Abstract
Entomopathogenic nematodes (EPN) (Steinernematidae and Heterorhabditidae) have a mutualistic partnership with Gram-negative Gamma-Proteobacteria in the family Enterobacteriaceae. Xenorhabdus bacteria are associated with steinernematids nematodes while Photorhabdus are symbionts of heterorhabditids. Together nematodes and bacteria form a potent insecticidal complex that kills a wide range of insect species in an intimate and specific partnership. Herein, we demonstrate in vivo and in vitro techniques commonly used in the rearing of these nematodes under laboratory conditions. Furthermore, these techniques represent key steps for the successful establishment of EPN cultures and also form the basis for other bioassays that utilize these organisms for research. The production of aposymbiotic (symbiont–free) nematodes is often critical for an in-depth and multifaceted approach to the study of symbiosis. This protocol does not require the addition of antibiotics and can be accomplished in a short amount of time with standard laboratory equipment. Nematodes produced in this manner are relatively robust, although their survivorship in storage may vary depending on the species used. The techniques detailed in this presentation correspond to those described by various authors and refined by P. Stock’s Laboratory, University of Arizona (Tucson, AZ, USA). These techniques are distinct from the body of techniques that are used in the mass production of these organisms for pest management purposes.
Keywords: Bioengineering, Issue 91, entomology, nematology, microbiology, entomopathogenic, nematodes, bacteria, rearing, in vivo, in vitro
Introduction
Entomopathogenic nematodes (EPN) Steinernema and Heterorhabditis spp. (Steinernematidae, Heterorhabditidae) and their bacterial symbionts, Xenorhabdus and Photorhabdus spp (Enterobacteriaceae) are considered an emergent model of terrestrial animal-microbe symbiotic relationships2-4,6,10,19. Xenorhabdus and Photorhabdus spp. are harbored as symbionts in the intestine of the only free-living stage of the nematodes, also known as the infective juvenile (IJ) or 3rd stage infective juvenile8,10,13. The bacterium-nematode pair is pathogenic for a wide range of insects and has successfully been implemented in biological control and integrated pest management programs worldwide6,8.
Herein we show a selection of in vivo and in vitro techniques that are frequently exercised for the rearing of EPN under laboratory conditions. In vivo methods contemplate an insect host for the rearing of the nematodes. Usually, immature stages of various insect orders (i.e. Lepidoptera, Coleoptera, Diptera, etc.) are considered suitable hosts. In vivo methods are usually considered for maintenance of nematode cultures in the lab. This method may not be suitable when considering mass production of the nematodes. Large quantities of insect hosts may be required for this purpose demanding more time and additional costs related to the insect rearing.
Entomopathogenic nematodes can also be cultured in vitro on several media. Depending on the goal of the study; in vitro methods may or consider the incorporation of the symbiotic bacteria in the media. In this presentation, we describe two commonly used methods for the propagation of EPN. The ingredients of the media provide a source of nutrients for the symbiotic bacterium and a sterol source for the nematodes. In vitro methods offer the advantage the rearing of EPN without an insect host.
Originally, many of the in vitro media developed were used for the multiplication of EPN when suitable insect hosts are not available. However, over the past years, in vitro rearing methods have become widely employed in research aiming to understand the mutualistic relationship between EPN and their symbiotic bacteria17,19.
The techniques detailed in this presentation correspond to those described by various authors and refined by the Stock Laboratory, University of Arizona (Tucson, AZ, USA). These techniques are distinct from the body of techniques that are used in the mass production of these organisms for pest management purposes.
Protocol
1. In vivo Rearing of Entomopathogenic Nematodes with their Symboitic Bacteria
Invert a 100 x 15 mm plastic Petri dish and place two discs of filter paper (90 mm) in the lid of the dish.
Evenly distribute 1 ml of the IJ (infective juveniles) water suspension (at a concentration of 1,000-2,000 IJ/ml) on the filter paper. NOTE: IJs do not need to be surface-sterilized.
Add 10 last instar larvae of the greater waxmoth Galleria mellonella to the dish. The goal is to provide approximately 100-200 IJs/larva.
Cover the lid with the bottom of the Petri dish (Figure 1) and label the Petri dish. Mention the following information: nematode species name (if known); isolate code/designation; date infection trap was set.
Place the dish inside a loosely sealed plastic bag (to conserve moisture) and keep it in the dark at either room temperature or in an incubator between 20-25 °C. Check dishes daily NOTE: Darkness facilitates nematode infection, as it mimics natural infection conditions in the soil.
