Lymph node removal for staging, as part of the initial surgical management of patients with endometrial carcinoma, remains controversial. Another evolving field in endometrial cancer staging is the interpretation of pathologic ultrastaging of sentinel lymph nodes, which can identify low-volume metastases. This article reviews clinical data related to lymphatic mapping for the staging and management of endometrial cancer and its role in clinical practice.
Keywords: Endometrial cancer, Sentinel lymph node, Sentinel lymph node mapping, Pathologic ultrastaging
Abstract
Lymph node removal for staging, as part of the initial surgical management of patients with endometrial carcinoma, remains a controversial topic in gynecologic oncology. There is currently wide variability among clinical practices, with surgical approaches ranging from no nodal evaluation to comprehensive pelvic and aortic lymphadenectomy. Lymphatic mapping has emerged as an increasingly popular option over the past few years, with several attractive features in its concept, innovative surgical approach, and encouraging preliminary results. At this time, however, several different techniques have been described and used for lymphatic mapping in endometrial cancer, incorporating a variety of mapping agents and injection sites. Although recently published results are encouraging, they are limited to single-institution series or multi-institutional collaborations undertaken without the aegis of a prospective randomized controlled trial. However, the surgical staging of endometrial cancer with lymphadenectomy was historically established based not on randomized trial data but on prospective clinicopathologic studies. Another evolving field in endometrial cancer staging is the interpretation of pathologic ultrastaging of sentinel lymph nodes (SLNs), which can identify low-volume metastases for which the clinical significance and the ideal management remain uncertain. This is particularly an issue with extremely low-volume nodal metastasis and isolated tumor cells. Furthermore, it has become apparent that applying a predefined SLN algorithm can decrease false-negative rates. The Memorial Sloan Kettering Cancer Center SLN algorithm can be used as a checklist to ensure standardization of care and to reduce the chance of missing nodal disease. Prospective trials are under way at many institutions to help establish the definitive role of SLN mapping for staging of endometrial cancer. The objective of this study was to provide an update on the latest clinical data related to lymphatic mapping for the staging and management of endometrial cancer and its role in clinical practice.
Implications for Practice:
Lymphatic mapping is an increasingly popular option in the surgical treatment of endometrial cancer. The aim of using this tool is to target the lymph nodes that are the most likely to be involved with metastatic cancer cells (sentinel lymph nodes) and thereby limit the extent of surgery needed and decrease surgical complications and long-term side effects associated with extensive lymph node removal. By examining a limited number of sentinel lymph nodes, a more detailed examination of the node can be done (ultrastaging). This allows for the detection of a small number of cancer cells (low-volume metastasis) that can be missed with standard techniques.
Introduction
Endometrial cancer is the most common gynecologic malignancy in the United States, with an estimated 54,870 new cases in 2015 [1]. Except in rare circumstances in which surgery is contraindicated, the initial management of apparent early-stage endometrial cancer (i.e., disease clinically assumed to be limited to the uterus) requires surgical staging, which includes a hysterectomy, bilateral salpingo-oophorectomy, and evaluation of regional lymph node involvement, specifically the pelvic and aortic lymph node basins. The decision whether to perform a lymph node dissection, and to what extent (pelvic vs. pelvic and aortic; below vs. above the inferior mesenteric artery; complete lymphadenectomy vs. lymph node sampling) has been one of the most controversial areas in the management of endometrial cancer. In this context, lymphatic mapping recently emerged as a promising new strategy and is increasingly being adopted by gynecologic oncology practices in the U.S. and worldwide.
Although initially described by Burke et al. in 1996 [2], lymphatic mapping did not garner much attention over the ensuing decade; only in recent years has lymphatic mapping in endometrial cancer gained increasing attention. A comprehensive review published in 2008 identified fewer than 20 articles regarding the subject, none of which included more than 30 patients [3]. At the time of the writing of this article, a PubMed search with the key words “sentinel node” and “endometrial cancer” resulted in more than 200 articles. The recent interest in this surgical strategy may be due to different factors that have influenced current practice. First, although criticized in methodology [4], the findings of two randomized prospective European trials evaluating the role of pelvic lymph node dissection in early-stage endometrial cancer showed no survival benefit in the lymphadenectomy group [5, 6]. Second, despite wide adoption of complete pelvic and aortic lymphadenectomy by many groups, there has been no prospective evidence of a survival benefit associated with this surgical strategy [7]. In addition, the last decade has seen the increasing adoption of laparoscopy and robotic-assisted surgery as a standard of care in the surgical management of endometrial cancer [8], and there is increasing awareness of the long-term side effects of lymphadenectomy, such as lymphocyst formation, neurovascular injury, and leg lymphedema. Finally, although complete pelvic and aortic lymphadenectomy can be performed via a minimally invasive or open approach, it can be technically challenging and often not feasible in a significant number of patients because of body habitus and comorbidities.
