Abstract
The mechanisms controlling stress-induced phenotypic plasticity in animals are frequently complex and difficult to study in vivo. A classic example of stress-induced plasticity is the dauer stage of C. elegans. Dauers are an alternative developmental larval stage formed under conditions of low concentrations of bacterial food and high concentrations of a dauer pheromone. Dauers display extensive developmental and behavioral plasticity. For example, a set of four inner-labial quadrant (IL2Q) neurons undergo extensive reversible remodeling during dauer formation. Utilizing the well-known environmental pathways regulating dauer entry, a previously established method for the production of crude dauer pheromone from large-scale liquid nematode cultures is demonstrated. With this method, a concentration of 50,000 - 75,000 nematodes/ml of liquid culture is sufficient to produce a highly potent crude dauer pheromone. The crude pheromone potency is determined by a dose-response bioassay. Finally, the methods used for in vivo time-lapse imaging of the IL2Qs during dauer formation are described.
Keywords: Neuroscience, Issue 91, C. elegans, dauer, dendrite, arborization, phenotypic plasticity, stress, imaging, pheromone
Introduction
Phenotypic plasticity, including developmental and behavioral plasticity, is important for adaptations to adverse environmental conditions and is considered a driving force in evolution1. C. elegans is a model organism frequently used for studies in development and neurobiology. Under ideal conditions, C. elegans develops through four larval stages prior to entering the adult reproductive stage. However, under conditions of low food availability and high population densities, C. elegans can undergo a developmental switch and enter into a long-lived and stress-resistant dauer stage2. Dauers display several morphological and behavioral differences from non-dauers which likely mediate this stress resistance. The environmental cues and genetic pathways regulating dauer entry have been extensively described3, 4, 5, 6, 7. However, the molecular mechanisms controlling individual phenotypic changes that occur during dauer formation are less well understood.
The examination of dauer-specific phenotypes requires the production of dauer animals. This can be accomplished in three ways: 1) Picking from a starved culture of C. elegans, 2) Use of dauer formation constitutive (daf-c) mutants, 3) Induction of dauer formation through purified pheromone. It is relatively simple to pick dauers from a standard NGM plate with a C. elegans population that has exhausted its bacterial food-supply. In our hands, a standard NGM plate originally seeded with 50 µl of OP50 Escherichia coli, allowed to grow for 3 days and subsequently inoculated with 3 - 4 adult hermaphrodites kept at 25 °C will exhaust the food supply within one week and produce several thousand dauers within an additional 3 days (in addition to numerous starved non-dauers). However, this method is unsatisfactory for examining processes occurring during the molt into dauer. It is difficult to determine which of the several thousand animals on a typical starved plate are entering into dauer. Furthermore, use of a starved plate offers no possibility for synchronization. The use of daf-c mutants allows for synchronization and the reliable generation of dauers. However, there is no guarantee that a particular daf-c mutation will not affect other dauer-specific phenotypes alone or in combination with additional mutations.
The presence of a dauer pheromone was first implied by Cassada and Russell and later demonstrated by Golden and Riddle. The dauer pheromone has since been chemically characterized2, 8, 9, 10. The purified pheromone consists of a complex blend of ascarosides, which in different forms regulate both behavioral and developmental processes9, 11. Use of a crude dauer pheromone extract allows for reliable controlled induction of synchronized dauers in a wild-type background.
The nervous system and associated glial cells undergo remodeling during dauer larva formation12, 13, 14. Some of these changes are irreversible, such as the remodeling of glia cells during dauer13, 14. However other remodeling events are reversible upon a return to favorable environmental conditions. For example, a set of four IL2Q neurons undergo rapid and reversible neuronal remodeling during dauer formation including: dendrite arborization, a switch from single dendritic to multidendritic neurons and axon remodeling12. The methods herein allow for the reliable in vivo imaging of stress-induced neuroplasticity in a model organism. The methods presented for the production and testing of crude dauer pheromone are previously described and compiled from several sources4, 8, 15, 16, 17, 18.
