Abstract
HIV-1 envelope glycoproteins (Env) mediate viral entry into target cells and are essential to the infectious cycle. Understanding how those glycoproteins are able to fuel the fusion process through their conformational changes could lead to the design of better, more effective immunogens for vaccine strategies. Here we describe a cell-based ELISA assay that allows studying the recognition of trimeric HIV-1 Env by monoclonal antibodies. Following expression of HIV-1 trimeric Env at the surface of transfected cells, conformation specific anti-Env antibodies are incubated with the cells. A horseradish peroxidase-conjugated secondary antibody and a simple chemiluminescence reaction are then used to detect bound antibodies. This system is highly flexible and can detect Env conformational changes induced by soluble CD4 or cellular proteins. It requires minimal amount of material and no highly-specialized equipment or know-how. Thus, this technique can be established for medium to high throughput screening of antigens and antibodies, such as newly-isolated antibodies.
Keywords: Infectious Diseases, Issue 91, HIV-1, envelope glycoproteins, gp120, gp41, neutralizing antibodies, non-neutralizing antibodies, CD4, cell-based ELISA
Introduction
Human immunodeficiency virus type 1 (HIV-1) entry, mediated by the trimeric viral envelope glycoproteins (Env) is the first step of the infectious cycle. Being the only exposed viral antigen presented at the surface of virions, the Env trimer elicits neutralizing and nonneutralizing antibodies. As such, it represents an interesting candidate for vaccine immunogen design. However, vaccination trials with Env in soluble or recombinant forms elicited responses with only minimal effectiveness against most primary HIV-1 isolates1-3. Nonetheless, partial efficacy observed in the RV144 vaccine trial4 renewed interest in HIV-1 Env as an immunogen candidate. This was corroborated by a recent study describing that vaccine-elicited anti-Env antibodies were sufficient to generate a certain degree of protection against SIV and HIV challenges5.
After being synthesized in the endoplasmic reticulum, the Env glycoprotein precursor, gp160, undergoes various post-translational modifications that are critical for its ability to fuel the viral fusion process. The Env precursor must fold properly and associate in trimers before being cleaved into its extra-cytoplasmic gp120 and transmembrane gp41 subunits6-10, with noncovalent interactions maintaining the gp120-gp41 liaison. The infected cell machinery is also responsible for heavily glycosylating Env, comprising about 50% of its total mass11,12. The resulting complex structure allows Env to be conformationally flexible13,14, while providing a metastability that is thought to allow Env to adapt and hide certain highly immunogenic epitopes that would otherwise be exposed15-19, highlighting the importance to better understand the different conformations sampled by the native Env trimer.
To date, several techniques have been developed and successfully used to study Env conformational changes. However, they vary in their limitations, being often restricted to specific Env contexts. For example, surface plasmon resonance or immunoprecipitation assays using conformation specific monoclonal antibodies (mAbs), rely either on monomeric soluble or solubilized Env molecules which are known to be immunogenetically different from their trimeric forms20,21. Recent studies also suggest that cleavage affects Env conformations resulting in the exposure of epitopes mainly recognized by nonneutralizing antibodies14,22,23.
Here we describe in detail a method that allows for fast and easy determination of the conformation of cellularly-expressed Env trimers18,24-26. Following transient transfection of Env in a human adherent cell line the binding of Env-specific antibodies is detected using a simple chemiluminescence reaction. This technique can also be used to characterize the conformational preference of conformation-dependent antibodies. Thus, this assay provides a robust and highly flexible detection method.
Protocol
1. Day 1 – Cell Culture
Plate 2 x 104 human osteosarcoma (HOS) cells per well in an opaque, 96-well cell-culture plate suitable for luminescence reading. Use Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin-streptomycin. Incubate until next day at 37 °C, 5% CO2.
2. Day 2 – Polyethylenimine (PEI) Transfection
Prepare transfection mix according to subsequent steps. Adjust reagents and DNA quantities according to the number of wells that are to be transfected with the same Env.
Tube A: Add 10 ng Tat-encoding plasmid (such as pTat-III27) and 150 ng Env-encoding plasmid to 5 µl DMEM supplemented with 25 mM HEPES.
The Tat-encoding plasmid is only required when using Tat-dependent Env-encoding plasmids such as pSVIII.
