Abstract
The encoding of experiences in the brain and the consolidation of long-term memories depend on gene transcription. Identifying the function of specific genes in encoding experience is one of the main objectives of molecular neuroscience. Furthermore, the functional association of defined genes with specific behaviors has implications for understanding the basis of neuropsychiatric disorders. Induction of robust transcription programs has been observed in the brains of mice following various behavioral manipulations. While some genetic elements are utilized recurrently following different behavioral manipulations and in different brain nuclei, transcriptional programs are overall unique to the inducing stimuli and the structure in which they are studied1,2.
In this publication, a protocol is described for robust and comprehensive transcriptional profiling from brain nuclei of mice in response to behavioral manipulation. The protocol is demonstrated in the context of analysis of gene expression dynamics in the nucleus accumbens following acute cocaine experience. Subsequent to a defined in vivo experience, the target neural tissue is dissected; followed by RNA purification, reverse transcription and utilization of microfluidic arrays for comprehensive qPCR analysis of multiple target genes. This protocol is geared towards comprehensive analysis (addressing 50-500 genes) of limiting quantities of starting material, such as small brain samples or even single cells.
The protocol is most advantageous for parallel analysis of multiple samples (e.g. single cells, dynamic analysis following pharmaceutical, viral or behavioral perturbations). However, the protocol could also serve for the characterization and quality assurance of samples prior to whole-genome studies by microarrays or RNAseq, as well as validation of data obtained from whole-genome studies.
Keywords: Behavior, Issue 90, Brain, behavior, RNA, transcription, nucleus accumbens, cocaine, high-throughput qPCR, experience-dependent plasticity, gene regulatory networks, microdissection
Introduction
The brain’s dynamic organization enables cognitive and behavioral flexibility. Experiences are encoded through modifications of the structure and strength of connections between neurons in the brain3. This “experience-dependent plasticity” is the result of induction of specific patterns of gene expression that provides the necessary proteins for modification of synaptic structure and strength4. The identification of gene regulatory networks mediating the formation of long-term memories is a central tenet of molecular neuroscience, with the expectation that identification of the dominant elements of transcriptional programs will provide insight into the fundamental principles regulating memory formation, as well as targets for treatment of neurodegenerative and neuropsychiatric disorders. Transcriptional programs unfold in temporally-defined waves, each of which encode genes of different character, which are important for different stages in the implementation of the outcome of the signalling event1,2. It is therefore important to address transcriptional dynamics on a detailed temporal timescale, so as to identify the full complement of genes induced, and gain insight into their potential function according to the dynamics of their induction.
Drug addiction is a robust form of experience-dependent plasticity caused by the long-lasting effects of drugs of abuse on neural circuits in the brain5,6. Initial, acute exposure to drugs may lead to the development of addiction and the transition to chronic use. Contextual information is a crucial element in the development of addiction. Drug-associated environmental cues are assigned significant importance in the minds of drug abusers. Contextual information reminding a drug abuser of past drug experience can induce relapse to drug craving even following long periods of abstinence from drug exposure7,8. Hence the great clinical challenge in addiction - the propensity of addicts to relapse even long after withdrawal symptoms have subsided9.
Behavioral sensitization to cocaine is a simple model of cocaine experience useful in the study of mechanisms of drug addiction. In this widely-studied model for the long-lasting sensitization induced by chronic exposure to drugs of abuse, rodents are first habituated to saline injections (intraperitoneal; IP) in a novel environment (an open field chamber in which their locomotor activity is monitored); then, they receive daily injections of cocaine in the open field chambers while their activity is monitored10 (Figure 1). This behavioral paradigm typically results in a robust sensitization of locomotor behavior (8-12 fold above baseline activity)11, which is maintained for a period of months following cessation of cocaine injections, demonstrating the formation of a pervasive memory trace of drug experience.
The neural circuitry of reward, naturally involved in reinforcing behaviors essential for the success of a species (e.g. feeding, sex), is exploited by drugs of abuse to reinforce drug-associated behaviors12,13. The molecular and cellular mechanisms by which the experience of drugs of abuse is enhanced appear to be similar to the mechanisms underlying the formation of declarative or semantic memories in other brain structures14. Therefore, the robustness of the behavioral sensitization model makes it an attractive model system to study mechanisms of experience-dependent plasticity.
The nucleus accumbens (NAc) is a central integrator of the brain’s reward circuitry, and has been extensively associated with the development of addiction5,6. The formation of addiction depends on transcription of novel proteins in the nucleus accumbens, and robust induction of clearly structured transcription programs is observed in the NAc following cocaine experience15-19. The acute transcriptional response to cocaine exposure is likely to function at multiple levels in order to adapt to the strong induction stimulus and to direct the production of new proteins that are responsible for the structural and electrophysiological changes induced by exposure to the drug 6,19-22.
In order to promote the study of molecular mechanisms of experience-dependent plasticity in the brain, a protocol is described for the comprehensive analysis of transcription dynamics in brain tissue samples following behavioral manipulation. The protocol is illustrated in the context of the behavioral experience studied in the Citri lab – behavioral sensitization to cocaine, utilizing microfluidic dynamic arrays for transcriptional analysis. The protocol described is obviously not limited to studying the nucleus accumbens in the context of behavioral sensitization, but could be applied to a large number of behavioral paradigms and brain regions. In fact, this protocol could be applied to body tissues outside the brain, and a variety of experiences or manipulations of the organism studied.
The protocol is roughly divided into four steps. In the first step, the animal is subjected to the behavioral paradigm; in the second step the tissue is microdissected; in the third step – mRNA is purified, reverse-transcribed and probed, and in the final step the data is analyzed.
In the context of studying transcriptional dynamics, the precise timing and definition of the experience are probably the most important experimental parameters to control. For this reason, our behavioral model of choice is that of behavioral sensitization to cocaine, a system which enables high level of experimenter control over the parameters of the experience. Additional behavioral paradigms that enable precise timing and address different models of experience-dependent plasticity or memory formation are available. These models include fear conditioning23, acute environmental enrichment24,25, novel object exploration26 and visual experience following dark rearing27. Still, behavioral sensitization to cocaine is a consistently robust behavioral manipulation, creating a highly pervasive memory trace which lasts for months following cocaine experience28.
