A fixed combination of netupitant and palonosetron was well-tolerated, with an adverse event profile as expected for the regimen. Sample size, demographic characteristics, study design, chemotherapy, and antiemetic regimen differences across the four studies may have contributed to differences in frequencies of neutropenia and alopecia. Adding a neurokinin-1 receptor antagonist to a chemotherapy-induced nausea and vomiting prophylaxis regimen can improve outcomes without additional toxicity.
Keywords: Antiemetics, Chemotherapy, Nausea, Neurokinin-1 receptor antagonists, Safety, Serotonin 5-hydroxytryptamine-3 receptor antagonists, Vomiting
Abstract
Background.
Standard prophylaxis for chemotherapy-induced nausea and vomiting (CINV) with highly emetogenic and anthracycline-cyclophosphamide-based chemotherapy includes a 5-hydroxytryptamine-3 receptor antagonist, a neurokinin-1 receptor antagonist (NK1RA), and corticosteroid therapy. NEPA is a fixed combination of netupitant and palonosetron. The primary objective of this analysis was to document the safety profile, including cardiac safety, of NEPA + dexamethasone in comparison with current therapies across all phase II/III trials.
Materials and Methods.
This pooled analysis was based on data from 3,280 patients in 4 randomized, double-blind clinical trials. Patients were categorized into 1 of 3 pooled groups on the basis of actual treatment received: NEPA + dexamethasone, palonosetron + dexamethasone, and aprepitant + ondansetron/palonosetron + dexamethasone. Safety was assessed by number and frequency of adverse events (AEs) and changes from baseline electrocardiogram measures.
Results.
Most patients were female and younger than 65 years of age. Demographic characteristics varied among studies and pooled groups. Frequencies of treatment-emergent AEs (TEAEs) and treatment-related AEs (TRAEs) were similar across groups. TEAEs were mostly mild and consistent with expected chemotherapy and disease-related AEs (hematologic events, hair loss, general weakness). TRAEs in ≥2% of patients were headache and constipation. Frequencies of cardiac TEAEs were similar across groups, with QT prolongation (1.6%), tachycardia (1.1%), and dyspnea (0.9%) the most common. Serious cardiac TEAEs were rare.
Conclusion.
NEPA was well-tolerated, with an AE profile as expected for the regimen. Sample size, demographic characteristics, study design, chemotherapy, and antiemetic regimen differences across the four studies may have contributed to differences in frequencies of neutropenia and alopecia. Adding an NK1RA to a CINV prophylaxis regimen can improve outcomes without additional toxicity.
Implications for Practice:
Supportive care for cancer should ideally be efficacious, convenient, and well-tolerated. There have been concerns about cardiac safety with current antiemetic prophylactic agents, namely dolasetron and ondansetron. This pooled safety analysis demonstrates that the new oral fixed combination therapy NEPA can be safely added to an antiemetic regimen without increased toxicity.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) can significantly interfere with a patient’s quality of life as well as a patient’s compliance with anticancer therapy [1]. Ideally, the optimal approaches to cancer supportive care are efficacious, convenient to administer, and well-tolerated. The current antiemetic standard of care for patients receiving highly emetogenic (including anthracycline-cyclophosphamide [AC]-based) chemotherapy involves therapy with a 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA), a neurokinin-1 receptor antagonist (NK1RA), and a corticosteroid [1].
5-HT3RAs [2, 3] and NK1RAs [4–6] are generally well-tolerated when given for CINV prevention, but there have been concerns about cardiac effects with some 5-HT3RAs. Intravenous formulations of the 5-HT3RAs dolasetron [7] and ondansetron [8] have been associated with electrocardiogram (ECG) abnormalities, including corrected QT interval (QTcI) prolongation. Because of these effects, intravenous (i.v.) dolasetron is no longer indicated for CINV, and i.v. ondansetron for CINV prevention has been restricted to a maximum of 16 mg per dose [8]. The effect of palonosetron (PALO) on ECG measures has been studied in both healthy volunteers [9] and cancer patients [10–12]. Two of the trials found significant differences in heart rate (HR) after administration of PALO compared with baseline [10, 12], but no HR-corrected cardiac measures significantly differed. All of the studies concluded that PALO does not result in any clinically significant cardiac changes and is safe to administer.
NEPA is a single-dose, oral, fixed combination of the highly selective NK1RA, netupitant (NETU), and the pharmacologically [13] and clinically [14] distinct 5-HT3RA, PALO. NEPA was developed to improve antiemetic control and guideline adherence by enhancing the convenience of administering antiemetic prophylaxis targeted at dual molecular pathways involved in emesis. Recently, NEPA was shown to be superior to oral PALO for prevention of CINV during a single-cycle study in highly emetogenic chemotherapy (HEC) [15], as well as in a multiple-cycle study in AC-based moderately emetogenic chemotherapy (MEC) [6, 16]. Studies have also shown NEPA to be well-tolerated over multiple cycles of MEC and HEC [16, 17].
Because of the concerns about cardiac toxicity raised with the aforementioned antiemetics, the cardiac safety of combination therapy with NETU and PALO has been previously investigated. An International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use E14 QT trial conducted in healthy volunteers found no signals for effects on QTcI, HR, PR interval, or QRS interval compared with placebo [18]. The primary goal of this analysis is to document the safety profile of this new antiemetic combination and to confirm that no significant cardiac effects are associated with its administration in the oncology setting.