Protocol
1. Crude Dauer Pheromone Production
Grow OP50 E. coli from a single colony O/N at 37 °C while shaking in 100 ml of LB broth at 200 rpm. NOTE: This O/N culture can be made in advance and stored at 4 °C.
Grow N2 C. elegans on 15 - 20 6 cm Petri dishes with NGM agar (See recipe in Table 1) seeded with 50 µl of OP50 E. coli until the bacteria is almost depleted19.
Make 5x 250 ml of S media (See recipe in Table 1) in 1 L Erlenmeyer flasks19. NOTE: This solution can be made in advance.
Grow a culture of OP50 E. coli in 4 L of LB broth O/N by inoculating with 1 ml of OP50 in E. coli and shaking at 37 °C for 16 hr.
Wash the nematodes from plates (step 1.2) with 1 ml of S basal solution (See recipe in Table 1) and transfer nematodes from 4-5 plates into each of 4 1 L Erlenmeyer flasks with the S media prepared in Step 1.3 (4-5 plates for each flask)19.
Centrifuge the OP50 from step 1.4 at 650 x g for 10 min and remove supernatant. Resuspend each bacterial pellet in 10 ml of S basal solution. Transfer equal amounts of resuspended bacteria to each flask of nematodes.
Place the flasks on an orbital shaker at room temperature (20 - 25 °C) and 100 - 150 rpm for 4 - 5 days until the solution begins to clear, indicating a depletion of food.
Once the food is depleted, grow another O/N culture of OP50 E. coli in 4x 1 L of LB broth. Centrifuge the bacteria at 650 x g for 10 min and add a pellet from one flask to each of the S media flasks with nematodes. Return the S media flasks to the orbital shaker, as in step 1.7, for an additional 3-4 days until the food is depleted again.
Take a ~1 ml aliquot of solution from each flask and estimate the nematode concentration by sub-sampling 1 µl from each 1 ml aliquot and counting the number of nematodes. NOTE: The nematode population should be 50,000 - 75,000 nematodes/ml. At this concentration the majority of nematodes are dauers.
Discard any contaminated flasks. NOTE: Contamination frequently manifests as fungal hyphae seen during step 1.9.
Centrifuge nematodes at 4,000 x g for 10 min at 4 °C. Discard the nematode pellet and retain the supernatant. Alternatively, see the discussion for using these animals to make a low-potency pheromone.
Filter the supernatant through #1 Whatman filter paper. NOTE: A vacuum flask will expedite this process.
Further filter the supernatant through 0.45 µm sterile vacuum filtration units. NOTE: Filtrate can be kept O/N at 4 °C or proceed to 1.14.
Add the filtrate to a 2 L beaker with a stir bar. Place in a fume hood on a heating stir plate. Bring to a boil while stirring and evaporate to approximately 200 ml. Transfer the solution to a 500 ml beaker. Continue boiling until approximately 50 ml are left. NOTE: Care should be taken during this step as it is possible for the solution to boil over. The color of the final 50 ml is a dark brown. At any time during the cooking process, the heat can be turned off and the filtrate stored O/N at RT or 4 °C.
Let cool and centrifuge for 5 min at 1,000 x g. Pour the supernatant into a 100 ml beaker and discard the pellet.
Boil the supernatant down to a brown crust. Remove from heat and break up the crust with a spatula. Add sufficient 200 proof ethanol to cover the crust. Cover the beaker with Parafilm and let it sit O/N at RT. Pour off the ethanol into a clean beaker and put to the side. Add fresh ethanol to cover the crust. Repeat O/N extractions 3 times. Pool the extracts.
Use a vacuum pump to dry the ethanol extracts. If a vacuum pump is not available, add the ethanol extract to a clean beaker and warm to 50 °C while stirring in a fume hood. NOTE: Once the ethanol has evaporated a sticky residue of light brown color should remain. If heating is used, care should be taken to not burn the solution by heating for too long.