After 3-5 days, remove cadavers with signs of nematode infection to a modified White trap7. NOTE: If the cadavers smell putrid, this may be an indication that the culturing was not successful and/or there was contamination. Set a new infection chamber with a new batch of nematodes and insects.
2. In vitro Rearing of Entomopathogenic Nematodes with their Symbiotic Bacteria
- Liver-kidney Agar Method9,19
- Chop liver and kidney into small pieces (2 cm3 or smaller) and place into a blender with NaCl, agar and 300 ml of water and blend until the meat becomes a liquid pulp, thick paste or puree. Then, transfer mixture to a 1 L Erlenmeyer flask.
- Add the final 200 ml of water to the blender vessel and decant any remaining media into the Erlenmeyer flask. Autoclave for 15 min at 121 °C. After autoclaving allow agar to cool to touch before pouring. NOTE: Pipetting of medium is not recommended because this medium tends to have chunks of meat.
- Pour ~20 ml of agar onto 5 (or 6) cm Petri dishes while hand stirring or swirling the Erlenmeyer flask between pours for a more homogenous media. Allow agar to solidify and store dishes at 4 °C until needed NOTE: Use a flame-sterilized metal spatula to break up any large chunks of meat poured into the Petri dish.
- Lipid Agar Method9,19
- Prepare lipid agar medium by mixing together nutrient broth, agar and yeast extract and pour mix into a 2 L Erlenmeyer flask. Add distilled H2O and MgCl2•6H2O and autoclave for 15 min at 121 °C.
- Add sterile mix of corn oil and corn syrup mix and stir or swirl vigorously and often to disperse oil droplets evenly. NOTE: Sterilize corn oil and syrup by placing the needed volume in a UV cross-linker. The oil will not dissolve into the liquid, but make sure it is in tiny droplets.
- Pour ~20 ml agar onto a 5 (or 6) cm Petri dishes and allow agar to solidify and store dishes at 4 °C until needed.
- Streak the desired symbiotic bacteria onto the lipid agar plate and incubate plates at 28 °C for 24-48 hr. NOTE: Spread 100-200 µl of overnight (12-16 hr) LB subculture.
- The day after, add approximately 0.4 ml of surface sterilized nematode suspension (eggs or infective juveniles) and incubate plates at room temperature in the dark or in an incubator at 22 ±3 °C. NOTE: Do not add more than 0.4 ml of nematode suspension as it can create an excessively wet environment that is unfavorable to nematode growth.
- Monitor cultures daily. Cultures should produce a new generation of IJs in about 12 days (Figure 2).
- Transfer bottom dish with agar when nematodes are seen crawling the sides of the dish (Figure 3A) to a modified White trap (see Orozco et al.12) to harvest IJs (Figure 3B). Perform this step under a laminar flow hood to reduce contamination of the media.
- Collect IJs from the modified White trap upon emergence and rinse IJ suspension to remove any medium debris. Store IJ in sterilized distilled water at 10 °C (in a cold-temperature incubator or cold room) or use immediately if needed. NOTE: Depending on the goal of the study, seed the plates with either symbiont-colonized or aposymbiotic nematodes.
3. In vitro Rearing of Aposymbiotic (Symbiont-free) Entomopathogenic Nematodes
Infect 10-20 G. mellonella larvae with IJs of the desired nematode species (see section 1). After 3 or 4 days (at this time IJs should have matured to first generation adults) collect cadavers. NOTE: Time to maturity and number of females needed to obtain an adequate number of eggs will vary by species. Infect more G. mellonella larvae if necessary and start dissections as early as the 48 hr post-infection to assess whether they are in the right developmental stage.
Place each cadaver individually in a Petri dish with saline solution (M9 buffer18). Dissect a cadaver by pulling its head with a fine tip forceps.
Collect at least 20 gravid (with eggs inside) females with a fine needle (L-shape preferably) and place them in a watch glass containing 10 ml of M9 buffer (Figure 4A).
Prepare an axenizing solution by considering the following ingredients: 6.75 ml distilled water, 1.25 ml 5 N NaOH, and 2.25 ml commercially available bleach solution diluted to 3% hypochlorite. NOTE: NaOH is a basic solution—gloves, goggles and other appropriate protective clothing should be worn. NOTE: Prepare fresh axenizing solution each time, as bleach degrades in light. Prepare several batches of axenizing solution and perform the surface sterilization and harvesting of eggs in multiple watch glasses.