The brain is sectioned, followed by manual microdissection of the nucleus accumbens. It has been our experience that manual microdissection from rapidly prepared brain slices provides the most reliable and rapid method of extracting the tissue relevant to the behavioral paradigm, and with experience, the boundaries of the tissue become evident and readily recognized. Alternatively, fine slices could be prepared, followed by laser-capture microdissection. Although this method enables highly defined delineation of the region of interest, it is slow (thus risking loss of labile mRNA), tedious and requires costly dedicated equipment (a microscope fitted with a laser-capture setup). The protocol defined herein could also be adapted to single-cell transcriptional analysis, by manual aspiration of cytoplasm of visually identified cells using patch pipettes29. It is important to note that the protocol described provides a population average, while it is highly likely that in most cases, only a subpopulation of the cells within the tissue are actually involved in responding to the experience. It is of interest to profile transcription in a selective manner from within specific cell populations responding to the experience, but discussion of these approaches is beyond the current scope.
For mRNA purification, reverse-transcription and qPCR querying, the tissue is disrupted by passing it through fine needles, followed by the utilization of commercially available kits (for more information, see Table 8). The choice is informed by experience with these methodologies, which ensure reliable extraction of high quality RNA and robust results from downstream applications.
While the protocol is described for high-throughput qPCR utilizing dynamic arrays, samples can be probed for gene expression using end-point PCR, low-throughput qPCR, gene expression microarrays or deep sequencing. The preference for high-throughput qPCR utilizing dynamic arrays is due to the fact that mRNA obtained from brain nuclei following behavioral paradigms is often of limiting quantities. Dynamic arrays provide a platform that enables efficient comprehensive analysis of transcripts from a large number of parallel samples in a single experiment. After the initial acquisition of the microfluidic system (commonly an institutional purchase), experiments are relatively inexpensive to run. Following this analysis, further querying of the samples could be performed using more costly platforms to search for novel transcripts (by microarrays or RNAseq) with the dynamic arrays providing a comprehensive reference for quality assurance. Finally, for data analysis, standard approaches are utilized. Specific pointers regarding issues that may arise will be discussed in the text of the protocol.
This protocol is most appropriate for investigators interested in a thorough investigation of their system of interest, studying multiple conditions and replicates. The protocol is also most suitable for investigators who have already honed in (through microarray or RNAseq experiments) on a subset of 50-500 genes of interest, which they are interested in querying repeatedly.
Protocol
NOTE: The protocol follows the animal care guidelines of The Hebrew University of Jerusalem.
1. Preparation of ACSF Solution
Prepare ACSF solution as described in Table 1. Make 1 L in ddH2O (>18 MΩ purity), bringing osmolarity to ~300 mOsm/L with appropriate addition of water or NaCl.
2. Equipment and Room Set Up
The equipment for monitoring of cocaine-induced locomotor behavior consists of behavior chambers (50 x 45 cm) wired with an inner light source, fan and camera (or infrared sensors), and fitted with an inner perspex box (30 x 30 cm) in which the mice are allowed to move freely while their locomotion is monitored. The behavioral testing should be performed between 1 hr after lights on to 1 hr before lights off, on a regular light/dark cycle. Animals should be trained consistently at the same time each day.
Connect camera or infrared system to an appropriate system for monitoring and recording the locomotor behavior of the mice.
Clean the chambers after each trial with disinfectant provided by mouse facility and/or odor removing reagent, in order to prevent olfactory cue bias and to ensure proper disinfection.
IMPORTANT: While handling the mice be quiet, calm and careful.
3. Animal Preparation
House 5 C57BL6 male mice (6-12 weeks old; ~25-35 g of weight) per cage, in a room with a 12 hr light/dark cycle and free access to food and water, according to standards and requirements of local Animal Care Committee guidance and protocols. Before initiating the experiment, weigh the mice and log the data. Cocaine is later administered according to the weight of the mice.
Transfer all the cages containing mice into the behavioral room 30 min before starting the first trial.
Habituate all of the mice used in the experiment to experimenter handling, injections and monitoring in the behavior setup. This is achieved by 3 consecutive daily intraperitoneal injections of 250 μl saline as described below.
Administer an intraperitoneal injection of 250 μl saline. Immediately following the injection, place the mouse in a behavior chamber to monitor locomotor activity for 15 min. Repeat this action for 3 consecutive days, at the same hour each day.
On the fourth day, divide the mice into two groups. Prepare a stock solution of cocaine at 2 mg/ml in saline. Administer a single injection of cocaine solution (final 20 mg/kg) to the experimental group according to the weight of the mouse (e.g. for a 25 g mouse – inject 250 μl, whereas a 30 g mouse will receive 300 μl). Administer saline to the control group. Immediately following the injection, place the mouse for 20 min in a behavior chamber for monitoring of locomotor activity (typically, the first 15 min are monitored).
After the cocaine injection, sacrifice the mice at different time points (0, 1, 2, 4, 8 and 24 hr following injection). Importantly, make sure that reference mice (0 time point) will not receive any injection on this day. Due to its significance, it is imperative to include replicates of the reference group.
4. Dissection of the Nucleus Accumbens
Anesthetize the mice with isoflurane at the appropriate time after the cocaine/saline injection. The isoflurane must be directly vented out of the room. Therefore, the procedure should be performed in a chemical fume hood.
Monitor the depth of anesthesia by testing the rear foot reflex. Pinch the paw to elicit a withdrawal response by the animal. An animal that shows a reflex is not at a surgical level of anesthesia and not in a state to euthanize.
- Extraction of the brain:
- Euthanize the mouse by decapitation.
- Remove the skin above the center of the skull and cut the skull with sharp scissors.
- Insert the scissors at the point where the spinal cord enters the brain (foramen magnum) and make two lateral cuts.
- From the same point, make a long sagittal cut along the sagittal suture. Upon reaching the olfactory bulb, clip to the left and to the right.