Tube B: Add 450 ng PEI (from a 1 µg/µl solution) to 5 µl DMEM.
Add content of tube B to tube A. Mix thoroughly by vortexing for 10 sec and incubate transfection mix 10 min at room temperature (22 °C).
Add 10 µl of the transfection mix per well of the 96-well plate. Incubate for 48 hr at 37 °C, 5% CO2.
3. Day 4 – ELISA
Perform all experiments at room temperature to minimize possible endocytosis of Env/antibodies complexes.
Prepare 250 ml of Washing Buffer per plate being used at the same time. Washing Buffer is 1x Tris-buffered saline (TBS) pH 7.5 (50 mM Tris-Cl, pH 7.5; 150 mM NaCl), supplemented with 1 mM MgCl2 and 1.8 mM CaCl2.
Prepare 125 ml Blocking Buffer per plate by adding 1% nonfat dry milk and 5 mM Tris pH 8.0 to Washing Buffer.
Remove cell culture media and transfection mix (supernatant) from 96-well plate.
Add 100 µl of Blocking Buffer per well and incubate 20 min at RT.
Remove supernatant and add 50 µl of antibody (or serum) per well, diluted to appropriate concentration in Blocking Buffer. Typically, use a concentration of 1 µg/ml. Incubate 1 hr at RT.
Wash 3x with 100 µl Blocking Buffer and then repeat washing process 3x with 100 µl Washing Buffer.
Remove supernatant, add 100 µl Blocking Buffer, and incubate 5 min at RT.
Remove supernatant and add 50 µl of secondary antibody, diluted 1/3,000 in Blocking Buffer. Vary optimal antibody dilution according to manufacturer differences. Incubate 40 min at RT.
Wash 3x with 100 µl Blocking Buffer and then repeat washing process 3x with 100 µl Washing Buffer.
4. Data Acquisition
Remove supernatant from the plate and add 30 µl 1x enhanced chemiluminescence (ECL) substrate per well.
Acquire chemiluminescence signal for 1 sec/well on a suitable plate-reader according to manufacturer instructions. Reading time may differ according to hardware differences.
Representative Results
Using the general procedure described above, we adapted the protocol to assay the impact of soluble CD4 (sCD4) and coexpressed cellular CD4 on the exposure of CD4i epitopes on either wild-type (wt) or mutated Env, as described previously18,24,25,28. Figure 1 schematically represents the general procedure and the exposure of CD4i epitopes following treatment with sCD4 or by coexpression of cellular CD418. In Figure 2, we used sCD4 to induce Env conformational changes that expose CD4i mAbs 17b and 48d epitopes which overlap the coreceptor binding site24,29, whereas the outer-domain recognizing mAb 2G12 is not affected by this treatment as expected18,24.
The impact of point mutations in Env conformation can also be assessed using this assay, as presented in Figure 3. Here we used either the layer 1 Env mutant H66A, known to have a decreased propensity to spontaneously sample the CD4-bound conformation18,24,30,31 or a mutant (S375W) which predisposes Env to the CD4-bound state32 and obtained concordant results (Figure 3A). In cases where different Env expressors are used, it is often necessary to normalize the raw data expressed as relative light units (RLU) according to expression levels. In this case, we used PGT121, a mAb recognizing part of the Env glycan shield33-35, as the normalizing antibody (Figure 3B).
As we recently described18, interaction of Env and CD4 in the same cell leads to Env conformational changes that expose CD4i epitopes. In Figure 4, we cotransfected increasing amounts of a CD4 expressor together with Env in the cell-based ELISA assay and obtained increasing signals for CD4i mAbs A32 and C1118,36-38, which recognize discontinuous epitopes in the inner domain of gp120, whereas Env recognition by the conformational-independent 2G12 antibody was not affected (Figure 4A). In order to control for transfection efficiency between conditions, raw data was normalized to 2G12 (Figure 4B). Increased signals obtained for A32 and C11 antibodies depended on Env-CD4 interaction as indicated by the absence of A32 and C11 modulation when Env was cotransfected with a CD4 mutant (F43H) with decreased ability to interact with Env39.