Rinse females at least three times with M9 buffer filling watch glass with buffer solution and sucking up buffer with a pipette. Make sure the females remain in the watch glass. Repeat this step two more times.
Immediately add the axenizing solution and allow the females to remain in this solution for no more than 10–15 min. Observe the nematode tissues beginning to disintegrate while the eggs (which are resistant to the axenizing solution) remain intact.
Manually disrupt female bodies to speed up the process, by cutting them with a needle or a scalpel (Figure 4B, C). Remove eggs by pipetting them into 1.5 ml microcentrifuge tubes, avoiding adding any undissolved tissue. NOTE: Use as many microcentrifuge tubes as necessary to gather eggs and work quickly, as the viability of the eggs will decrease over a prolonged period of exposure to axenizing solution.
Vortex tubes for 15 sec using the “high speed setting” to further remove nematode tissues attached to the eggs. Alternatively, if a vortex is unavailable, pipet up and down the solution several times.
Pellet eggs by centrifugation at approximately 18,000 g for 2 min. Increase speed if eggs do not sufficiently pellet. Remove axenizing solution and rinse twice by filling the microcentrifuge tube with sterile distilled water and centrifuge one more time at 900 x g for 1 min.
After the last wash, resuspend eggs in 300–500 µl of sterile distilled water and place them in a sterile 3 cm Petri dish or sterilized watch glass. Remember that eggs are now surface-sterilized, so use sterile pipettes and/or pippetter tips and water to maintain cleanness of the eggs.
Examine eggs under a dissecting microscope (30-50X magnification) to confirm they are intact (Figure 4D) and transfer them to a 5 cm plate with liver-kidney agar (see section 2.1) NOTE: Do not add more than 400 µl of the egg suspension on the plates as it will make the agar excessively wet.
Label plates with appropriate information and store them upright in the dark at 28 °C. Hatching of eggs should be evident overnight. NOTE: Water may condense on the lid of the dish but it will evaporate after 1 or 2 days. At this time, place the dish in plastic bag to retain moisture of the agar and avoid its drying.
Observe dishes periodically and discard any dishes that show signs of fungal or bacterial contamination. Once IJs are seen migrating up the wall of the Petri dish, remove the lid and transfer the bottom dish with agar to a modified White trap12. Consider sterile conditions when transferring the dish into the modified White trap to avoid contamination of the exposed media.
Continue monitoring the White Traps and harvest IJ as needed. NOTE: Aposymbiotic nematodes will continue to migrate into the water of the White trap for many days and even weeks.
Representative Results
The in vivo rearing method uses live insects as hosts for nematode growth and reproduction. Infection chambers are an efficient method for exposing insects IJs. This is the only stage in the nematodes’ life cycle that vectors the bacterial symbionts from one insect host to another. Figure 1 shows the set up for an infection chamber as well as the materials needed to build this chamber. In vitro rearing methods allow EPN to grow without an insect host but are also implemented for the rearing of aposymbiotic (symbiont-free) nematodes. Figure 2 shows a lipid agar plate with different nematode stages. Lipid agar plates may also be considered in cross-hybridization tests which are required to validate nematode identity based on the biological species concept. The liver kidney agar method was originally described by Poinar & Thomas (1966)14. This method allows the nematodes to mature and reproduce without an insect host and can be used to rear nematodes without their symbiotic bacteria (i.e. produce aposymbiotic nematodes). A disadvantage of this method is that it is prone to contamination because of their rich nature, allowing unwanted bacteria or fungi to grow. Figure 3A shows liver-kidney agar plates with IJS crawling on the side of the plate. At this stage the bottom portion of the Petri dish can be transferred to a modified White trap. Figure 3B demonstrates a liver-kidney plate with Steinernema nematodes in a modified White trap for the harvesting of IJs progeny. Figure 4A shows a watch glass with gravid Steinernema females prior to their axenization. Figure 4B displays the disruption of the females with a dissecting needle. Figure 4C shows disrupted females in the axenizing solution. Figure 4D shows an intact egg of the nematode Steinernema carpocapsae.
 Figure 1. Set up of an infection chamber for in vivo rearing of EPN. Top row shows a 5 cm Petri dish and filter paper. The bottom row displays a 10 cm Petri dish and filter paper. Notice number of insect larvae added to each infection chamber.
