Abstract
In current protocol, a combinatorial approach has been developed to simplify the design and production of sensing materials for the construction of electronic tongues (eT) for protein analysis. By mixing a small number of simple and easily accessible molecules with different physicochemical properties, used as building blocks (BBs), in varying and controlled proportions and allowing the mixtures to self-assemble on the gold surface of a prism, an array of combinatorial surfaces featuring appropriate properties for protein sensing was created. In this way, a great number of cross-reactive receptors can be rapidly and efficiently obtained. By combining such an array of combinatorial cross-reactive receptors (CoCRRs) with an optical detection system such as surface plasmon resonance imaging (SPRi), the obtained eT can monitor the binding events in real-time and generate continuous recognition patterns including 2D continuous evolution profile (CEP) and 3D continuous evolution landscape (CEL) for samples in liquid. Such an eT system is efficient for discrimination of common purified proteins.
Keywords: Bioengineering, Issue 91, electronic tongue, combinatorial cross-reactive receptor, surface plasmon resonance imaging, pattern recognition, continuous evolution profiles, continuous evolution landscapes, protein analysis
Introduction
Precise and rapid protein sensing methods are very important in medical diagnostics and proteomics. Classical protein-detecting arrays, such as biochips, are based on the “lock-and-key” recognition principle and require specific receptors such as aptamers, antibodies, or mimetics.
In recent years, differential sensing inspired by the human olfaction and gustation has emerged as an alternative1. This electronic nose/tongue (eN/eT) approach is based on differential binding of analytes to an array of cross-reactive receptors (CRRs), that do not need to be highly specific or selective for the target molecules thus allow to surmount the laborious process of developing highly selective receptors. It is the combined response of all the receptors that creates a distinct pattern for each sample, like a fingerprint, allowing its identification.
The two key challenges for the development of electronic nose/tongue for effective protein sensing are the production of sensing elements that have the ability to distinguish among structurally similar analytes and the appropriate transduction system for the binding event. Up to now, studies have reported various approaches to array development2. For example in one study an array-based identification of proteins was developed using CRRs prepared from tetra-carboxyphenylporphyrin derivatives by coupling the carboxyl groups to various amino acids or dipeptides to provide differential receptors possessing a hydrophobic core for affinity for proteins and distinct charged peripheries for imparting differential binding. Using this system, different proteins and protein mixtures were identified by measuring fluorescence quenching of the receptors upon interaction with the analytes3,4. In another study, a library of 29 CRRs containing tripeptide and boronic acid moieties synthesized in a combinatorial way on a hexasubstituted benzene scaffold was developed for sensing proteins with an indicator-uptake colorimetric detection5,6. With such a design, each receptor showed differential binding capacity with proteins based on the variance in the peptide arms, and the boronic acids assisted in differentiation of proteins from glycoproteins. More recently, an array composed of different cationic functionalized gold nanoparticles conjugated with an anionic fluorescent polymer poly(p-phenyleneethynylene) (PPE) has been created to detect and identify proteins7. The competitive binding between protein analytes and quenched PPE/gold nanoparticle complexes regenerated fluorescence, producing distinct recognition patterns for proteins. In this study, the functionalized nanoparticle-protein interactions were tuned by varying physicochemical properties of nanoparticle end groups. Furthermore, it was shown that this approach is effective for protein analysis in complex and protein-rich medium such as human serum at physiologically relevant concentrations, thus showing the potential of eT in profiling real samples for diagnosing disease states8.
Though very promising, these systems have some inherent limitations. They require designing and synthesizing from 5 to 29 CRRs with quite complicated structures. In addition, unlike the olfactory system that is reset following each measurement, protein sensing requires preparing an array per sample. Finally, monitoring real-time binding events are extremely difficult.
