Abstract
Dopaminergic neurons represent less than 1% of the total number of neurons in the brain. This low amount of neurons regulates important brain functions such as motor control, motivation, and working memory. Nigrostriatal dopaminergic neurons selectively degenerate in Parkinson's disease (PD). This progressive neuronal loss is unequivocally associated with the motors symptoms of the pathology (bradykinesia, resting tremor, and muscular rigidity). The main agent responsible of dopaminergic neuron degeneration is still unknown. However, these neurons appear to be extremely vulnerable in diverse conditions. Primary cultures constitute one of the most relevant models to investigate properties and characteristics of dopaminergic neurons. These cultures can be submitted to various stress agents that mimic PD pathology and to neuroprotective compounds in order to stop or slow down neuronal degeneration. The numerous transgenic mouse models of PD that have been generated during the last decade further increased the interest of researchers for dopaminergic neuron cultures. Here, the video protocol focuses on the delicate dissection of embryonic mouse brains. Precise excision of ventral mesencephalon is crucial to obtain neuronal cultures sufficiently rich in dopaminergic cells to allow subsequent studies. This protocol can be realized with embryonic transgenic mice and is suitable for immunofluorescence staining, quantitative PCR, second messenger quantification, or neuronal death/survival assessment.
Keywords: Neurobiology, Issue 91, Mus musculus, mesencephalon, embryonic, tyrosine hydroxylase, dopamine transporter, Parkinson's disease in vitro model
Introduction
Dopamine, one of the essential brain neurotransmitters1,2, is mainly released by midbrain dopaminergic (DA) neurons. The majority of DA neurons reside in the ventral part of the mesencephalon2-6. Schematically, midbrain DA neurons can be divided in three anatomically and functionally distinct projection systems: mesostriatal, mesolimbic, and mesocortical pathways2,5. The nigrostriatal pathway is involved in motor behavior, the mesolimbic pathways play an important role in reinforcement, motivation, and learning, whereas the dopaminergic pathways projecting to the prefrontal cortex are implicated in cognition2.
DA neurons are involved in several human neurological disorders such as schizophrenia, attention deficit, hyper activity disorder, and Parkinson’s disease (PD)2,4. PD is characterized by a progressive and selective degeneration of DA neurons connecting substantia nigra pars compacta (SNc) to the striatum. The loss of nigro-striatal DA neurons results in severe dopamine depletion in the striatum that is responsible of the motor symptoms of PD (bradykinesia, resting tremor, and rigidity)7. The initial cause of the idiopathic PD has not been established and the current treatments are only symptomatic, aiming at restoring dopamine level in the striatum. The most prescribed drug is L-Dopa (Levodopa), the natural precursor of dopamine. Though administration of Levodopa compensates for the loss of dopamine for a certain time, motor complications occur after long-term treatments (dyskinesia and on/off states)8,9.
Research on dopaminergic neurons and PD is in constant progression and intense efforts are being made to develop treatments based on cell transplantation, gene therapy, or neuroprotective agents10,11. However, a major issue remains non-elucidated: what is the cause of the extreme vulnerability of DA neurons? Part of the answer can be found in the activity of DA neurons. A reduction in the electrical activity and of the excitability of DA neurons seems to augment their propensity to degenerate12. Nevertheless, the complexity of PD pathogenesis requires further studies to identify the mechanisms involved in DA neurons degeneration13-15.
Primary cultures are especially relevant to study DA neuron properties16-19 and to challenge these neurons to various stresses for evaluation of neuroprotective agents20-24. Rat culture models are most often used, as the dissection of rat embryo mesencephalon is easier, compared with the mouse, and higher amounts of neurons can be obtained in the rat. However, generation of transgenic mouse models of the disease25 has considerably increased the interest of the neuroscientist community for primary cultures from the mouse26-29. Although cultures prepared from newborn animals can be used, it is better to prepare them from embryos at the post-mitotic stage (E13.5 for mesencephalon neurons), when neurons have retained their capacity to differentiate. The following protocol presents isolated mesencephalon neurons in primary culture from mouse embryos (E13.5), which are the most difficult to prepare. Notably, we provide a protocol using serum-free culture medium for a better reproducibility. The two most critical steps in culture preparation (dissection and mechanical dissociation) will be carefully detailed in the associated video.
Protocol
The mice used in this work were cared for and handled in accordance with the guidelines of the European Union Council (86/609/EU) for the use of laboratory animals.
1. Preparation of Required Solutions
- Stock Solutions
- 10x Poly-L-Ornithine (PLO) Solution: weigh out 10 mg of PLO hydrobromide (molecular weight = 30,000-70,000) and dissolve in 70 ml of sterile water. Filter the solution using 0.2 µm syringe filter, aliquot, and store at -20 °C.
- 30% Glucose Solution: weigh out 30 g of D(+)-Glucose and dissolve in sterile water to a total volume of 100 ml. Filter, aliquot, and store at 4 °C.
- 5x Dulbecco’s Modified Eagle’s Medium (DMEM)/Nutrient Mixture F-12 Ham: weigh out 6 g of DMEM/F-12 Ham powder and dissolve in sterile water to a total volume of 100 ml. Filter, aliquot, and store at 4 °C.