Materials and Methods
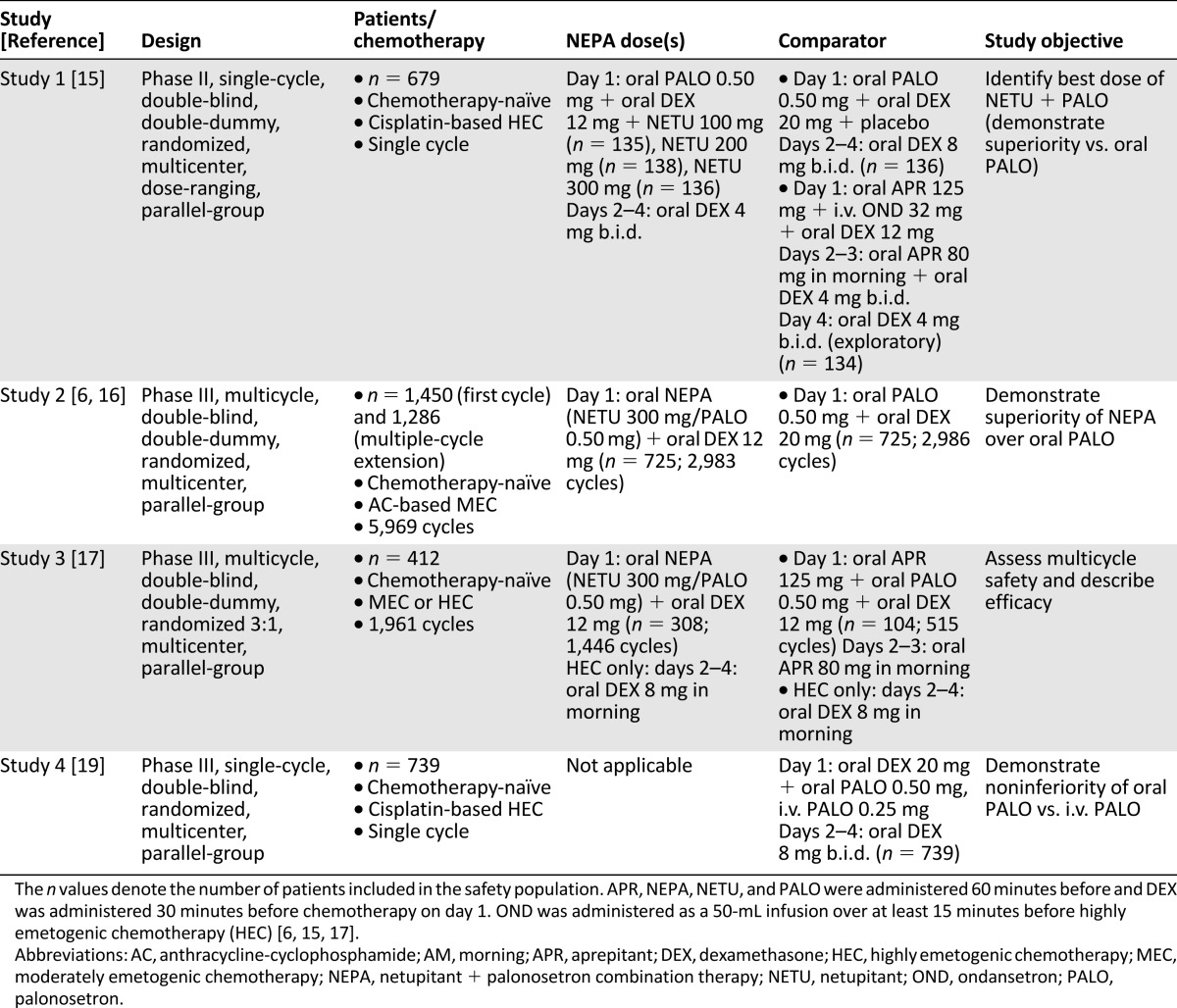
The objective of this integrated summary of safety is to describe the general and cardiac safety and tolerability of a single oral dose of NEPA + dexamethasone (DEX) compared with oral/i.v. PALO + DEX and with aprepitant (APR) + PALO/ondansetron (OND) + DEX during initial and repeated cycles of both MEC and HEC. The analysis is based on pooled adverse event (AE) and ECG data from 3,280 patients (9,348 chemotherapy cycles) who participated in 4 randomized, double-blind, multinational clinical trials. Of the 4 studies, 2 were single-cycle [15, 19] and 2 were multicycle [6, 16, 17].
Patients assigned to the NEPA treatment group received NEPA + DEX before HEC or MEC on day 1. For patients receiving HEC, DEX was also administered on days 2–4. Comparators in three of the trials included oral PALO 0.50 mg + DEX or APR + PALO/OND + DEX. One of the trials was a dose-ranging study that compared different doses of NETU in combination with PALO, and another compared oral and i.v. PALO. In all 4 studies, patients were not allowed to receive any drug with potential antiemetic efficacy within 24 hours before day 1 or systemic corticosteroids within 72 hours before day 1. A different steroid dosing was permitted in study 3 only if associated with taxane therapy. Patients in the NEPA studies were not permitted to receive CYP3A4 substrates or inhibitors within 1 week; to receive CYP3A4 inducers within 4 weeks; or to have long-term use of any CYP3A4 substrates, inhibitors, or inducers before day 1. Patients in the NEPA studies were also excluded if they had any history of serious cardiovascular disease or predisposition to cardiac conduction abnormalities, with the exception of incomplete right bundle-branch block [6, 15–17, 19].
The individual study results were previously published (study 1, no registry number available [15]; study 2, NCT01339260 [6, 16]; study 3, NCT01376297 [17]; study 4, NCT01363479 [19]), and the study designs are summarized in Table 1. All the original studies were approved by ethical review committees for each study center; all patients provided written informed consent; and all study sites followed Good Clinical Practice, International Conference on Harmonization, and Declaration of Helsinki principles, local laws, and regulations.
Table 1.
Study designs
Statistical Analysis
Data from the aforementioned studies were pooled into an integrated summary of safety that was conducted post hoc. The safety population for the summary was defined as the total of the safety populations of the single studies and included all patients who had received at least one study treatment. For all safety analyses, patients were included in treatment groups on the basis of what they actually received. Data for NEPA doses of 100, 200 (study 1 only), and 300 mg (studies 1, 2, and 3) were combined into the NEPA + DEX group; data for PALO given orally (studies 1, 2, and 4) and via i.v. route (study 4 only) were combined into the i.v./oral PALO + DEX group; and data for APR given with OND (study 1) or PALO (study 3) were combined into the APR + OND/PALO + DEX group.
Safety was assessed by descriptive statistics and is displayed as the number and frequency of treatment-emergent AEs (TEAEs) and treatment-related AEs (TRAEs) by treatment group. TEAEs were defined as any AE reported after the administration of the first study drug. TRAEs were AEs deemed possibly, probably, or definitely related to study drug by the investigator. TEAEs were coded by using MedDRA, version 14.0 (MedDRA MSSO, McLean, Virginia; https://www.meddra.org/). To identify common TEAEs and TRAEs, additional tables display only TEAEs that occurred in ≥5% and those TRAEs that occurred in ≥2% of patients in any treatment group, respectively. Frequencies of AEs are also provided with 95% confidence intervals (CIs) for percentages of patients, calculated by using the Wilson score method.
In all 4 studies, 12-lead ECG was performed at each cycle: screening; predose; and 5, 24, and 120 hours after study drug administration. ECG recordings were centrally evaluated and interpreted by a blinded cardiologist. Descriptive statistics for observed values and changes from baseline were calculated for ECG measures, including HR, PR interval, QRS interval, QT interval, QT interval corrected for HR according to Bazett’s formula, and QT interval corrected for HR according to Fridericia’s formula (QTcF). In addition, the numbers and percentages of patients with new QTcF values >500 milliseconds and with a QTcF increase >60 milliseconds from baseline were calculated.