In this context, a combinatorial approach was proposed by using a small number of simple and easily accessible molecules with different physicochemical properties (hydrophilic, hydrophobic, positively charged, negatively charged, neutral, etc.) as building blocks (BBs)9. By mixing BBs in varying and controlled proportions and allowing the mixtures to self-assemble on the gold surface of a prism, an array of combinatorial surfaces featuring appropriate properties for binding protein was created. Notably, the self-assembled monolayers on this system allow easy tuning of a range of surface properties in a highly divergent fashion, enabling diverse combinatorial cross-reactive receptors (CoCRRs) to be rapidly and efficiently produced. Protein sensing was performed using an optical detection system, surface plasmon resonance imaging (SPRi). Briefly, a broad-beam monochromatic polarized light from a LED illuminates the whole CoCRR array area on the surface of the prism. A high resolution CCD video camera provides real-time difference images across all the spots of the CoCRR array. It captures all of the local changes at the surface of the CoCRR array providing detailed information on binding events and kinetic processes10. Meanwhile, with the help of imaging software, SPR images corresponding to spots are automatically converted to variations of reflectivity versus time, generating a series of kinetic binding curves called sensorgrams. Thus, SPRi allows a label-free, synchronous, parallel, and real-time observation of binding events. Additionally, the obtained CoCRR array is regenerable and reusable for protein analysis.
This protocol describes the construction of the electronic tongue by using only two small molecules as building blocks and illustrates its application for analysis of common proteins based on continuous recognition patterns obtained with SPRi.
Protocol
1. Preparation of Various Solutions and Protein Samples
Prepare 100 ml of phosphate buffer solution (PBS-G) containing 50 mM NaH2PO4, 50 mM NaCl, and 10% glycerol at pH 6.8.
Prepare 250 ml of HEPES buffer solution containing 10 mM HEPES, 150 mM NaCl, 0.005% Tween 20 at pH 7.4.
Prepare stock solution of building block 1 (BB1) lactose and building block 2 (BB2) sulfated lactose (Figure 1) at 0.2 mM in PBS-G.
Prepare 1 ml of protein solutions in HEPES: Arachis hypogaea lectin (AHL) at 500 nM, myoglobin at 1 µM, and lysozyme at 500 nM.
Prepare 20 ml of 1% SDS in ultrapure water.
2. Preparation of the CoCRR Array
Clean the gold surface of the prism 48 hr prior to use with a plasma cleaner for 3 min under these conditions: 75% Oxygen, 25% Argon, 0.6 mbar, power 40 W.
Prepare eleven pure and mixed solutions of BB1 and BB2 with [BB1]/([BB1]+[BB2]) ratios between 0 and 100% by increments of 10% at a total BB concentration of 0.1 mM in PBS-G.
Deposit 8 nl droplets of these pure and mixed solutions on the prism surface using a non-contact spotter in quadruplicate for each ratio with 44 spots in total (Figure 2). NOTE: 10% glycerol added in PBS-G is very important to reduce solvent evaporation and the change of BB concentration after deposition.
Place the prism inside a Petri dish containing 1 ml of ultrapure water and leave it O/N at RT for self-assembly of BB1 and BB2 on the gold surface. Herein, it is assumed that the average surface composition in the mixed SAMs reflects the composition of the deposition mixed solution (Figure 3)11.
Wash thoroughly the prism with ultrapure water and then dry it under a flow of argon.
3. Protein Sensing by SPRi
Set the incubator in which the SPRi apparatus is placed at 25 °C to avoid refractive index changes induced by temperature variation during protein sensing.
Insert a non-functionalized rinsing prism in the 10 µl Polyether ether ketone (PEEK) flow cell connected to a computer controlled syringe pump, a degasser and a 6-port medium pressure injection valve (Figure 4). Fill in the flow system with freshly filtered and degassed running buffer HEPES.
Remove the rinsing prism and insert the prism containing the CoCRR array in the flow cell. Run HEPES at a 100 µl/min flow rate. With the help of the CCD camera remove any air bubbles present on the prism surface by passing running buffer quickly.