- 7.5% Sodium Bicarbonate (NaHCO3) Solution: weigh out 7.5 g of NaHCO3 and dissolve in sterile water to a total volume of 100 ml. Filter, aliquot, and store at 4 °C.
- 1 M HEPES Solution: Weigh out 23.8 g of HEPES and dissolve in sterile water to a total volume of 100 ml. Filter, aliquot, and store at 4 °C.
- 70% (v/v) Ethanol: Dilute 70 ml of absolute ethanol with 30 ml of sterile water.
Phosphate Buffered Saline with Glucose and Antibiotics (PBS-GAB): add 10 ml of 30% glucose solution and 5 ml of penicillin-streptomycin solution to 500 ml of Dulbecco’s Phosphate Buffered Saline. Store at 4 °C.
Inactivated Fetal Bovine Serum (iFBS): Thaw bovine serum overnight at 4 °C and inactivate at 56 °C for 30 min. Aliquot into 50 ml and store at -20 °C.
Culture Medium: In sterile water, mix successively 40 ml of 5x DMEM/F12-Ham, 1 ml of 1 M HEPES, 2 ml of 200 mM L-Glutamine, 2 ml of penicillin-streptomycin, 4 ml of 30% glucose and 3 ml of 7.5% NaHCO3 to a final volume of 180 ml. Filter and use the same day.
- Hormone Mix
- Prepare 180 ml of culture medium. Dissolve 200 mg of apo-transferrin in this medium.
- Weigh out 50 mg of insulin and dissolve in 2 ml of 0.1 M HCl. Add slowly 8 ml of sterile water while mixing gently. Dilute this solution in the culture medium.
- Weigh out 19.3 mg of Putrescine dihydrochloride and dissolve in 10 ml of sterile water. Add the 10 ml solution to the hormone mix.
- Prepare a 3 mM sodium selenite solution in sterile water and a 2 mM progesterone solution in absolute ethanol. Add 20 μl of each solution to the hormone mix.
- Filter the hormone mix, aliquot into 10 ml and store at -20 °C.
2. Preparation of Culture Plates and Instruments
FBS Coating of the Culture Plates: The day prior the dissection, prepare 180 ml of culture medium. Add 10 ml of iFBS to 90 ml of culture medium. Add 300 μl/well of culture medium/10% iFBS into poly-D-lysine precoated 24-well plates. Incubate overnight at 37 °C. Store the remaining media (with and without iFBS) at 4 °C.
Sterilize the instruments and Pasteur pipettes. Gather the equipment listed in Materials table as well as a Class II laminar flow hood and a CO2 incubator.
3. Dissection of Mouse Mesencephalon
Sacrifice an E13.5 pregnant mouse (according to institutional guidelines). Clean the abdomen of the mouse with 70% ethanol and open the abdomen wall. Collect the uterine horns, open them using delicate scissors, dissect each embryo from the uterine horns, and remove the amniotic membranes. Place the embryos in a 100 mm Petri dish containing sterile PBS-GAB and wash them by transfer in 3 successive identical baths using forceps. For dissection, place the embryos by 3 in a 60 mm sterile Petri dish.
- Move the final Petri dish under the stereomicroscope. Do not behead the embryos. Using Vannas scissors, excise the brains.
- Carefully remove and discard the fore- and hindbrain regions. To the rostral side, cut close to the thalamic region, to the caudal side cut at the isthmus region, remove the superior colliculus.
- Once the ventral midbrain is isolated, carefully remove meninges using ultra fine forceps. Collect the dissected segments, without the meninges, in a sterile 13 ml tube filled with PBS-GAB.
Clean the tube containing the mesencephala thoroughly with 70% ethanol and bring it under the hood.
4. Cell Dissociation
Wash mesencephala 3x with sterile PBS-GAB. Allow brain fragments to settle between each wash and avoid disrupting the tissue. After the last wash, remove carefully as much solution as possible and incubate brain segments in 3 ml of Trypsin/EDTA for 15 min at 37 °C in a CO2 incubator.
Fire-polish the Pasteur pipettes to maximize the survival of the neurons as the end of a non-polished glass pipet is sharp and can damage the cells during the dissociation steps. Slightly reduce the extremity diameter (up 0.5 mm) of the Pasteur pipette while fire-polishing it.
Carefully remove as much Trypsin/EDTA solution as possible and add 10 ml of culture medium/10% iFBS. Wash brain segments 3x with culture medium/10% iFBS.
Using the fire-polished Pasteur pipette, begin dissociation of mesencephala in 6 ml of culture medium/10% iFBS. Triturate 10x (avoid air bubbles), allow the chunks to settle, then collect the medium in a new sterile 13 ml tube.
Add 6 ml of culture medium, repeat the previous step once and collect the medium containing dissociated cells in the same 13 ml tube.
Centrifuge cells at 160 g for 5 min. Discard the supernatant and resuspend the cells in culture medium supplemented with 10% hormone mix.