- With a forceps, remove the skull carefully, grasping each half of the skull firmly and pulling to the side, taking care not to press onto or damage the brain.
- Remove the brain while severing the cranial nerves by using an inverted micro spoon spatula.
Place the brain in an ice-cold ACSF solution for 1-2 min to chill and harden.
Remove brain from the ACSF, wick excess fluid with a paper towel, create a coronal cut between the cerebellum and the rest of the brain and glue the brain, with the rostral part facing up, onto the vibratome stage.
- Cutting the NAc:
- Maintain ACSF solutions at ice-cold temperatures in the slicing chamber of the vibratome. Section at 200 μm until the nucleus accumbens (NAc) is clearly identified according to the Paxinos and Franklin Mouse Brain Atlas (approximately 1.8 mm anterior to Bregma; pay attention to the shape of the corpus callosum, the location and shape of the anterior commissure and the ventricles, as well as the overall morphology of the brain section). At this point, create two 400 μm sections, from which the NAc will be dissected.
- Under a stereoscope, use a fine blade to dissect the NAc area out from the slices. The definition of the NAc is according to the Paxinos and Franklin mouse brain atlas, and could conveniently be dissected in appropriate sections by four quick passes of the blade on the tissue. (Figure 2).
- Place NAc sections immediately in 900 μl Trizol lysis reagent in a microcentrifuge tube and store at -80 °C until RNA extraction.
5. RNA Extraction and cDNA Reverse Transcription
Thaw the tubes containing nucleus accumbens sections in trizol lysis reagent at room temperature and homogenize the lysate with a 1 ml syringe connected to 25 G needle until the tissue is completely homogenized (at least 15-20 times). The first few rounds are difficult, and require pushing the tissue against the wall of the tube in order to break it to small pieces that could enter the tip of the needle.
To ensure homogenization, pipet the lysate into a QIAshredder mini spin column and centrifuge at 12,000 x g for 1 min.
Pipet the lysate into new microcentrifuge tube and extract the RNA according to the manufacturer’s instructions. Store the RNA at -80 °C until use.
Measure the RNA concentration, analyze integrity and quality using a Bioanalyzer or equivalent equipment. Use 100-500 ng RNA for each round of cDNA preparation with random hexamer primers.
6. Primer Design and Primer Testing
- Choosing good primers: For an optimal primer pair for qPCR of mRNA transcripts, follow these requirements (Figure 3):
- Design primers containing no longer than 17-28 bases. Avoid nucleotide repeats as they promote mispriming.
- Design primers with melting temperature (Tm) between 56-68°C (optimally 60-64°C). The Tm within the primer pair should be within 1 °C of each other.
- Be aware that the product size should ideally be between 80 and 150bp.
- Ensure that at least one of the primers covers an exon-exon junction, or the amplicon contains a large intron (intron-spanning), to avoid amplification of genomic DNA contaminants.
- Use a reliable software based on Primer3 (for examples see Table 2) in order to improve primer design.
- Testing the primers: In order to ensure the specificity and efficiency of the primers, a control qPCR reaction should be performed:
- Use a positive sample (containing cDNA template for all relevant primer pairs) and titrate the cDNA (e.g. eight three-fold dilutions from an initial concentration of ~10 ng) in duplicate parallel reactions. Include a no-template control, which controls for contamination within the solutions used for the reaction.
- Calculate primer efficiency by using the formula: (10(−1/slope)-1) × 100, such that the curve of the optimal slope is − 3.333 (i.e. 10(− 1/3.333) = 2) (Figure 4).
- Determine the specificity of the amplification. Specific amplification is demonstrated by a single prominent peak common to all the melting curves of the amplified products, whereas off-target amplification would appear as an obvious deviation from the single large peak.
7. qPCR Analysis
- Pre-amplification:
- Comprehensive qPCR analysis is based on parallel querying of limiting quantities of RNA with a large number of primer pairs. Therefore, a step of pre-amplification is essential. In this step, the cDNA undergoes 14-18 cycles of pre-amplification with a mixture of all the primers that will be utilized for querying the sample in the future (up to 800 primer pairs).
- Dilute all primers in TE (low EDTA) to 50 μM for Specific Target Amplification (STA).
- Pool together 1 μl aliquots of all of the 50 μM primer sets to be included in the STA reaction, up to 800 assays.
- Prepare pre-mix and samples for STA as described in Table 3. Allow for error by preparing mix for 110% of the number of samples that need to be amplified.
- Aliquot 3.75 μl STA pre-mix for each sample. Add 1.25 μl of cDNA to each.
- Vortex to mix the reactions and centrifuge for 10 sec.
- Place the STA reactions in a thermal cycler and cycle as indicated at Table 4.
- Exonuclease I (ExoI) treatment:
- Dilute the Exo I as indicated in Table 5.
- Add 2 μl of diluted ExoI (4 U/μl) to each 5 μl STA reaction, vortex, spin down (>6,000 x g) and place in a thermal cycler at 37°C for 30 min and then at 80 °C for 15 min.
- Dilute the final products to an appropriate concentration for testing. Use TE Buffer (add 43 μl TE buffer to the 7 μl TSA sample).
- Store the diluted STA products at -20 °C or use immediately.
- Primer and sample setup for qPCR:
- Prepare samples as shown in Table 6. Prepare mix according to the chip chosen for the experiment, and add samples as appropriate.
- Agitate the Sample Mix solutions for a minimum of 20 sec, and centrifuge (>6,000 x g) for at least 30 sec. Prepared reactions can be stored for short durations at 4 °C until the chip is ready for loading.
- Prepare the assays as described in Table 7.
- Agitate the Assay Mix for a minimum of 20 sec and centrifuge (>6,000 x g) for at least 30 sec to spin down all components. IMPORTANT: Vortex thoroughly and centrifuge all sample and assay solutions before pipetting into the chip inlets. Failure to do so may result in a decrease in data quality. NOTE: The final concentration of each primer is 5 μM in the inlet and 500 nM in the final reaction.