Injection Technique
For any given surgical procedure, different techniques and approaches are initially attempted to achieve a certain goal. As different teams and surgeons believe in the validity of the goal, there is typically a continuous improvement that follows, and serial modifications in the surgical technique take place, mostly derived from understanding complications and analyzing whether the observed outcomes achieve the desired goals. During this process, there is usually a concomitant simplification of the technique, and improvement in the instruments and technology involved.
Lymphatic mapping in endometrial cancer has followed a similar path since its first description in 1996 [2]. The initial goal was to investigate whether what was to become the standard of care in breast cancer and melanoma could be applicable to endometrial cancer, specifically identifying the sentinel lymph nodes (SLNs) initially involved in the metastatic spread of cancer cells, making a complete pelvic and aortic lymphadenectomy for staging purposes unnecessary. The two technical aspects that needed to be initially evaluated involved determining which injection site was the most appropriate to allow the dye or radiocolloid to follow the route of potential metastatic spread and which marker (radiocolloid vs. blue dye) was associated with the highest detection rates.
Three injection sites were mainly used: the uterine fundus, the endometrium using hysteroscopy, and the cervix [3]. The fundal injection site was not associated with high detection rates, and it is often not technically feasible when there is distortion of the uterus secondary to leiomyomas, which is not an uncommon occurrence [9]. The hysteroscopic approach focused on a peritumoral injection concept and involved identifying the tumor by direct visualization and injecting the underlying endometrium. The initial published reports with this approach were encouraging, indicating a high rate of detection in both the pelvic and aortic nodal areas mimicking the distribution of lymph node metastasis [10, 11]. Despite these encouraging initial results, however, this technique has not been widely adopted. Compared with the other injection methods, this is a technically more involved approach (hysteroscopic visualization