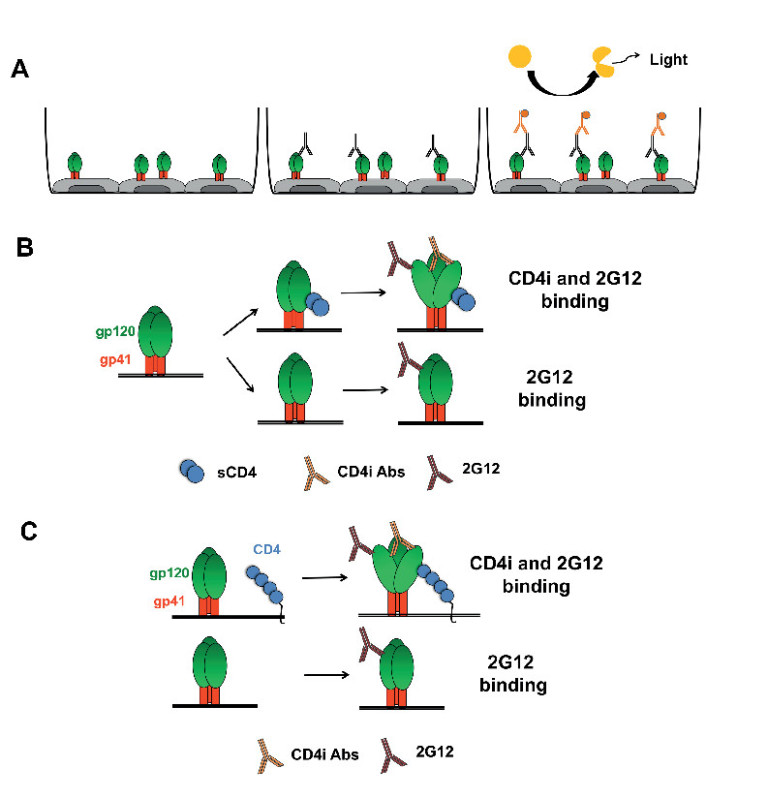 Figure 1. Schematic representation of the anti-Env cell-based ELISA. (A) General scheme of the procedure in which HOS cells are transfected to express trimeric Env at the cell surface. Env conformation can then be sampled by using different antibodies recognizing specific conformations (such as CD4i mAbs). Signals are detected by chemiluminescence after staining with HRP-conjugated anti-human mAbs. sCD4 (B) or coexpression of cellular CD4 (C) can be used to induce Env conformational changes that lead to exposure of CD4i epitopes.
Figure 1. Schematic representation of the anti-Env cell-based ELISA. (A) General scheme of the procedure in which HOS cells are transfected to express trimeric Env at the cell surface. Env conformation can then be sampled by using different antibodies recognizing specific conformations (such as CD4i mAbs). Signals are detected by chemiluminescence after staining with HRP-conjugated anti-human mAbs. sCD4 (B) or coexpression of cellular CD4 (C) can be used to induce Env conformational changes that lead to exposure of CD4i epitopes.
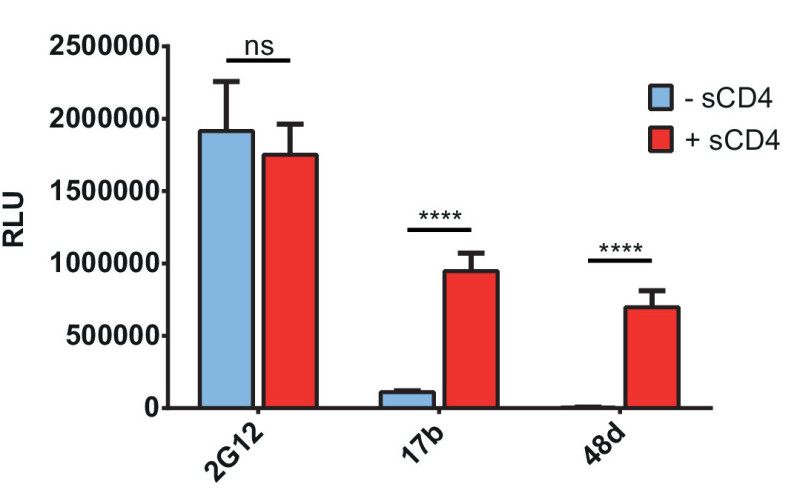 Figure 2. sCD4 induces Env conformational changes leading to exposure of CD4i epitopes. Interaction of sCD4 with HIV-1CO-JRFLΔCT Env enhances recognition by antibodies targeting CD4i epitopes (17b, 48d), but not by the gp120 outer-domain recognizing antibody 2G12. A plasmid encoding HIV-1CO-JRFLΔCT Env was transfected in each well and 48 hr later the cells were washed and incubated in presence or absence of 4 µg/ml sCD4 for 30 min at RT before continuing with the standard protocol (Day 4 – ELISA). Env conformation was then probed by incubating with 0.25 µg/ml 2G12, 1 µg/ml 17b or 48d anti-Env mAbs for 1 hr at RT. Signals were detected by chemiluminescence after incubation with an HRP-conjugated anti-human antibody for 45 min at RT. Shown are the mean RLU values ± SD of six replicates with signal obtained from wells transfected with an irrelevant plasmid (no Env) subtracted. Data is representative of results obtained in three independent experiments, with significance tested by two-way ANOVA (ns, not significant; ****, p <0.0001).
