Obesity is an important risk factor for breast cancer (BC) in postmenopausal women; interlinked molecular mechanisms might be involved in the pathogenesis. The structural defects of typical genes related to both BC and obesity have been associated with a high or low risk of BC development. The identification of both genetic and pharmacological implications on the prevention and management of BC is the ultimate aim of these studies.
Keywords: Obesity, Breast cancer, Risk factors, Adipokines, Insulin resistance, Polymorphisms
Abstract
Obesity is an important risk factor for breast cancer (BC) in postmenopausal women; interlinked molecular mechanisms might be involved in the pathogenesis. Increased levels of estrogens due to aromatization of the adipose tissue, inflammatory cytokines such as tumor necrosis factor-α, interleukin-6, and prostaglandin E2, insulin resistance and hyperactivation of insulin-like growth factors pathways, adipokines, and oxidative stress are all abnormally regulated in obese women and contribute to cancerogenesis. These molecular factors interfere with intracellular signaling in the mitogen-activated protein kinase and phosphatydilinositol-3-phosphate/mammalian target of rapamycin (mTOR) pathways, which regulate the progression of the cell cycle, apoptosis, and protein synthesis. In this context, structural defects of typical genes related to both BC and obesity, such as leptin, leptin receptor, serum paraoxonase/arylesterase 1, the fat mass and obesity-associated gene and melanocortin receptor 4, have been associated with a high or low risk of BC development. The early detection of these gene alterations might be useful as risk predictors in obese women, and targeting these pathways involved in the BC pathogenesis in obese women is a potential therapeutic tool. In particular, mTOR pathway deregulation concurs in both obesity and BC, and inhibition of this might disrupt the molecular interlinks in a similar manner to that of metformin, which exerts definite anticancer activity and is currently used as an antidiabetic drug with a weight-reducing property. The identification of both genetic and pharmacological implications on the prevention and management of BC is the ultimate aim of these studies.
Implications for Practice:
Obese women are at risk of breast cancer, but clinicians lack concrete tools for the prevention or early diagnosis of this risk. The present study, starting from the biology and the molecular defects characterizing both obesity and breast cancer, analyzed the potential molecules and genetic defects whose early identification could delineate a risk profile. Three steps are proposed that are potentially achievable in the clinical assessment of obese women, namely the evaluation of altered levels of serum molecules, the identification of genetic polymorphisms, and the study of the transcriptomic profile of premalignant lesions. Finally, the therapeutic implications of this molecular assessment were evaluated.
Abstract
摘要
肥胖是绝经后女性乳腺癌的重要危险因素, 发病机制可能涉及共通的分子机制。肥胖女性的脂肪组织芳香化导致的雌激素水平升高、炎性细胞因子 (如肿瘤坏死因子α、白介素6、前列腺素E2) 、胰岛素抵抗和胰岛素样生长因子旁路超活化、脂肪细胞因子及氧化应激均处于调节异常状态并可促进癌症形成。这些分子因素干扰细胞内信号转导中的丝裂原活化蛋白激酶和磷脂酰肌醇-3-磷酸/哺乳动物雷帕霉素靶蛋白 (mTOR) 通路, 而这些通路可调控细胞周期进展、凋亡和蛋白质合成。因此, 与乳腺癌和脂肪均相关的典型基因 (如瘦素、瘦素受体、血清对氧磷酶/芳香酯酶1、脂肪量与肥胖相关基因和黑素皮质素受体4) 的结构缺陷与乳腺癌发生风险升高或降低有关。早期发现这些基因改变可能有助于预测肥胖女性的风险, 针对肥胖女性中这些乳腺癌发病机制涉及的通路进行治疗也可能具有潜力。特别是肥胖和乳腺癌都会发生mTOR通路调节紊乱, 对其加以抑制也许能破坏二者的分子内在联系, 类似当前作为具有减轻体重作用的降糖药使用的二甲双胍却能发挥确切的抗癌活性那样。这些研究的终极目标是确定乳腺癌管理和预防措施的遗传和药理学意义。The Oncologist 2016;21:404–417
对临床实践的提示: 肥胖女性有发生乳腺癌的风险, 但临床医生缺乏具体的工具以预防或早期诊断这种风险。本研究中, 开篇讲述了肥胖和乳腺癌的生物学和分子缺陷特征, 分析了通过早期鉴别分子和基因缺陷可能绘制出风险谱。我们对肥胖女性的临床评估提出了三个可能达到的步骤, 即对血清分子水平的改变进行评价、鉴别基因多态性, 以及对癌前病变的转录组学特征进行研究。最后, 我们评价了这一分子评估的治疗意义。
Introduction
Breast cancer (BC) is the highest incidence tumor and the most common cause of death from cancer in women [1]. Obesity is a known risk factor in postmenopausal women [2, 3] and is present in up to 50% of all BC cases in older women [4]. It has been estimated that by preventing overweight, the annual incidence would be reduced by 50%, to less than 13,000 cases in the European Union [5]. Nevertheless, the BC risk in obesity is different among ethnic groups; the association of an increased body mass index (BMI) with BC appears to be particularly strong among the Asia-Pacific populations [6].
A meta-analysis of observational studies suggested that the risk of BC is 12% higher for each 5 kg/m2 increase in BMI in postmenopausal women [4]. In contrast, a recent meta-analysis examining prospective cohort and case-control studies showed that obesity exerts minor effects on BC development after data adjustment for age, race, and marital status [7]. Unlike in postmenopausal women, a high BMI is apparently protective in premenopausal women [4], because a U.K. population-based cohort study revealed that, independently of smoking, each 5-unit BMI increase was inversely associated with BC risk [8].
Pathology studies support the influence of obesity on the BC histopathology. It has been reported that among 1,177 women with invasive ductal BC, those in the highest BMI quartile developed more malignant tumors in terms of histological grade, mitotic cell count, and tumor size [9], and these obese patients showed increased lymph node involvement and a higher propensity to distant metastases [10, 11]. Obesity has also been linked to BC recurrence and a lower overall survival (OS) in pre- and postmenopausal women with BC [12, 13]. Weight change might influence the risk of BC and its complications, and the degree of weight increase before menopause might enhance the postmenopausal BC risk [14].
At least in postmenopausal women, the link between obesity and BC is related to the hormonal balance, based on high aromatase levels and the release of growth factors and inflammatory cytokines by adipocytes [15, 16]. This interlink is dependent on several molecular pathways, such as the phosphatydilinositol-3-phosphate/mammalian target of rapamycin (PI3K/mTOR) pathway, natively activated as a checkpoint for nutrient/hormonal cell signaling that regulates the proliferation of both adipocytes and mammary epithelial ductal cells [17]. In this regard, genetic defects in obesity-related genes definitely appear linked to BC [18, 19], and drug targeting of molecular pathways that are deregulated in obesity might help to prevent this cancer in obese women. We review both biological and molecular mechanisms linking obesity to BC in women and the relative implications in patient management.
Molecular Mechanisms Linking Obesity With Breast Cancer
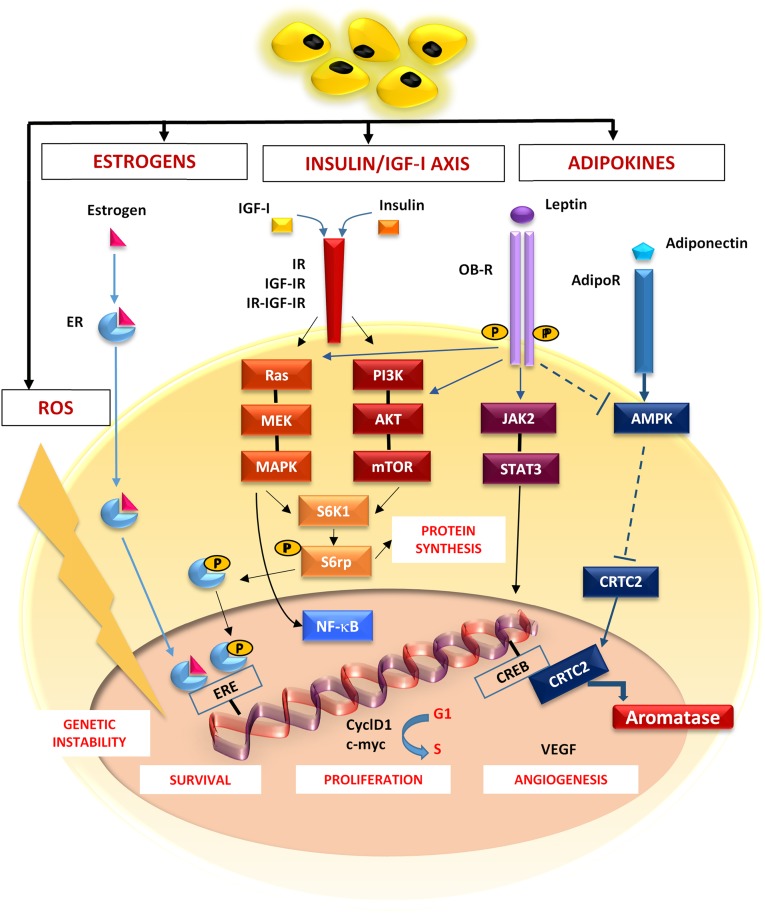
The molecular mechanisms include hormones, adipocytokines, inflammatory cytokines, and reactive oxygen species (ROS), as summarized in Figure 1.