- Priming and Loading the Dynamic Array IFC (integrated fluidic circuit) chip. The IFC chip has inlets for samples and assays, as well as specified inlets (accumulators) for control line fluid (mineral oil) used to pressurize the microfluidic chambers (Figure 4A).
- Inject control line fluid into each accumulator on the chip.
- Remove and discard the blue protective film from the bottom of the chip.
- Place the chip into the appropriate IFC controller (controller MX for the 48.48 Dynamic Array IFC or the IFC controller HX for the 96.96 Dynamic Array IFC), then run the Prime (113x) script for the 48.48 Dynamic Array IFC or the Prime (136x) script for the 96.96 Dynamic Array IFC in order to prime the chip.
- When the script has finished, remove the primed chip from the IFC controller.
- Pipette 5 μl of each Assay Mix and 5 μl of each Sample Mix into their inlets.
- Return the chip to the IFC controller.
- Using the IFC controller software, run the Load Mix (113x) script for the 48.48 Dynamic Array IFC) or Load Mix (136x) script for the 96.96 Dynamic Array IFC to load the samples and assays into the chip. When the Load Mix script has finished, remove the loaded chip from the IFC controller.
- Load the chip on the thermal cycler, create a new chip run (according to manufacturer guidelines), including melting curve analysis. Using the thermal cycler software, ensure that the reaction chambers are recognized properly, and allow the reaction to run to completion.
8. Data Analysis
Quality assurance: In order to ensure data quality, verify the quality of the primers by addressing dilution curves (as described above). Monitor the specificity of amplification by viewing the melting curves of amplicons, the peaks of which should optimally be tightly aligned29.
Visualization: For visualization of large quantities of data obtained by high-throughput qPCR, use software generated heatmaps, in which expression is color-coded by intensity. In addition, display the transcriptional dynamics of single genes as lined scatter plots.
Publication: In publishing the gene expression results, it is advised to follow the MIQE Guidelines for publication of quantitative Real-Time PCR experiments, detailing the minimum information that must be reported for a qPCR experiment to ensure its relevance, accuracy, correct interpretation, and reproducibility.
Representative Results
The quality of the results obtained by applying this protocol crucially depends upon a number of parameters. Proper experimental planning will result in minimal disturbance to the experimental mice, such that the experience tested (in this example, that of exposure to cocaine) will be the most dominant experience in their recent history, and therefore will result in a robust and specific transcriptional program. Figure 1 describes the experimental plan for behavioral sensitization to cocaine, defining the time points following cocaine experience at which the nucleus accumbens is dissected from mice and analyzed. The next crucial point is that of defining the precise boundaries for excision of the proper tissue for analysis. Figure 2 describes the boundaries of the nucleus accumbens in coronal and sagittal sections. Preferably the dissection should be performed such that a thin margin of NAc tissue is excluded from the region taken for profiling. This ensures consistent profiling only of the NAc, reducing variability and the potential for artifacts arising from inclusion of surrounding tissue.
When amplifying limiting amounts of RNA, as in the dissection of small brain nuclei, many cycles of amplification are necessary for proper detection of the signal. Therefore a crucial point in any quantitative PCR experiment, further amplified when working with small quantities of starting material, is characterization of the primers used for amplification. Figure 3 describes key features in defining optimal primers, and Figure 4 demonstrates the amplification curves of primers directed against c-Fos and FosB, demonstrating that these primer pairs amplify their target transcript with ~100% efficiency in every cycle. Following primer validation and establishment of the proper experimental paradigm to enable clear assessment of transcription from the target tissue, comprehensive analysis can be performed on the Fluidigm Biomark platform, enabling up to 9,216 parallel qPCR reactions (in the 96.96 format; Figure 5). This high-throughput platform enables parallel analysis of multiple experimental repetitions, using identical experimental conditions on a single chip. Data for quadruple experimental repetitions, addressing the transcriptional induction of c-Fos and FosB following acute cocaine experience, are demonstrated in Figure 6.
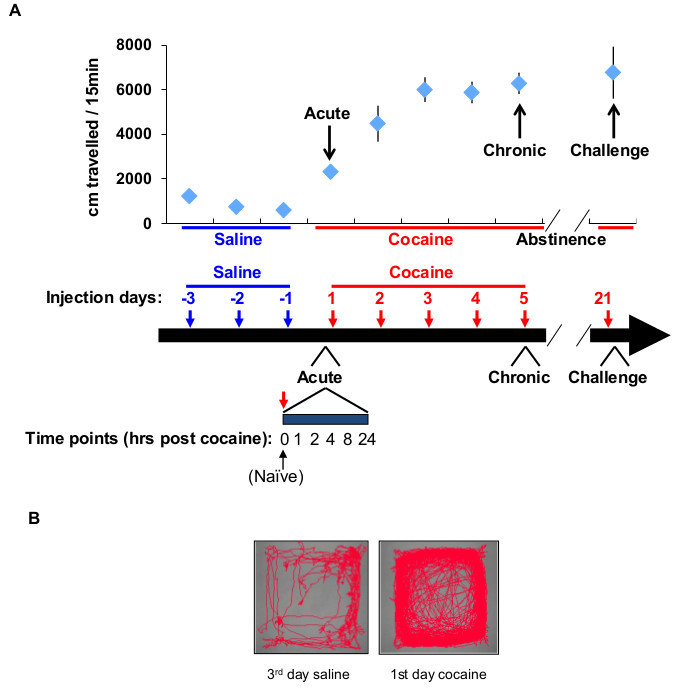 Figure 1. Experimental paradigm for dynamic transcriptional analysis following cocaine experience. (A) Experimental protocol – mice are habituated to handling and injection for 3 days, while monitoring locomotor activity by video tracking in a sound attenuating behavior chamber. Mice are then subject to cocaine experience; a single exposure = acute cocaine experience; 5 consecutive injections are termed chronic exposure. A single injection following ≥16 days of abstinence from cocaine is termed a cocaine challenge. Transcriptional dynamics are addressed at different experimental time points following cocaine experience (1, 2, 4, 8, 24 hr). (B) Plot of the mouse track within the behavior chamber following 3 days of saline injection or 3 days of saline + a single acute cocaine exposure. Please click here to view a larger version of this figure.