of the tumor and the surrounding endometrium is not easy when the tumor is either too large and occupying the whole endometrial cavity, or too small/microscopic and harder to identify) and frequently involves a separate procedure the day before the hysterectomy, ultimately creating a logistical challenge and understandably minimal enthusiasm from patients [12]. The third injection approach, which has gained the most popularity in recent years, involves injection into the cervix. The cervix is almost always clearly visualized and accessible despite major distortion of the uterine corpus (by tumors or leiomyomas). The most recent national and international reports mostly use the cervix as their injection site, and a 2011 meta-analysis described improved SLN detection rates with the cervical injection compared with the other injection sites [13]. With this approach, the reported SLN detection rates have surpassed 80% [14]. The major criticism of the cervical injection approach is the low rates of mapping in the aortic area, which is discussed later in this article.
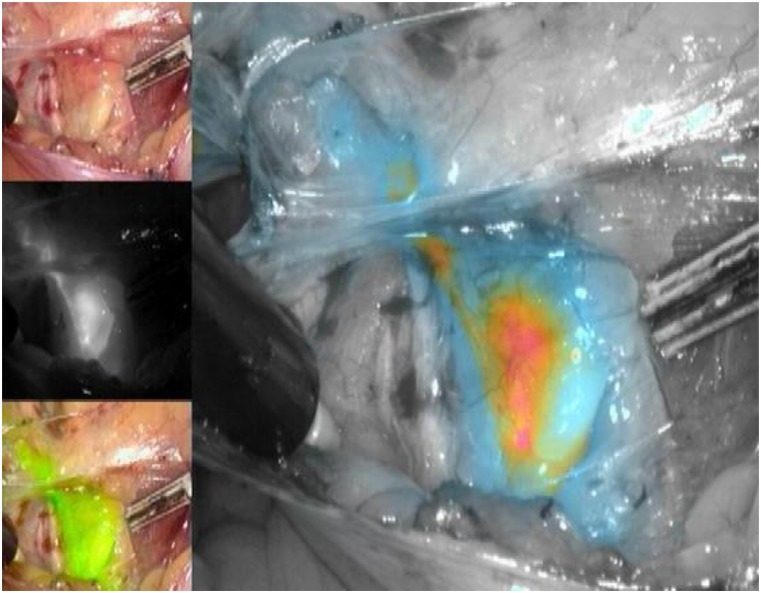
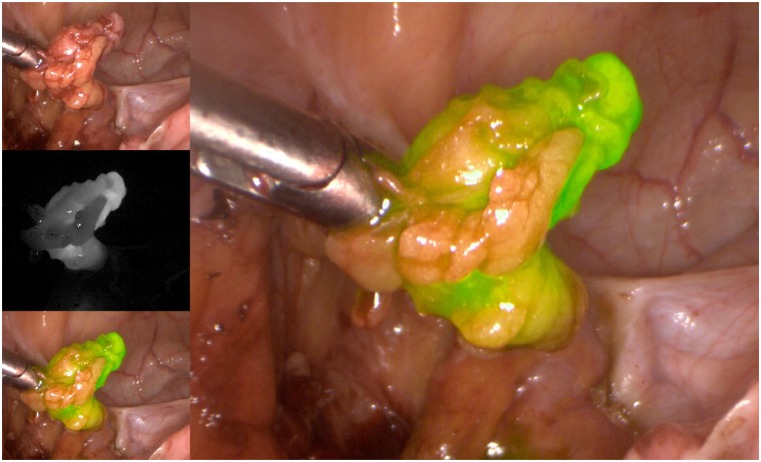
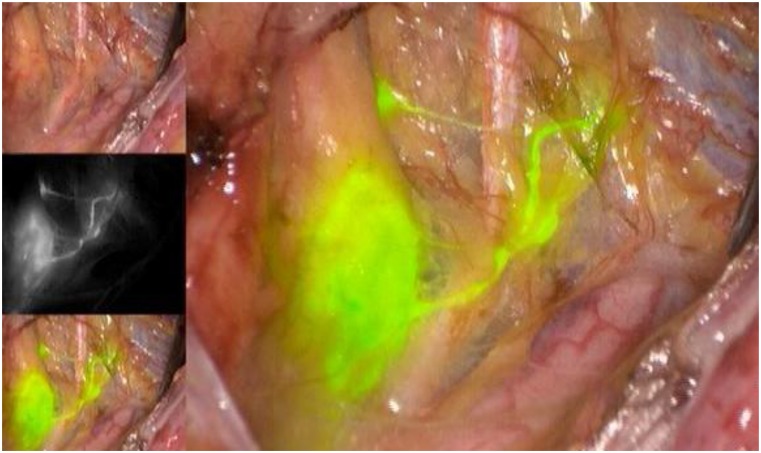
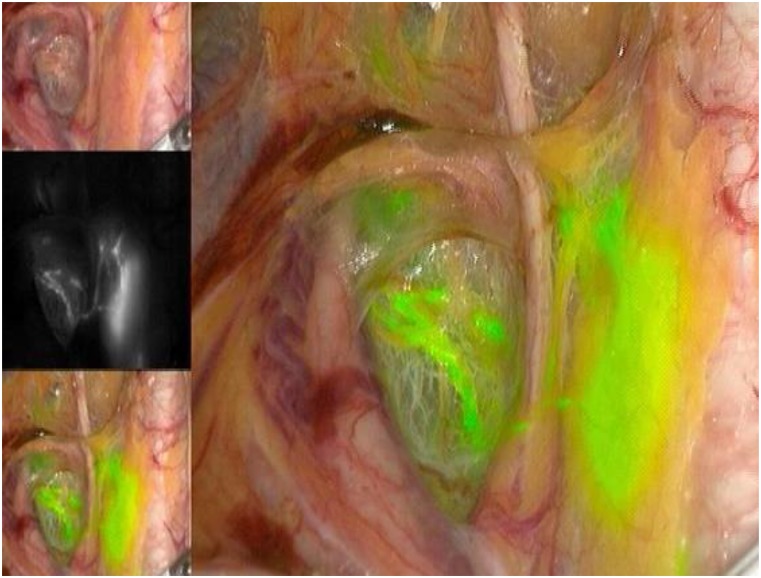
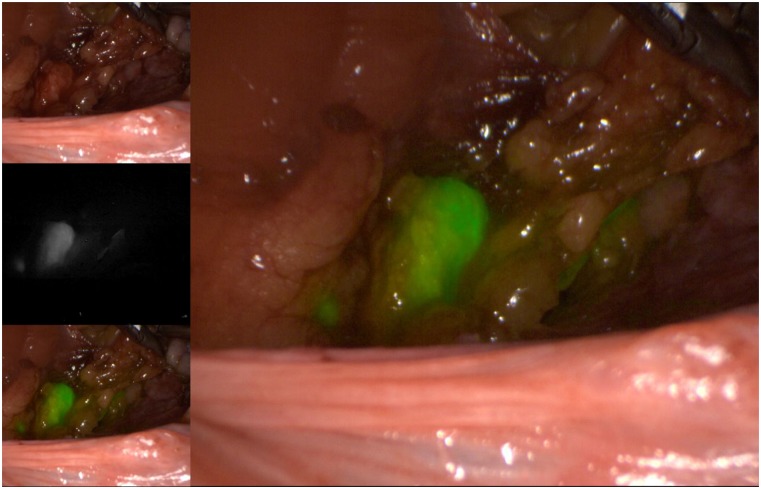
As for the agents used to identify the SLNs, the three available options include radioactive technetium-99 (Tc99), blue dye (isosulfan blue or methylene blue), and, more recently, indocyanine green (ICG). Although all markers are acceptable options to use in isolation, the recent experience of most groups indicates that ICG, with SLN identification using a near-infrared camera, is associated with the highest bilateral detection rate. ICG is rapidly becoming the marker of choice, when available and when cost permits (Figs. 1–5) [15]. The radioactive option requires a preoperative injection of Tc99 and lymphoscintigraphy before surgery and an intraoperative gamma probe to identify the hot nodes. The blue dye injection is the simplest, as no special equipment is needed, but seems to be associated with a low rate of bilateral mapping compared with the other two techniques [14]. Combining more than one injection agent is also used by some groups and may be associated with a higher detection rate [14].