Pooling data from these four studies can lead to certain limitations because of the different study objectives, patient populations enrolled (e.g., number of patients enrolled, gender distribution, and study locations), different study durations (i.e., number of exposures to each antiemetic regimen), and different chemotherapy and antiemetic regimens used. These limitations should be considered when interpreting the results of this integrated analysis.
Results
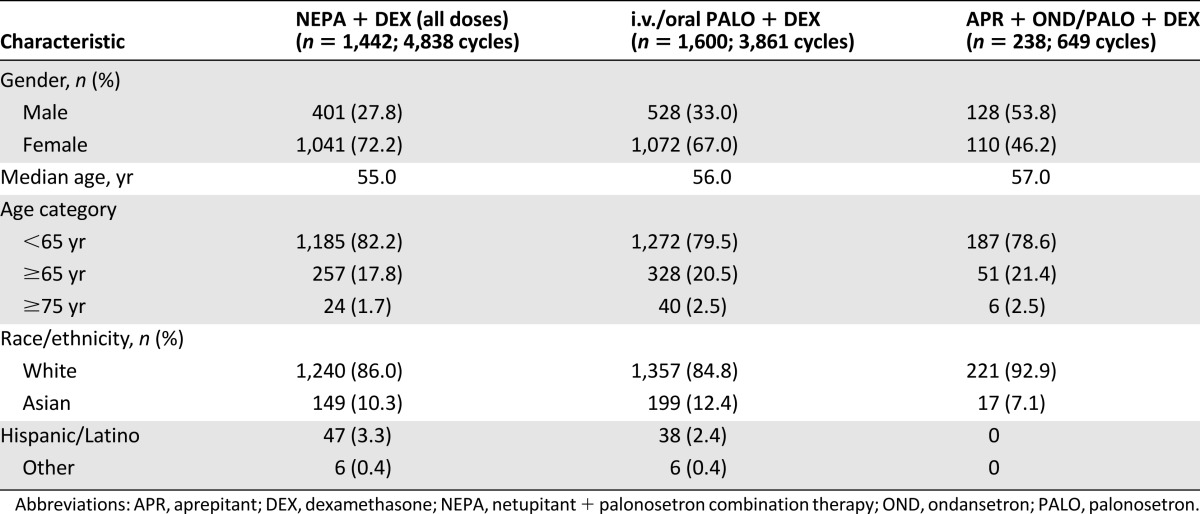
A majority of the 3,280 patients included in the safety analysis were female (68%), white (86%), and younger than 65 years of age (81%). Patient demographic characteristics in each study varied, with fewer women in studies 1 and 4 (43% and 41%, respectively) [15, 19], a high percentage of women in study 2 (98%) [16], and the same percentage of men and women in study 3 [17]. Although most patients in the pooled NEPA and PALO groups were female, 53.8% of the APR group was male (Table 2).
Table 2.
Patient demographic characteristics
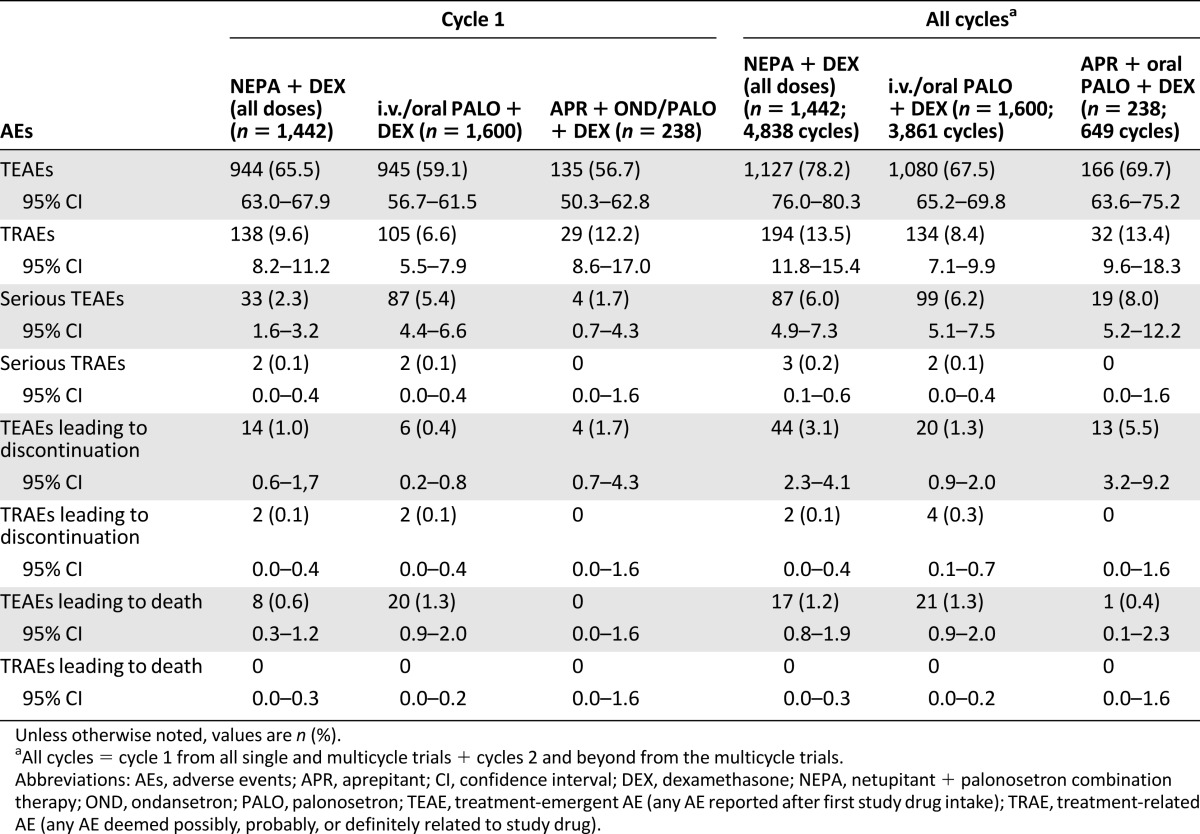
The percentages of patients with at least one TEAE in cycle 1 and in all cycles were similar in all three groups, with slightly higher percentages in the NEPA group. The percentages of patients reporting TRAEs were also similar, with the lowest seen in the PALO group. The incidence of serious TEAEs was similar across treatment groups during all cycles, and few patients in any group experienced AEs that led to discontinuation or death (Table 3).
Table 3.