Figure 1.
Molecular breast cancer (BC)-related pathways activated in obesity. In obese patients, estrogens, insulin, IGFs, and adipokines activate pathways shown to be deregulated in BC. The estrogen receptor complex migrates to the nucleus and binds the EREs in promoters of target genes that regulate cell survival. Obesity causes increased levels of insulin, IGF-I, and IGF-II that bind the IR, IGF-IR, and a hybrid form (IR-IGF-IR), and thus activate both the RAS/MEK/MAPK and PI3K/Akt/mTOR pathways converging on S6K1. Phosphorylation of S6rp by S6K1 promotes both protein synthesis and cell proliferation. Leptin activates JAK/STAT signaling by its receptor (OB-R). Adiponectin triggers AMPK through its receptor AdipoR. AMPK phosphorylation decreases the nuclear translocation of CRTC2 that binds CREB, thus increasing the aromatase activity. These pathways, converging on NF-κB, lead to the expression of CyclD1 and c-Myc, which promotes cell survival and proliferation, as well as the secretion of VEGF and tumor angiogenesis. ROS contribute to the tumor progression, favoring the genomic instability.
Abbreviations: AdipoR, adiponectin receptor; AMPK, AMP-activated protein kinase; CREB, cAMP response element-binding protein; CRTC2, CREB-regulated transcription coactivator 2; CyclD1, cyclin D1; EREs, estrogen response elements; IGF, insulin-like growth factor; IGF-IR, IGF-I receptor; IR, insulin receptor; JAK2/STAT3, janus kinase 2/signal transducer and activator of transcription 3; NF-κB, nuclear factor κB; PI3K, phosphatydilinositol-3-phosphate; ROS, reactive oxygen species; S6K1, S6 kinase-1; S6rp, S6 ribosomal protein; VEGF, vascular endothelial growth factor.
Hormonal Status
Estrogens
The higher estrogen levels in postmenopausal women derive from the aromatization of androstenedione and testosterone in the adipose tissue [20, 21]. Aromatase is increased twofold in obese women [22], and its activity is strongly influenced by both tumor necrosis factor-α (TNF-α) and interleukin (IL)-6, which are usually abundant within the adipose tissue [23, 24]. Aromatase is expressed by undifferentiated adipose fibroblasts but not by mature adipocytes, and the ratio of stromal tissue to adipocytes in breast quadrants is higher in BC [25]. Epithelial tumor cells also produce both TNF-α and IL-11 [24] as anti-adipogenic cytokines and prostaglandin E2 (PGE2), resulting in higher aromatase production due to the effect on the promoter I.3/II region (PI.3/PII) promoter region of the aromatase gene [26, 27] via protein kinase (PK) A and PKC signaling [28] (Fig. 2). Also, estrogens regulate the G1/S phase progression by both c-MYC and cyclin D1 (CCND1), enabling the CCNE-cyclin dependent kinase-2 complexes necessary for phosphorylation of the retinoblastoma gene (RB), resulting in the activation of E2F transcription factors. This has been supported by antiestrogen treatment of BC cell lines, which downregulates both c-MYC and CCND1, leading to a quiescent state [29]. Finally, estrogens regulate the insulin receptor substrate-1 (IRS-1) in the breast [30], induce free radical-mediated DNA damage, genetic instability, and gene mutations, and inhibit both DNA repair and apoptosis [31, 32].
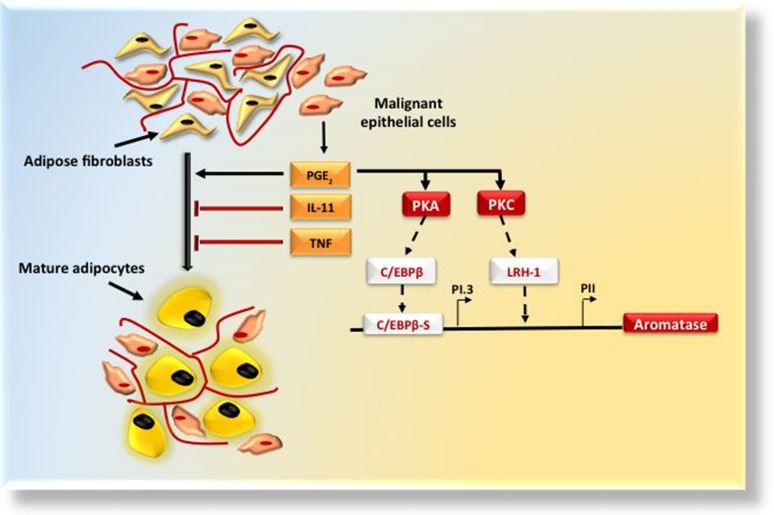
Figure 2.
Breast tissue microenvironment: stromal-epithelial interactions. Adipose tissue is a permissive environment for breast cancer (BC). Malignant epithelial cells establish close interactions with adipose fibroblasts that differentiate into mature adipocytes. BC cells produce IL-11, TNF-α, and PGE2. IL-11 and TNF-α act as antiadipogenic cytokines and PGE2 reinforces the aromatase expression priming PKA and PKC. PKA activates transcription factor C/EBPβ that binds the key cis-regulatory element C/EBPβS in proximity of the PI.3, and PKC activates the LRH-1 transcription factor whose binding site is located more proximal to PII.
Abbreviations: IL-11, interleukin-11; LRH-1, liver receptor homolog-1; PGE2, prostaglandin E2; PI.3, promoter I.3 region; PII, promoter II; PKA, protein kinase A; PKC, protein kinase C; TNF-α, tumor necrosis factor-α.
Insulin Resistance and Hyperinsulinemia
Excess body weight and adiposity are directly correlated with insulin resistance and compensated by an increased secretion of insulin that ultimately primes both the growth and the aggressiveness of postmenopausal BC [33]. Hyperinsulinemia is also responsible for increased levels of insulin-like growth factor (IGF)-I and -II and reduced hepatic expression of IGF-I binding proteins (IGFBP)-1 and -2, leading to higher circulating levels of free IGF-I [34, 35]. IGF-I receptors, overexpressed by BC cells, and a third receptor, in hybrid form (IR-IGFIR), mediate the effects of both insulin and IGF-I [36, 37]. In this regard, it has been shown that IGFBP-1 inhibits the tumor cell growth in mice transplanted with MCF-7 cells [38]; decreased IGFBP-1 levels in obesity suggest the existence of a mechanism enhancing the growth of BC cells.
IGF-I signaling interacts with estrogens to synergistically induce the mitogenic response in breast epithelial cells by c-MYC and CCND1 [29] and primes the canonic mitogenic RAS/MEK/MAPK/ERK1/2 (mitogen-activated protein kinase/extracellular signal-related kinase 1/2) and PI3K/AKT/mTOR (phoshatidylinositol-3 kinase/murine thymoma viral oncogene homolog/mammalian target of rapamycin) pathways [39]. mTOR and ERK activate S6 kinase-1 (S6K1) and the subsequent phosphorylation of S6 ribosomal protein (S6rp), resulting in increased cell proliferation [40]. S6K1 is also responsible for the phosphorylation of estrogen receptors (ERs) and, in turn, both estrogens and ERs increase IGF-IR signaling [40]. High mTOR activity has been definitely correlated with higher risks of disease progression [41], recurrence [42], short disease-free survival (DFS), and a lower response to tamoxifen [43].
Both hyperinsulinemia and hyperglycemia increase the BC risk through the WNT pathway, which induces the translocation of β-catenin to the nuclei via the canonical WNT/β-catenin loop and primes the transcription of target genes, such as CCND1 and c-MYC [44, 45]. Furthermore, variants of the transcription factor 7-like 2 (TCF7L2), which regulates hepatic glucose production as a part of the WNT/β-catenin signaling cascade, are associated with an increased risk of type 2 diabetes and BC [46]. That high glucose levels are able to amplify WNT/β-catenin signaling provides the link between hyperglycemia and cancer.