Dissolve residue in 10 ml of distilled sterile water. Filter sterilize this solution with a 0.22 µm syringe filter and store at -20 °C in 1 ml aliquots. NOTE: 1 ml of water is used for dissolving the residue for every 100 ml of the original liquid nematode culture. For example, if one of the liquid culture flasks is discarded due to contamination, then adjust the amount of water used to dissolve the residue.
2. Determination of Pheromone Dose Response
Prepare dauer pheromone by making 6x 5 ml of NGM media with Noble agar, without peptone, and supplemented with 50 µg/ml streptomycin sulfate and a range of dauer pheromone (see recipe in Table 1)4, 19. Mix components in a 13x100 mm glass culture tube, boil over a Bunsen burner, and then pour into 3 cm Petri dishes.
Weigh a microcentrifuge tube. Add 1 ml of O/N culture of E. coli OP50 to the tube and centrifuge at ~17,000 x g in a microcentrifuge tube. Remove all of the supernatant. Weigh the tube again and determine the weight of the bacterial pellet. Resuspend the pellet with M9 buffer (See recipe in Table 1) for a final concentration of 4% (w/v). Aliquot 10 µl of the bacterial suspension onto the center of each pheromone plate4. Store plates O/N at RT.
Pick 10-15 gravid N2 wild-type hermaphrodites one day after the molt into adulthood onto the pheromone plates4. Store the plates at 25 °C for 3 - 4 hr.
Pick off all of the adult hermaphrodites. Record the number of eggs laid for each plate. Seal the plates with Parafilm and return to 25 °C for 3 days17.
Count the percentage of dauers (Figure 2). Note: For both methods it is important to note that dauers (and to a lesser extent non-dauers) will climb up the walls and onto the lid of Petri dishes. It is therefore important to examine all surfaces of the dish for nematodes.
Fit the data to a dose-response curve and estimate the EC90 concentration of pheromone (sufficient pheromone to induce 90% of animals to form dauers) as shown in Figure 3. Note: We typically use a logistic regression model to estimate the EC90, but different statistical software packages may have alternative methods that produce similar estimates.
3. Live Imaging of Dauer IL2 Neurons
Prepare dauer pheromone plates as in protocol 2 using the EC90 value determined in step 2.6.
Induce dauers using the methods in protocol 2 with a strain carrying an integrated IL2 reporter transgene (myIs13[Pklp-6::gfp], myIs14[Pklp-6::gfp], myIs16[Pklp-6::tdTomato] or qIs56[Plag-2::gfp])12, 20, 21. NOTE: If qIs56 is used, expression of GFP will not be visible in the IL2Qs until several hours into the dauer molt and some of the initial remodeling events will not be visible. Additionally, while the fluorescent signal from the Plag-2::gfp transgene is ultimately brighter in the IL2s during dauer than the Pklp-6 fluorescentreporters, the Plag-2::gfp transgene also expresses weakly in the head muscles which can mask the fine IL2 branches.
Following egg-laying, remove the adult hermaphrodites, seal the plates with Parafilm and incubate for 34 hr at 25 °C17. NOTE: The length of time to the dauer molt varies slightly among individuals. The most rapid period of dendrite arborization begins 4 - 5 hr following the onset of the dauer molt (39 - 44 hr post egg-laying at 25 °C)12.
The day before imaging, make up a solution of 10% (w/v) agarose in M9 buffer with dauer pheromone at the EC90 concentration. Aliquot approximately 100 µl of agarose onto a microscope slide and quickly, but gently, place another slide on top to create a flat agarose surface. After the agarose has solidified, wrap the slides in saran wrap and store in a humidity chamber (pipette tip box with wet paper towels).
After the 34 hr incubation period, examine pheromone plates for nematodes that have stopped pharyngeal pumping. Separate slides such that only one surface of one slide has agarose on it. Pick one or more nematodes onto a 10% agarose pad and 1 µl of 0.1 micron polystyrene beads22. NOTE: With a well-synchronized population, the majority of the nematodes on the plate will be entering into the molt from L2d to dauer. However, there may be variation among individuals. It is therefore important to note additional features (stoma occlusion, pharyngeal remodeling) to estimate the amount of time the animals have been within the dauer molt (see representative results for example).