Define study area for each spot by drawing a circle with the same diameter for the area of interest based on a well-contrasted image of the array and with the help of integrated software in SPRi system.
Trace plasmon curves, which represent reflectivity curves in function of the incident angle, for all the spots.
Choose the working angle (position at which kinetic curves will be recorded) at the highest slope of the reflectivity curves. Rotate the scanning mirror to fix the selected working angle for kinetic measurements.
Continue to run HEPES through the flow system until reflectivity signal for all the spots is stable and constant.
Inject 1 ml AHL solution with a syringe into the sample loop (500 µl) with the injection valve on position “load”. Then put the injection valve to position “injection”. The flow rate used for all the protein injection was 100 µl/min.
Start kinetic measurements by monitoring reflectivity variations against time simultaneously on all the spots.
At the end of protein injection, rinse the array with running buffer for 8 min. Finally, inject 1 ml 1% SDS to regenerate it.
Repeat the same procedure for the other proteins.
4. Data Processing and Analysis
Following entry of protein solution into the flow cell, molecular binding occurs and induces a shift of the plasmon curves and a variation of reflectivity. The image acquisition software converts the measured light intensity values to gray scale levels, giving SPR images Figure 5A, and generates the variation of reflectivity versus time, giving sensorgrams (Figure 5B).
Use a math computing software to plot the reflectivity (R%) at the end of each protein injection versus the [BB1]/([BB1]+[BB2]) ratio to generate 2D continuous evolution profile (CEP) for each sample (Figure 5C).
Add the [BB1]/([BB1]+[BB2]) ratio in sensorgrams (Figure 5B) to generate 3D continuous evolution landscape (CEL) for each protein (Figure 5D).
Representative Results
To probe the ability of the electronic tongue for common protein analysis, three proteins were used: AHL, myoglobin and lysozyme. For each protein, a distinct 2D continuous evolution profile, CEP, was generated by the eT, as shown in Figure 6.
In addition, thanks to SPRi, which is able to monitor the real-time adsorption and desorption kinetics, for each protein a time dependent continuous recognition pattern, called 3D continuous evolution landscape (CEL), was generated. In Figure 7, characteristic CELs of these three proteins are shown.
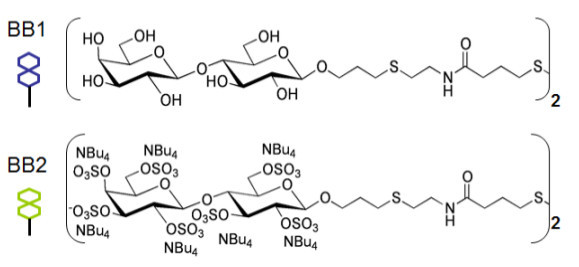 Figure 1.
Chemical structures of the two building blocks used in this protocol to prepare the CoCRR array.
Please click here to view a larger version of this figure.
Figure 1.
Chemical structures of the two building blocks used in this protocol to prepare the CoCRR array.
Please click here to view a larger version of this figure.
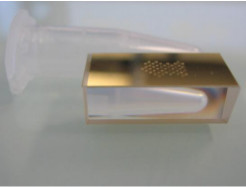 Figure 2.A photo of a prism after depositing the 44 droplets of the pure and mixed solutions of BB1 and BB2 using a non-contact piezoelectric spotter with each ratio in quadruplicate. The prism was placed next to an Eppendorf tube of 1.5 ml.
Figure 2.A photo of a prism after depositing the 44 droplets of the pure and mixed solutions of BB1 and BB2 using a non-contact piezoelectric spotter with each ratio in quadruplicate. The prism was placed next to an Eppendorf tube of 1.5 ml.
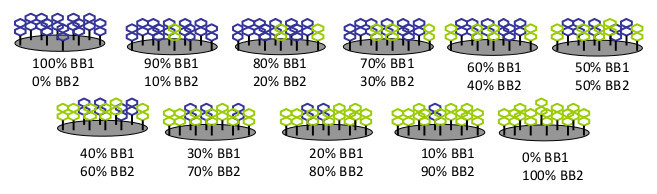 Figure 3.