Overview of adverse events
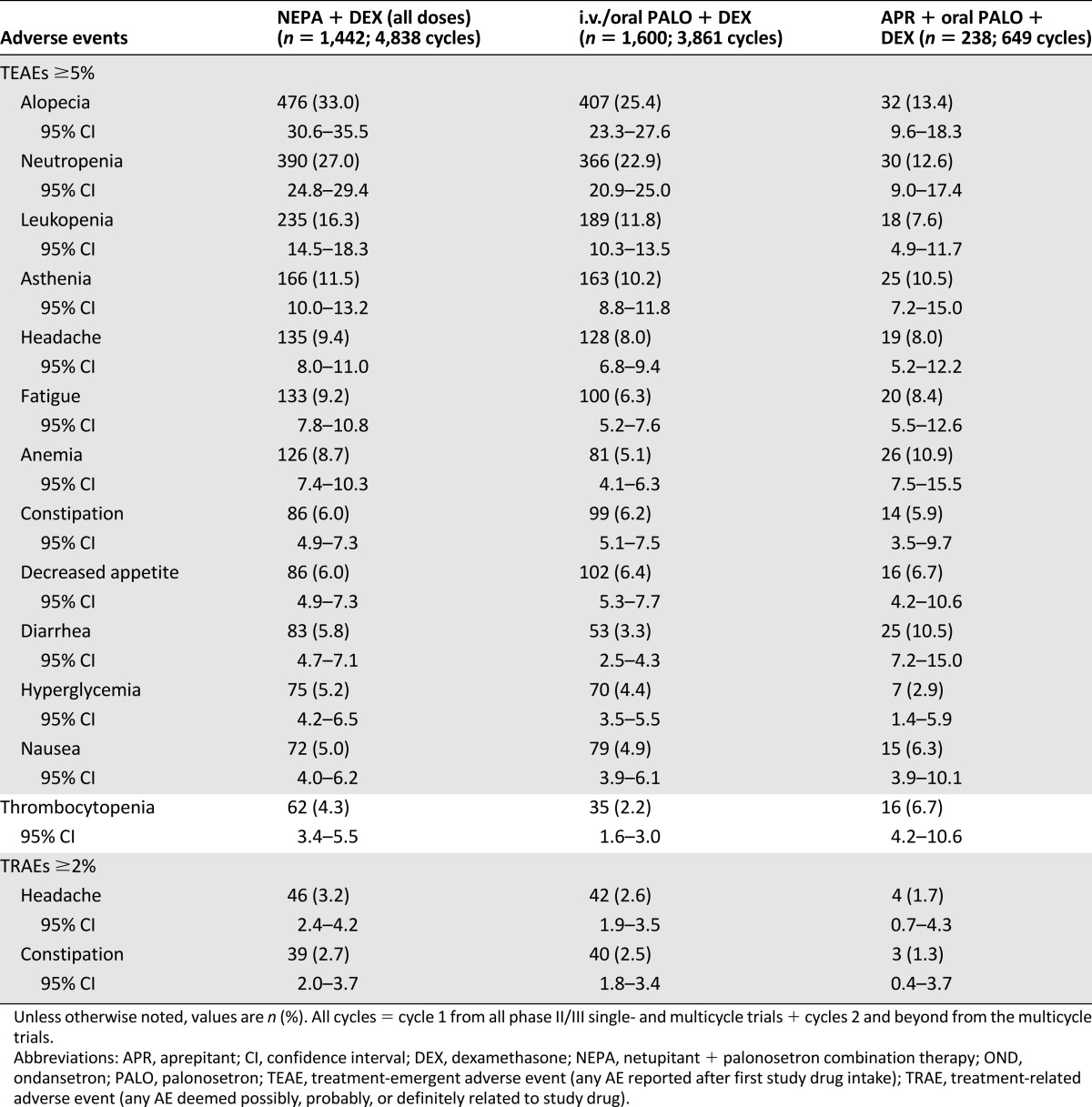
The most commonly reported TEAEs (reported by ≥5% of patients in any treatment group) in cycle 1 were consistent with those expected with the toxic effects of chemotherapy and disease-related processes. These included hematological events, such as neutropenia, as well as hair loss and general weakness (Table 4). For all cycles of chemotherapy, a similar pattern was observed with the most commonly reported TEAEs across all treatment groups; TEAEs did not increase over multiple cycles (Table 5). The only TRAEs reported by ≥2% of patients in any treatment group for cycle 1 or all cycles were headache and constipation. There was a relatively low incidence of treatment-emergent constipation (4.0% with NEPA, 4.4% with PALO, and 3.4% with APR) and treatment-related constipation (1.9%, 1.8%, and 0.8%, respectively) across all treatment groups in cycle 1. This pattern continued throughout all cycles.
Table 4.
Treatment-emergent (≥5%) and treatment-related (≥2%) adverse events in cycle 1
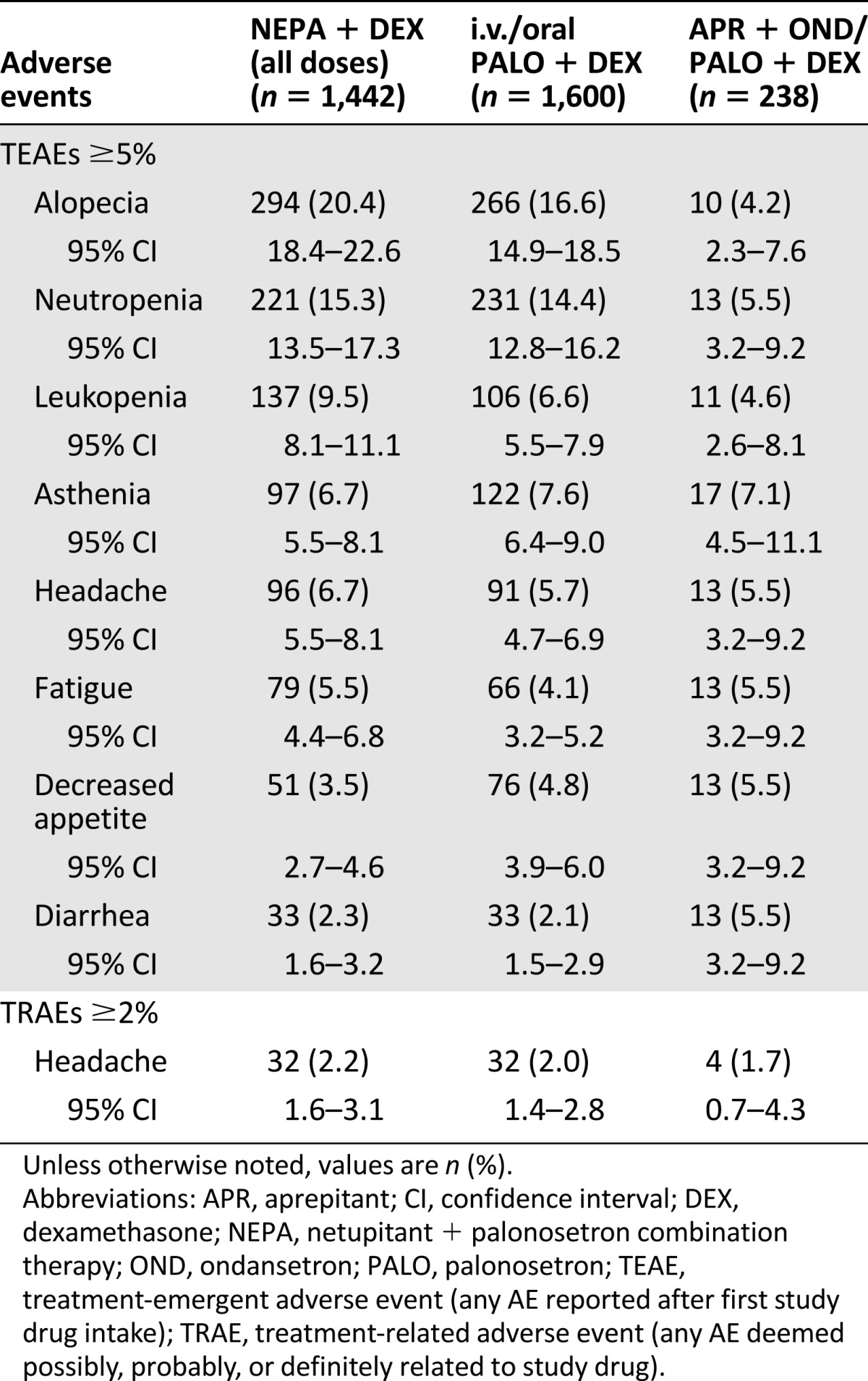
Table 5.