5. Cell Plating
Count cells in suspension in a Malassez cell and adjust the volume of medium to a concentration of 600,000 cells/ml. Around 1,700,000 cells can be obtained from one mouse mesencephalon.
Remove FBS-containing coating medium from 24-well plates and add in each well 1 ml of cell suspension (600,000 cells/well, 2-4% of TH+ cells).
Incubate the plate at 37 °C and 5% CO2/95% air. No medium change is necessary. Dopaminergic neurons are mature after around 5-7 days in vitro (consistent expression of the dopamine transporter). Keep cells in culture up to 15 days without medium replacement.
6. Immunofluorescence Protocol
- Cleaning of the Coverslips
- The day before the dissection, add 10 ml of 1 M HCl into a Petri dish. Lay at the surface of the liquid 12-15 glass coverslips and wait 15 min.
- Sink the coverslip into the liquid and wait for 15 min.
- Remove HCl and wash 3x with water.
- Wash the coverslips quickly with pure ethanol once, then add pure ethanol to the dish and wait for 30 min.
- Under the hood, remove cleaned coverslips from ethanol and add 1 coverslip per well of a 12-well cell culture plate using sterilized forceps.
- Wash coverslips twice with sterile water (1 ml/well). Then, wash them once with sterile PBS.
- Coating of the Coverslips
- Remove PBS and add 500 μl/well of 2x PLO (diluted in PBS). Incubate for 4 hr at 37 °C in the CO2 incubator.
- Remove the PLO solution and wash 3x with sterile PBS.
- Remove PBS and add 500 μl/well of 20% iFBS/1 g/ml Laminin. Incubate overnight at 37 °C in the CO2 incubator.
- Plating the Cells for Immunofluorescence
- Remove coating medium from 12-well plates.
- Add 900,000 cells/well in 2 ml volume and grow the cells at 37 °C in the incubator. Cells can be kept in culture up to 15 days without medium replacement.
- Immunostaining
- Remove medium from 12-well plates and wash with 500 μl/well DMEM preheated at 37 °C.
- Add 500 μl/well of DMEM preheated at 37 °C, then add gently 160 μl/well of 8% paraformaldehyde (PFA, 2% final concentration) and incubate for 10 min at 37 °C.
- Remove PFA and wash 3x with PBS/0.1 M glycine for 10 min.
- Remove PBS, add 300 μl/well of PBS/0.05% Triton X-100/20% Goat serum and incubate for 30 min at room temperature.
- Remove medium, add 300 μl/well of primary antibody diluted in PBS/0.05% Triton X-100/1% Goat serum and incubate for 2 hr at room temperature. Primary antibodies that can be used for the detection of dopaminergic neurons and characterization of the culture are indicated in the Materials table. Keep 1 well without primary antibody to assess for background of the different secondary antibodies.
- Remove medium and wash 3x with PBS/0.2% gelatine for 10 min.
- Remove medium, add 300 μl/well of secondary antibody diluted in PBS/0.05% Triton X-100/1% Goat serum and incubate for 1 hr at room temperature, in the dark. Secondary antibodies used are indicated in the Materials table.
- Remove medium and wash 3x with PBS/0.2% gelatine for 10 min.
- Remove medium and wash 3x with PBS.
- Mount the coverslips on glass slides cell side down in a drop of VECTASHIELD. Keep the slides at room temperature overnight to let the mounting medium dry, then store them at 4 °C.
- Acquire images using a confocal microscope or a phase-contrast microscope equipped for epifluorescence. Representative images were acquired with a Leica SP2 UV confocal microscope.
Representative Results
An illustrated flow chart of the mesencephalon culture steps is shown in Figure 1. Briefly, after collecting E13.5 embryos from a pregnant Swiss mouse, ventral mesencephalon is dissected from the entire embryo. The isolated brain fragments are successively submitted to enzymatic digestion and mechanical dissociation. Dissociated cells are pelleted by centrifugation, resuspended in culture medium and plated in pre-coated 12- or 24-well plates. Cells are maintained up to 15 days without medium replacement.
A detailed flow chart of the ventral mesencephalon dissection, corresponding to step 3.2, is shown in Figure 2. Dashed red lines indicate the 3 main cutting lines on a schematic representation of E13.5 mouse brain (adapted from Prestoz et al.30) and cutting steps are illustrated.
Phase contrast images of the culture after 1, 4, and 7 days in vitro (DIV) are shown in Figure 3. Neurons quickly develop extensions and sprouting (Figure 3A). Branching and ramifications are more extended at DIV4 and DIV7 (Figures 3B-3C).
Dopaminergic neurons are detected by immunocytochemistry using an anti-TH antibody (Figure 4A). The number of TH+ cells is expressed per 2.5 cm2 coverslip (Figure 4B). We do not express the number of TH+ cells per well as the density of cells is much higher on the borders of the coated plastic well than on the coated glass coverslip, that is on the center on the well. DA neurons (TH+ cells) represent 2-4% of the whole cell population on the coverslip. TH-positive (TH+) cells appear as early as DIV1 and their number increases rapidly to reach a maximum at DIV6.