Figure 1. Set up of an infection chamber for in vivo rearing of EPN. Top row shows a 5 cm Petri dish and filter paper. The bottom row displays a 10 cm Petri dish and filter paper. Notice number of insect larvae added to each infection chamber.
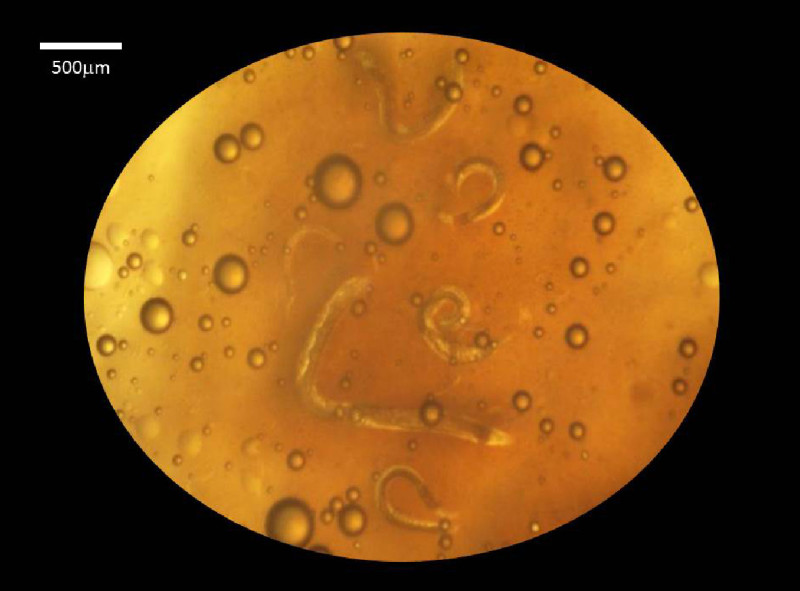 Figure 2. Lipid agar method for in vitro rearing of EPN. The image shows a close-up (20X magnification) of a dish with adult nematodes developing on the medium.
Figure 2. Lipid agar method for in vitro rearing of EPN. The image shows a close-up (20X magnification) of a dish with adult nematodes developing on the medium.
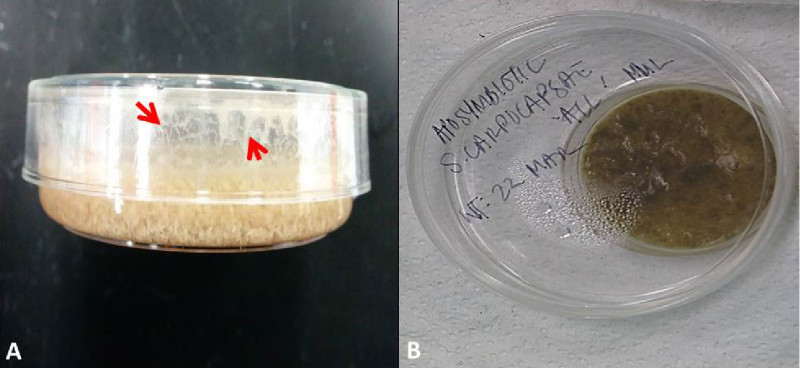 Figure 3. Liver-kidney agar plate. Image A on the left shows a dish with successful growth of nematodes. Notice nematodes crawling on the side of the dish. Image B shows a liver-kidney agar plate placed in a modified White trap for the harvesting of IJ nematodes.
Figure 3. Liver-kidney agar plate. Image A on the left shows a dish with successful growth of nematodes. Notice nematodes crawling on the side of the dish. Image B shows a liver-kidney agar plate placed in a modified White trap for the harvesting of IJ nematodes.