Figure 1.
Right iliac sentinel lymph node using indocyanine green injection into the cervix and viewed using color-segmented fluorescence.
Figure 5.
Excised sentinel lymph node.
Figure 2.
Left external iliac sentinel lymph node.
Figure 3.
Right external iliac sentinel lymph node.
Figure 4.
Left obturator sentinel lymph node.
Predictors of Success: Surgeon, Patient, Technique
As mentioned, it seems that the use of ICG is associated with a higher rate of mapping compared with the other agents. In addition, other factors may influence the detection rate and, if not taken into account, can significantly affect mapping results. Surgeon experience is one of those factors. The number of cases needed to achieve a certain level of proficiency with any given technique depends on the individual surgeon, volume of cases, and, likely, the technique used [16]. As lymphatic mapping has increased in popularity, many fellows in training are being taught this technique as part of their training. However, it remains important for individual surgeons to establish their own detection rates and false-negative rates (FNRs), regardless of how many cases they performed during their training, to determine as precisely as possible whether they can offer this option to their patients, and in which circumstances. It is not uncommon for new graduates to experience frustration in identifying SLNs once they start independent practices after having felt extremely comfortable doing so under supervision and guidance during their training.
It remains important for individual surgeons to establish their own detection rates and false-negative rates, regardless of how many cases they performed during their training, to determine as precisely as possible whether they can offer this option to their patients, and in which circumstances.
In addition to factors related to the technique and to the surgeon, successful mapping may also be affected by patient factors. Obesity has been reported to affect successful mapping [17, 18]. ICG was recently reported to perform better than blue dye in obese patients [18].
False-Negative Rates: Relevance, Alternatives
The ideal goal of lymphatic mapping is to identify one or a limited number of SLNs that will be the first involved with metastatic disease and, therefore, representative of the rest of the nodal basin. Specifically, an SLN negative for metastatic disease would then indicate with high confidence (usually in the 90%–95% range) that the rest of the lymph nodes are not involved, rendering a lymph node dissection unnecessary. The typical measure that will establish whether the spread of metastatic cells follows this predictable pattern is the FNR of the lymphatic mapping protocol. In other words, the FNR indicates the probability of having metastatic cells in non-SLNs when the SLN is negative. To calculate the FNR accurately, the population used for this calculation should have undergone removal of the SLN as well as non-SLNs in the regional basins at risk. For endometrial cancer, calculating an accurate FNR would require removal of the SLNs and then performing a systematic pelvic and aortic lymph node dissection. In the absence of this, it could theoretically be concluded that lymphatic mapping should not be used for endometrial cancer staging before a reliable FNR is established [19].
However, this would be a premature conclusion if we do not analyze current data and look at the benefits of lymphatic mapping, acknowledging the peculiarities of endometrial cancer. In this aspect, any discussion of the current role and benefit of lymphatic mapping would not be accurate if not done within the context of discussing the role of lymph node dissection in endometrial cancer, which is the larger issue of controversy that lymphatic mapping is trying to resolve. Simply put, we cannot conclude that lymphatic mapping is inferior to complete lymph node dissection and, therefore, should not be used, if we do not first accept that a complete pelvic and aortic lymph node dissection is the standard that should be performed in all cases and is associated with improved patient outcomes.