Adipokines
Adipokines are small peptide hormonal growth factors; they include leptin, adiponectin, and hepatocyte growth factor (HGF). All of them might contribute to BC development [47]. Leptin directly promotes cell proliferation by its receptor and then through either canonical pathways, such as MAPK/ERK1-2, PI3K/AKT/mTOR, Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3), or noncanonical pathways, such as Jun N-terminal kinase (JNK), PKC, and p38 MAPK, nuclear factor-κB (NF-κB), is activated [48]. Thus, NF-κB is translocated to the nucleus for the transcription of CCND1, c-MYC, JUN, FOS, and BCL2 [49], regulating cell proliferation. Leptin can also induce the direct activation of ERs in MCF-7 cells, even in the absence of its natural ligand estradiol [50], whereas, as an indirect effect, it intensifies the expression of aromatase [51]. In contrast, leptin decreases AMP-activated protein kinase (AMPK) phosphorylation and increases the nuclear translocation of cAMP response element-binding protein (CREB)-regulated transcription coactivator 2 (CRTC2), thus increasing CYP19A1 expression and aromatase activity [52]. Finally, leptin acts as a proinflammatory protein that promotes monocyte proliferation and macrophage function and reinforces T helper-1 (Th-1) cell proliferation, together with overproduction of TNF-α and other cytokines such as IL-6 and IL-12 [50, 52].
Adiponectin is the most abundant adipokine and enhances cell sensitivity to insulin. This cytokine inhibits the proliferation of a number of cell types and exerts proapoptotic effects, while inhibiting aromatase expression in BC cells [53]. Moreover, adiponectin stimulates intracellular pathways, including AMPK, having an inhibitory effect on the cell cycle via p53 expression. It inhibits protein synthesis through the activation of the tuberous sclerosis complex (TSC)1/TSC2, which is a tumor suppressor complex with a growth inhibitory activity through mTOR suppression. Adiponectin also activates the peroxisome proliferator activated receptor-γ (PPAR-γ) pathway, which, in turn, drives the transcription of other genes that regulate both cell proliferation and differentiation [54]. Therefore, a low adiponectin availability modulates PPAR-γ signaling, causing a subsequent decrease of nuclear levels of the breast cancer gene-1 (BRCA1) and alterations of the cellular mechanisms for DNA repair [32, 49].
Inflammation
In obese women, both systemic and local inflammation are usually associated with high levels of inflammatory cytokines (TNF-α, IL-1β, IL-6, and monocyte chemotactic protein 1 [MCP-1]) that activate the transcription of NF-κB in breast tissue [16]. Thus, inflammation of adipose tissue plays a key role in tumor development and progression. Macrophages recruited after adipocyte death [55] form crown-like structures and are detectable both in visceral fat [56] and in mammary glands of obese mice [22], in the presence of high levels of proinflammatory cytokines (IL-1β, IL-6, PGE2), and with increased CYP19A1 gene transcription, thereby encoding aromatase [57]. Obesity is also associated with hypertrophy and lipolysis of adipocytes that release free fatty acids and activates NF-κB through Toll-like receptor 4, an immune receptor, ultimately inducing a high bioavailability of TNF-α, IL-6, and other cytokines [55, 58].
A deregulation of PPAR-γ, as a key regulator of adipogenesis in obese patients has been associated with an increased production of the plasminogen-activator inhibitor 1, which is involved in angiogenesis, enhancement of cell adhesion, migration, and inhibition of apoptosis and is suspected of modifying the breast microenvironment and facilitating local cancer development and/or metastasis [59].
Oxidative Stress
Obesity is associated with increased oxidative stress and is characterized by high levels of ROS that variably denature lipids, proteins, and nucleic acids and lead to genetic instability, driving both tumor progression and metastasis by triggering the PI3K/AKT pathway in BC [60]. In addition, ROS and nitric oxide species generated in cancer-associated fibroblasts promote genomic defects in adjacent cancer cells that characterize their typical aggressive behavior [49]. BC cells provoke oxidative stress in adjacent fibroblasts as an engine to fuel their own survival through the production of nutrients from the stromal cells [49].
Gene Polymorphisms Shared by Obesity and BC
Gene polymorphisms and BC risk haven been increasingly described. The most important and penetrant mutations for inherited BC include BRCA1 and BRCA2 [61], phosphatase and tensin homologs (PTEN) [62], serine-threonine kinase-11 (STK11/LKB1) [63], and cadherin 1-type 1 (CDH1) genes [64]. However, the association of the defined mutations in these genes with obesity is unclear. Hereditary factors might account for approximately one quarter of interindividual differences that cluster the susceptibility to BC in developed countries, although high-risk mutations appear to be implicated in fewer than 10% of all BC cases [65]. Therefore, a substantial component of the BC risk seems to be the combined effect of a number of low-risk polymorphisms. The role of obesity-related gene polymorphisms in BC development is under investigation, and only a few studies have reported that obesity-related genotypes play a role in determining the progression of benign breast disease (BBD) to invasive cancer. Because polymorphisms in obesity-related genes can modify the associations between BMI and BC, several polymorphisms in obesity-related genes appear to be significantly associated with a variable risk of BC development. These are summarized in Table 1.
Table 1.
Polymorphisms in obesity-related genes
Leptin and Leptin Receptor Single Nucleotide Polymorphisms
Polymorphisms of both leptin (LEP) and leptin receptor (LEPR) genes have been associated with the development of BC in patients with higher leptin serum levels and leptin overexpression in adipocytes [66]. For example, the LEPR*6920A allele is associated with decreased risk in women with BBD [67], and the LEPR Q223R polymorphism is associated with BC development in East Asians [68] and Tunisians [67], but not in whites or Africans [68]. A significant risk of BC was observed for carriers of the LEP-2548A/A genotype (p = .001) and carriers of the LEP-2548 G/A genotype (p = .04) [66, 69] that can be explained by the proximal position of LEP-2548G/A to a binding site for the transcriptional factor Sp1 [70]. The occurrence of LEP-2548A/A or LEP-2548G/A coincides with higher or intermediate leptin mRNA expression, respectively, and cells with LEP-2548G/G contain low mRNA leptin levels [70]. In a study relating these associations with the clinical evolution, a shorter DFS was related to higher frequency of LEP (−2548) A allele, and OS was reduced in patients carrying the LEPR Gln223Arg allele [67]. In contrast, LEPR single nucleotide polymorphism (SNP) rs11585329 (622G>T) has recently been associated with improved DFS in women with stage I-II BC [71].
Serum Paraoxonase/Arylesterase 1 SNPs
Paraoxonase/arylesterase 1 (PON1) is an esterase bound to high-density lipoproteins that is disabled in obese subjects [72]. Women carrying at least one copy of the variant PON1 Gln192Arg allele are at a lower risk of BC compared with women carrying the reference Gln/Gln genotype [67].
TNF-α SNPs
Second-grade obese patients with BC are carriers of allele A of TNF-α SNP −308G>A [73], which is responsible for increased serum levels of TNF-α whose contribution to BC development has been definitely shown [74]. Thus, the GA-AA genotypes define the risk factor in HER2+ BC patients with a BMI of 30.0 to >40 kg/m2 [73].
Fat Mass and Obesity-Associated Gene and Melanocortin Receptor 4 SNPs
Fat mass and obesity-associated gene (FTO) is apparently related to obesity and type 2 diabetes [75]. A significant expression of both rs1121980 (T/C) and rs9939609 (A/T) SNPs has been reported in overweight and obese women, even if it has not been associated with BC development [76, 77]. However, Kaklamani et al. found that SNPs in the first intron of FTO (rs1477196, rs9939609, rs7206790, and rs8047395) were associated with BC risk [78]. In particular, rs7206790 is predictive for hereditary hormone receptor-positive (HR+) BC in young women.
Mutations in the melanocortin receptor 4 (MC4R) gene, such as rs17782313 (T/C), are associated with a monogenic form of extreme, early-onset obesity and are suspected of inducing an increased risk of BC [78]. Women showing the allele combination C/T/C (FTO rs1121980/FTO rs9939609/MC4R rs17782313) have a 4.59-fold increased risk of developing this type of cancer, independently of age and BMI [77].
Adiponectin (ADIPOQ) and Adiponectin Receptor-1 (ADIPOR1) SNPs
A functional adiponectin (ADIPOQ) SNP, rs1501299, is significantly linked to high serum adiponectin levels [79] and BC [80], and rs1501299 (+276C>A) and rs2241766 (+45T>G) are associated with this tumor in whites and African Americans [81] and populations from Kuwait [81] and South India [82]. However, ADIPOQ SNP rs1063539 is correlated with improved DFS in women with stage I-II BC [81]; however, a further polymorphism in the ADIPOR1 SNP rs7539542 is apparently associated with BC risk [83].
β2-Adrenergic Receptor Gene and β3-Adrenergic Receptor Gene SNPs
Polymorphisms in the β2-adrenergic receptor gene (ADRB2) are associated with both obesity and type 2 diabetes. Two ADRB2 variants (rs 1042713G/A and rs1042714G/C) have been related to an increased risk in non-Hispanic women and, in contrast, to a lower risk of BC in Hispanic women [84]. The pro-BC effect of these two variants was higher in women with a BMI ≥25.0 kg/m2 and in Hispanic women with a history of diabetes [85], suggesting that ethnicity modifies the association between the ADRB2 G–G haplotype and BC risk and that overweight or obesity might enhance the differing risk between Hispanic and non-Hispanic women.