Determine the orientation (left/right, dorsal/ventral) of the nematode. Capture Z-stack images of an IL2Q dendrite at 100x magnification with the lowest possible fluorescence intensity every 15 min or as needed for the specific experiment. Continue capturing images until the nematode has separated from the L2d cuticle and immobilization is impossible or if the animal recovers from dauer (pharynx begins pumping). When not actively imaging, place the slide in a humidity chamber. NOTE: We use an epifluorescent microscope with a motorized stage and differential interference contrast (DIC) optics.
Representative Results
The production of crude dauer pheromone results in a yellow liquid (Figure 1) that is both heat and cold stable. Aliquots of crude pheromone are stored at -20 °C indefinitely with no obvious loss in activity. Following the production of pheromone, a single dose-response bioassay is sufficient for a rough estimate of potency. However, for most experiments it is necessary to repeat the bioassay 2 - 3 times and take an average from each experiment. Representative results from two pheromone bioassays are given in Figure 3. From these data an EC50 and EC90 can be calculated.
In addition to resistance to sodium dodecyl sulfate2, dauers can be recognized by several morphological features including an occluded stoma, lateral alae, refractile bodies in the anterior hypodermis and a shrunken pharynx (Figure 2 and Figure 4A for L2 larvae). Dauers also have several distinctive behavioral characteristics including: a propensity to remain behaviorally quiescent, the presence of an occasional dispersal strategy called nictation, suppression of head foraging movements, suppression of pharyngeal pumping and the induction of excretory duct pulsing2, 5, 23. The behavioral characteristics show variability among individuals.
The molt into dauer takes approximately 12 hr from the end of the pre-dauer L2 stage (L2d)4. This period corresponds with stereotypical growth of the IL2Q arbors. The first noticeable event of the molt into dauer is the suppression of pharyngeal pumping. Suppression of pumping is common to all molts in C. elegans. However, suppression of pharyngeal pumping continues throughout the dauer stage and ends approximately 4 hr following a return of the animal to favorable environmental conditions. During the initial period of pharyngeal pumping suppression, a layer of cuticle forms over the stoma. These changes occur during the first hour following the onset of the molt into dauer. During this initial period there is frequently the formation of an additional neurite process extending from the IL2Q cell bodies. However, this initial additional process usually retracts within 2 hr following the onset of the molt into dauer12.
During hours 2 - 5 following the onset of the molt into dauer the pharynx begins to remodel showing a gradual reduction in size (Figure 4B). Concomitantly, short puncta form and retract along the IL2Q primary dendrite (Figure 5). During hours 4 - 8 following the onset of the molt, the body undergoes a gradual radial shrinkage resulting in the pulling away of the nematode from the L2d cuticle (Figure 4C). This period is correlated with outgrowth of 2° dendrites from the primary dendrites and the establishment of additional dendrites from the IL2Q cell bodies (dauer-specific primary dendrites—1d°). The dendritic growth is not constant, but rather characterized by both the dynamic formation and retraction of processes as illustrated in Figure 5. The 2° and 1d° dendrites generally extend to the ventral (for IL2Vs) or dorsal (for IL2Ds) midlines. Once the dauer cuticle is completely separated from the L2d cuticle, the application of fluorescent light causes the animal to rapidly spin on its longitudinal axis, making subsequent imaging challenging. Attempts to remove the developing animal from the slide, mechanically remove the L2D cuticle, and mount the animal on a new slide while still maintaining dauer development were not successful.
 Figure 1. Crude dauer pheromone is a yellow liquid that can be stored indefinitely at -20 °C.
Please click here to view a larger version of this figure.
Figure 1. Crude dauer pheromone is a yellow liquid that can be stored indefinitely at -20 °C.
Please click here to view a larger version of this figure.