Treatment-emergent (≥5%) and treatment-related (≥2%) adverse events in all cycles
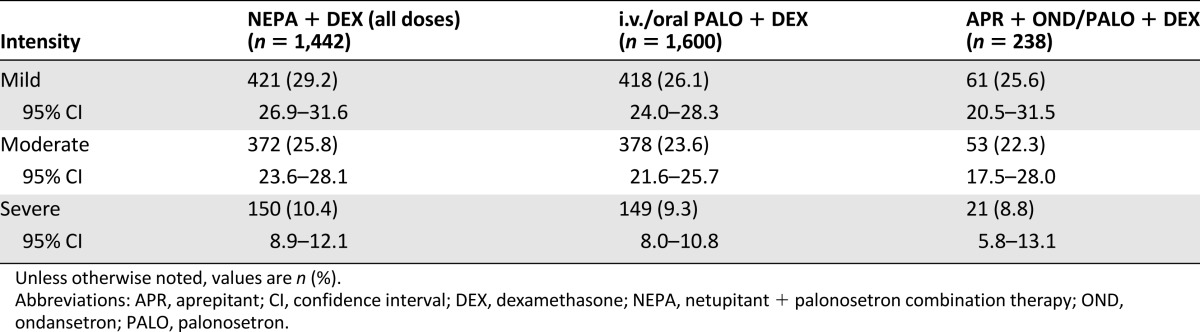
Of the 61.7% of patients (2,024 of 3,280) who experienced a TEAE across all treatment groups in cycle 1, most reported them to be of mild (27.4% [900 of 3,280]) or moderate (24.5% [803 of 3,280]) intensity rather than severe (9.8% [320 of 3,280]). The frequencies of mild, moderate, and severe events were similar across the treatment groups (Table 6).
Table 6.
Intensity of treatment-emergent adverse events in cycle 1
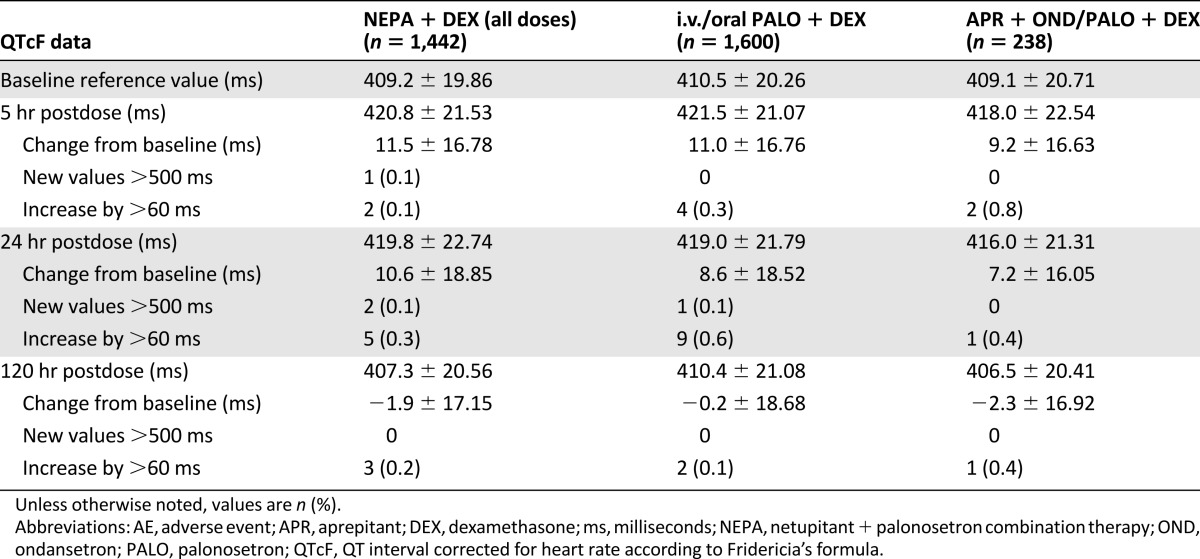
Similar frequencies of cardiac AEs were reported throughout all cycles in all the treatment groups: 174 (12.1%) in the NEPA group, 139 (8.7%) in the PALO group, and 32 (13.4%) in the APR group. The most common cardiac TEAEs in all groups were QT prolongation (1.6%), tachycardia (1.1%), and dyspnea (0.9%). Serious cardiac TEAEs were rare and similar across treatment groups, and mean changes from baseline in ECG measures (HR, PR interval, QRS interval, QT interval, QT interval corrected for HR according to Bazett’s formula, and QTcF) were small and also generally similar across treatment groups at each time point. Mean QTcF values returned to baseline or lower at 120 hours. Overall, a low percentage of patients experienced QTcF >500 milliseconds or increases >60 milliseconds from baseline or same cycle predose in all 3 groups (Table 7).