Glutathione S-transferase P1 and M1 SNPs
Obesity decreases the antioxidant defenses by lowering the levels of enzymes, such as catalase, glutathione peroxidase, and glutathione reductase and, concurrently, by altering the activity of cytochrome P-450 [86]. The frequency of heterozygous glutathione S-transferase P1 (GSTP1) SNP Ile105Val is 1.5 higher in patients with BC, and the frequency of the homozygous form Val/Val is 1.6 higher in BC patients than in control subjects [87]. Finally, an increased risk of BC was found in women with the GSTM1 null genotype [87].
IGF-I SNPs
Recent studies have shown that IGF-I levels in plasma are correlated with the length of (CA)n repeats in the IGF-I gene, although the direction of the correlation remains controversial [88–90]. One study reported a significantly increased BC risk in Chinese women with a higher BMI carrying the (CA)19 allele [91]. However, an increased risk of BC was not found in two other studies [89, 92]. Recently, Pande et al. found an association between the IGF-I SNP rs1520220 and poor DFS in women with stage I-II BC [71].
PIK3CA SNPs
The SNP rs2677760 in PIK3CA is associated with reduced DFS in women with stage I-II BC [71]. Moreover, a recent meta-analysis showed that PIK3CA gene mutations correlate with ER/progesterone receptor (PR) expression (p < .00001) and relapse-free survival (p = .03), but not OS in unsorted BC patients [92]. It is conceivable that SNPs in PI3K-AKT-mTOR genes, such as ADIPOQ, IGF1, INS, IRS1, LEP, LEPR, LEPROT, PIK3CA, PTEN, TSC1, TSC2, and AKT1, might simultaneously affect body weight and decrease the responsiveness to BC treatment [71].
PR and Steroid Hormone Receptor Coactivator (AIB1) SNPs
The combined inheritance of the polymorphic variant PROGINS A1/A1 (PR SNPs), AIB1 long polyglutamine genotypes and an early age of menarche (12 years) are risk factors for obesity. The absence of the PR polymorphism PROGINS, together with longer polyglutamine repeats in AIB1, was associated with postmenopausal obesity in women with BC [93]. Moreover, De Vivo et al. observed a significantly higher risk of BC among carriers of the PR +331 A allele compared with subjects with the GG genotype [94]. They also observed a potential interaction between the genotype and BMI among postmenopausal women, with the highest risk for obese women (BMI >30 kg/m2) with the GA or AA genotype compared with lean women (BMI <25 kg/m2) with the GG genotype [94]. Their findings suggest that an increased production of polymorphic variant hPR-B by the PR +331 G/A might predispose women to BC through an increased hPR-B-dependent stimulation of mammary cell growth [94].
Obese Women at Risk of BC
The chronic inflammatory state typical of obesity is due to increased levels of C-reactive protein, inflammatory cytokines, such as TNF-α, IL-6, IL-8, and MCP-1, and leptin [22]. Accumulating evidence has suggested a positive correlation between their high bioavailability and BC incidence. High levels of leptin, in particular, might apparently define a generally increased risk of, and poor prognosis in, BC [95], because postmenopausal patients express high leptin mRNA in adipose tissue and elevated serum levels in association with increased estradiol [96]. Moreover, ER-positive (ER+) tumors have been shown to express high intratumoral levels of leptin, which is specifically involved in cancer growth through an autocrine mechanism [95, 97]. Leptin has been identified as an independent predictive variable of BC pathological tumor size and TNM stage in several studies [52]. In line with these results, clinical studies have recently demonstrated that serum leptin levels correlate with total body aromatase activity in postmenopausal BC patients [98].
The chronic inflammatory state typical of obesity is due to increased levels of C-reactive protein, inflammatory cytokines such as TNF-α, IL-6, IL-8, and MCP-1, and leptin. Accumulating evidence has suggested a positive correlation between their high bioavailability and the BC incidence.
Unlike leptin, adiponectin levels are inversely related to cancer occurrence and stage [49]. Two case-control studies also proved this inverse relation with the risk of BC in pre- and postmenopausal women [79, 99, 100]. Also, serum adiponectin levels negatively correlate with BMI and visceral adiposity, and both insulin and estrogens might suppress its secretion [52, 101]. Adipocytes and stromal cells in adipose tissue are the main sources of another adipokine, namely the HGF. Serum HGF levels correlated positively with the BMI, high stage, ER−, degree of differentiation, and presence of lymph node and distant metastases in patients with locally advanced BC [102]. In a murine model, obesity correlated with the activation, by HGF, of the c-MET axis, involved in the pathogenesis of basal-like BC [103]. Moreover, in a case-cohort study, hyperinsulinemia was an independent risk factor for BC in postmenopausal women, and fasting levels of total IGF-I, free IGF-I, IGFBP-3, and glucose were not associated with the risk of BC [104].
Because several studies showed a specific transcriptomic profile in samples of BC from obese women, the detection of particular signatures in premalignant dysplastic lesions of obese women might serve as a tool for risk prediction, as demonstrated for ERα mutations [105]. Analysis of the transcriptomic profile of pretreatment biopsies from a prospective cohort of 137 ER+ BC patients revealed that 62 genes were significantly overexpressed in obese patients and 50 additional genes were downregulated (p < .01) [106]. These genes are involved in several biological processes such as the epithelial-to-mesenchymal transition (EMT), protein synthesis, Notch signaling, inflammation, proliferation, lipid and cholesterol metabolism, adipokines, energy production, cell adhesion, and the suppression of STAT1 signaling (Table 2). In the same study, gene set enrichment analysis revealed an association between obesity and an upregulation of AKT target genes involved in glucose metabolism, EMT, and metastasization [106]. The evidence that hyperactivity of the AKT/mTOR pathway in obese mice accelerates mammary tumor growth supports this finding. Microarray data on transgenic mouse tumors identified 1,603 genes with a statistically significantly altered expression, related to 42 biological processes [106]. Statistical evaluation led to the identification of many biological functions concordantly affected by obesity and linked to hallmarks of cancer, in both humans and mice [106]. These processes are primarily associated with metastasis, tumor-promoting inflammation, resistance to cell death, and, above all, a reinforced cell proliferation in ER+ BC.
Table 2.
A potential breast cancer risk profile in obese women

Furthermore, an obesity-associated BC transcriptome signature has been delineated in a set of 103 tumors, and elevated IGF gene scores were observed in the obese tumor group compared with the other tumor group. Also, within the same cohort, an inverse correlation was found between ER gene (ESR1) mRNA levels and obesity signature scores [107]. In that study, the investigators also evaluated the overlap between obesity and gene signatures of oncogenic pathways, including IGF-I, PI3K, MAPK, and estrogen (17β-estradiol) and found a significant overlap between underexpressed genes in tumors from obese patients and genes repressed by these pathways, but no overlap was found between overexpressed genes that are high in obesity-associated tumors and the genes activated by these intracellular pathways. They concluded that the obesity signature was distinct from the previously characterized oncogenic pathway signatures [107, 108].
BC development in obese women is also related to a genetic susceptibility, that is specific to each ethnicity, as revealed by the analysis of several gene SNPs (Table 1). In whites, major polymorphisms associated with the BC risk in obese women include LEP-2548 AA [66], PON1 Leu55Met [68], TNF-α-308G>A [73], FTO-MC4R (FTO rs1121980; FTO rs9939609; MC4R rs17782313) [77], ADIPOQ (rs1501299 T; rs2241766 C; rs1063539 A/C) [71, 80, 81], IGF-I (CA)19 allele [87], and PR (rs10895068 A) [94]. In contrast, other polymorphisms of obesity-related genes exert a protective effect (e.g., LEPR*6920A, associated with a decreased risk of BC in women with BBD [67]; LEP-2548G/G, related to the expression of low leptin mRNA levels [70]; and LEPR SNP rs11585329 [622G>T]) and have been associated with improved DFS in women with early-stage BC [71].
Thus, the detection of soluble molecules, expression profiles, and the identification of SNPs could depict a risk signature for BC in obese women, as proposed in Table 2, that could help to plan adequate prevention strategies for obese patients.
Targeting BC and Obesity Interlinked Pathways
The metabolic changes associated with obesity lead to alterations of several pathways targeted by drugs used in BC treatment (Fig. 3). Because estrogens are major inducers of HR+ BC, aromatase inhibitors (AIs) are the standard of care for postmenopausal women, in both the adjuvant and the metastatic setting [109], although anastrozole and letrozole incompletely suppress both estrone and estradiol levels in overweight and obese women [110]. In the ATAC trial (Arimidex, Tamoxifen, Alone or in Combination) comparing anastrozole to tamoxifen in the adjuvant setting, a higher BMI was associated with a higher recurrence in the overall population and in the population receiving anastrozole. The investigators observed that the benefits of anastrozole were lower in women with a higher BMI but that those of tamoxifen were similar, regardless of the BMI [111]. In the ABCSG 12 trial (Austrian Breast and Colorectal Cancer Study Group trial 12), performed in premenopausal women, both overweight and obese patients receiving anastrozole had an increased risk of disease recurrence and death. However, this result was not detected in the group of patients treated with tamoxifen [112]. In contrast, a side effect of all AIs is the weight gain related to the reduction of estrogen levels [113].