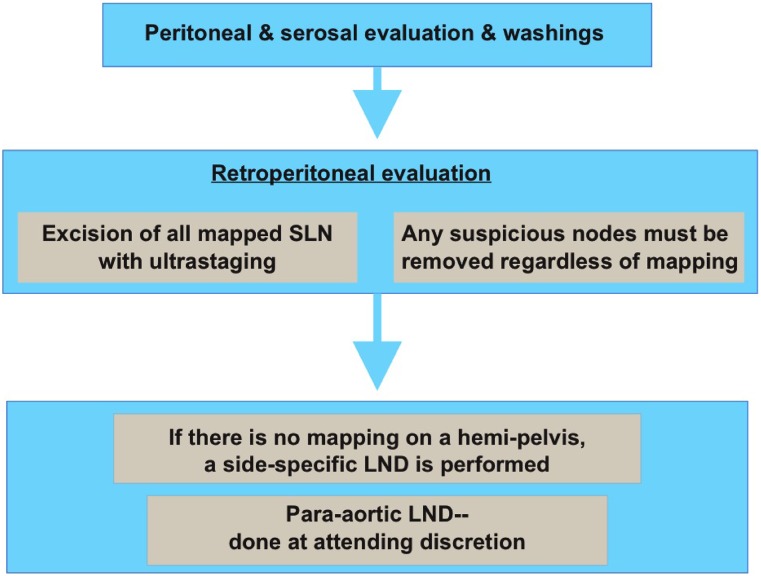
The question, therefore, is not merely whether lymphatic mapping is associated with very low FNRs but also whether a strategy of lymphatic mapping is more beneficial than a strategy of complete lymphadenectomy, even if associated with a higher FNR than what is acceptable in other solid tumors. In that regard, the data appear encouraging, which probably explains the adoption of this method of surgical staging by a growing number of groups worldwide. Data from Memorial Sloan Kettering Cancer Center (MSK) initially showed that SLNs are three times more likely to harbor metastatic cells than non-SLNs [20]. This was among the first evidence showing that, at least partially, the concept of lymphatic mapping is valid in endometrial cancer and should be evaluated further. Following that, applying a structured systematic algorithm was then shown to have the potential to further decrease the incidence of false-negatives, albeit in a retrospective data analysis (Fig. 6) [21]. Soon after, a separate analysis from the same data and institution showed that over a 3-year period, as the number of lymph nodes removed decreased and lymphatic mapping use increased, the incidence of patients with stage IIIC disease did not decrease [22]. In other words, the data suggested that identifying an SLN compared with performing a more extensive lymphadenectomy led to a quantitatively similar detection of nodal metastasis. These benefits of lymphatic mapping are valid and clinically useful, even without a prospectively established acceptably low FNR. More recently, an additional approach was reported using an algorithm incorporating lymphatic mapping and frozen section of the uterus, which can potentially be another valid surgical staging alternative [23]. It is important to note, however, that although encouraging, the majority of the currently available data on SLN mapping are based on retrospective or prospective, uncontrolled studies in which surgical decisions were based on surgeon preference and not on a strict, predefined surgical protocol. Nevertheless, the data were compelling enough that the National Comprehensive Cancer Network guidelines incorporated lymphatic mapping as a surgical staging option in 2014 based on lower-level evidence (Fig. 6) [24].
Figure 6.
Memorial Sloan Kettering Cancer Center sentinel lymph node algorithm [21]. Reprinted with permission from Elsevier.
Abbreviations: LND, lymph node dissection; SLN, sentinel lymph node.
Although a low FNR is needed to conclude that SLN mapping can confidently replace complete pelvic and aortic lymphadenectomy, the controversy surrounding the role of pelvic and aortic lymphadenectomy in endometrial cancer has allowed lymphatic mapping to become an acceptable alternative, even in the absence of prospective data to calculate the FNR.
Evaluation of Aortic Nodes: Mandatory or Optional?
An integral part of the controversy regarding lymph node evaluation in the surgical staging of endometrial cancer relates to the evaluation of aortic nodes [25]. There is little debate that performing a complete aortic lymph node dissection to the level of the renal veins will identify a significant proportion of patients with aortic nodal metastases [26]. The question, however, is whether identifying and removing these involved nodes is associated with improved survival, either directly as a result of their surgical removal or by allowing for a more tailored adjuvant treatment [27].
For lymphatic mapping, the cervical site of injection is the most commonly used in published reports. The growing experience in lymphatic mapping from different groups seems to indicate that once a certain level of competency is achieved, a very high rate of mapping can be achieved in the pelvis. However, mapping of the aortic area has been less successful. Although SLNs can be identified in the aortic area after a cervical injection, this is an infrequent occurrence, not surpassing 5% [14]. As discussed earlier, the highest rates of mapping in the aortic area have been associated with the hysteroscopic injection. At this time, however, this approach has not gained popularity for the reasons mentioned above.