Figure 2. sCD4 induces Env conformational changes leading to exposure of CD4i epitopes. Interaction of sCD4 with HIV-1CO-JRFLΔCT Env enhances recognition by antibodies targeting CD4i epitopes (17b, 48d), but not by the gp120 outer-domain recognizing antibody 2G12. A plasmid encoding HIV-1CO-JRFLΔCT Env was transfected in each well and 48 hr later the cells were washed and incubated in presence or absence of 4 µg/ml sCD4 for 30 min at RT before continuing with the standard protocol (Day 4 – ELISA). Env conformation was then probed by incubating with 0.25 µg/ml 2G12, 1 µg/ml 17b or 48d anti-Env mAbs for 1 hr at RT. Signals were detected by chemiluminescence after incubation with an HRP-conjugated anti-human antibody for 45 min at RT. Shown are the mean RLU values ± SD of six replicates with signal obtained from wells transfected with an irrelevant plasmid (no Env) subtracted. Data is representative of results obtained in three independent experiments, with significance tested by two-way ANOVA (ns, not significant; ****, p <0.0001).
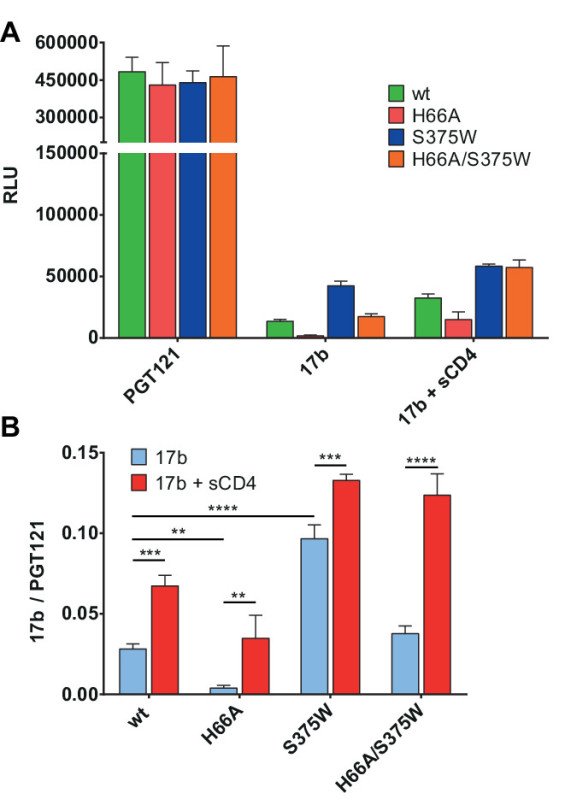 Figure 3. Modulation of Env conformation. HIV-1 YU2ΔCT layer 1 Env mutant (H66A) diminishes CD4i 17b recognition whereas the S375W variant exhibits increased 17b signal and is sufficient to restore the phenotype of the layer 1 mutant. (A) RLU values of the signals obtained using anti-Env PGT121 and 17b mAbs. (B) PGT121-normalized signals of CD4i mAb 17b following treatment with or without sCD4. Shown are the mean values ± SD of triplicates with signal obtained from wells transfected with an irrelevant plasmid (no Env) subtracted. Data is representative of results obtained in three independent experiments, with significance tested by two-way ANOVA (**, p <0.01; ***, p <0.001; ****, p <0.0001).