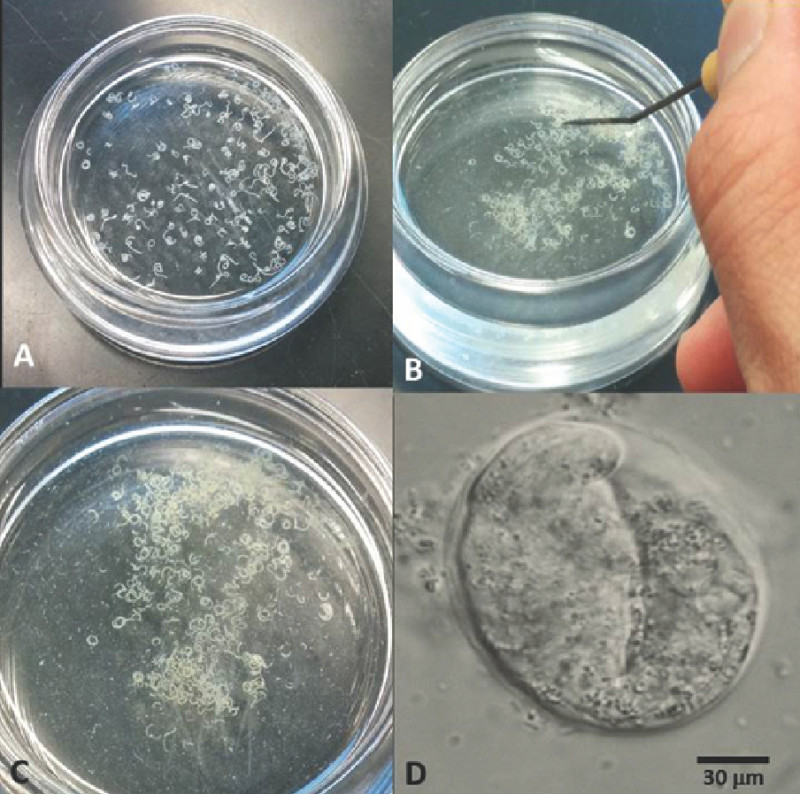 Figure 4. Watch glass with gravid females in axenizing solution. Image A shows gravid females in the axenizing solution. Image B shows the grinding of the females with a dissecting needle. Image C displays disrupted females. Image D shows an intact egg of the nematode Steinernema carpocapsae.
Figure 4. Watch glass with gravid females in axenizing solution. Image A shows gravid females in the axenizing solution. Image B shows the grinding of the females with a dissecting needle. Image C displays disrupted females. Image D shows an intact egg of the nematode Steinernema carpocapsae.
Discussion
Using a suitable host is a key factor for the successful in vivo rearing of EPN. Usually, both steinernematids and heterorhabditids can reproduce and successfully complete their life cycle in larvae of the greater wax moth, Galleria mellonella (Lepidoptera: Pyralidae). However, other insect species from different families and/or orders can be considered. A few of the currently described nematode species are known to have specificity for a particular insect host. For example, S. kushidai and S. scarabaei are not very virulent to lepidopteran larvae, and need coleopteran larvae such as scarab beetle larvae (Scarabaeidae) for successful rearing in the lab. Another species, S. scapterisci, prefers orthopteran insects such as crickets or mole crickets.
When a suitable host is not available or when experimental conditions require it, in vitro methods can be employed for the rearing of EPN. These methods use media that offer a rich source of nutrients for nematodes and their bacterial symbionts. In this presentation we described two types of agar (liver-kidney and lipid) that can be utilized for the successful growth and reproduction of EPN with or without their symbiotic bacteria.
Users should be aware that the liver-kidney agar is a rich media and is therefore easily contaminated. Sterile technique is recommended in handling both the axenized eggs and the inoculated plates during all steps of the procedure. Plates that are contaminated with bacteria or fungus should be discarded immediately.
When rearing aposymbiotic Steinernema IJs, care should be taken to properly lyse the female nematode tissues, as they could harbor Xenorhabdus bacteria. Also, to validate that IJ are indeed free of symbiotic bacteria, we recommend the grinding of a sample of freshly harvested IJs in LB with a hand-held motor-driven pestle as per (Heungens et al. 2002)7 and plate the suspension onto NBTA media1. If bacteria colonies are found (after overnight incubation) it will be either an indication of a poor axenization technique or the result of contamination.
