This study found that after implementation of a cancer care equity program (CCEP), cancer clinical trial enrollment increased. A greater proportion of CCEP participants were younger, female, and in phase I trials, lived farther away from the trial site, and had lower incomes and metastatic disease. CCEP participants were more likely than those not enrolled to report concerns regarding finances, medical costs, travel, lodging, and insurance coverage related to trial participation.
Keywords: Cost of illness, Health care costs, Clinical trial, Financial support, Quality of life
Abstract
Introduction.
Cancer clinical trial (CT) participation rates are low and financial barriers likely play a role. We implemented a cancer care equity program (CCEP) to address financial burden associated with trial participation. We sought to examine the impact of the CCEP on CT enrollment and to assess barriers to participation.
Methods.
We used an interrupted time series design to determine trends in CT enrollment before and after CCEP implementation. Linear regression models compared trial enrollment before and after the CCEP. We also compared patient characteristics before and after the CCEP and between CCEP and non-CCEP participants. We surveyed CCEP and non-CCEP participants to compare pre-enrollment financial barriers.
Results.
After accounting for increased trial availability and the trends in accrual for prior years, we found that enrollment increased after CCEP implementation (18.97 participants per month greater than expected; p < .001). A greater proportion of CCEP participants were younger, female, in phase I trials, lived farther away, had lower incomes, and had metastatic disease. Of 87 participants who completed the financial barriers survey, 49 CCEP and 38 matched, non-CCEP participants responded (63% response rate). CCEP participants were more likely to report concerns regarding finances (56% vs. 11%), medical costs (47% vs. 14%), travel (69% vs. 11%), lodging (60% vs. 9%), and insurance coverage (43% vs. 14%) related to trial participation (all p < .01).
Conclusion.
CT participation increased following implementation of the CCEP and the program enrolled patients experiencing greater financial burden. These findings highlight the need to address the financial burden associated with CT participation.
Implications for Practice:
Financial barriers likely discourage patients from participating in clinical trials. Implementation of a cancer care equity program (CCEP) seeking to reduce financial barriers by assisting with travel and lodging costs was associated with increased trial accrual. The CCEP provided assistance to patients particularly in need, including those living farther away, those with lower incomes, and those reporting financial barriers related to trial participation. These findings suggest that financial concerns represent a major barrier to patient participation in clinical trials and underscore the importance of efforts to address these concerns.
Introduction
Cancer clinical trials help advance the standard of care and often represent the best available treatment option for patients with cancer, yet only a small fraction of eligible patients participate [1–4]. The reasons for these low trial participation rates are myriad and include financial and logistical barriers [3, 5–8]. In particular, groups with historically lower financial resources, such as uninsured and minority patients, are frequently underrepresented in cancer clinical trials [1, 9–11]. Conversely, study participants of higher socioeconomic status (SES) are over-represented in cancer clinical trials [12–15]. If the distribution of trial participants does not include a representative cross-section of the target population, the results are of uncertain relevance to patients not included in the study [16]. Additionally, clinical trials offer the possibility of early access to novel treatments; therefore, lack of access because of financial constraints represents a disparity in care.
Although there is considerable evidence that routine cancer care places a significant financial strain on patients and their families [17–21], little has been done to address the added financial burden of clinical trial participation. Patients with cancer experience greater financial burden, higher out-of-pocket expenses, and are at increased risk for bankruptcy compared with patients without cancer [17, 21]. Additionally, studies have demonstrated that patients experiencing financial burden may jeopardize their medical care by forgoing recommended treatments [22–24]. Thus, the financial burden experienced by patients with cancer has been called a toxic side effect of their care [19]. Because of the added expenses related to trial participation, research subjects are especially vulnerable to this financial toxicity. Not only do cancer clinical trial participants encounter the same financial issues that all cancer patients face but they endure the additional costs of more frequent clinical visits and travel to trial sites [5, 19, 25, 26]. However, few efforts have focused on the direct costs to the research participant; instead, most of the literature discussing the costs of clinical trials considers the costs borne by the study sponsor and payers [27–29]. Therefore, efforts to improve cancer clinical trial enrollment need to focus on ways to effectively recognize and address the financial barriers that likely play a critical role in the poor accrual.
We sought to examine the impact of a cancer care equity program (CCEP) that provides financial assistance for trial-related expenses, such as travel and lodging, on clinical trial participation and to assess patient-reported barriers to trial participation. Our findings will help define the impact of such programs and highlight the need to address the financial burden of cancer clinical trial participants.
