO nanostructured thin films. Superlattice Microst. 2006;39:267–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