The relative proportion of cells is assessed by immunofluorescence labeling of DA cells with an anti-DAT antibody (Figure 5A) or an anti-TH antibody (Figure 5B) and concomitant staining of neuronal cells with anti-MAP2 antibody. Representative immunostaining of several neuronal populations present in the culture is also shown in Figure 5. GABAergic neurons are stained using an anti-GAD67 antibody (Figure 5C) and represent around 50% of the cells. Serotonergic neurons are detected with an anti-serotonin antibody (Figure 5D) and represent less than 1% of the cells in the culture. The mesencephalon cell culture also contains glutamatergic neurons (40% of the culture), cholinergic neurons, and rare glial cells (around 2-3%). Proportion of of glial cells is determined using glial fibrillary acidic protein (GFAP) staining (Figure 5E). Unambiguous identification of mesencephalon dopaminergic neurons can also be achieved using specific markers such as Pitx3 or Foxa231,32.
Higher magnification immunostaining of several neuronal populations present in the culture is shown in Figure 6. Dopaminergic neurons are detected using an anti-DAT antibody (Figure 6A). DAT staining reflects DA neuron maturation state. DAT expression begins at DIV3-4. GABAergic neurons and serotonergic neurons are stained using an anti-GAD67 antibody (Figure 6B) or an anti-serotonin antibody (Figure 6C), respectively.
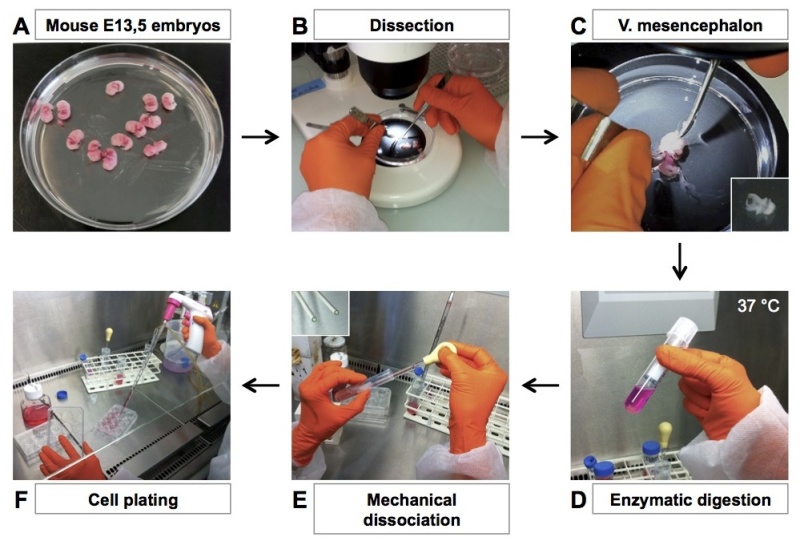 Figure 1. Illustrated flow chart of the mesencephalon culture. Main culture steps are indicated. (A) Sacrifice a pregnant Swiss mouse, collect E13.5 embryos in Petri dishes and wash them by successive PBS baths. (B-C) Under dissection microscope, dissect ventral mesencephalon from the entire embryos and place them in a tube. (D) Add Trypsin-EDTA to the dissected brain fragments and digest at 37 °C for 15 min. (E) Remove trypsin, add serum containing-culture medium and perform mechanical cell dissociation (10 trituration movements, repeated twice). (F) Pellet the cell, resuspend them in serum-free medium supplemented with hormones and plate them in precoated 12- or 24-well plates. Please click here to view a larger version of this figure.
Figure 1. Illustrated flow chart of the mesencephalon culture. Main culture steps are indicated. (A) Sacrifice a pregnant Swiss mouse, collect E13.5 embryos in Petri dishes and wash them by successive PBS baths. (B-C) Under dissection microscope, dissect ventral mesencephalon from the entire embryos and place them in a tube. (D) Add Trypsin-EDTA to the dissected brain fragments and digest at 37 °C for 15 min. (E) Remove trypsin, add serum containing-culture medium and perform mechanical cell dissociation (10 trituration movements, repeated twice). (F) Pellet the cell, resuspend them in serum-free medium supplemented with hormones and plate them in precoated 12- or 24-well plates. Please click here to view a larger version of this figure.