Table 7.
Changes in QT interval corrected for heart rate according to Fridericia’s formula interval and outlier analysis in cycle 1
Discussion
In the 3,280 patients whose data are presented in this integrated summary, NEPA was generally well-tolerated. The AE profile was as expected for this drug regimen and consistent with the administration of cytotoxic chemotherapy. Most TEAEs were of mild or moderate intensity, with alopecia and hematological events being among the most frequent. Alopecia and neutropenia, which are more prevalent with AC-based chemotherapy regimens, were more often reported in the NEPA and PALO groups. This was likely due to the difference in sample size and the fact that the APR regimen was not included in the large trial with AC-based chemotherapy. The incidence of TRAEs was low; the most common TRAEs were headache and constipation, which are typical for these drug classes, but were reported rarely in these studies. These findings are consistent with other antiemetic trials that concentrate on rare, serious AEs (SAEs).
A meta-analysis by Popovic et al. [20] comparing PALO to other 5-HT3RAs (i.e., OND, granisetron [GRA], and dolasetron) in HEC and MEC settings showed similarities between safety profiles. The most common TRAEs consisted of constipation (odds ratio [OR]: 0.80; 95% CI: 0.63–1.01), headache (OR: 1.10; 95% CI: 0.84–1.44), and diarrhea (OR: 1.82; 95% CI: 0.74–4.53). PALO-treated patients had less dizziness (OR: 2.15; 95% CI: 1.05–4.41).
In a systematic review comparing other NK1RAs (i.e., APR, casopitant, and ezlopitant), hiccups and fatigue occurred more commonly in patients who received NK1RA therapy and constipation was more common in patients who received standard dual antiemetic therapy [21]. Of note, the incidence of neutropenia and infection-related SAEs was higher with APR [21], although that may have been due to increased plasma levels of DEX from a drug interaction with APR, per a study by Chawla et al. [22] and as demonstrated by McCrea et al. [23]. More recent clinical trials [4, 24–26] evaluating the efficacy of three-drug antiemetic therapy with APR found safety profiles similar to that in the current analysis. In a study by Miura et al. [4], chemotherapy-naïve lung cancer patients receiving cisplatin-based HEC were given antiemetic therapy, including APR, PALO, and DEX. The most common AEs associated with this therapy were constipation and diarrhea. A similar study in gastric cancer patients receiving APR, GRA, and DEX [24] found that this antiemetic regimen was also well-tolerated, with such TRAEs as anorexia, diarrhea, hiccups, and constipation.
A study conducted in chemotherapy-naïve Chinese patients also receiving cisplatin-based HEC [25] compared an APR regimen (APR + GRA + DEX) with standard therapy (placebo + GRA + DEX). AEs were similar in frequency in both groups (40.0% with APR compared with 44.3% with standard therapy) and included fatigue, dizziness, anemia, insomnia, upper abdominal pain, and noncardiac chest pain, most of which were similar between the groups. There were also similar incidences of TRAEs with APR (11.7%) and standard therapy (13.3%). A recent study by Choi et al. evaluated therapy with APR, ramosetron, and DEX in patients receiving MEC with paclitaxel and carboplatin [26]. This study found the most common AEs to be constipation and headache. The investigators also reported at least one TRAE in 38.9% of patients, 4 cases of infection, 2 cases of neutropenic fever, and 30 cycles (6.5%) of grade 3 or 4 hematologic toxicities.
Similar results have been found with the NK1RA rolapitant. A phase II clinical trial with rolapitant in patients receiving cisplatin-based HEC found that TRAEs were mild and that SAEs were similar across all treatment groups and were related to chemotherapy or the underlying cancer [27]. The safety of rolapitant was further investigated in two phase III studies [28] involving patients receiving MEC or AC-based chemotherapy [29] and HEC [30]. The incidences of AEs for MEC (63.9% with rolapitant vs. 66.0% with control) and HEC (64.7% vs. 60.2%, respectively). In addition, SAEs for MEC (6.6% vs. 7.1%, respectively) and HEC (12.5% vs. 14.2%, respectively) were similar across groups in cycle 1. The most common AEs in both groups for MEC were fatigue, constipation, and alopecia; for HEC they were constipation, asthenia, and neutropenia. The most common SAEs in the MEC and HEC studies were febrile neutropenia, neutropenia, neutrophil count decrease, and thrombocytopenia.
An important objective of this pooled safety analysis was to ensure that no significant cardiac effects were associated with the NEPA combination therapy. In this analysis, cardiac AEs and changes in ECG measures were similar in all groups, with a low incidence of serious cardiac TEAEs, indicating an acceptable cardiac safety profile. Few patients experienced QTcF >500 milliseconds or increases >60 milliseconds from baseline across all 3 groups. Patients in the NEPA studies were excluded if they had any history of serious cardiovascular disease or predisposition to cardiac conduction abnormalities, which may have affected these results.
A previous study with NEPA in healthy volunteers did not find any signals for effects on QTcI, HR, PR interval, or QRS interval compared with placebo, even at supratherapeutic doses [18]. Other studies in patients receiving PALO for CINV also found no evidence of cardiac effects from therapy. A study by Yavas et al. in 76 patients revealed a higher median minimal QT interval value after PALO administration, which was not statistically significant [11]. Another study by Dogan et al. among 50 patients receiving PALO showed that minimum and maximum QT intervals increased significantly, but there were no significant changes in any HR-corrected measures [12]. Gonullu et al. also showed a significant decrease in HR (p < .001) and increase in PR distances (p < .001), but not in any other intervals [10]. The above-mentioned meta-analysis by Popovic et al. found that increases in the weighted mean corrected QT interval were significantly lower (p = .002) with PALO (2.45 milliseconds in 1,213 patients) when compared with other 5-HT3RAs (5.13 milliseconds in 604 patients) in patients receiving HEC and MEC [20]. The results from this analysis further support the findings of these previous studies and indicate that NEPA does not increase the risk for cardiac adverse events, including prolongation of QT interval.
Although this integrated summary was able to evaluate the safety of 3-drug and 2-drug antiemetic regimens in 3,280 patients for 9,348 cycles, the results were pooled from 4 separate clinical trials involving different patient populations and study designs and using different chemotherapy and antiemetic regimens. In particular, the number of patients enrolled in each study ranged from 412 in study 3 to 1,450 in study 2, and only studies 2 and 3 treated patients over multiple cycles. This led to reduced exposure to the APR regimen in the integrated analysis (238 patients in 649 cycles). The pooled NEPA group had the most exposure, with 4,838 cycles. The proportion of female patients was much higher in study 2 (98% vs. 43%, 50%, and 41% in studies 1, 3, and 4, respectively) because of the high number of patients with breast cancer, and lower percentages were assigned to receive APR in studies 1 (44%) and 3 (49%). This resulted in a lower percentage of women in the integrated APR treatment group (46% vs. 72% for NEPA and 67% for PALO) and may have contributed to the increased incidence of alopecia in the NEPA (20% in cycle 1 and 33% in all cycles) and PALO (17% and 25%, respectively) groups compared with the incidence in the APR group (4% and 13%, respectively).