Figure 3. Modulation of Env conformation. HIV-1 YU2ΔCT layer 1 Env mutant (H66A) diminishes CD4i 17b recognition whereas the S375W variant exhibits increased 17b signal and is sufficient to restore the phenotype of the layer 1 mutant. (A) RLU values of the signals obtained using anti-Env PGT121 and 17b mAbs. (B) PGT121-normalized signals of CD4i mAb 17b following treatment with or without sCD4. Shown are the mean values ± SD of triplicates with signal obtained from wells transfected with an irrelevant plasmid (no Env) subtracted. Data is representative of results obtained in three independent experiments, with significance tested by two-way ANOVA (**, p <0.01; ***, p <0.001; ****, p <0.0001).
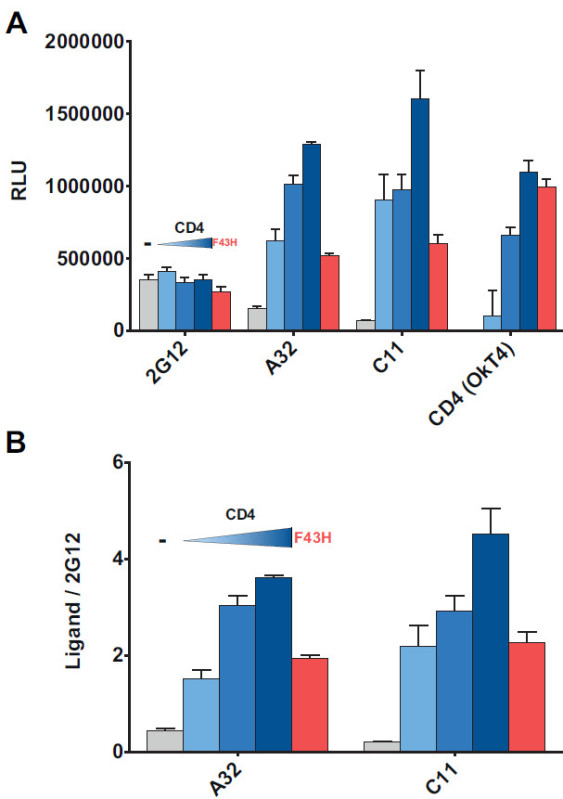 Figure 4. Coexpression of cellular CD4 enhances recognition by CD4i antibodies. A CD4-encoding plasmid was cotransfected with HIV1YU2ΔCT Env in order to favor the CD4-bound conformation18.(A) RLU values of the signals obtained using anti-Env 2G12, A32 or C11 mAbs and the anti-CD4 OkT4 mAb. (B) 2G12-normalized signals of CD4i mAbs A32 and C11. Shown are the mean values ± SD of triplicates with signal obtained from wells transfected with an irrelevant plasmid (no Env) subtracted. Data is representative of results obtained in three independent experiments. Grey bar indicates in absence of CD4, whereas the increasing blue bar indicates a step-wise increase in the amount of CD4 expressor being transfected (1.7 ng, 3.5 ng, and 7 ng) and red bar indicates the transfection of a CD4 mutant (F43H, 7 ng) with decreased capacity to interact with gp120.
Figure 4. Coexpression of cellular CD4 enhances recognition by CD4i antibodies. A CD4-encoding plasmid was cotransfected with HIV1YU2ΔCT Env in order to favor the CD4-bound conformation18.(A) RLU values of the signals obtained using anti-Env 2G12, A32 or C11 mAbs and the anti-CD4 OkT4 mAb. (B) 2G12-normalized signals of CD4i mAbs A32 and C11. Shown are the mean values ± SD of triplicates with signal obtained from wells transfected with an irrelevant plasmid (no Env) subtracted. Data is representative of results obtained in three independent experiments. Grey bar indicates in absence of CD4, whereas the increasing blue bar indicates a step-wise increase in the amount of CD4 expressor being transfected (1.7 ng, 3.5 ng, and 7 ng) and red bar indicates the transfection of a CD4 mutant (F43H, 7 ng) with decreased capacity to interact with gp120.
Discussion
This assay is optimized to detect the interaction of specific mAbs with HIV-1 trimeric Env expressed at the cell surface. Once the protocol has been established, it can be used at medium to high throughputs with low overall material costs and little amounts of antibodies. Since this assay is transfection-based, it can easily be adapted for coexpression of cellular proteins such as CD4 in order to study their effects on Env conformation.