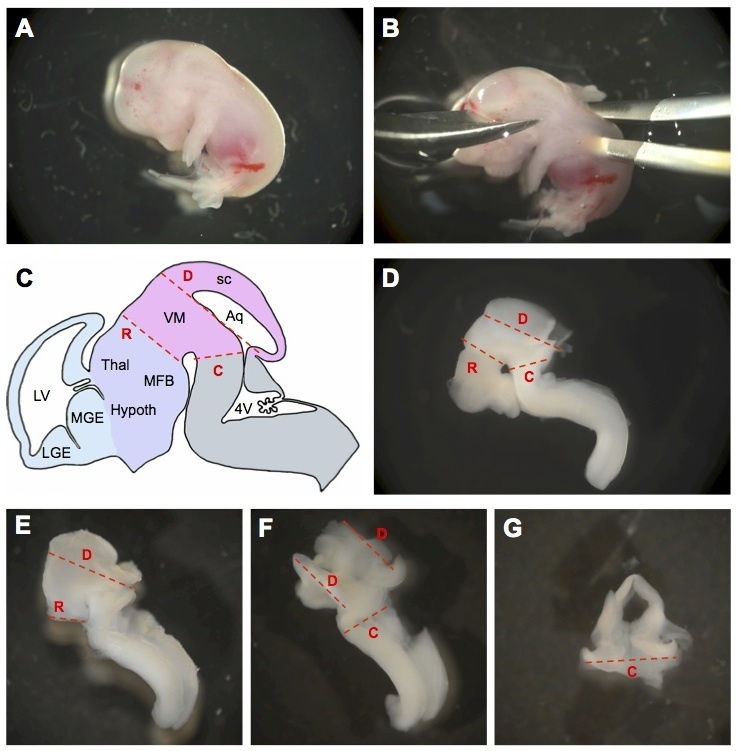 Figure 2. Detailed flow chart of the mesencephalon dissection. Main dissection steps are indicated. (A) Mouse embryo at E13.5 after removal from the uterine horns. (B) Without beheading the embryo, excise the brain. (C) Schematic representation of embryonic mouse brain at E13.5. Red dashed lines indicate where the brain should be cut to isolate ventral mesencephalon (R, rostral cut; C, caudal cut; D, dorsal cut). The cephalic vesicles telencephalon, diencephalon, mesencephalon, and rhombencephalon are delimited in blue, purple, pink, and gray, respectively. Aq, aqueduct; Hypoth, hypothalamus; LGE, lateral ganglionic eminence; LV, lateral ventricle; MFB, medial forebrain bundle; MGE, medial ganglionic eminence; sc, superior colliculus; Thal, thalamus; VM, ventral mesencephalon; 4V, fourth ventricle (adapted from30). (D) Cutting lines are indicated by red dashed lines on an E13.5 mouse brain. (E) Mouse brain after removal of the fore- and hindbrain regions (i.e. after cut R). (F) Mouse brain after removal of the superior colliculus (i.e. after cuts R and D). (G) Ventral view of an isolated mesencephalon (i.e. after cuts R, C, D). Please click here to view a larger version of this figure.
Figure 2. Detailed flow chart of the mesencephalon dissection. Main dissection steps are indicated. (A) Mouse embryo at E13.5 after removal from the uterine horns. (B) Without beheading the embryo, excise the brain. (C) Schematic representation of embryonic mouse brain at E13.5. Red dashed lines indicate where the brain should be cut to isolate ventral mesencephalon (R, rostral cut; C, caudal cut; D, dorsal cut). The cephalic vesicles telencephalon, diencephalon, mesencephalon, and rhombencephalon are delimited in blue, purple, pink, and gray, respectively. Aq, aqueduct; Hypoth, hypothalamus; LGE, lateral ganglionic eminence; LV, lateral ventricle; MFB, medial forebrain bundle; MGE, medial ganglionic eminence; sc, superior colliculus; Thal, thalamus; VM, ventral mesencephalon; 4V, fourth ventricle (adapted from30). (D) Cutting lines are indicated by red dashed lines on an E13.5 mouse brain. (E) Mouse brain after removal of the fore- and hindbrain regions (i.e. after cut R). (F) Mouse brain after removal of the superior colliculus (i.e. after cuts R and D). (G) Ventral view of an isolated mesencephalon (i.e. after cuts R, C, D). Please click here to view a larger version of this figure.
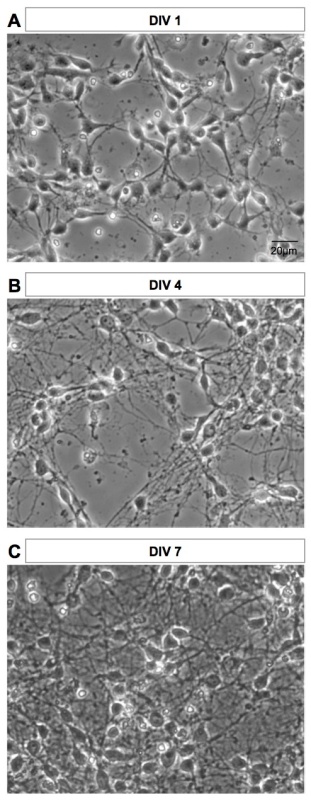 Figure 3. Phase contrast images of the culture at different stages of development. (A) DIV1, (B) DIV4, and (C) DIV7 images of the same culture are shown. Images were acquired on live cells with an inverted microscope. Please click here to view a larger version of this figure.
Figure 3. Phase contrast images of the culture at different stages of development. (A) DIV1, (B) DIV4, and (C) DIV7 images of the same culture are shown. Images were acquired on live cells with an inverted microscope. Please click here to view a larger version of this figure.