Of the four studies, study 1 was the only one not conducted worldwide; it also had a lower incidence of TEAEs in the NEPA groups (47%) compared with study 2 (76% in cycle 1) and study 3 (86%). Chemotherapy regimens varied: Patients received cisplatin-based HEC in studies 1 and 4, AC-based MEC in study 2, and non-AC-based MEC and HEC in study 3. This may have affected the chemotherapy-related AEs observed in the trials. In addition, study 1 was a phase II dose-ranging study evaluating three different doses of NETU in combination with PALO, and study 4 did not evaluate therapy with NETU at all. These studies also had different objectives, with safety being a primary objective only in study 3. Safety assessments were similar in all four trials; however, the differences in primary objectives may have contributed to a bias in data collection [15–17, 19].
Medications with the potential for drug-drug interactions with NETU were not allowed in the studies included in this analysis. Drug-drug interactions with NEPA have been previously evaluated in clinical trials [31]. NETU is mainly metabolized by CYP3A4 and is a moderate inhibitor of CYP3A4. Concomitant use with a chronic strong CYP3A4 inducer should be avoided because it can decrease the efficacy of NEPA. A strong CYP3A4 inhibitor can significantly increase systemic exposure to NETU, although dose adjustments are not required for a single dose of NEPA. NEPA can increase plasma concentrations of CYP3A4 substrates and so should be used with caution in patients receiving medications primarily metabolized through CYP3A4, including DEX, midazolam, and relevant chemotherapeutic agents [31].
Conclusion
Combining an NK1RA and a 5-HT3RA into one oral dosage form can make administration of antiemetic therapy more convenient for patients and can promote greater medication compliance and guideline adherence. More important, this combined safety analysis showed no signs of any increased toxicity with the addition of an NK1RA (netupitant or APR) to two-drug antiemetic regimens consisting of a 5-HT3RA and a corticosteroid. The individual studies also found that therapy with NEPA provided significantly improved CINV prevention compared with PALO [6, 15] and similar prevention compared with APR [17]. This analysis demonstrates that NK1RAs contribute to increased prevention of emesis without additional AEs and that NEPA may be a beneficial option for patients.
Acknowledgments
We thank Rozena Varghese of MedVal Scientific Information Services, LLC (Skillman, NJ), for providing medical writing and editorial assistance. This assistance was funded by Eisai Inc. This report was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company Sponsored Medical Research: The GPP2 Guidelines.”
Footnotes
For Further Reading: Matti Aapro, Alexandra Carides, Bernardo L. Rapoport et al. Aprepitant and Fosaprepitant: A 10-Year Review of Efficacy and Safety. The Oncologist 2015;20:450-458.
Implications for Practice: Aprepitant (and its prodrug fosaprepitant) is a neurokinin-1 receptor antagonist approved more than a decade ago for the prevention of chemotherapy-induced nausea and vomiting (CINV). Its alternative mechanism of action complements traditional antiemetic drugs, enhancing control of CINV. This review examined safety and efficacy data for aprepitant and fosaprepitant accumulated since the first regulatory approval and explores recommendations in current guidelines for their use. The review serves as a useful reminder for the practitioner that aprepitant and fosaprepitant are valuable additions to the therapeutic armamentarium for the prevention of CINV. Future perspectives on potential uses of aprepitant and fosaprepitant for indications other than CINV are discussed.
Author Contributions
Conception/Design: Matti Aapro, Paul J. Hesketh, Karin Jordan, Richard J. Gralla, Giorgia Rossi, Giada Rizzi, Marco Palmas
Provision of study material or patients: Giorgia Rossi, Giada Rizzi, Marco Palmas
Collection and/or assembly of data: Giorgia Rossi, Giada Rizzi, Marco Palmas
Data analysis and interpretation: Matti Aapro, Paul J. Hesketh, Karin Jordan, Richard J. Gralla, Giorgia Rossi, Giada Rizzi, Marco Palmas
Manuscript writing: Matti Aapro, Paul J. Hesketh, Karin Jordan, Richard J. Gralla, Giorgia Rossi, Giada Rizzi, Marco Palmas
Final approval of manuscript: Matti Aapro, Paul J. Hesketh, Karin Jordan, Richard J. Gralla, Giorgia Rossi, Giada Rizzi, Marco Palmas
Disclosures
Matti Aapro: Eisai, Helsinn Healthcare SA, Merck Sharp & Dohme, Tesaro (C/A, H); Paul J. Hesketh: Helsinn Healthcare SA, Tesaro (C/A); Karin Jordan: Merck Sharp & Dohme, Merck, Helsinn Healthcare SA, ProStrakan (C/A); Richard J. Gralla: Merck Sharpe & Dohme, Helsinn Healthcare SA, Tesaro, Eisai (C/A), Merck, Helsinn, Esai (H); Giorgia Rossi: Helsinn Healthcare SA (E); Giada Rizzi: Helsinn Healthcare SA (E); Marco Palmas: Helsinn Healthcare SA (E).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.National Comprehensive Cancer Network . Fort Washington, PA: National Comprehensive Cancer Network; 2015. [Google Scholar]
- 2.Cancer Care Ontario. Use of 5-HT3 receptor antagonists in patients receiving moderately or highly emetogenic chemotherapy. Practice Guideline Report 12-3. Available at https://http://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=154911. Accessed December 6, 2014.
- 3.Schwartzberg L, Barbour SY, Morrow GR, et al. Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting (CINV) Support Care Cancer. 2014;22:469–477. doi: 10.1007/s00520-013-1999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miura S, Watanabe S, Sato K, et al. The efficacy of triplet antiemetic therapy with 0.75 mg of palonosetron for chemotherapy-induced nausea and vomiting in lung cancer patients receiving highly emetogenic chemotherapy. Support Care Cancer. 2013;21:2575–2581. doi: 10.1007/s00520-013-1835-2. [DOI] [PubMed] [Google Scholar]
- 5.Gao HF, Liang Y, Zhou NN, et al. Aprepitant plus palonosetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving multiple-day cisplatin chemotherapy. Intern Med J. 2013;43:73–76. doi: 10.1111/j.1445-5994.2011.02637.x. [DOI] [PubMed] [Google Scholar]
- 6.Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25:1328–1333. doi: 10.1093/annonc/mdu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anzemet (dolasetron mesylate) injection [prescribing information]. Bridgewater, NJ: sanofi-aventis US; October 2014.