However, the transfection base of this protocol also implies that it is one of its most important pitfalls. First off, antigens to be studied with this technique are required to be available in an independent expression vector. As such, Env genes from various clinical sources or proviral constructs would need to be subcloned into mammalian expression vectors. While full-length proviral constructs can also be used in this technique (see Veillette et al.18), this also implies active viral particles production thus requiring work in appropriate biocontainment facilities.
Moreover, success of this technique is intimately linked with transfection efficiency. Low signals obtained are often due to poor expression of transfected antigens. Typical sources of problems are plasmid DNA quality, transfection reagents. and cells viability. If required, optimization of transfection conditions could also be performed using other techniques, such as flow cytometry or western blotting. Of note, it is also important to be aware that expression of some Env constructs could be suboptimal and could therefore affect the technique’s outcome.
Here we focused on probing HIV-1 Env conformation using previously described CD4i mAbs. This setting allows for a broad range of analysis such as probing the effect of Env point mutations or the conformational consequences of coexpressed proteins. Moreover, the technique described here can also be used with well-characterized Env mutants with different conformational propensity in order to probe the specificity of different mAbs for various Env conformations. This allows an easy and rapid characterization of newly isolated mAbs while not requiring highly-specialized equipment or know-how.
Although we only used this method against Env from various HIV-1 clades and other close relative lineages (HIV-2, SIV/Mac)18, we believe this assay could be adapted for additional surface antigens, such as ones from other virus families.
Disclosures
The authors declare no conflicts of interest.
Acknowledgments
We thank Dr. James Robinson for his generous gift of A32, 17b, 48d, and C11 mAbs. PGT 121 was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH (Cat#12343). This work was supported by a Canada Foundation for Innovation Program Leader #29866, by a CIHR operating #257792, by a FRQS Establishment of Young Scientist grant #24639 to AF and by a CRCHUM continuum grant as well as by a CIHR catalyst grant #126630 to AF and MR. AF is the recipient of a FRSQ Chercheur Boursier Junior 1 fellowship #24639. MV was supported by a CIHR Doctoral Research Award #291485.
References
- Bures R, et al. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Human Retroviruses. 2000;16:2019–2035. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- Koff WC. HIV vaccine development: challenges and opportunities towards solving the HIV vaccine-neutralizing antibody problem. Vaccine. 2012;30:4310–4315. doi: 10.1016/j.vaccine.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Mascola JR, et al. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Roederer M, et al. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature. 2014;505:502–508. doi: 10.1038/nature12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennie C, Lasky LA. Model for intracellular folding of the human immunodeficiency virus type 1 gp120. J. Virol. 1989;63:639–646. doi: 10.1128/jvi.63.2.639-646.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey RL, Bonifacino JS, Potts BJ, Martin MA, Klausner RD. Biosynthesis cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc. Natl. Acad. Sci. U.S.A. 1988;85:9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch V, Pawlita M. Mutational analysis of the human immunodeficiency virus type 1 env gene product proteolytic cleavage site. J. Virol. 1990;64:2337–2344. doi: 10.1128/jvi.64.5.2337-2344.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenberger S, et al. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature. 1038;360:358–361. doi: 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Luo L, Thomas DY, Kang CY. The HIV-1 Env protein signal sequence retards its cleavage and down-regulates the glycoprotein folding. Virology. 2000;272:417–428. doi: 10.1006/viro.2000.0357. [DOI] [PubMed] [Google Scholar]
- Leonard CK, et al. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- Wang W, et al. A systematic study of the N-glycosylation sites of HIV-1 envelope protein on infectivity and antibody-mediated neutralization. Retrovirology. 2013;10(14) doi: 10.1186/1742-4690-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth E, Olshevsky U, Furman C, Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J. Virol. 1991;65:2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim H, Salas I, Sodroski J. Proteolytic processing of the human immunodeficiency virus envelope glycoprotein precursor decreases conformational flexibility. J. Virol. 2013;87:1884–1889. doi: 10.1128/JVI.02765-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, et al. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science. 2009;326:1123–1127. doi: 10.1126/science.1175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- Sakai K, Takiguchi M. Toward an effective AIDS vaccine development. Eur. J. Immunol. 2013;43:3087–3089. doi: 10.1002/eji.201370125. [DOI] [PubMed] [Google Scholar]