Figure 1. Experimental paradigm for dynamic transcriptional analysis following cocaine experience. (A) Experimental protocol – mice are habituated to handling and injection for 3 days, while monitoring locomotor activity by video tracking in a sound attenuating behavior chamber. Mice are then subject to cocaine experience; a single exposure = acute cocaine experience; 5 consecutive injections are termed chronic exposure. A single injection following ≥16 days of abstinence from cocaine is termed a cocaine challenge. Transcriptional dynamics are addressed at different experimental time points following cocaine experience (1, 2, 4, 8, 24 hr). (B) Plot of the mouse track within the behavior chamber following 3 days of saline injection or 3 days of saline + a single acute cocaine exposure. Please click here to view a larger version of this figure.
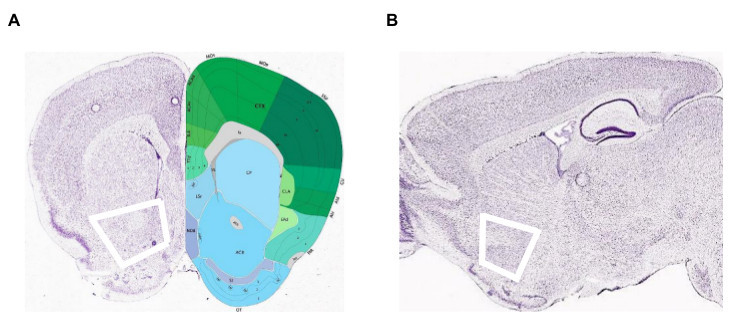 Figure 2. Location of the Nucleus Accumbens in the mouse brain. Thick white lines mark the location of cuts defining the boundaries of the NAc. (A) Coronal section; (B) Sagittal section. (Pictures adapted from the Allen Brain Atlas Reference Atlas. Images: http://atlas.brain-map.org/atlas?atlas=1&plate=100960352; http://atlas.brain-map.org/atlas?atlas=2&plate=100883869). Please click here to view a larger version of this figure.
Figure 2. Location of the Nucleus Accumbens in the mouse brain. Thick white lines mark the location of cuts defining the boundaries of the NAc. (A) Coronal section; (B) Sagittal section. (Pictures adapted from the Allen Brain Atlas Reference Atlas. Images: http://atlas.brain-map.org/atlas?atlas=1&plate=100960352; http://atlas.brain-map.org/atlas?atlas=2&plate=100883869). Please click here to view a larger version of this figure.
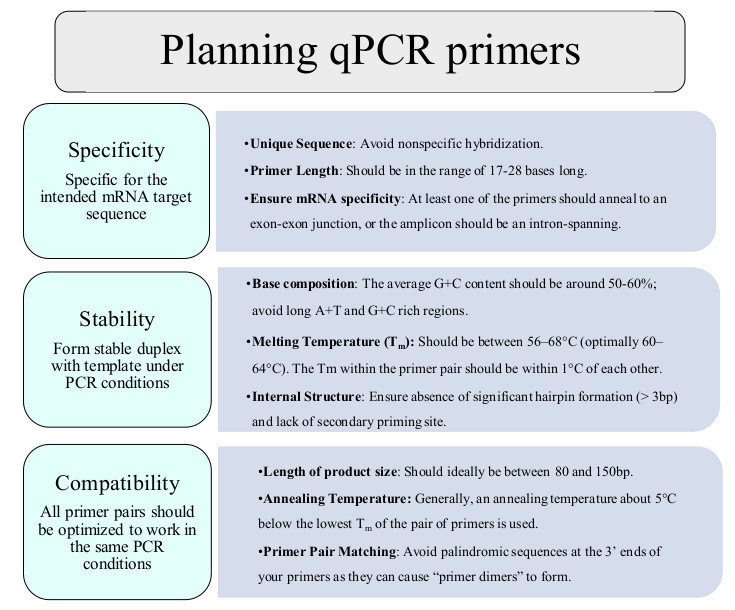 Figure 3. Planning qPCR primers. Guidelines for planning qPCR primers, with definitions for Specificity, Stability and Compatibility. Please click here to view a larger version of this figure.
Figure 3. Planning qPCR primers. Guidelines for planning qPCR primers, with definitions for Specificity, Stability and Compatibility. Please click here to view a larger version of this figure.
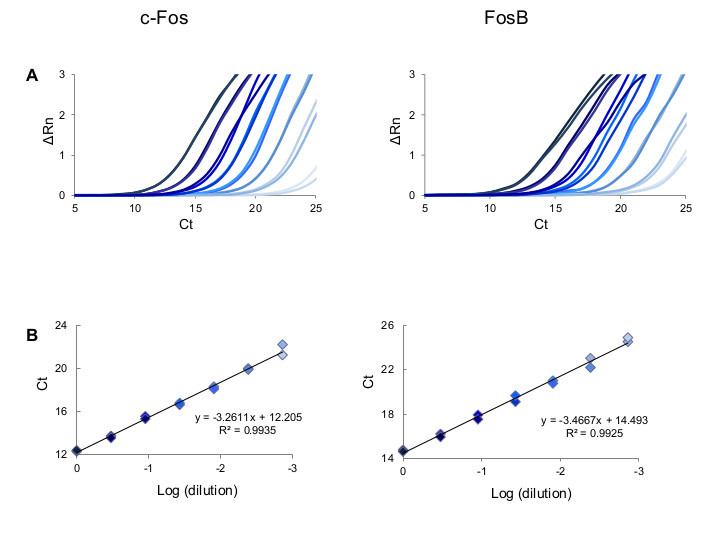 Figure 4. Assessment of primer efficiency. (A) Results of amplification curves of Fos and FosB using Fluidigm IFC arrays. A standard curve was generated using seven serial 3-fold dilutions of total RNA extracted from mouse NAc and probed for expression of Fos and FosB. (B) The efficiency of the primer pair for Fos and FosB was evaluated by plotting the cycle threshold value (Ct) at each dilution against the logarithm of the fold dilution of the sample. The efficiency of the primers are calculated from the slope of the standard curve, as defined in the text. The amplification curves and points on the dilution curve are color-matched. Please click here to view a larger version of this figure.