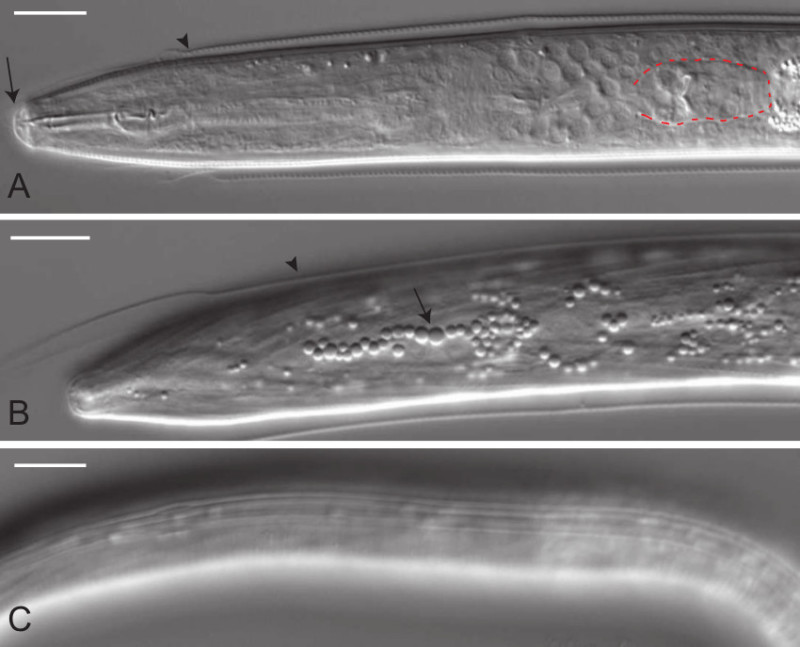 Figure 2. DIC micrographs of C. elegans dauers with typical dauer morphological features. (A) Dorsal mid-body view of a dauer demonstrating an occluded stoma (arrow) and a shrunken pharynx with rectangular terminal bulb (outlined in red, compare with L2 larva shown in Figure 4A). Newly formed dauers will frequently retain part of the L2d cuticle (arrowhead). (B) Dorsal view of hypodermis showing highly refractile bodies (arrow) typical of dauers. Again the L2d cuticle is evident (arrowhead). (C) Lateral cuticle view showing lateral alae, a set of four prominent longitudinal ridges that run from the anus to anterior of the nerve ring. Scale bars, 10 µm. Please click here to view a larger version of this figure.
Figure 2. DIC micrographs of C. elegans dauers with typical dauer morphological features. (A) Dorsal mid-body view of a dauer demonstrating an occluded stoma (arrow) and a shrunken pharynx with rectangular terminal bulb (outlined in red, compare with L2 larva shown in Figure 4A). Newly formed dauers will frequently retain part of the L2d cuticle (arrowhead). (B) Dorsal view of hypodermis showing highly refractile bodies (arrow) typical of dauers. Again the L2d cuticle is evident (arrowhead). (C) Lateral cuticle view showing lateral alae, a set of four prominent longitudinal ridges that run from the anus to anterior of the nerve ring. Scale bars, 10 µm. Please click here to view a larger version of this figure.