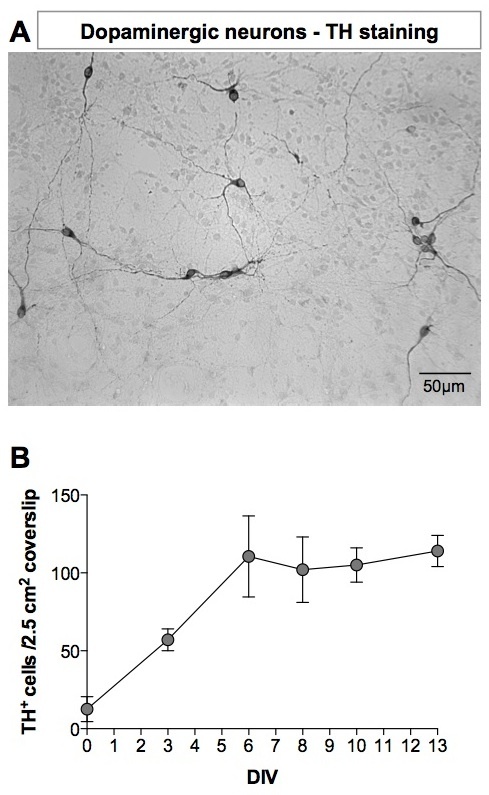 Figure 4. Tyrosine hydroxylase staining of dopaminergic neurons. (A) At DIV8, DA neurons were detected using anti-TH antibody. Revelation was performed using a 3,3'-Diaminobenzidine (DAB) kit. The image was acquired with an inverted microscope. (B) Number of TH+ neurons in mesencephalon cultures as a function of the age of the culture. Please click here to view a larger version of this figure.
Figure 4. Tyrosine hydroxylase staining of dopaminergic neurons. (A) At DIV8, DA neurons were detected using anti-TH antibody. Revelation was performed using a 3,3'-Diaminobenzidine (DAB) kit. The image was acquired with an inverted microscope. (B) Number of TH+ neurons in mesencephalon cultures as a function of the age of the culture. Please click here to view a larger version of this figure.
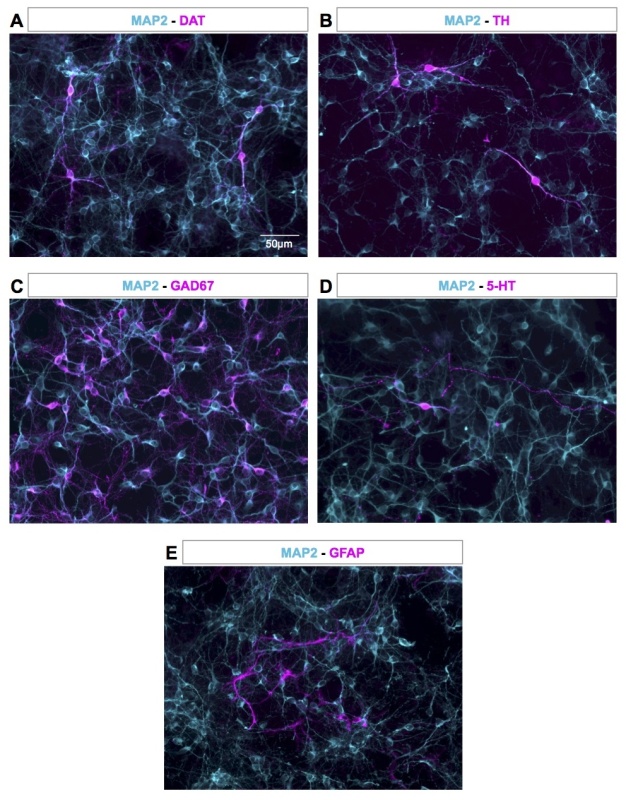 Figure 5. Representative proportion of neurons and astrocytes in the culture. At DIV4, DA neurons (in magenta) were detected using anti-DAT (A) or anti-TH antibody (B) and GABAergic neurons, serotonergic neurons and astrocytes (in magenta) were detected using anti-GAD67 (C), anti-serotonin (D), and anti-GFAP (E) antibodies, respectively. Neuronal cells (in cyan) were stained with an anti-MAP2 antibody (A-E). Images were acquired with an inverted microscope. Please click here to view a larger version of this figure.
Figure 5. Representative proportion of neurons and astrocytes in the culture. At DIV4, DA neurons (in magenta) were detected using anti-DAT (A) or anti-TH antibody (B) and GABAergic neurons, serotonergic neurons and astrocytes (in magenta) were detected using anti-GAD67 (C), anti-serotonin (D), and anti-GFAP (E) antibodies, respectively. Neuronal cells (in cyan) were stained with an anti-MAP2 antibody (A-E). Images were acquired with an inverted microscope. Please click here to view a larger version of this figure.