The question then becomes whether there is value in identifying SLNs in the pelvis if mapping of the aortic area is suboptimal. This also can only be answered within the broader aspect of the role of aortic lymph node dissection in the surgical staging of endometrial carcinoma. This is not the subject of this article and, therefore, we will not review in detail the evidence for or against aortic lymphadenectomy but will limit the discussion to relevant points with regard to lymphatic mapping. At this time, multiple retrospective and prospective series have demonstrated that the incidence of isolated aortic lymph node metastasis in the absence of pelvic nodal metastasis is not greater than 5%, even among patients with disease of high-risk histology [26, 28, 29]. In one of the most detailed descriptions of the topographic distribution of lymph node metastasis, researchers at the Mayo Clinic showed that aortic nodal metastases, when present, are most commonly encountered above the inferior mesenteric artery when the pelvic nodes are negative [30]. In other words, sampling the lower aortic nodes (below the inferior mesenteric artery) in these cases is less likely to identify nodal metastasis and more likely to result in increased operative time and complications, with minimal clinical benefit.
The options for aortic nodal evaluation using lymphatic mapping are largely provider dependent and do not necessarily conflict with the incorporation of a lymphatic mapping protocol. Specifically, in the absence of mapping in the high aortic area (which current data seem to indicate is rare), the surgeon can still perform an aortic lymph node dissection regardless of mapping results in the pelvis. For surgeons who routinely perform lower aortic nodal dissection, pelvic mapping may be informative of both the pelvic and lower aortic nodal basins, and may be an acceptable substitute. It is particularly important to mention that current practice is already variable regarding lymph node evaluation [31], and incorporating lymphatic mapping constitutes an additional option, similar to the other surgical strategies.
Role of Ultrastaging
One of the potential advantages of lymphatic mapping is the possibility of identifying low-volume metastasis by additional serial sectioning and immunohistochemical staining (ultrastaging) of the SLN. Low-volume metastases include micrometastases and isolated tumor cells (ITCs), similar to what has been described in the breast cancer literature. Large series have identified a significant percentage of low-volume metastases in SLNs submitted for ultrastaging, including in patients with low-grade, early-stage endometrial cancer, a group in whom lymph node dissection arguably could have been omitted [32].
At this time, the clinical significance of these low-volume metastases is unknown, as is the optimal postsurgical treatment when identified. Choosing to ignore them is difficult in the absence of reassuring clinical data, and treating them as macrometastases may submit the patient to the complications of adjuvant treatment unnecessarily, especially when these low-volume metastases are identified in low-risk patients with typically excellent outcomes. In 1 large retrospective cohort study of 844 patients who underwent staging with SLN mapping for endometrial cancer, 44 patients (5.2%) had low-volume metastasis; 23 (2.7%) had ITCs alone, and 21 (2.5%) had micrometastases. Adjuvant treatment, including chemotherapy, was given to greater than 80% of these patients and, therefore, the authors were not able to comment on the natural history of untreated low-volume nodal disease. However, the 3-year progression-free survival for patients with ITCs or micrometastasis was 86%, compared with 71% for those with macrometastasis (p < .001), suggesting that patients with low-volume nodal metastasis may behave as a “better node positive” group [33]. It is imperative to evaluate this population prospectively to further inform the discussion regarding adjuvant therapy in this setting. Absent this, treatment decisions are based on the overall oncologic picture and other tumor factors after patient counseling. Pending additional study, the authors currently recommend individualizing the adjuvant treatment of patients with ITC node-positive disease.
Applying the MSK Algorithm
The algorithm developed at MSK allows for a systematic approach to incorporating lymphatic mapping in the surgical staging of endometrial cancer (Fig. 6). Following the algorithm decreases the FNR. Key components to this algorithm are a contralateral lymphadenectomy in cases of unilateral mapping and removal of any suspicious nodes regardless of mapping; moreover, aortic nodal evaluation is optional and based on surgeon preference [22]. Additional modifications have been published, including a frozen-section evaluation of the endometrium, which will further decrease the number of patients submitted to unnecessary lymph node dissections [23]. In this area too, it is important to emphasize that these algorithms are based on retrospective data and need to be validated in prospective trials with patient outcomes as the primary endpoint (see below) to solidify their role in current clinical practice.
Current Unanswered Questions: Role of Prospective Trials
The popularity of lymphatic mapping is most certainly related to the controversy surrounding the role of lymph node evaluation in endometrial carcinoma. With the lack of prospective trials demonstrating a survival benefit for extensive lymphadenectomies, the rise of the minimally invasive surgical approach (laparoscopic or robotically assisted), the increasing availability of novel near-IR imaging technologies, and an increased focus on decreasing surgical morbidity, it is not surprising to see that the last few years have witnessed a decrease in the frequency of lymph node dissection in the management of endometrial cancer [34]. Critics of lymphatic mapping frequently put forward the argument that there are no controlled data demonstrating an acceptable low FNR for SLNs, especially in the aortic area. However, it is noteworthy to emphasize that the same may be said about systematic pelvic and aortic lymph node dissection; to date, there are no prospective trials demonstrating its survival benefit.