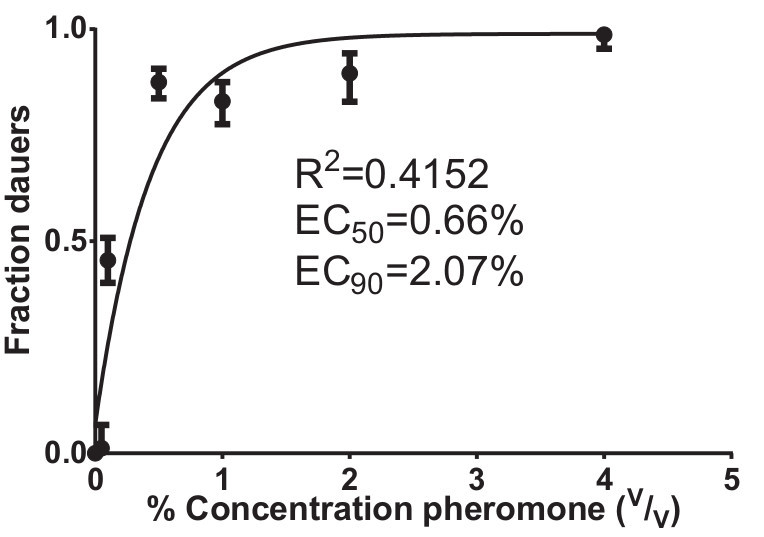 Figure 3. Dose response assay to crude dauer pheromone. L1 animals exposed to increasing levels of dauer pheromone incorporated into modified NGM media are more likely to form dauers. Data were collected and pooled from two separate experiments except for the following concentrations, which were only tested once: 0.05%, 2%, and 4%. Data were fit to a sigmoidal dose response curve. The R2 and EC values were calculated using logistic regression. The number of animals examined for each concentration is as follows: 0% n=299, 0.05% n=81, 0.1% n=352, 0.5% n=368, 1% n=241, 2% n=125, 4% n=155. Error bars represent 95% confidence intervals.
Figure 3. Dose response assay to crude dauer pheromone. L1 animals exposed to increasing levels of dauer pheromone incorporated into modified NGM media are more likely to form dauers. Data were collected and pooled from two separate experiments except for the following concentrations, which were only tested once: 0.05%, 2%, and 4%. Data were fit to a sigmoidal dose response curve. The R2 and EC values were calculated using logistic regression. The number of animals examined for each concentration is as follows: 0% n=299, 0.05% n=81, 0.1% n=352, 0.5% n=368, 1% n=241, 2% n=125, 4% n=155. Error bars represent 95% confidence intervals.
 Figure 4. DIC lateral right view micrographs of C. elegans during different stages of the molt into dauer. (A) L2 animal with typical rounded terminal pharyngeal bulb (outlined in red). (B) Approximately 3 hr following the molt into dauer, the terminal bulb is shrinking and the L2d cuticle is beginning to detach from the newly formed dauer cuticle (arrowhead). (C) Approximately 10 hr following the onset of the dauer molt, the animal has undergone extensive radial shrinkage and detachment from the L2d cuticle (arrowhead). Occasionally, the cuticle lined excretory duct of the L2d stage (arrow) can be seen still attached to the developing dauer. Scale bars, 10 µm. Please click here to view a larger version of this figure.
Figure 4. DIC lateral right view micrographs of C. elegans during different stages of the molt into dauer. (A) L2 animal with typical rounded terminal pharyngeal bulb (outlined in red). (B) Approximately 3 hr following the molt into dauer, the terminal bulb is shrinking and the L2d cuticle is beginning to detach from the newly formed dauer cuticle (arrowhead). (C) Approximately 10 hr following the onset of the dauer molt, the animal has undergone extensive radial shrinkage and detachment from the L2d cuticle (arrowhead). Occasionally, the cuticle lined excretory duct of the L2d stage (arrow) can be seen still attached to the developing dauer. Scale bars, 10 µm. Please click here to view a larger version of this figure.