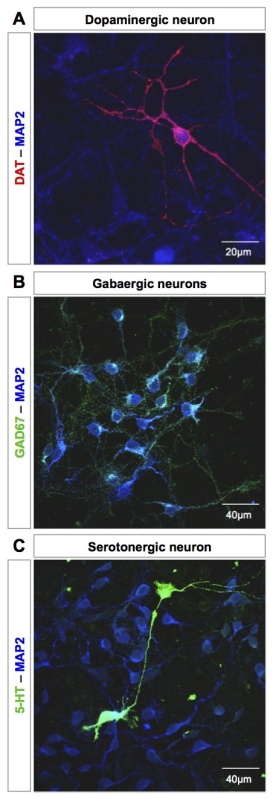 Figure 6. Representative staining of several cell populations of the mesencephalon culture. (A) Dopaminergic neuron detected by DAT staining at DIV9 (in red). (B) GABAergic neurons visualized by GAD-67 staining at DIV9 (in green). (C) Serotonergic neuron stained with an anti-serotonin antibody at DIV9 (in green). Neurons are identified by MAP2 staining (in blue, A-C). Images were acquired with a UV confocal microscope. Please click here to view a larger version of this figure.
Figure 6. Representative staining of several cell populations of the mesencephalon culture. (A) Dopaminergic neuron detected by DAT staining at DIV9 (in red). (B) GABAergic neurons visualized by GAD-67 staining at DIV9 (in green). (C) Serotonergic neuron stained with an anti-serotonin antibody at DIV9 (in green). Neurons are identified by MAP2 staining (in blue, A-C). Images were acquired with a UV confocal microscope. Please click here to view a larger version of this figure.
Discussion
This protocol presents the procedures and reagents necessary to prepare a primary culture of mesencephalic neurons from the embryonic mouse and the immunofluorescence procedure to detect dopaminergic neurons. Critical steps of the procedure are the dissection of the embryos and the mechanical dissociation of the collected brain fragments. High quality dissection instruments helps to master the dissection technique. DA neurons constitute a small proportion of mesencephalon. Accordingly, collecting the right part of the ventral mesencephalon is essential to obtain a culture that contains 2-4% of DA neurons. Mechanical dissociation should be performed carefully and gently. If the culture contains clusters of non-dissociated neurons, add one or two more trituration steps.
This protocol uses serum-free medium for neuron culture. However, coating with serum is necessary to enhance attachment of the cells. Hormone mix, which replaces the serum, is defined to allow neuron growth and to minimize glial cells survival and proliferation. Consequently, non-neuronal cells represent around 2-3% of the culture (quantification using GFAP-staining). Alternatively, the cultures can be grown in the presence of serum with the addition, two-days after seeding, of cytosine-β-D-arabinofuranoside (ara-C, 5-8 μM) to suppress the proliferation of glial cells26. Another alternative to the hormone mix is the use of defined media and supplements29.
This technique is suitable to perform immunofluorescence staining, quantitative PCR, second messenger quantification and various types of toxicology/survival screens. It should also be possible to conduct electrophysiological studies on these preparations33,34. These cultures can identify pre-candidate neuroprotective molecules and help to characterize DA neurons properties, while minimizing the number of animals used. However, final confirmation using in vivo models is requested, as cultured neurons do not receive inputs from other brain regions, which might strongly influence their maturation.
This technique is not widely used, compared with rat culture models, despite its first descriptions in the early eighties16,17. The lack of images presenting the delicate dissection may restrain researchers to develop this mouse culture model. However, due to the generation of numerous transgenic mouse models of neurodegenerative disorders25 with specific gene invalidation or labeling of specific neuron types, DA neuron cultures from mouse are now of great interest.
Mastering this technique allows the study of DA neurons isolated from any type of transgenic mouse and to explore the perturbation induced by gene modification. Moreover, these cultures can be used as reference models to be compared with DA neurons derived from mouse stem cells35 or from induced pluripotent stem cells36.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Supported by grants from CNRS and INSERM. PM acknowledges support from the Fondation pour la Recherche Médicale en France (Equipe FRM 2009). SC acknowledges support from the Fondation de France.