Schematic illustration of the CoCRR array containing 11 differential receptors that were prepared with pure and mixed solutions of BB1 and BB2 at various ratios and allowing the mixtures to self-assemble on the gold surface of the prism.
Figure 3.
Schematic illustration of the CoCRR array containing 11 differential receptors that were prepared with pure and mixed solutions of BB1 and BB2 at various ratios and allowing the mixtures to self-assemble on the gold surface of the prism.
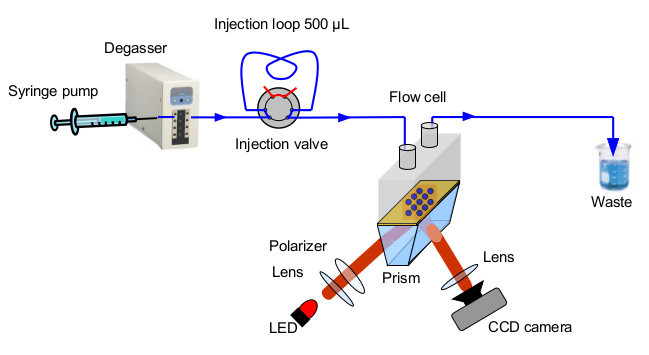 Figure 4.
Schematic illustration of the SPRi detection system combined with a microfluidic system.
Figure 4.
Schematic illustration of the SPRi detection system combined with a microfluidic system.
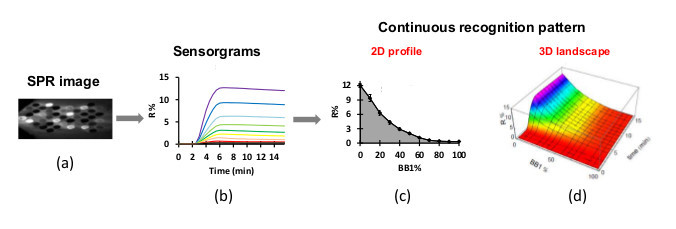 Figure 5.
Schematic illustration of data treatment for generation of two types of continuous recognition pattern with developed electronic tongue: 2D continuous evolution profile and 3D continuous evolution landscape.
Figure 5.
Schematic illustration of data treatment for generation of two types of continuous recognition pattern with developed electronic tongue: 2D continuous evolution profile and 3D continuous evolution landscape.
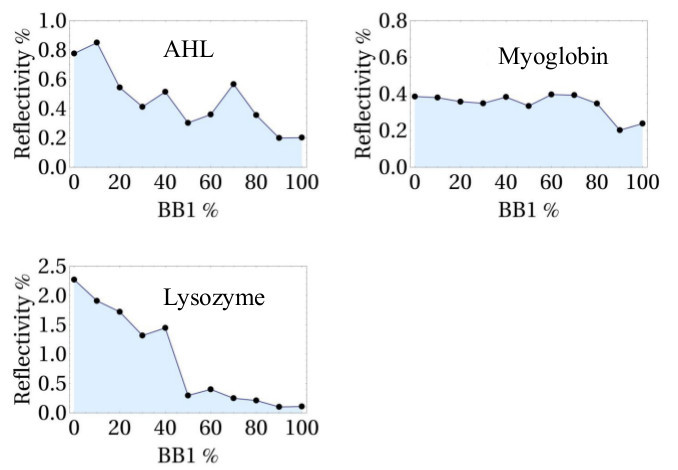 Figure 6.Continuous evolution profiles for three pure proteins: AHL (500 nM), myoglobin (1 µM), and lysozyme (500 nM). They were generated by plotting the variation of reflectivity (R%) at the end of protein injection versus the [BB1]/([BB1]+[BB2]) ratio.