Methods
CCEP Intervention
A single academic institution (Massachusetts General Hospital [MGH]) partnered with the Lazarex Cancer Foundation, a 501 (c)(3) nonprofit organization, in 2013 to form the CCEP [30]. The goal of the CCEP is to ensure that all patients with cancer have access to the best care possible, regardless of SES. The CCEP consists of three key components: (a) community outreach and education to build awareness of available cancer care options, especially clinical trials; (b) patient navigation for cancer screening and diagnosis; and (c) a financial assistance program for clinical trial participants. The current study focuses on the impact of the financial assistance program, which provides financial reimbursement for clinical trial participants struggling with the added costs of travel and lodging.
Patient Selection
From December 1, 2013 to November 30, 2014, patients in the process of being screened for a clinical trial or already participating in a clinical trial, age 18 years and older, were referred to the CCEP by a member of their cancer team, such as their primary oncologist, disease-center new-patient access nurse, research nurse, or social worker. Referral was based on patients expressing interest in a trial, but concern regarding the costs of trial participation. After referral to the CCEP, patients filled out an application to request financial assistance. Eligibility for assistance was determined on a case-by-case basis by the Lazarex Cancer Foundation, which took into consideration basic information about family income, expenses, debt, and the anticipated expenses related to travel and lodging for trial visits. For patients with incomes ≤400% of the federal poverty level (FPL), the foundation reimbursed 100% of their travel and lodging expenses. For incomes between 401% and 550% of the FPL, 75% was reimbursed; for incomes between 551% and 700% of the FPL, 50% was reimbursed; and for incomes >700% of the FPL, reimbursement was considered if there were extenuating circumstances (e.g., excessive debt, loss of income). Once approved, patients were required to provide proof of their trial-related travel and lodging out-of-pocket expenses (e.g., receipts for gasoline, tolls, parking, flights, and hotels) and were reimbursed monthly.
Study Design
To determine the impact of the CCEP on cancer clinical trial enrollment, we used an interrupted time-series design to evaluate rates of participation in cancer clinical trials before and after implementation of the CCEP. We collected data regarding the total number of clinical trial enrollees at MGH from October 2005 through September 2013 (before implementation of the CCEP) and from December 2013 through November 2014 (after implementation of the CCEP).
To compare demographics and clinical characteristics of trial enrollees before and after implementation of the CCEP, and between CCEP and non-CCEP participants, we reviewed patients’ electronic medical records. We performed retrospective chart reviews on all patients enrolled in cancer clinical trials at MGH in the 2 years before the implementation of the CCEP (October 1, 2011, to September 30, 2013). We prospectively collected data about clinical trial enrollees following implementation of the CCEP (December 1, 2013, to November 30, 2014). To derive patients’ incomes, we used zip code information combined with 2013 census data [31]. The Dana-Farber/Harvard Cancer Center Institutional Review Board approved the study protocol.
Survey Data
To assess patient-reported barriers to trial participation, we mailed surveys to all living, English-speaking CCEP participants and a systematically matched group of cancer clinical trial participants who were not enrolled in the CCEP. We matched participants by age, sex, the specific clinical trial patients were enrolled in, and the year they enrolled in that trial. We mailed the surveys to CCEP participants within 3 months of their enrollment in the CCEP. After we received a CCEP participant’s completed survey, we sent a survey to a matched, non-CCEP trial participant. We asked participants to report their financial concerns in the prior 3 months and barriers to clinical trial participation they experienced when considering trial enrollment. The survey contained items from previously validated questions about barriers to clinical trial participation [32–37].
Statistical Analysis
To assess the impact of the CCEP on trial enrollment, we fit a linear regression model to the monthly enrollment data from December 2005 to November 2014. This allowed us to determine the average enrollment size increase per month over time and to estimate and test the significance of increased enrollment after implementation of the CCEP. These models also accounted for the trends in annual trial availability. To test for the presence of significant autocorrelation in our modeling, we used the Durbin-Watson statistic.
To determine if differences in clinical trial enrollment before and after CCEP could be related to changes in demographics or clinical characteristics, we compared differences in these characteristics between participants in the 2 years before the CCEP and participants the year following implementation of the CCEP. For continuous variables, we used independent-samples t tests or nonparametric Mann-Whitney U test, as appropriate. We compared categorical variables using chi-square or Fisher's exact test, as appropriate. Similarly, we compared the demographic and clinical characteristics of the CCEP and the non-CCEP clinical trial participants for the 1-year period following implementation of the CCEP. We also calculated differences between these groups with multivariable logistic regression, accounting for the cancer type, enrollment in phase I trials, and presence of metastatic disease.