- 8.Zofran (ondansetron hydrochloride) tablets; Zofran ODT (ondansetron) orally disintegrating tablets; Zofran (ondansetron hydrochloride) oral solution [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; September 2014.
- 9.Morganroth J, Flaharty KK, Parisi S, et al. Effect of single doses of IV palonosetron, up to 2.25 mg, on the QTc interval duration: A double-blind, randomized, parallel group study in healthy volunteers. Support Care Cancer. 2015 doi: 10.1007/s00520-015-2822-6. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonullu G, Demircan S, Demirag MK, et al. Electrocardiographic findings of palonosetron in cancer patients. Support Care Cancer. 2012;20:1435–1439. doi: 10.1007/s00520-011-1226-5. [DOI] [PubMed] [Google Scholar]
- 11.Yavas C, Dogan U, Yavas G, et al. Acute effect of palonosetron on electrocardiographic parameters in cancer patients: A prospective study. Support Care Cancer. 2012;20:2343–2347. doi: 10.1007/s00520-011-1348-9. [DOI] [PubMed] [Google Scholar]
- 12.Dogan U, Yavas G, Tekinalp M, et al. Evaluation of the acute effect of palonosetron on transmural dispersion of myocardial repolarization. Eur Rev Med Pharmacol Sci. 2012;16:462–468. [PubMed] [Google Scholar]
- 13.Rojas C, Slusher BS. Pharmacological mechanisms of 5-HT₃ and tachykinin NK₁ receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol. 2012;684:1–7. doi: 10.1016/j.ejphar.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Jordan K, Jahn F, Aapro M. Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Ann Oncol. 2015;26:1081–1090. doi: 10.1093/annonc/mdv138. [DOI] [PubMed] [Google Scholar]
- 15.Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: A randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340–1346. doi: 10.1093/annonc/mdu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aapro MS, Karthaus M, Schwartzberg S, et al. Phase 3 study of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting during repeated moderately emetogenic chemotherapy (MEC) cycles. J Clin Oncol. 2014;32(suppl 5s):9502a. doi: 10.1093/annonc/mdu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gralla RJ, Bosnjak SM, Hontsa A, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. 2014;25:1333–1339. doi: 10.1093/annonc/mdu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinelli T, Moresino C, Baumann S, et al. Effects of combined netupitant and palonosetron (NEPA), a cancer supportive care antiemetic, on the ECG of healthy subjects: An ICH E14 thorough QT trial. Springerplus. 2014;3:389. doi: 10.1186/2193-1801-3-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karthaus M, Tibor C, Lorusso V, et al. Efficacy and safety of oral palonosetron compared with IV palonosetron administered with dexamethasone for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with solid tumors receiving cisplatin-based highly emetogenic chemotherapy (HEC) Support Care Cancer. 2015;23:2917–2923. doi: 10.1007/s00520-015-2657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popovic M, Warr DG, Deangelis C, et al. Efficacy and safety of palonosetron for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV): A systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2014;22:1685–1697. doi: 10.1007/s00520-014-2175-6. [DOI] [PubMed] [Google Scholar]
- 21.dos Santos LV, Souza FH, Brunetto AT, et al. Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting: A systematic review. J Natl Cancer Inst. 2012;104:1280–1292. doi: 10.1093/jnci/djs335. [DOI] [PubMed] [Google Scholar]
- 22.Chawla SP, Grunberg SM, Gralla RJ, et al. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer. 2003;97:2290–2300. doi: 10.1002/cncr.11320. [DOI] [PubMed] [Google Scholar]
- 23.McCrea JB, Majumdar AK, Goldberg MR, et al. Effects of the neurokinin1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolone. Clin Pharmacol Ther. 2003;74:17–24. doi: 10.1016/S0009-9236(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 24.Oyama K, Fushida S, Kaji M, et al. Aprepitant plus granisetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients with gastric cancer treated with S-1 plus cisplatin. J Gastroenterol. 2013;48:1234–1241. doi: 10.1007/s00535-012-0746-1. [DOI] [PubMed] [Google Scholar]
- 25.Hu Z, Cheng Y, Zhang H, et al. Aprepitant triple therapy for the prevention of chemotherapy-induced nausea and vomiting following high-dose cisplatin in Chinese patients: a randomized, double-blind, placebo-controlled phase III trial. Support Care Cancer. 2014;22:979–987. doi: 10.1007/s00520-013-2043-9. [DOI] [PubMed] [Google Scholar]
- 26.Choi CH, Kim MK, Park JY, et al. Safety and efficacy of aprepitant, ramosetron, and dexamethasone for chemotherapy-induced nausea and vomiting in patients with ovarian cancer treated with paclitaxel/carboplatin. Support Care Cancer. 2014;22:1181–1187. doi: 10.1007/s00520-013-2070-6. [DOI] [PubMed] [Google Scholar]
- 27.Rapoport B, Chua D, Poma A, et al. Study of rolapitant, a novel, long-acting, NK-1 receptor antagonist, for the prevention of chemotherapy-induced nausea and vomiting (CINV) due to highly emetogenic chemotherapy (HEC) Support Care Cancer. 2015;23:3281–3288. doi: 10.1007/s00520-015-2738-1. [DOI] [PubMed] [Google Scholar]
- 28.Urban L, Poma A, Dardeno MM, et al. Safety of rolapitant, a novel NK-1 receptor antagonist, for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic chemotherapy (MEC or HEC) J Clin Oncol. 2014;32(5 suppl):9636a. [Google Scholar]
- 29.Schnadig ID, Modiano MR, Poma A, et al. Phase 3 trial results for rolapitant, a novel NK-1 receptor antagonist, in the prevention of chemotherapy-induced nausea and vomiting (CINV) in subjects receiving moderately emetogenic chemotherapy (MEC) 2014;32(5 suppl):9633a. [Google Scholar]
- 30.Rapoport BL, Poma A, Hedley ML, et al. Phase 3 trial results for rolapitant, a novel NK-1 receptor antagonist, in the prevention of chemotherapy-induced nausea and vomiting (CINV) in subjects receiving highly emetogenic chemotherapy (HEC) J Clin Oncol. 2014;32(5 suppl):9638a. [Google Scholar]
- 31.Akynzeo (netupitant and palonosetron) [prescribing information]. Woodcliff Lake, NJ: Eisai Inc.; April 2015.