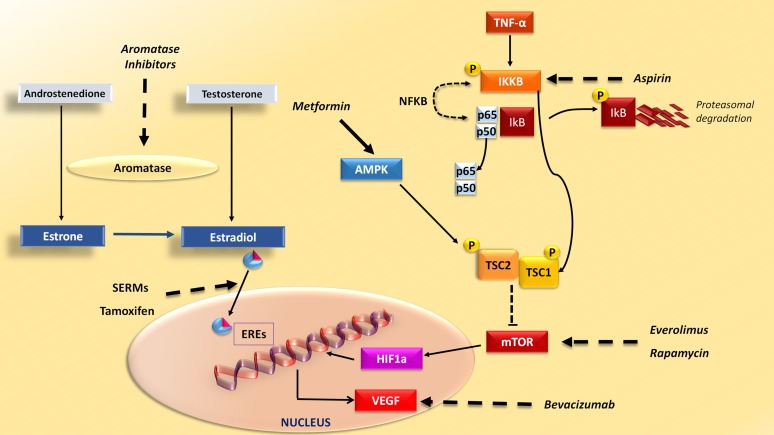
Figure 3.
Drugs interfering with breast cancer (BC)- and obesity-related pathways. Drugs commonly used in BC, obesity, and diabetes interfere with intracellular signaling hyperactivation in BC. Aromatase inhibitors inhibit enzyme aromatase in the conversion of androgens, such as testosterone and androstenedione, to estrogens, such as estradiol and estrone. SERMs, such as tamoxifen, bind to estrogen receptors and induce conformational changes that inhibit the expression of estrogen-related genes. In BC cells, high levels of proinflammatory cytokines such as TNF-α, typical in obese patients, activate IKKB that phosphorylates IkB, thus activating NF-κB, composed of its subunits p50 and p65. Furthermore, phosphorylation of TSC1 by IKKB activated by mTOR kinase is involved in cell growth and angiogenesis. Drugs targeting IKKB, such as aspirin, and mTOR inhibitors, such as everolimus and rapamycin, are potentially effective as anti-BC drugs in obese women. Metformin, an antidiabetes drug that can effectively reduce body weight, activates AMPK, which phosphorylates TSC2, having an inhibitory effect on mTOR. Activation of the mTOR pathway leads to the production of VEGF, mediated by transcription factor HIF1α, which could be targeted by bevacizumab.
Abbreviations: AMPK, AMP-activated protein kinase; EREs, estrogen response elements; HIF1α, hypoxia inducible factor-1α, IKKB, IkB kinase β; NF-κB, nuclear factor κB; SERMs, selective estrogen receptor modulators; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
Because estrogens are major inducers of HR+ BC, aromatase inhibitors are the standard of care for postmenopausal women, in both the adjuvant and the metastatic setting, although anastrozole and letrozole incompletely suppress both estrone and estradiol levels in overweight and obese women.
Another signaling pathway involved in the BC pathogenesis is the PI3K/AKT/mTOR pathway, which is targeted by mTOR inhibitors such as everolimus. This drug was approved for the treatment of advanced metastatic ER+, HER2− BC, in association with exemestane. Everolimus can overcome the resistance to endocrine therapy, thus prolonging progression-free survival [114]. The results from the GINECO study (Groupe d'Investigateurs Nationaux pour l'Étude des Cancers Ovariens et du sein) have confirmed the importance of this therapeutic approach, demonstrating the improved clinical benefit rate and improved time to progression and OS [115]. Combined therapy against mTOR and IGFI-R is currently being investigated in other clinical trials [116].
In obese patients, activation of the mTOR pathway was related to high levels of cytokines such as TNF-α that, by activating IkB kinase β (IKKβ), lead to the phosphorylation of TSC1, mTOR activation, and production of high levels of vascular endothelial growth factor (VEGF), whose expression was associated with a poor outcome for BC patients [117]. The establishment of mammary tumors in mouse models of genetic and diet-induced obesity revealed higher tumor growth in obese than in lean mice. It is noteworthy that high levels of phosphorylated IKKβ and S6, as indicators of mTOR pathway activation, were detected by immunofluorescence staining in mammary tumors in these mice [118]. However, targeting the IKKβ/mTOR/VEGF pathway with aspirin (IKKβ inhibitor), rapamycin (mTOR inhibitor), or bevacizumab (VEGF antagonist) induced a significant reduction of mammary tumor size only in obese but not in control mice [118]. In a model of ovariectomized obese mouse with orthotopic BC, the normalization of body weight did not affect AKT/mTOR signaling, which remained continuously activated even after appropriate weight loss. The finding of high levels of phosphorylated-mTOR and phosphorylated-p70S6K in tumor tissue even after calorie restriction suggests a persistent effect of obesity on tumor activity through AKT/mTOR signaling. In this mouse model, everolimus exerted an antiproliferative effect and blocked mTOR activation, thus prompting the speculation that obese patients could benefit from treatment with mTOR inhibitors [119]. Furthermore, the phosphorylation of IRS-1 by S6K was associated with impairment of the activation of PI3K/AKT by insulin, resulting in insulin resistance [120]. Despite the potential role of mTOR inhibitors in disabling this effect, everolimus and rapamycin per se induce metabolic side effects such as hyperglycemia and diabetes, in addition to dyslipidemia [121]. Thus, in obese and diabetic patients, mTOR inhibition primarily worsens insulin resistance [122].
Metformin is an antidiabetic drug that reduces the body weight and reinforces the anticancer properties through a direct or an indirect insulin-dependent mechanism. Through the activation of AMPK, metformin is also able to directly inhibit mTOR and modulate cell growth [123]. This effect is mediated by phosphorylation of TSC2 and raptor, inducing a direct inhibitory action on mTOR kinase [124]. In vitro studies showed the inhibitory effect of metformin in BC cell lines [125], in ER+ cells in particular, and, recently, in BC stem cells [126]. Additional in vivo studies support the antitumor effect of metformin in murine models of ER− BC [127].
Several clinical studies have suggested that metformin reduced the risk and the development of breast and gynecological cancer [128]. In 2009, an epidemiological study revealed that BC patients receiving neoadjuvant treatment with metformin obtained a higher pathology-demonstrated complete response rate (24%) than did the controls (8.0% in the non-metformin group and 16% in the nondiabetic group) [129]. The recent introduction of mTOR inhibitors in BC treatment and the reported evidence of an antitumor effect of metformin support their potential benefit, especially in obese patients, and several ongoing clinical trials are addressing this topic.
Conclusion
BC is a major health problem worldwide, and both overweight and obesity definitely increase the risk for this tumor. Some ongoing studies are evaluating the potential efficacy of behavioral and pharmacological interventions in preventing the incidence and/or recurrence of BC in overweight women (Table 3). Several molecules link obesity with BC and variably affect intracellular pathways such as MAPK, PI3K/AKT/mTOR, and WNT involved in adipogenesis and cancerogenesis. Obese women at risk of BC are characterized by high circulating levels of estrogens and molecules related to inflammation, insulin metabolism, and other intracellular processes. Monitoring the blood levels of these factors and the early detection of intracellular signaling hyperactivation, such as the IGF pathway, along with the identification of polymorphisms of BC and obesity-related genes, will offer a new diagnostic and prognostic tool for the treatment of BC obese patients (Fig. 4), although other genomic studies are needed to identify a clear, obesity-associated BC transcriptional signature. Early recognition of such a signature, together with compliance to a low-calorie diet in high-risk subjects, might be a prevention strategy that is potentially applicable in clinical practice. Obesity is a BC risk factor that can be corrected with both diet and physical exercise, and obesity-associated cancer gene activation might be modulated by small molecules interfering with intracellular pathways that are deregulated in cancer, such as IGFR, PI3K, and mTOR inhibitors. Because the hyperactivity of these oncogenic pathways is related to a worse prognosis of BC in obese patients, these drugs could potentially be effective in this patient setting, alone or in association with conventional treatments. However, in vivo studies are needed to prove that these compounds are really useful in counteracting tumor growth in obese patients and to ascertain whether a lifestyle change might have a true impact on genetic tumor features or could prevent oncogenesis. Thus, the steps that might affect the growing global problem of obesity-related cancer could include the following points: (a) educational nutrition programs for children and young people, (b) information on healthy lifestyles, (c) early recognition of clinical conditions increasing the risk of cancer in general practice outpatients, and (d) application of genomic tools to identify risk signatures for prevention of BC in obese women.