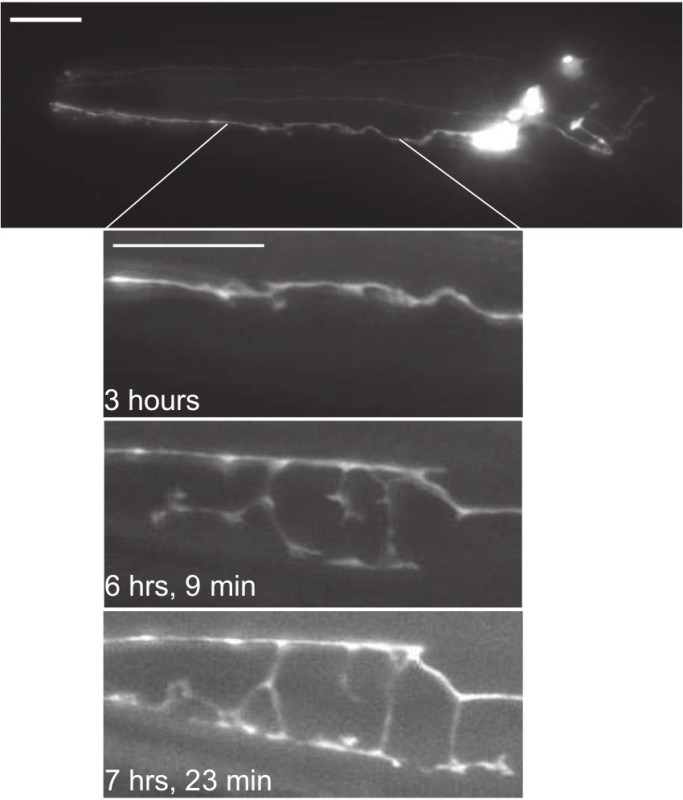 Figure 5. Lateral view epifluorescent micrographs of a single animal expressing the IL2 reporter Pklp-6::tdTomato. Top, full-length image of IL2 neurons approximately 3 hr following the onset of the molt into dauer. Inset shows portion of IL2Q dendrite at various time-points following the onset of the molt into dauer. A rapid extension of the IL2Q branches occurs starting approximately 5 hr following the onset of the molt into dauer. Scale bars, 10 µm. Please click here to view a larger version of this figure.
Figure 5. Lateral view epifluorescent micrographs of a single animal expressing the IL2 reporter Pklp-6::tdTomato. Top, full-length image of IL2 neurons approximately 3 hr following the onset of the molt into dauer. Inset shows portion of IL2Q dendrite at various time-points following the onset of the molt into dauer. A rapid extension of the IL2Q branches occurs starting approximately 5 hr following the onset of the molt into dauer. Scale bars, 10 µm. Please click here to view a larger version of this figure.
Discussion
An examination of dauer-specific neuroplasticity and other dauer-associated morphological changes requires controlled and reliable formation of dauers either through genetic mutation (i.e., daf-c mutants) or by exposure of wild-type animals to pheromone. While the use of a genetic mutation to induce dauers is convenient, it may confound results. Therefore data collected with daf-c mutations should be subsequently confirmed with wild-type animals induced to enter the dauer stage by exposure to dauer pheromone.
The production of crude dauer pheromone is relatively simple and variations of this protocol are published18. With the possible exception of contamination, the method presented here is very reliable. To produce the extract, we use a standard method of culturing large densities of nematodes in S media liquid culture19. There is a large amount of flexibility in the quantity of initial nematode inoculum added to the liquid culture. Starting with larger numbers of nematodes will expedite the procedure. However, it is important to ensure that all of the initial nematode inoculum is free of contamination. Both fungal and bacterial contamination may arise during growth in the liquid media. Fungal contamination can be easily detected by the presence of hyphae. Bacterial contamination is more difficult to assess. Typically a bacterial contaminant may be observed if the liquid culture does not clear soon after the listed incubation periods. There is flexibility in the total nematode population density needed to produce sufficient pheromone. For example, a concentration of 25,000 nematodes/ml is sufficient to produce a pheromone effective at inducing dauers. An alternative method consists of transferring the nematodes from a liquid culture to a small volume of M9 buffer (~50 ml) followed by an O/N incubation at RT on an orbital shaker and subsequent purification of the pheromone by the methods described herein. This alternative is effective, but produces a pheromone with a higher EC50 (i.e., less potent).
As each batch of crude pheromone may differ in potency, it is important to test a subsample for effectiveness in inducing dauer. For many applications a single bioassay with one replicate for each pheromone concentration is sufficient to provide a general estimate of potency. However, there can be significant variation between independently performed dose-response bioassays. Therefore, if experiments require a delicate balance between dauer formation and non-dauer development, it will be necessary to perform 2 - 3 replicate bioassays to determine an accurate effective dose. In the future, as the importance of ascarosides in nematode biology is more widely recognized, it may be possible to purchase synthesized dauer pheromone at reasonable cost.