References
- Glowinski J, Cheramy A, Romo R, Barbeito L. Presynaptic regulation of dopaminergic transmission in the striatum. Cell Mol Neurobiol. 1988;8:7–17. doi: 10.1007/BF00712906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Dahlstroem A, Fuxe K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta Physiol Scand Suppl. SUPPL. 1964. pp. 231–255. [PubMed]
- Chinta SJ, Andersen JK. Dopaminergic neurons. Int J Biochem Cell Biol. 2005;37:942–946. doi: 10.1016/j.biocel.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Hegarty SV, Sullivan AM, O'Keeffe GW. Midbrain dopaminergic neurons: a review of the molecular circuitry that regulates their development. Dev Biol. 2013;379:123–138. doi: 10.1016/j.ydbio.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson's disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;2 doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
- Ohlin KE, et al. Vascular endothelial growth factor is upregulated by L-dopa in the parkinsonian brain: implications for the development of dyskinesia. Brain. 2011;134:2339–2357. doi: 10.1093/brain/awr165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, et al. Missing pieces in the Parkinson's disease puzzle. Nat Med. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- Cooper O, et al. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson's disease. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel PP, Toulorge D, Guerreiro S, Hirsch EC. Specific needs of dopamine neurons for stimulation in order to survive: implication for Parkinson disease. FASEB J. 2013;27:3414–3423. doi: 10.1096/fj.12-220418. [DOI] [PubMed] [Google Scholar]
- Decressac M, Volakakis N, Bjorklund A, Perlmann T. NURR1 in Parkinson disease-from pathogenesis to therapeutic potential. Nat Rev Neurol. 2013. [DOI] [PubMed]
- Jouve L, Salin P, Melon C, Le Goff LKerkerian- Deep brain stimulation of the center median-parafascicular complex of the thalamus has efficient anti-parkinsonian action associated with widespread cellular responses in the basal ganglia network in a rat model of Parkinson's disease. J Neurosci. 2010;30:9919–9928. doi: 10.1523/JNEUROSCI.1404-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Jenner P, Przedborski S. Pathogenesis of Parkinson's disease. Mov Disord. 2013;28:24–30. doi: 10.1002/mds.25032. [DOI] [PubMed] [Google Scholar]
- di Porzio U, Daguet MC, Glowinski J, Prochiantz A. Effect of striatal cells on in vitro maturation of mesencephalic dopaminergic neurones grown in serum-free conditions. Nature. 1980;288:370–373. doi: 10.1038/288370a0. [DOI] [PubMed] [Google Scholar]
- Denis-Donini S, Glowinski J, Prochiantz A. Glial heterogeneity may define the three-dimensional shape of mouse mesencephalic dopaminergic neurones. Nature. 1984;307:641–643. doi: 10.1038/307641a0. [DOI] [PubMed] [Google Scholar]
- Barbin G, Mallat M, Prochiantz A. In vitro studies on the maturation of mesencephalic dopaminergic neurons. Dev Neurosci. 1985;7:296–307. doi: 10.1159/000112298. [DOI] [PubMed] [Google Scholar]
- Marey-Semper I, Gelman M, Levi-Strauss M. A selective toxicity toward cultured mesencephalic dopaminergic neurons is induced by the synergistic effects of energetic metabolism impairment and NMDA receptor activation. J Neurosci. 1995;15:5912–5918. doi: 10.1523/JNEUROSCI.15-09-05912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthun-Lassalle B, Hirsch EC, Wolfart J, Ruberg M, Michel PP. Rescue of mesencephalic dopaminergic neurons in culture by low-level stimulation of voltage-gated sodium channels. J Neurosci. 2004;24:5922–5930. doi: 10.1523/JNEUROSCI.5668-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulorge D, et al. Neuroprotection of midbrain dopamine neurons by nicotine is gated by cytoplasmic Ca2. FASEB J. 2011;25:2563–2573. doi: 10.1096/fj.11-182824. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Michel PP, Hirsch EC. The Iron-Binding Protein Lactoferrin Protects Vulnerable Dopamine Neurons from Degeneration by Preserving Mitochondrial Calcium Homeostasis. Mol Pharmacol. 2013;84 doi: 10.1124/mol.113.087965. [DOI] [PubMed] [Google Scholar]
- Orme RP, Bhangal MS, Fricker RA. Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS One. 2013;8(e62040) doi: 10.1371/journal.pone.0062040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Kruse SE, Palmiter RD, Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trancikova A, Ramonet D, Moore DJ. Genetic mouse models of neurodegenerative diseases. Prog Mol Biol Transl Sci. 2011;100:419–482. doi: 10.1016/B978-0-12-384878-9.00012-1. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson's disease. FASEB J. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Lin X, et al. Conditional expression of Parkinson's disease-related mutant alpha-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci. 2012;32:9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye CR, Thompson LH, Parish CL. Birth dating of midbrain dopamine neurons identifies A9 enriched tissue for transplantation into parkinsonian mice. Exp Neurol. 2012;236:58–68. doi: 10.1016/j.expneurol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Ramonet D, et al. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One. 2011;6(e18568) doi: 10.1371/journal.pone.0018568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestoz L, Jaber M, Gaillard A. Dopaminergic axon guidance: which makes what. Front Cell Neurosci. 2012;6 doi: 10.3389/fncel.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci U S A. 1073;100:4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri AL, et al. Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development. 2007;134:2761–2769. doi: 10.1242/dev.000141. [DOI] [PubMed] [Google Scholar]
- Rayport S, et al. Identified postnatal mesolimbic dopamine neurons in culture: morphology and electrophysiology. J Neurosci. 1992;12:4264–4280. doi: 10.1523/JNEUROSCI.12-11-04264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Nakajima S, Nakajima Y. Dopamine and GABA receptors in cultured substantia nigra neurons: correlation of electrophysiology and immunocytochemistry. Neuroscience. 1997;78:759–769. doi: 10.1016/s0306-4522(96)00585-4. [DOI] [PubMed] [Google Scholar]
- Nefzger CM, et al. Lmx1a allows context-specific isolation of progenitors of GABAergic or dopaminergic neurons during neural differentiation of embryonic stem cells. Stem Cells. 2012;30:1349–1361. doi: 10.1002/stem.1105. [DOI] [PubMed] [Google Scholar]
- Su H, et al. Immediate expression of Cdh2 is essential for efficient neural differentiation of mouse induced pluripotent stem cells. Stem Cell Res. 2013;10:338–348. doi: 10.1016/j.scr.2013.01.003. [DOI] [PubMed] [Google Scholar]