Table 3.
Ongoing trials evaluating behavioral or medical interventions for BC in obese women
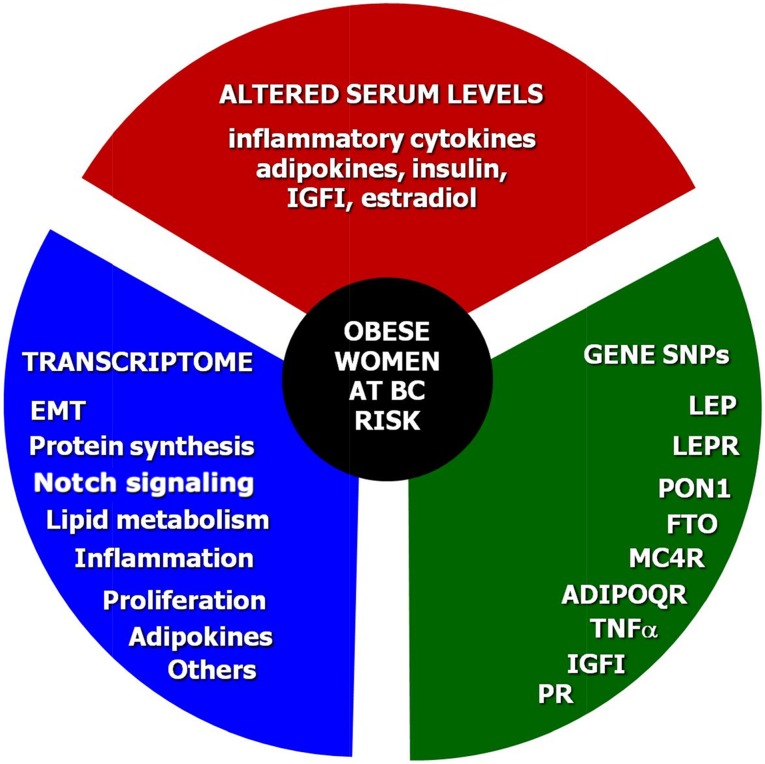
Figure 4.
Arbitrary BC risk grading in obese women. Considering the molecular interlinks between obesity and BC, clinical assessment of the BC risk in obese women should include three steps. The first step is to measure the serum levels of inflammatory cytokines, adipokines, insulin, IGFI, and estradiol. The second step should include the assessment of both obesity and BC-related genetic derangements such as SNPs of LEP, LEPR, PON1, FTO, MC4R, ADIPOQ, TNFα, IGFI, and PR. In the case of premalignant or suspicious lesions, the third step would include the analysis of the transcriptomic profile related to biological processes such as EMT, protein synthesis, Notch signaling, the lipid and cholesterol metabolism, inflammation, proliferation, adipokines, cell adhesion, estrogen receptor signaling, and others.
Abbreviations: EMT, epithelial-to-mesenchymal transition; IGFI, insulin-like growth factor I; SNPs, single nucleotide polymorphisms.
Acknowledgment
This work was supported by Italian Association for Cancer Research (AIRC) Grant IG 11647.
Author Contributions
Conception/design: Valeria Simone, Franco Silvestris
Provision of study material or patients: Antonella Argentiero, Claudia Felici, Francesca Maria Rizzo, Giovanni De Pergola
Collection and/or assembly of data: Morena D’Avenia, Claudia Felici, Francesca Maria Rizzo, Giovanni De Pergola
Data analysis and interpretation: Antonella Argentiero, Giovanni De Pergola
Manuscript writing: Valeria Simone, Morena D’Avenia, Antonella Argentiero, Giovanni De Pergola, Franco Silvestris
Final approval of manuscript: Franco Silvestris
Disclosures
The authors indicated no financial relationships.
References
- 1.World Cancer Research Fund/American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 2.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 3.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 5.Vainio H, Bianchini F.Weight Control and Physical Activity, Vol. 6. IARC Handbook of Cancer Prevention Lyon, France: IARC Press; 2002:1–315. [Google Scholar]
- 6.John EM, Phipps AI, Sangaramoorthy M. Body size, modifying factors, and postmenopausal breast cancer risk in a multiethnic population: The San Francisco Bay Area breast cancer study. Springerplus. 2013;2:239. doi: 10.1186/2193-1801-2-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheraghi Z, Poorolajal J, Hashem T, et al. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: A meta-analysis. PLoS One. 2012;7:e51446. doi: 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhaskaran K, Douglas I, Forbes H, et al. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daling JR, Malone KE, Doody DR, et al. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001;92:720–729. doi: 10.1002/1097-0142(20010815)92:4<720::aid-cncr1375>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Olivotto IA, Jackson JS, Mates D, et al. Prediction of axillary lymph node involvement of women with invasive breast carcinoma: A multivariate analysis. Cancer. 1998;83:948–955. [PubMed] [Google Scholar]
- 11.Ewertz M, Jensen MB, Gunnarsdóttir KÁ, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 12.Berclaz G, Li S, Price KN, et al. Body mass index as a prognostic feature in operable breast cancer: The International Breast Cancer Study Group experience. Ann Oncol. 2004;15:875–884. doi: 10.1093/annonc/mdh222. [DOI] [PubMed] [Google Scholar]
- 13.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan K, Bassett JK, MacInnis RJ, et al. Associations between weight in early adulthood, change in weight, and breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22:1409–1416. doi: 10.1158/1055-9965.EPI-13-0136. [DOI] [PubMed] [Google Scholar]
- 15.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: A cross-sectional study in healthy women. Eur J Endocrinol. 2004;150:161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 16.Rose DP, Vona-Davis L. Biochemical and molecular mechanisms for the association between obesity, chronic inflammation, and breast cancer. Biofactors. 2014;40:1–12. doi: 10.1002/biof.1109. [DOI] [PubMed] [Google Scholar]
- 17.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu F, Zhou G, Han S, et al. Association of TNF-α, TNFRSF1A and TNFRSF1B gene polymorphisms with the risk of sporadic breast cancer in northeast Chinese Han women. PLoS One. 2014;9:e101138. doi: 10.1371/journal.pone.0101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi N, Kannan S, Kotian N, et al. Interleukin 6 −174G>C polymorphism and cancer risk: Meta-analysis reveals a site dependent differential influence in ancestral North Indians. Hum Immunol. 2014;75:901–908. doi: 10.1016/j.humimm.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Kirschner MA, Schneider G, Ertel NH, et al. Obesity, androgens, estrogens, and cancer risk. Cancer Res. 1982;42(suppl):3281s–3285s. [PubMed] [Google Scholar]
- 21.Simpson ER, Brown KA. Obesity and breast cancer: Role of inflammation and aromatase. J Mol Endocrinol. 2013;51:T51–T59. doi: 10.1530/JME-13-0217. [DOI] [PubMed] [Google Scholar]
- 22.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 24.Skurk T, Alberti-Huber C, Herder C, et al. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 25.Bulun SE, Price TM, Aitken J, et al. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome P450 transcripts using competitive polymerase chain reaction after reverse transcription. J Clin Endocrinol Metab. 1993;77:1622–1628. doi: 10.1210/jcem.77.6.8117355. [DOI] [PubMed] [Google Scholar]
- 26.Chen D, Reierstad S, Fang F, et al. JunD and JunB integrate prostaglandin E2 activation of breast cancer-associated proximal aromatase promoters. Mol Endocrinol. 2011;25:767–775. doi: 10.1210/me.2010-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng L, Zhou J, Sasano H, et al. Tumor necrosis factor alpha and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein alpha and peroxisome proliferator-activated receptor gamma: Mechanism of desmoplastic reaction. Cancer Res. 2001;61:2250–2255. [PubMed] [Google Scholar]
- 28.Zhou J, Gurates B, Yang S, et al. Malignant breast epithelial cells stimulate aromatase expression via promoter II in human adipose fibroblasts: An epithelial-stromal interaction in breast tumors mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2001;61:2328–2334. [PubMed] [Google Scholar]
- 29.Mawson A, Lai A, Carroll JS, et al. Estrogen and insulin/IGF-1 cooperatively stimulate cell cycle progression in MCF-7 breast cancer cells through differential regulation of c-Myc and cyclin D1. Mol Cell Endocrinol. 2005;229:161–173. doi: 10.1016/j.mce.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Lee AV, Jackson JG, Gooch JL, et al. Enhancement of insulin-like growth factor signaling in human breast cancer: Estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol. 1999;13:787–796. doi: 10.1210/mend.13.5.0274. [DOI] [PubMed] [Google Scholar]
- 31.Caldon CE. Estrogen signaling and the DNA damage response in hormone dependent breast cancers. Front Oncol. 2014;4:106. doi: 10.3389/fonc.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 36.Frasca F, Pandini G, Sciacca L, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 37.Frasca F, Pandini G, Vigneri R, et al. Insulin and hybrid insulin/IGF receptors are major regulators of breast cancer cells. Breast Dis. 2003;17:73–89. doi: 10.3233/bd-2003-17108. [DOI] [PubMed] [Google Scholar]
- 38.Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21:610–618. doi: 10.1016/j.tem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novosyadlyy R, Lann DE, Vijayakumar A, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70:741–751. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 41.Zhou X, Tan M, Stone Hawthorne V, et al. Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor progression in breast cancers. Clin Cancer Res. 2004;10:6779–6788. doi: 10.1158/1078-0432.CCR-04-0112. [DOI] [PubMed] [Google Scholar]
- 42.Bose S, Chandran S, Mirocha JM, et al. The Akt pathway in human breast cancer: A tissue-array-based analysis. Mod Pathol. 2006;19:238–245. doi: 10.1038/modpathol.3800525. [DOI] [PubMed] [Google Scholar]