- Veillette M, et al. Interaction with Cellular CD4 Exposes HIV-1 Envelope Epitopes Targeted by Antibody-Dependent Cell-Mediated Cytotoxicity. J. Virol. 2014;88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibmer CK, et al. Viral escape from HIV-1 neutralizing antibodies drives increased plasma neutralization breadth through sequential recognition of multiple epitopes and immunotypes. PLoS Pathogens. 2013;9(e1003738) doi: 10.1371/journal.ppat.1003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JM, et al. HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc. Natl. Acad. Sci. U.S.A. 2012;109:12111–12116. doi: 10.1073/pnas.1204533109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Bazick J, Sodroski J. Characterization of the multiple conformational States of free monomeric and trimeric human immunodeficiency virus envelope glycoproteins after fixation by cross-linker. J. Virol. 2006;80:6725–6737. doi: 10.1128/JVI.00118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, Lee KK. A functional interaction between gp41 and gp120 is observed for monomeric but not oligomeric, uncleaved HIV-1 Env gp140. J. Virol. 2013;87:11462–11475. doi: 10.1128/JVI.01681-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringe RP, et al. Cleavage strongly influences whether soluble HIV-1 envelope glycoprotein trimers adopt a native-like conformation. Proc. Natl. Acad. Sci. U.S.A. 2013;110:18256–18261. doi: 10.1073/pnas.1314351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desormeaux A, et al. The highly conserved layer-3 component of the HIV-1 gp120 inner domain is critical for CD4-required conformational transitions. J. Virol. 2013;87:2549–2562. doi: 10.1128/JVI.03104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim H, et al. Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state. PLoS Pathogens. 2009;5(e1000360) doi: 10.1371/journal.ppat.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim H, et al. Contribution of intrinsic reactivity of the HIV-1 envelope glycoproteins to CD4-independent infection and global inhibitor sensitivity. PLoS Pathogens. 2011;7(e1002101) doi: 10.1371/journal.ppat.1002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M, et al. Effects of changes in gp120-CD4 binding affinity on human immunodeficiency virus type 1 envelope glycoprotein function and soluble CD4 sensitivity. J. Virol. 1991;65:5007–5012. doi: 10.1128/jvi.65.9.5007-5012.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medjahed H, Pacheco B, Desormeaux A, Sodroski J, Finzi A. The HIV-1 gp120 major variable regions modulate cold inactivation. J. Virol. 2013;87:4103–4111. doi: 10.1128/JVI.03124-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M, et al. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi A, et al. Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol. Cell. 2010;37:656–667. doi: 10.1016/j.molcel.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassa A, et al. Transitions to and from the CD4-bound conformation are modulated by a single-residue change in the human immunodeficiency virus type 1 gp120 inner domain. J. Virol. 2009;83:8364–8378. doi: 10.1128/JVI.00594-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang SH, et al. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 2002;76:9888–9899. doi: 10.1128/JVI.76.19.9888-9899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. U.S.A. 2012;109:3268–3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien JP, et al. Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans. PLoS Pathogens. 2013;9(e1003342) doi: 10.1371/journal.ppat.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Willey RL, Lewis GK, Robinson J, Sodroski J. Immunological evidence for interactions between the first, second, and fifth conserved domains of the gp120 surface glycoprotein of human immunodeficiency virus type 1. J. Virol. 1994;68:6836–6847. doi: 10.1128/jvi.68.11.6836-6847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Yoshiyama H, Holton D, Elliott S, Ho DD. Distinct antigenic sites on HIV gp120 identified by a panel of human monoclonal antibodies. J. Cell. Biochem. Suppl. 1992;16E(Q449) [Google Scholar]
- Bonsignori M, et al. Antibody-Dependent Cellular Cytotoxicity-Mediating Antibodies from an HIV-1 Vaccine Efficacy Trial Target Multiple Epitopes and Preferentially Use the VH1 Gene Family. J. Virol. 2012;86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand D, Srinivasan K, Sodroski J. Determinants of human immunodeficiency virus type 1 entry in the CDR2 loop of the CD4 glycoprotein. J. Virol. 1995;69:166–171. doi: 10.1128/jvi.69.1.166-171.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