With the lack of prospective trials demonstrating a survival benefit for extensive lymphadenectomies, the rise of the minimally invasive surgical approach (laparoscopic or robotically assisted), the increasing availability of novel near-IR imaging technologies, and an increased focus on decreasing surgical morbidity, it is not surprising to see that the last few years have witnessed a decrease in the frequency of lymph node dissection in the management of endometrial cancer.
Therefore, the main question is not whether lymphatic mapping can replace standard lymphadenectomy templates (pelvic and aortic) but rather which approach is associated with the most useful information to guide adjuvant therapy, at the lowest cost of surgical morbidity, and ideally associated with the best survival. Undoubtedly, as our understanding of tumor biology and molecular drivers increases, the classification and risk stratification of uterine malignancy will continue to evolve, and adjuvant therapy may become guided by uterine and tumoral factors. Will there be any fundamental role for lymph node evaluation if adjuvant treatment decisions can be completely derived from the primary tumor characteristics in the uterus? This remains to be determined. Prospective controlled trials are needed to address these questions. At this time, several ongoing trials are evaluating the predictive values of SLNs correlated with systematic pelvic and aortic lymphadenectomies, the role of metastasis detected by ultrastaging, and the role of 18F-fluorodeoxyglucose imaging in detecting SLNs. These prospective trials should add to the current single-institution series that have conclusively demonstrated the feasibility of SLN mapping in uterine carcinoma. Until then, lymphatic mapping is a reasonable surgical staging strategy that seems to maximize the likelihood of identifying pelvic lymph node metastasis while having the potential to decrease the surgical morbidity associated with complete pelvic and aortic lymphadenectomy.
Acknowledgment
This study was supported in part by the MSK Cancer Center Support Grant P30 CA008748.
Author Contributions
Conception/Design: Fady Khoury-Collado, Caryn St. Clair, Nadeem R. Abu-Rustum
Collection and/or assembly of data: Fady Khoury-Collado
Data analysis and interpretation: Fady Khoury-Collado, Caryn St. Clair, Nadeem R. Abu-Rustum
Manuscript writing: Fady Khoury-Collado, Caryn St. Clair, Nadeem R. Abu-Rustum
Final approval of manuscript: Fady Khoury-Collado, Caryn St. Clair, Nadeem R. Abu-Rustum
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Burke TW, Levenback C, Tornos C, et al. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: Results of a pilot study. Gynecol Oncol. 1996;62:169–173. doi: 10.1006/gyno.1996.0211. [DOI] [PubMed] [Google Scholar]
- 3.Khoury-Collado F, Abu-Rustum NR. Lymphatic mapping in endometrial cancer: A literature review of current techniques and results. Int J Gynecol Cancer. 2008;18:1163–1168. doi: 10.1111/j.1525-1438.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT, Mutch DE, Herzog TJ. ASTEC lymphadenectomy and radiation therapy studies: Are conclusions valid? Gynecol Oncol. 2010;116:293–294. doi: 10.1016/j.ygyno.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 7.Dowdy SC. Improving oncologic outcomes for women with endometrial cancer: Realigning our sights. Gynecol Oncol. 2014;133:370–374. doi: 10.1016/j.ygyno.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frumovitz M, Bodurka DC, Broaddus RR, et al. Lymphatic mapping and sentinel node biopsy in women with high-risk endometrial cancer. Gynecol Oncol. 2007;104:100–103. doi: 10.1016/j.ygyno.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Niikura H, Okamura C, Utsunomiya H, et al. Sentinel lymph node detection in patients with endometrial cancer. Gynecol Oncol. 2004;92:669–674. doi: 10.1016/j.ygyno.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8) suppl:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Lécuru F, Bats AS, Faraggi M. Sentinel node of endometrial cancer after hysteroscopic injection. Gynecol Oncol. 2009;113:296–297; author reply 297. doi: 10.1016/j.ygyno.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Kang S, Yoo HJ, Hwang JH, et al. Sentinel lymph node biopsy in endometrial cancer: meta-analysis of 26 studies. Gynecol Oncol. 2011;123:522–527. doi: 10.1016/j.ygyno.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 14.Cormier B, Rozenholc AT, Gotlieb W, et al. Sentinel lymph node procedure in endometrial cancer: A systematic review and proposal for standardization of future research. Gynecol Oncol. 2015;138:478–485. doi: 10.1016/j.ygyno.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Rustum NR. Sentinel lymph node mapping for endometrial cancer: A modern approach to surgical staging. J Natl Compr Canc Netw. 2014;12:288–297. doi: 10.6004/jnccn.2014.0026. [DOI] [PubMed] [Google Scholar]