Figure 4. Assessment of primer efficiency. (A) Results of amplification curves of Fos and FosB using Fluidigm IFC arrays. A standard curve was generated using seven serial 3-fold dilutions of total RNA extracted from mouse NAc and probed for expression of Fos and FosB. (B) The efficiency of the primer pair for Fos and FosB was evaluated by plotting the cycle threshold value (Ct) at each dilution against the logarithm of the fold dilution of the sample. The efficiency of the primers are calculated from the slope of the standard curve, as defined in the text. The amplification curves and points on the dilution curve are color-matched. Please click here to view a larger version of this figure.
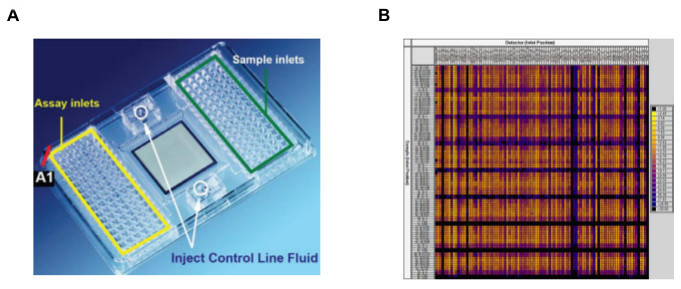 Figure 5. Comprehensive qPCR profiling applying the Fluidigm platform. (A) 96.96 Dynamic Array IFC for Real-Time Quantitative PCR. The primers are loaded in the inlets located in the right side, and the samples are loaded in the inlets located in the left side of the chip. This figure has been modified with permission from Fluidigm Real Time PCR Analysis User Guide. (B) Heat map of the qPCR results, representing Ct values for each well on the chip. Please click here to view a larger version of this figure.
Figure 5. Comprehensive qPCR profiling applying the Fluidigm platform. (A) 96.96 Dynamic Array IFC for Real-Time Quantitative PCR. The primers are loaded in the inlets located in the right side, and the samples are loaded in the inlets located in the left side of the chip. This figure has been modified with permission from Fluidigm Real Time PCR Analysis User Guide. (B) Heat map of the qPCR results, representing Ct values for each well on the chip. Please click here to view a larger version of this figure.
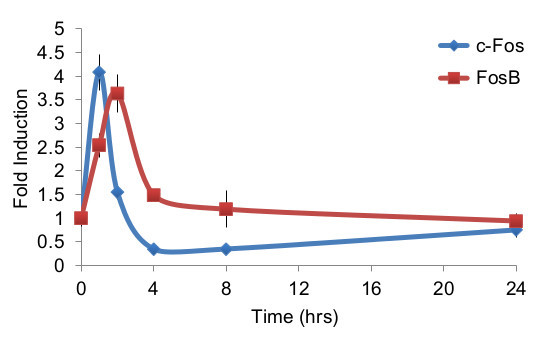 Figure 6. Sample results of expression dynamics following an acute cocaine exposure. Cocaine-induced expression of c-Fos and FosB are displayed as a function of time. Averages of quadruple experimental repetitions, performed according to the protocol defined herein, are displayed, along with error bars portraying standard error of the mean.
Figure 6. Sample results of expression dynamics following an acute cocaine exposure. Cocaine-induced expression of c-Fos and FosB are displayed as a function of time. Averages of quadruple experimental repetitions, performed according to the protocol defined herein, are displayed, along with error bars portraying standard error of the mean.
| Reagent | MW | mM | g/L |
| NaCl (Sigma-Aldrich,71376) | 58.44 | 119 | 6.95 |
| NaHCO3 (Sigma-Aldrich, S6014) | 84.01 | 26 | 2.18 |
| Glucose (Sigma-Aldrich,G8270) | 180.16 | 10 | 1.8 |
| KCl (Sigma-Aldrich, P9541) | 74.55 | 2.5 | 0.186 |
| NaH2PO4 (Sigma-Aldrich, S9638) | 137.99 | 1 | 0.138 |
| MgSO4⋅7H2O (Sigma-Aldrich,M1880) | 246.5 | 4 | 0.99 |
| CaCl2 (Sigma-Aldrich, C3011) | 147 | 4 | 0.59 |
Table 1. ACSF solution.
| Software name | Application | web location |
| Roche Probefinder | primers design | http://qpcr.probefinder.com/organism.jsp |
| NCBI primer-blast | primers design | http://www.ncbi.nlm.nih.gov/tools/primer-blast/ |
Table 2. Software list.
| Reagent | Volume / Reaction (μl) | Volume for 48 Reactions + 10% overage (μl) | Volume for 96 Reactions + 10% overage (μl) |
| 2X TaqMan PreAmp Master Mix (Applied BioSystems) | 2.5 | 132 | 264 |
| 500 nM (10X) pooled primer mixture | 1 | 52.8 | 105.6 |
| Water | 0.25 | 13.2 | 26.4 |
| cDNA | 1.25 | ||
| Total Volume | 5 |
Table 3. STA Reaction solution.
| Condition | Hold | 10-14 Cycles | Hold | |
| Temperature | 95 ºC | 95 ºC | 60 ºC | 4 ºC |
| Time | 10 min | 15 sec | 4 min | ∞ |
Table 4. Thermal cycle conditions for pre-amplification.
| component | Per 5 μL Sample (μl) | 48 Samples with Overage (μl) | 96 Samples with Overage (μl) |
| Water | 1.4 | 84 | 168 |
| Exonuclease I Reaction Buffer | 0.2 | 12 | 24 |
| Exonuclease I at 20 units/μl | 0.4 | 24 | 48 |
| Total Volume | 2.0 | 120 | 240 |
Table 5. ExoI reaction solution.