- 43.Bostner J, Karlsson E, Pandiyan MJ, et al. Activation of Akt, mTOR, and the estrogen receptor as a signature to predict tamoxifen treatment benefit. Breast Cancer Res Treat. 2013;137:397–406. doi: 10.1007/s10549-012-2376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.García-Jiménez C, García-Martínez JM, Chocarro-Calvo A, et al. A new link between diabetes and cancer: Enhanced WNT/β-catenin signaling by high glucose. J Mol Endocrinol. 2014;52:R51–R66. doi: 10.1530/JME-13-0152. [DOI] [PubMed] [Google Scholar]
- 45.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Naidu R, Yip CH, Taib NA. Genetic variations in transcription factor 7-like 2 (TCF7L2) gene: Association of TCF7L2 rs12255372(G/T) or rs7903146(C/T) with breast cancer risk and clinico-pathological parameters. Med Oncol. 2012;29:411–417. doi: 10.1007/s12032-011-9837-8. [DOI] [PubMed] [Google Scholar]
- 47.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 48.Guo S, Liu M, Wang G, et al. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim Biophys Acta. 2012;1825: 207–222. doi: 10.1016/j.bbcan.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalamaga M. Obesity, insulin resistance, adipocytokines and breast cancer: New biomarkers and attractive therapeutic targets. World J Exp Med. 2013;3:34–42. doi: 10.5493/wjem.v3.i3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin N, Wang D, Zhang H, et al. Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Res. 2004;64:5870–5875. doi: 10.1158/0008-5472.CAN-04-0655. [DOI] [PubMed] [Google Scholar]
- 51.Catalano S, Marsico S, Giordano C, et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278:28668–28676. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- 52.Macciò A, Madeddu C, Gramignano G, et al. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: Preliminary results and therapeutic implications. J Mol Med (Berl) 2010;88:677–686. doi: 10.1007/s00109-010-0611-8. [DOI] [PubMed] [Google Scholar]
- 53.Brown KA, McInnes KJ, Hunger NI, et al. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 2009;69:5392–5399. doi: 10.1158/0008-5472.CAN-09-0108. [DOI] [PubMed] [Google Scholar]
- 54.Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes. 2008;32(suppl 7):S13–S18. doi: 10.1038/ijo.2008.233. [DOI] [PubMed] [Google Scholar]
- 55.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: New insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book. 2013:46–51. doi: 10.1200/EdBook_AM.2013.33.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Subbaramaiah K, Howe LR, Bhardwaj P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carter JC, Church FC. Obesity and breast cancer: The roles of peroxisome proliferator-activated receptor-γ and plasminogen activator inhibitor-1. PPAR Res. 2009;2009:345320. doi: 10.1155/2009/345320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seibold P, Hein R, Schmezer P, et al. Polymorphisms in oxidative stress-related genes and postmenopausal breast cancer risk. Int J Cancer. 2011;129:1467–1476. doi: 10.1002/ijc.25761. [DOI] [PubMed] [Google Scholar]
- 61.Newman B, Austin MA, Lee M, et al. Inheritance of human breast cancer: Evidence for autosomal dominant transmission in high-risk families. Proc Natl Acad Sci USA. 1988;85:3044–3048. doi: 10.1073/pnas.85.9.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.García JM, Silva J, Peña C, et al. Promoter methylation of the PTEN gene is a common molecular change in breast cancer. Genes Chromosomes Cancer. 2004;41:117–124. doi: 10.1002/gcc.20062. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Lindblom A. Germline mutation screening of the STK11/LKB1 gene in familial breast cancer with LOH on 19p. Clin Genet. 2000;57:394–397. doi: 10.1034/j.1399-0004.2000.570511.x. [DOI] [PubMed] [Google Scholar]
- 64.Kuusisto KM, Bebel A, Vihinen M, et al. Screening for BRCA1, BRCA2, CHEK2, PALB2, BRIP1, RAD50, and CDH1 mutations in high-risk Finnish BRCA1/2-founder mutation-negative breast and/or ovarian cancer individuals. Breast Cancer Res. 2011;13:R20. doi: 10.1186/bcr2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—Analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 66.Snoussi K, Strosberg AD, Bouaouina N, et al. Leptin and leptin receptor polymorphisms are associated with increased risk and poor prognosis of breast carcinoma. BMC Cancer. 2006;6:38. doi: 10.1186/1471-2407-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gallicchio L, McSorley MA, Newschaffer CJ, et al. Body mass, polymorphisms in obesity-related genes, and the risk of developing breast cancer among women with benign breast disease. Cancer Detect Prev. 2007;31:95–101. doi: 10.1016/j.cdp.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Liu C, Liu L. Polymorphisms in three obesity-related genes (LEP, LEPR, and PON1) and breast cancer risk: A meta-analysis. Tumour Biol. 2011;32:1233–1240. doi: 10.1007/s13277-011-0227-9. [DOI] [PubMed] [Google Scholar]
- 69.Cleveland RJ, Gammon MD, Long CM, et al. Common genetic variations in the LEP and LEPR genes, obesity and breast cancer incidence and survival. Breast Cancer Res Treat. 2010;120:745–752. doi: 10.1007/s10549-009-0503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terrasi M, Fiorio E, Mercanti A, et al. Functional analysis of the -2548G/A leptin gene polymorphism in breast cancer cells. Int J Cancer. 2009;125:1038–1044. doi: 10.1002/ijc.24372. [DOI] [PubMed] [Google Scholar]
- 71.Pande M, Bondy ML, Do KA, et al. Association between germline single nucleotide polymorphisms in the PI3K-AKT-mTOR pathway, obesity, and breast cancer disease-free survival. Breast Cancer Res Treat. 2014;147:381–387. doi: 10.1007/s10549-014-3081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferretti G, Bacchetti T, Moroni C, et al. Paraoxonase activity in high-density lipoproteins: A comparison between healthy and obese females. J Clin Endocrinol Metab. 2005;90:1728–1733. doi: 10.1210/jc.2004-0486. [DOI] [PubMed] [Google Scholar]
- 73.Gómez Flores-Ramos L, Escoto-De Dios A, Puebla-Pérez AM, et al. Association of the tumor necrosis factor-alpha −308G>A polymorphism with breast cancer in Mexican women. Genet Mol Res. 2013;12:5680–5693. doi: 10.4238/2013.November.18.17. [DOI] [PubMed] [Google Scholar]
- 74.Alokail MS, Al-Daghri NM, Al-Attas OS, et al. Combined effects of obesity and type 2 diabetes contribute to increased breast cancer risk in premenopausal women. Cardiovasc Diabetol. 2009;8:33. doi: 10.1186/1475-2840-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kusinska R, Górniak P, Pastorczak A, et al. Influence of genomic variation in FTO at 16q12.2, MC4R at 18q22 and NRXN3 at 14q31 genes on breast cancer risk. Mol Biol Rep. 2012;39:2915–2919. doi: 10.1007/s11033-011-1053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.da Cunha PA, de Carlos Back LK, Sereia AF, et al. Interaction between obesity-related genes, FTO and MC4R, associated to an increase of breast cancer risk. Mol Biol Rep. 2013;40:6657–6664. doi: 10.1007/s11033-013-2780-3. [DOI] [PubMed] [Google Scholar]
- 78.Kaklamani V, Yi N, Sadim M, et al. The role of the fat mass and obesity associated gene (FTO) in breast cancer risk. BMC Med Genet. 2011;12:52. doi: 10.1186/1471-2350-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- 80.Kaklamani VG, Hoffmann TJ, Thornton TA, et al. Adiponectin pathway polymorphisms and risk of breast cancer in African Americans and Hispanics in the Women’s Health Initiative. Breast Cancer Res Treat. 2013;139:461–468. doi: 10.1007/s10549-013-2546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al Khaldi RM, Al Mulla F, Al Awadhi S, et al. Associations of single nucleotide polymorphisms in the adiponectin gene with adiponectin levels and cardio-metabolic risk factors in patients with cancer. Dis Markers. 2011;30:197–212. doi: 10.3233/DMA-2011-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohan Reddy N, Kalyana Kumar CH, Kaiser J. Association of adiponectin gene functional polymorphisms (+45T/G and 276G/T) with obese breast cancer. J Mol Biomark Diagn. 2012;3:6. [Google Scholar]