Figure 6.Continuous evolution profiles for three pure proteins: AHL (500 nM), myoglobin (1 µM), and lysozyme (500 nM). They were generated by plotting the variation of reflectivity (R%) at the end of protein injection versus the [BB1]/([BB1]+[BB2]) ratio.
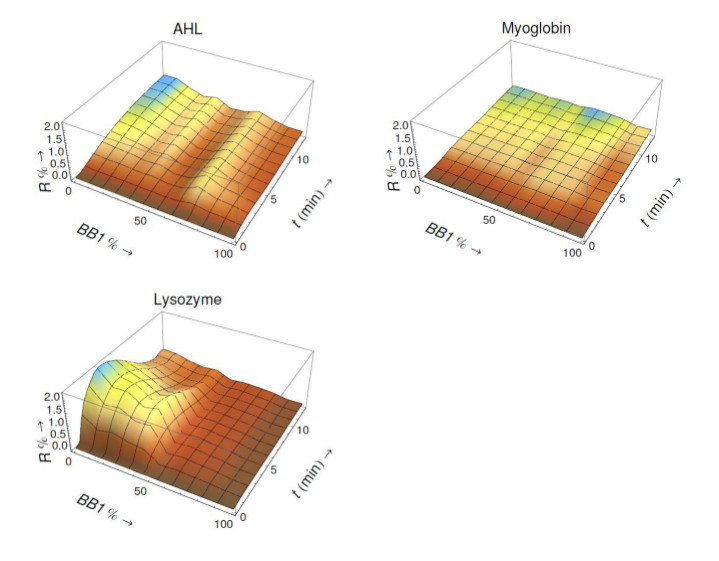 Figure 7.
Landscape of taste: characteristic 3D CELs of AHL, myoglobin and lysozyme generated by the electronic tongue.
Figure 7.
Landscape of taste: characteristic 3D CELs of AHL, myoglobin and lysozyme generated by the electronic tongue.
Discussion
The most critical steps for construction of this eT are dedicated to ensure good reproducibility of the system. For example, cleaning the gold surface of the prism with a standardized procedure before use, adding 10% of glycerol in the eleven pure and mixed solutions of BB1 and BB2 to eliminate solvent evaporation during BBs self-assembling on the gold surface of the prism, depositing multiple replicates for each [BB1]/([BB1]+[BB2]) ratio, etc. As for protein sensing by SPRi, the choice of the working angle is critical. Usually, it is fixed at the highest slope of the reflectivity curves. In addition, it is very important to use standardized experimental conditions for each protein sensing, such as working temperature, flow rate, etc. After each protein injection, the CoCRR array must be regenerated for reuse with an appropriate solution that allows complete regeneration of the system without any damage to receptors.
To demonstrate that the developed eT is not only effective for the study of sugar binding proteins as previously reported9, but also for common proteins, one lectin AHL together with two non-sugar-binding proteins myoglobin and lysozyme were used. They were chosen to have a variety of charges in HEPES (pH 7.4): AHL (isoelectric point (pI) 6.0), myoglobin (pI 7.2) and lysozyme (pI 11). Notably, as shown in Figure 6, each protein possesses a unique response pattern. AHL is slightly negatively charged in HEPES. Its CEP shows that it has stronger interaction with the negatively charged BB2-rich CoCRRs. Most likely, it is due to the interaction between the positively charged domain of the protein and BB2-rich CoCRRs. For myoglobin, which is globally neutral in HEPES, there is not much difference between signals obtained with all CoCRRs. When comparing myoglobin in CEP to the CEPs of AHL and lysozyme, even at a higher concentration the signal is much lower. As for lysozyme, which is positively charged in HEPES, the maximal signal was obtained with the CoCRR containing pure BB2.