| SAMPLE MIX | Component Volume per Inlet (μl) | Volume per Inlet with Overage (μl) | Volume for 48.48 Dynamic Array IFC (μl) (120 samples) | Volume for 96.96 Dynamic Array IFC (μl) (120 samples) |
| 2X SsoFast EvaGreen Supermix with Low ROX (Bio-Rad, PN 172- 5211) | 2.5 | 3 | 180 | 360 |
| 20X DNA Binding Dye Sample Loading Reagent (Fluidigm, PN 100- 3738) green cap | 0.25 | 0.3 | 18 | 36 |
| STA and ExoI treated sample | 2.25 | 2.7 | ||
| Total | 5 | 6 |
Table 6. Sample Pre-Mix solution.
| Component | Volume per Inlet (μl) | Volume per Inlet with Overage (μl) | Volume for 48.48 Dynamic Array IFC (μl) | Volume for 96.96 Dynamic Array IFC (μl) |
| 2X Assay Loading Reagent | 2.5 | 3 | 180 | 360 |
| 1X DNA Suspension Buffer (TE low EDTA) | 2 | 2.4 | 144 | 288 |
| 50 μM each mixed Forward and Reverse Primers | 0.5 | 0.6 | ||
| Total Volume | 5 | 6 |
Table 7. Assay Mix solution.
| Name of the Reagent/Material | Company | Catalog Number |
| Virusol | Oriek Medical | J29D |
| Isoflurane, USP 100% | MINRAD INC | NDC 60307-110-25 |
| RNeasy plus Universal Mini Kit | QIAGENE | 73404 |
| QIAshredder | QIAGENE | 79654 |
| High Capacity cDNA Reverse Transcription kit | Invitrogene | AB-4368814 |
| TE Buffer | Invitrogene | 1355656 |
Table 8. List of chemicals/reagents.
| Name of Equipment | Company | Catalog Number |
| Behavior Chamber (MDF; 50 x 45 cm) | Self assembled | |
| Inner Perspex box (30 x 30 cm) | Self assembled | |
| Camera and video recorder | Campden Inst | CMD-80051 |
| Media Recorder software | Noldus | NDS-NMR3-00M |
| Iris Scissors | FST | FST-14062-09 |
| Vibratome | Campden Inst. | 7000SMZ-2 |
| Bioanalyzer | Agilent Technologies | The Agilent 2100 Bioanalyzer |
| Thermal cycler | Bio-Rad | 1852048 |
| Inverted microspun spatula | Bochem Instrument GmbH | 3213 |
| Biomark HD Reader | Fluidigm | BMHD-BMKHD |
| Dynamic array Chip for 96.96 gene expression | Fluidigm | BMK-M-96.96 |
Table 9. Equipment list.
Discussion
Successful characterization of gene expression from brain tissue following behavioral paradigms depends upon: 1) Careful handling of mice during the behavioral paradigm; 2) Quick and precise dissection of tissue of interest; 3) RNA-safe measures to ensure the integrity of RNA; and 4) Careful planning of primers and experimental layout as well as precision and attention to detail in preparation for the qPCR analysis.
The aim of the procedure described is to characterize dynamics of transcriptional events induced by a behavioral experience; therefore, careful attention is necessary in order to ensure that the behavioral paradigm experienced by the mouse indeed represents the experience intended by the researcher. For example, even in such a simple paradigm as behavioral sensitization to cocaine, the experience of the mouse could be influenced by many confounding factors: the prior experience of the mouse in the home-cage prior to the experiment; the method of transfer to the experimental room; the light, sound and odor exposure in the experimental room; the experience of handling by the experimenter; the pain/discomfort caused by the IP injection; exposure to a novel experiment, and the experience following the specified behavior up to the point of euthanasia. Each one of these experiences may, in isolation, promote induction of transcriptional programs in various brain regions of the mouse. Therefore, care must be taken to ensure that the transcriptional program measures the desired paradigm, and not epiphenomena associated with the paradigm. In the case of cocaine experience, this is easily achieved by careful experimentation and attention to detail, as well as a simple control experiment, in which all the parameters are maintained constant, but vehicle (saline) is injected to the mouse rather than cocaine. In our experience, saline injection induces a transcriptional program of very limited scale in comparison to cocaine exposure, demonstrating that the paradigm overwhelmingly enables investigation of the phenomena of interest.
Utmost care should be taken to limit the range of experiences of the mouse while it is still active, necessitating calm and careful handling of the mice. In contrast, following anesthesia, fast and precise work is essential due to the potential for rapid changes in the representation of RNA (on the timescale of minutes) and the intrinsic lability of RNA, which could be degraded rapidly once the cells are disrupted, potentially disrupting the transcriptional profile induced by experience. It is therefore important to remove the brain from the skull quickly to a chilled environment (within 1-2 min) and rapidly dissect the NAc in ice-cold conditions. Once the brain has been chilled, the potential for transcriptional changes or RNA degradation are significantly reduced, but only when the relevant sections are placed in trizol reagent and frozen, is the integrity of the tissue and the representation of the transcriptional profile ensured.
In this context, it is worthwhile mentioning that transcriptional dynamics of immediate-early gene expression occurs on a rapid time-scale, often peaking prior to 1 hr following stimulation. In the context of the experimental paradigm described, the interrogation of transcriptional dynamics is limited to the scale of hours in order to reduce variability between samples. For times shorter than 1 hr, small variations in the timing could result in large variation in the magnitude of transcriptional changes observed. Therefore the 1 hr time point has been chosen for analysis, since this time point is experimentally easier to perform with precision, and is less susceptible to variation at the level of a few minutes difference in the experiment.
Traditionally, a tissue micropunch has been used by many labs for dissection of tissue samples. There are a number of reasons to prefer manual microdissection of the tissue. The punch is normally made of stainless steel and is not transparent. Therefore positioning the punch in a precise and consistent location can be tricky. Furthermore, the punch does not allow any correction to be made if there was a mistake in the initial positioning. Finally, extraction of tissue from within the punch can sometimes prove tricky, and there is potential for losing tissue, or carry-over between samples. Microdissection of the tissue using a fine scalpel enables the experimenter more precise control and provides security regarding the purity of the samples.