- 16.Khoury-Collado F, Glaser GE, Zivanovic O, et al. Improving sentinel lymph node detection rates in endometrial cancer: How many cases are needed? Gynecol Oncol. 2009;115:453–455. doi: 10.1016/j.ygyno.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Rustum NR, Khoury-Collado F, Pandit-Taskar N, et al. Sentinel lymph node mapping for grade 1 endometrial cancer: Is it the answer to the surgical staging dilemma? Gynecol Oncol. 2009;113:163–169. doi: 10.1016/j.ygyno.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanner EJ, Sinno AK, Stone RL, et al. Factors associated with successful bilateral sentinel lymph node mapping in endometrial cancer. Gynecol Oncol. 2015;138:542–547. doi: 10.1016/j.ygyno.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Frumovitz M, Levenback CF. Is lymphatic mapping in uterine cancer feasible? Ann Surg Oncol. 2008;15:1815–1817. doi: 10.1245/s10434-008-9956-4. [DOI] [PubMed] [Google Scholar]
- 20.Khoury-Collado F, Murray MP, Hensley ML, et al. Sentinel lymph node mapping for endometrial cancer improves the detection of metastatic disease to regional lymph nodes. Gynecol Oncol. 2011;122:251–254. doi: 10.1016/j.ygyno.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–535. doi: 10.1016/j.ygyno.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Leitao MM, Jr, Khoury-Collado F, Gardner G, et al. Impact of incorporating an algorithm that utilizes sentinel lymph node mapping during minimally invasive procedures on the detection of stage IIIC endometrial cancer. Gynecol Oncol. 2013;129:38–41. doi: 10.1016/j.ygyno.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Sinno AK, Fader AN, Roche KL, et al. A comparison of colorimetric versus fluorometric sentinel lymph node mapping during robotic surgery for endometrial cancer. Gynecol Oncol. 2014;134:281–286. doi: 10.1016/j.ygyno.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Koh WJ, Greer BE, Abu-Rustum NR, et al. Uterine neoplasms. J Natl Compr Canc Netw. 2014;12:248–280. doi: 10.6004/jnccn.2014.0025. [DOI] [PubMed] [Google Scholar]
- 25.Mariani A, El-Nashar SA, Dowdy SC. Lymphadenectomy in endometrial cancer: Which is the right question? Int J Gynecol Cancer. 2010;20(suppl 2):S52–S54. doi: 10.1111/IGC.0b013e3181f60d0f. [DOI] [PubMed] [Google Scholar]
- 26.Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlin JN, Zhou Q, St Clair CM, et al. Classification and regression tree (CART) analysis of endometrial carcinoma: Seeing the forest for the trees. Gynecol Oncol. 2013;130:452–456. doi: 10.1016/j.ygyno.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu-Rustum NR, Gomez JD, Alektiar KM, et al. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol Oncol. 2009;115:236–238. doi: 10.1016/j.ygyno.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Milam MR, Java J, Walker JL, et al. Nodal metastasis risk in endometrioid endometrial cancer. Obstet Gynecol. 2012;119:286–292. doi: 10.1097/AOG.0b013e318240de51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Podratz KC, Bakkum-Gamez JN, et al. Prospective assessment of the prevalence of pelvic, paraaortic and high paraaortic lymph node metastasis in endometrial cancer. Gynecol Oncol. 2014;132:38–43. doi: 10.1016/j.ygyno.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soliman PT, Frumovitz M, Spannuth W, et al. Lymphadenectomy during endometrial cancer staging: Practice patterns among gynecologic oncologists. Gynecol Oncol. 2010;119:291–294. doi: 10.1016/j.ygyno.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim CH, Khoury-Collado F, Barber EL, et al. Sentinel lymph node mapping with pathologic ultrastaging: A valuable tool for assessing nodal metastasis in low-grade endometrial cancer with superficial myoinvasion. Gynecol Oncol. 2013;131:714–719. doi: 10.1016/j.ygyno.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St. Clair CM, Eriksson AG, Ducie JA et al. Low-volume lymph node metastasis discovered during sentinel node mapping for endometrial carcinoma. Society of Gynecologic Oncology Plenary Session, Chicago 2015. 10.1245/s10434-015-5040-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melamed A, Rauh-Hain JA, Clemmer JT, et al. Changing trends in lymphadenectomy for endometrioid adenocarcinoma of the endometrium. Obstet Gynecol. 2015;126:815–822. doi: 10.1097/AOG.0000000000001063. [DOI] [PubMed] [Google Scholar]