Thanks to the advantages of SPRi capable of monitoring the real-time adsorption and desorption kinetics, for each protein a 3D continuous evolution landscape was generated. This kind of time-dependent recognition pattern brings supplementary differentiation parameters for protein analysis. Particularly, it could be extremely useful for analyzing two analytes presenting the similar affinity for a set of CoCRRs but differing in their kinetics of interaction. As shown in Figure 7, the CELs are easily distinguishable. These results show that the interaction of the three proteins with the CoCRRs is dependent on their surface characteristics such as the distribution of hydrophobic, neutral and charged amino-acid residues. Both CEP and CEL can be used as “fingerprints” for the protein discrimination and prospective identification. Thus, the eT is effective for analysis of common proteins.
The protocol described herein uses a combinatorial approach to construct the eT for protein analysis. Unlike other approaches that use a number of molecules which are structurally complicated and laborious to synthesize, only two small molecules were used to prepare the cross-reactive receptor array capable of differentiating proteins with good resolution in present study. Additionally, according to the previous investigation, the eT is stable, reproducible and reusable9. Furthermore, SPRi allows monitoring binding events in real-time and generating novel continuous recognition patterns with unprecedented reliability. In the near future, additional BBs with complementary physicochemical properties will be introduced to increase the diversity of the CoCRR in the arrays for analysis of complex mixtures in liquid or in gas.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgments
The authors would like to acknowledge Ph.D. grant of LANEF in Grenoble for support of Laurie-Amandine Garçon. This work was financially supported by the French National Research Agency (ANR-grant 06-NANO-045).
References
- Margulies D, Hamilton AD. Combinatorial protein recognition as an alternative approach to antibody-mimetics. Current Opinion in Chemical Biology. 2010;14:705–712. doi: 10.1016/j.cbpa.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Umali AP, Anslyn EV. A general approach to differential sensing using synthetic molecular receptors. Current Opinion in Chemical Biology. 2010;14:685–692. doi: 10.1016/j.cbpa.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini L, Wilson AJ, Hong J, Hamilton AD. Pattern-based detection of different proteins using an array of fluorescent protein surface receptors. J. Am. Chem. Soc. 2004;126:5656–5657. doi: 10.1021/ja039562j. [DOI] [PubMed] [Google Scholar]
- Zhou HC, Baldini L, Hong J, Wilson AJ, Hamilton AD. Pattern recognition of proteins based on an array of functionalized porphyrins. J. Am. Chem. Soc. 2006;128:2421–2425. doi: 10.1021/ja056833c. [DOI] [PubMed] [Google Scholar]
- Wright AT, Griffin MJ, Zhong Z, McCleskey SC, Anslyn EV, McDevitt JT. Differential receptors create patterns that distinguish various proteins. Angew. Chem. Int. Ed. 2005;44:6375–6378. doi: 10.1002/anie.200501137. [DOI] [PubMed] [Google Scholar]
- Wright AT, Anslyn EV. Differential receptor arrays and assays for solution-based molecular recognition. Chem. Soc. Rev. 2006;35:14–28. doi: 10.1039/b505518k. [DOI] [PubMed] [Google Scholar]
- You CC, et al. Detection and identification of proteins using nanoparticle–fluorescent polymer ‘chemical nose’ sensors. Nature Nanotechnology. 2007;2:318–323. doi: 10.1038/nnano.2007.99. [DOI] [PubMed] [Google Scholar]
- De M, et al. Sensing of proteins in human serum using conjugates of nanoparticles and green fluorescent protein. Nature Chemistry. 2009;1:461–465. doi: 10.1038/nchem.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, et al. Continuous evolution profiles for electronic-tongue-based analysis. Angew. Chem. Int. Ed. 2012;51:10394–10398. doi: 10.1002/anie.201205346. [DOI] [PubMed] [Google Scholar]
- Campbell CT, Kim G. SPR microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics. Biomaterials. 2007;28:2380–2392. doi: 10.1016/j.biomaterials.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Stranick SJ, et al. Nanometer-scale phase separation in mixed composition self-assembled monolayers. Nanotechnology. 1996;7:438–442. [Google Scholar]