During RNA extraction and up until cDNA synthesis, the working environment needs to be kept free from sources of RNase contaminants, and work should be performed using RNase free tools, disposables and reagents. At this stage, the smallest contamination could easily corrupt hard-earned RNA samples. Keeping the samples ice cold is crucial for minimizing neural activity that could modify the transcriptional readout, as well as to minimize enzymatic activity that could affect sample integrity. Additional pharmacological inhibitors are sometimes added to reduce neural excitability, but often these are not essential.
Following reverse-transcription to cDNA, the emphasis turns to ensuring the validity of the results obtained from the comprehensive profiling of transcripts. The first step is to ensure that appropriate qPCR primers are utilized, for which it is highly recommended to test primer efficiency and specificity on dilution curves of a defined source of RNA. Optimal PCR primers provide specific and efficient amplification of the target. Specific amplification implies that amplification is only of the target sequence, evident as a single discrete peak in melting curve analysis, while efficient amplification implies that in each cycle, the quantity of the target sequence is duplicated, with an efficiency approximating 100%. Because of the high-throughput nature of the microfluidic qPCR chips, these experiments require careful planning to ensure the inclusion of a significant number of positive and negative controls. It is preferable to include consistent controls in every chip run, enabling direct comparison of the results from control and experimental conditions. During the setup of the experiment, it is crucial not to cross-contaminate wells. Since tiny amount of primers in the wrong well could cause the amplification of the unwanted target, extra care should be taken when pipetting primers.
For most tissue samples from the brain it is difficult to be certain that during dissection only the tissue of interest was extracted, with no contamination of irrelevant brain regions, which could confound the analysis of the results. To this end, an important control is probing for genes whose expression is expected to be excluded from the tissue of interest. A useful tool for identifying spatial patterns of gene expression is the Allen Brain Atlas (http://www.brain-map.org/).
While a thorough discussion of data analysis is beyond the scope of this publication, it should be noted that careful experimentation warrants careful analysis. It is important to ensure that a number of relevant reference genes are included in every round of qPCR. These genes will serve for normalization in order to ensure that similar quantities of RNA are being assayed. Normalization is performed first to the reference genes within the sample, and then to the reference mice for the experiment (“time 0”), applying the “ΔΔCt method”.
The protocol described in this manuscript is aimed at studying transcriptional dynamics following cocaine experience in the nucleus accumbens of mice. However, it is very easily adapted for the study of any other experience of the mouse, as long as proper controls are implemented, and the timing of the experience is well defined. Obviously, this protocol could also be implemented for the study of dynamics of transcription in tissues outside the nervous system as well.
It should be emphasized that comprehensive transcriptional profiling using microfluidic-based qPCR is a cost-effective method of profiling transcriptional events, but depends upon prior knowledge of the target genes of interest. These are normally obtained through unbiased profiling methods, such as microarrays and deep sequencing. Therefore, in the workflow of a large project, comprehensive qPCR profiling could provide the bulk of the data, but preferentially should follow a prior unbiased characterization of the system.
It is also worth stating that the process described herein for obtaining tissue samples could also be implemented towards studying a variety of other cellular events – proteomics and protein modifications, metabolomics, lipidomics and epigenetic marks.
Disclosures
We have nothing to disclose.
Acknowledgments
This work has been funded by the Israel Science Foundation Grant (ISF # 393/12), Israel Centers of Research Excellence Grant (I-CORE 1796/12), German-Israel Foundation Grant (GIF # 2299-2291.1/2011) and the Marie Curie Career Integration Grant (FP7-PEOPLE-2013-CIG #618201). Initial steps in the project were funded by an AXA postdoctoral fellowship to AC. We acknowledge the generous startup funds provided by the Edmond and Lily Safra Center for Brain Sciences.
Critical reading by members of the Citri lab is greatly appreciated.
References
- Amit I, et al. A module of negative feedback regulators defines growth factor signaling. Nature genetics. 2007;39:503–512. doi: 10.1038/ng1987. [DOI] [PubMed] [Google Scholar]
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nature reviews. Molecular cell biology. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nature reviews. Neuroscience. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. Journal of speech, language, and hearing research. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature reviews. Neuroscience. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Current opinion in neurobiology. 2012;22:545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Koob GF, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience and biobehavioral reviews. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual review of neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Malenka RC. Enhanced inhibition of synaptic transmission by dopamine in the nucleus accumbens during behavioral sensitization to cocaine. The Journal of neuroscience. 2002;22:5817–5822. doi: 10.1523/JNEUROSCI.22-14-05817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Boening JA. Neurobiology of an addiction memory. Journal of neural transmission. 2001;108:755–765. doi: 10.1007/s007020170050. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. The Journal of clinical investigation. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, et al. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nature reviews. Neuroscience. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nature reviews. Neuroscience. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. The neurobiology of cocaine addiction. Science & practice perspectives / a publication of the. National Institute on Drug Abuse, National Institutes of Health. 2005;3:4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Current opinion in neurobiology. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Moore KA. The use of cognitive enhancers in animal models of fear extinction. Pharmacology, biochemistry, and behavior. 2011;99:217–228. doi: 10.1016/j.pbb.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Goldberg SR, Jaber M, Solinas M. Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology. 2012;63:635–641. doi: 10.1016/j.neuropharm.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nature reviews. Neuroscience. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Silingardi D, et al. ERK pathway activation bidirectionally affects visual recognition memory and synaptic plasticity in the perirhinal cortex. Frontiers in behavioral neuroscience. 2011;5:84. doi: 10.3389/fnbeh.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Majewska AK, Garcia R, Sur M. Structural dynamics of synapses in vivo correlate with functional changes during experience-dependent plasticity in visual cortex. The Journal of neuroscience. 2010;30:11086–11095. doi: 10.1523/JNEUROSCI.1661-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacological reviews. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Pang ZP, Sudhof TC, Wernig M, Malenka RC. Comprehensive qPCR profiling of gene expression in single neuronal cells. Nature protocols. 2012;7:118–127. doi: 10.1038/nprot.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]