The rearing of aposymbiotic Steinernema nematodes is a technique that can also be considered in a wide array of procedures and experiments. Readers should be aware that the impact of rearing Steinernema nematodes without symbionts over multiple generations may have an impact on nematode fitness and survival over generation times. A few studies suggest the necessity of their pairing in natural systems5,11,15-17. However, these studies have been done on a limited number of species and further exploration is needed.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors wish to thank past members of the Stock lab: Ming-Min Lee, Kathryn Plichta, Victoria Miranda-Thompson and Sam-Kyu Kim for their contributions to the improvement of many of these protocols. This work was funded in part by the National Science Foundation grant IOS-0840932 and IOS-0724978 to S. P. Stock
References
- Akhurst RJ. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes, Neoaplectana and Heterorhabditis. J. Gen. Microbiol. 1980;121:303–309. [Google Scholar]
- Dunphy GB, Webster JM. The monoxenic culture of Neoaplectana carpocapsae DD 136 and Heterorhabditis heliothidis. Revue Nematol. 1989;12:113–123. [Google Scholar]
- Boemare N. Biology, taxonomy and systematics of Photorhabdus and Xenorhabdus. In: Gaugler R, editor. Entomopathogenic Nematology. Wallingford: CABI Publishing; 2002. [Google Scholar]
- Boemare NE, Akhurst RA. The Genera Photorhabdus and Xenorhabdus. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The Prokaryotes. New York, NY: Springer; 2002. pp. 473–488. [Google Scholar]
- Ehlers RU, Wulff A, Peters A. Pathogenicity of axenic Steinernema feltiae. Xenorhabdus bovienii. and the bacto-helminthic complex to larvae of Tipula oleracea (Diptera) and Galleria mellonella (Lepidoptera) Journal Invertebrate Pathology. 1997;69:212–217. doi: 10.1006/jipa.1996.4647. [DOI] [PubMed] [Google Scholar]
- Gaugler R, Kaya HK. Entomopathogenic Nematodes in Biological Control. Boca. Raton, FL: CRC Press; 1990. [Google Scholar]
- Heungens K, Cowles CE, Goodrich-Blair H. Identification of Xenorhabdus nematophila genes required for mutualistic colonization of Steinernema carpocapsae nematodes. Molecular Microbiology. 2002;45:1337–1353. doi: 10.1046/j.1365-2958.2002.03100.x. [DOI] [PubMed] [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Stock SP. Techniques in insect nematology. In: Lackey LA, editor. Manual of techniques in insect pathology. London: Academic Press; 1997. pp. 281–324. [Google Scholar]
- Koppenhöfer H. Bacterial Symbionts of Steinernema and Heterorhabditis. In: Nguyen KB, Hunt DJ, editors. Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts. Leiden-Boston: Brill; 2007. pp. 735–759. [Google Scholar]
- Lunau S, Stoessel S, Schmidt Peisker AJ, Ehlers RU. Establishment of monoxenic inocula for scaling up in vitro cultures of the entomopathogenic nematodes Steinernema spp and Heterorhabditis spp. Nematologica. 1993;39:385–399. [Google Scholar]
- Orozco RA, Lee M, Stock SP. Soil sampling and isolation of entomopathogenic nematodes (Steinernematidae, Heterorhabditidae) J. Vis. Exp. 2014. [DOI] [PMC free article] [PubMed]
- Poinar GO. The presence of Achromobacter nematophilus in the infective stage of a Neoaplectana sp (Steinernematidae: Nematoda) Nematologica. 1966;12:105–108. [Google Scholar]
- Poinar GO, Jr, Thomas GM. Significance of Achromobacter nematophilus sp. nov. (Achromobacteriaceae: Eubacteriales) associated with a nematode. Int. Bull. Bacteriol. Nomencl. Taxon. 1966;15:249–252. [Google Scholar]
- Sicard M, Brugirard-Ricaud K, Pagès S, Lanois A, Boemare NE, Brehéli M, Givaudan A. Stages of infection during the tripartite interaction between Xenorhabdus nematophila, its nematode vector, and insect hosts. Appl. Environ. Microbiol. 2004;70:6473–6480. doi: 10.1128/AEM.70.11.6473-6480.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard M, Hinsinger J, LeBrun N, Pagès S, Boemare N, Moulia C. Interspecific competition between entomopathogenic nematodes (Steinernema) is modified by their bacterial symbionts (Xenorhabdus) BMC Evolutionary Biology. 2006;6:68–78. doi: 10.1186/1471-2148-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H, Stock SP, Kim SK, Flores-Lara Y, Forst S. New insights into the colonization and release processes of Xenorhabdus nematophila and the morphology and ultrastructure of the bacterial receptacle of its nematode host, Steinernema carpocapsae. Applied and environmental microbiology. 2007;73:5338–5346. doi: 10.1128/AEM.02947-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. ed C.elegans.WormBook., editor. Maintenance of C. elegans. WormBook. 2006. Available from: http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Stock SP, Goodrich-Blair H. Nematode parasites, pathogens and associated of insects and invertebrates of economic importance. In: Lacey LA, editor. Manual of Techniques in Invertebrate Pathology. Yakima, WA: Elsevier; 2012. pp. 373–426. [Google Scholar]