- 83.Kaklamani VG, Sadim M, Hsi A, et al. Variants of the adiponectin and adiponectin receptor 1 genes and breast cancer risk. Cancer Res. 2008;68:3178–3184. doi: 10.1158/0008-5472.CAN-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Connor A, Baumgartner RN, Kerber RA, et al. ADRB2 G-G haplotype associated with breast cancer risk among Hispanic and non-Hispanic white women: Interaction with type 2 diabetes and obesity. Cancer Causes Control. 2012;23:1653–1663. doi: 10.1007/s10552-012-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ng ST, Zhou J, Adesanya OO, et al. Growth hormone treatment induces mammary gland hyperplasia in aging primates. Nat Med. 1997;3:1141–1144. doi: 10.1038/nm1097-1141. [DOI] [PubMed] [Google Scholar]
- 86.Faber P, Johnstone AM, Gibney ER, et al. The effect of rate of weight loss on erythrocyte glutathione concentration and synthesis in healthy obese men. Clin Sci (Lond) 2002;102:569–577. doi: 10.1042/cs1020569. [DOI] [PubMed] [Google Scholar]
- 87.Helzlsouer KJ, Selmin O, Huang HY, et al. Association between glutathione S-transferase M1, P1, and T1 genetic polymorphisms and development of breast cancer. J Natl Cancer Inst. 1998;90:512–518. doi: 10.1093/jnci/90.7.512. [DOI] [PubMed] [Google Scholar]
- 88.Yu H, Li BD, Smith M, et al. Polymorphic CA repeats in the IGF-I gene and breast cancer. Breast Cancer Res Treat. 2001;70:117–122. doi: 10.1023/a:1012947027213. [DOI] [PubMed] [Google Scholar]
- 89.Missmer SA, Haiman CA, Hunter DJ, et al. A sequence repeat in the insulin-like growth factor-1 gene and risk of breast cancer. Int J Cancer. 2002;100:332–336. doi: 10.1002/ijc.10473. [DOI] [PubMed] [Google Scholar]
- 90.DeLellis K, Ingles S, Kolonel L, et al. IGF1 genotype, mean plasma level and breast cancer risk in the Hawaii/Los Angeles multiethnic cohort. Br J Cancer. 2003;88:277–282. doi: 10.1038/sj.bjc.6600728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wen W, Gao YT, Shu XO, et al. Insulin-like growth factor-I gene polymorphism and breast cancer risk in Chinese women. Int J Cancer. 2005;113:307–311. doi: 10.1002/ijc.20571. [DOI] [PubMed] [Google Scholar]
- 92.Pang B, Cheng S, Sun SP, et al. Prognostic role of PIK3CA mutations and their association with hormone receptor expression in breast cancer: A meta-analysis. Sci Rep. 2014;4:6255. doi: 10.1038/srep06255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wasserman L, Flatt SW, Natarajan L, et al. Correlates of obesity in postmenopausal women with breast cancer: Comparison of genetic, demographic, disease-related, life history and dietary factors. Int J Obes Relat Metab Disord. 2004;28:49–56. doi: 10.1038/sj.ijo.0802481. [DOI] [PubMed] [Google Scholar]
- 94.De Vivo I, Hankinson SE, Colditz GA, et al. A functional polymorphism in the progesterone receptor gene is associated with an increase in breast cancer risk. Cancer Res. 2003;63:5236–5238. [PubMed] [Google Scholar]
- 95.Miyoshi Y, Funahashi T, Tanaka S, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118:1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 96.Tessitore L, Vizio B, Pesola D, et al. Adipocyte expression and circulating levels of leptin increase in both gynaecological and breast cancer patients. Int J Oncol. 2004;24:1529–1535. [PubMed] [Google Scholar]
- 97.Machinal-Quélin F, Dieudonné MN, Pecquery R, et al. Direct in vitro effects of androgens and estrogens on ob gene expression and leptin secretion in human adipose tissue. Endocrine. 2002;18:179–184. doi: 10.1385/ENDO:18:2:179. [DOI] [PubMed] [Google Scholar]
- 98.Geisler J, Haynes B, Ekse D, et al. Total body aromatization in postmenopausal breast cancer patients is strongly correlated to plasma leptin levels. J Steroid Biochem Mol Biol. 2007;104:27–34. doi: 10.1016/j.jsbmb.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 99.Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 100.Tworoger SS, Eliassen AH, Kelesidis T, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 101.Fasshauer M, Klein J, Neumann S, et al. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 102.Sheen-Chen SM, Liu YW, Eng HL, et al. Serum levels of hepatocyte growth factor in patients with breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:715–717. doi: 10.1158/1055-9965.EPI-04-0340. [DOI] [PubMed] [Google Scholar]
- 103.Sundaram S, Freemerman AJ, Johnson AR, et al. Role of HGF in obesity-associated tumorigenesis: C3(1)-TAg mice as a model for human basal-like breast cancer. Breast Cancer Res Treat. 2013;142:489–503. doi: 10.1007/s10549-013-2741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alluri PG, Speers C, Chinnaiyan AM. Estrogen receptor mutations and their role in breast cancer progression. Breast Cancer Res. 2014;16:494. doi: 10.1186/s13058-014-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fuentes-Mattei E, Velazquez-Torres G, Phan L, et al. Effects of obesity on transcriptomic changes and cancer hallmarks in estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2014;106:dju158. doi: 10.1093/jnci/dju158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Creighton CJ, Sada YH, Zhang Y, et al. A gene transcription signature of obesity in breast cancer. Breast Cancer Res Treat. 2012;132:993–1000. doi: 10.1007/s10549-011-1595-y. [DOI] [PubMed] [Google Scholar]
- 108.Creighton CJ, Casa A, Lazard Z, et al. Insulin-like growth factor-I activates gene transcription programs strongly associated with poor breast cancer prognosis. J Clin Oncol. 2008;26:4078–4085. doi: 10.1200/JCO.2007.13.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dalmau E, Armengol-Alonso A, Muñoz M, et al. Current status of hormone therapy in patients with hormone receptor positive (HR+) advanced breast cancer. Breast. 2014;23:710–720. doi: 10.1016/j.breast.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 110.Goodwin PJ. Obesity and endocrine therapy: Host factors and breast cancer outcome. Breast. 2013;22(suppl 2):S44–S47. doi: 10.1016/j.breast.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 111.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 112.Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 113.Garreau JR, Delamelena T, Walts D, et al. Side effects of aromatase inhibitors versus tamoxifen: The patients’ perspective. Am J Surg. 2006;192:496–498. doi: 10.1016/j.amjsurg.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 114.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: A GINECO study. J Clin Oncol. 2012;30:2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 116.Ma CX, Suman VJ, Goetz M, et al. A phase I trial of the IGF-1R antibody cixutumumab in combination with temsirolimus in patients with metastatic breast cancer. Breast Cancer Res Treat. 2013;139:145–153. doi: 10.1007/s10549-013-2528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee DF, Kuo HP, Chen CT, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 118.Chen CT, Du Y, Yamaguchi H, et al. Targeting the IKKβ/mTOR/VEGF signaling pathway as a potential therapeutic strategy for obesity-related breast cancer. Mol Cancer Ther. 2012;11:2212–2221. doi: 10.1158/1535-7163.MCT-12-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Angel RE, Conti CJ, Wheatley KE, et al. The enhancing effects of obesity on mammary tumor growth and Akt/mTOR pathway activation persist after weight loss and are reversed by RAD001. Mol Carcinog. 2013;52:446–458. doi: 10.1002/mc.21878. [DOI] [PubMed] [Google Scholar]
- 120.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 121.Sivendran S, Agarwal N, Gartrell B, et al. Metabolic complications with the use of mTOR inhibitors for cancer therapy. Cancer Treat Rev. 2014;40:190–196. doi: 10.1016/j.ctrv.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fraenkel M, Ketzinel-Gilad M, Ariav Y, et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945–957. doi: 10.2337/db07-0922. [DOI] [PubMed] [Google Scholar]
- 123.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 125.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 126.Hirsch HA, Iliopoulos D, Tsichlis PN, et al. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Phoenix KN, Vumbaca F, Claffey KP. Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERalpha negative MDA-MB-435 breast cancer model. Breast Cancer Res Treat. 2009;113:101–111. doi: 10.1007/s10549-008-9916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sivalingam VN, Myers J, Nicholas S, et al. Metformin in reproductive health, pregnancy and gynaecological cancer: Established and emerging indications. Hum Reprod Update. 2014;20:853–868. doi: 10.1093/humupd/dmu037. [DOI] [PubMed] [Google Scholar]
- 129.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]