The finding of dauer-specific neuronal remodeling in the IL2s provides a platform for studying the molecular mechanisms of stress-induced neuroplasticity. To identify the mechanism by which specific genes regulate neuroplasticity, it may be necessary to follow the remodeling process in real-time. The method of IL2 in vivo imaging builds upon previous C. elegans methods22, 24, 25. One obstacle to imaging during dauer formation is the possibility of dauer recovery. The easiest solution is to initially use a daf-c mutation. Confirmation of any results can then be performed in a non-daf-c background using the methods described herein.
Singh and Sulston noted several obstacles for the in vivo imaging of dauer formation25. These included newly formed dauers escaping off the microscope slide and uncontrolled movement following radial shrinkage. The use of microsphere beads has eliminated the possibility of escape22. However the radial shrinkage that occurs approximately 10 hr into the dauer molt still creates difficulties for subsequent imaging.
Disclosures
The authors have nothing to disclose
Acknowledgments
I thank Dr. Maureen Barr, under whose supervision the dauer IL2 remodeling phenotype was initially characterized. Thanks to Dr. C. Britt Carlson for critical reading of the manuscript. Funding in the Barr lab was from the NIH (5R01DK59418) and postdoctoral fellowships from the USDA (2010-65106-20587) and the New Jersey Commission on Spinal Cord Research (CSCR12FEL004). Current funding for this work is from a University of Illinois Urbana-Champaign College of ACES FIRE grant.
References
- West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford University Press; 2003. [Google Scholar]
- Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Albert PS. In: C. elegans II. Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. Plainview, NY: Cold Spring Harbor Laboratory Press; 1998. pp. 739–768. [Google Scholar]
- Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- Riddle DL. In: The Nematode C. elegans. Wood WB, editor. Plainview, NY: Cold Spring Harbor Laboratory Press; 1988. pp. 393–412. [Google Scholar]
- Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu PJ. WormBook. 2007. pp. 1–19. [DOI] [PMC free article] [PubMed]
- Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nature Chemical Biology. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- Jeong P, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, et al. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder N, et al. Dauer-Specific dendrite arborization in C. regulated by KPC-1/Furin. Curr. Biol. 2013;16:1527–1535. doi: 10.1016/j.cub.2013.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procko C, Lu Y, Shaham S. Glia delimit shape changes of sensory neuron receptive endings in C. elegans. Development. 2011;138:1371–1381. doi: 10.1242/dev.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PS, Riddle DL. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J. Comp. Neurol. 1983;219:461–481. doi: 10.1002/cne.902190407. [DOI] [PubMed] [Google Scholar]
- Vowels JJ, Thomas JH. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics. 1992;130:105–123. doi: 10.1093/genetics/130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowels JJ, Thomas JH. Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics. 1994;138:303–316. doi: 10.1093/genetics/138.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailion M, Thomas JH. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics. 2000;156:1047–1067. doi: 10.1093/genetics/156.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal SJ, Kim K, Sengupta P. Pheromone Signaling. Springer; 2013. pp. 273–283. [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet J, Li S, Roy R. Notch signalling is required for both dauer maintenance and recovery in C. elegans. Development. 2008;135:2583–2592. doi: 10.1242/dev.012435. [DOI] [PubMed] [Google Scholar]
- Blelloch R, et al. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev. Biol. 1999;216:382–393. doi: 10.1006/dbio.1999.9491. [DOI] [PubMed] [Google Scholar]
- Fang-Yen C, Gabel CV, Samuel AD, Bargmann CI, Avery L. Laser microsurgery in Caenorhabditis elegans. Methods Cell Biol. 2012;107 doi: 10.1016/B978-0-12-394620-1.00006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson FK, Riddle DL. Functional study of the Caenorhabditis elegans secretory-excretory system using laser microsurgery. J. Exp. Zool. 1984;231:45–56. doi: 10.1002/jez.1402310107. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Singh R, Sulston J. Some observations on moulting in Caenorhabditis elegans. Nematologica. 1978;24:63–71. [Google Scholar]