We used descriptive statistics to summarize responses to each of the survey items. For survey items with Likert scales asking participants to specify their level of agreement or disagreement, we used “strongly agree” and “agree” responses to represent agreement. For Likert scales asking participants to respond on a scale from “never” to “always,” we compared the “always” responses to all other responses. To assess differences between responses for CCEP and non-CCEP participants, we used Fisher’s exact test.
Results
Clinical Trial Enrollment Before and After Implementation of the CCEP
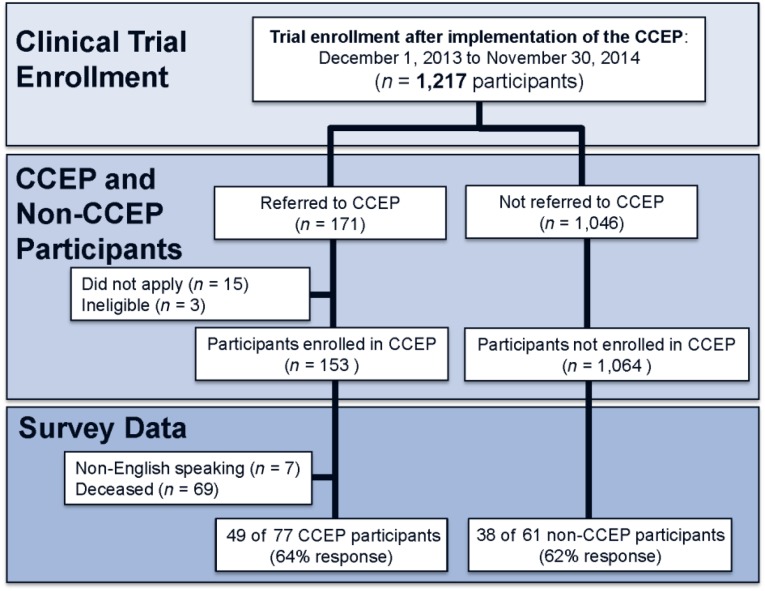
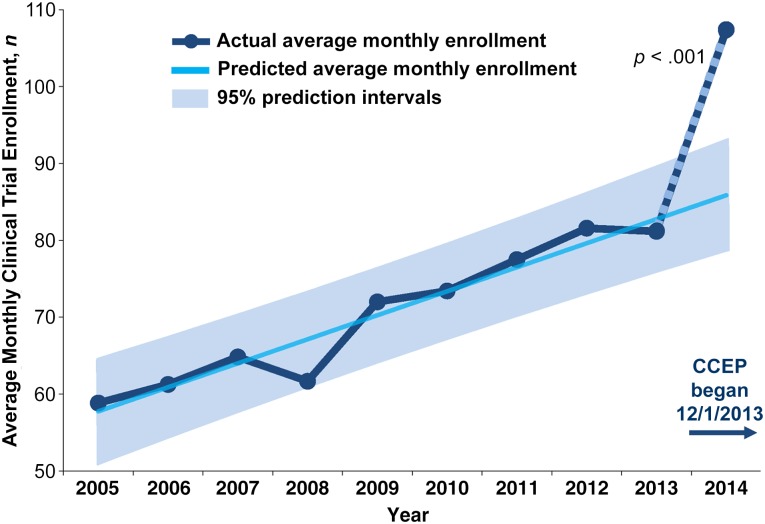
A total of 1,217 patients, age 18 years and older, enrolled in cancer clinical trials at MGH after implementation of the CCEP (from December 1, 2013, to November 30, 2014) (Fig. 1). Accounting for the increased number of clinical trials available to patients over time and the trend of increased trial enrollment prior to implementation of the CCEP, trial participation following implementation of the CCEP was greater than expected (β = 18.97 participants per month greater than expected; SE 4.31; 95% confidence interval: 10.43–25.51; p < .001) (Fig. 2). We found no substantial positive or negative autocorrelation in our modeling according to the Durbin-Watson statistic (1.70).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Abbreviation: CCEP, cancer care equity program.
Figure 2.
Average monthly clinical trial enrollment by year.
Abbreviation: CCEP, cancer care equity program.
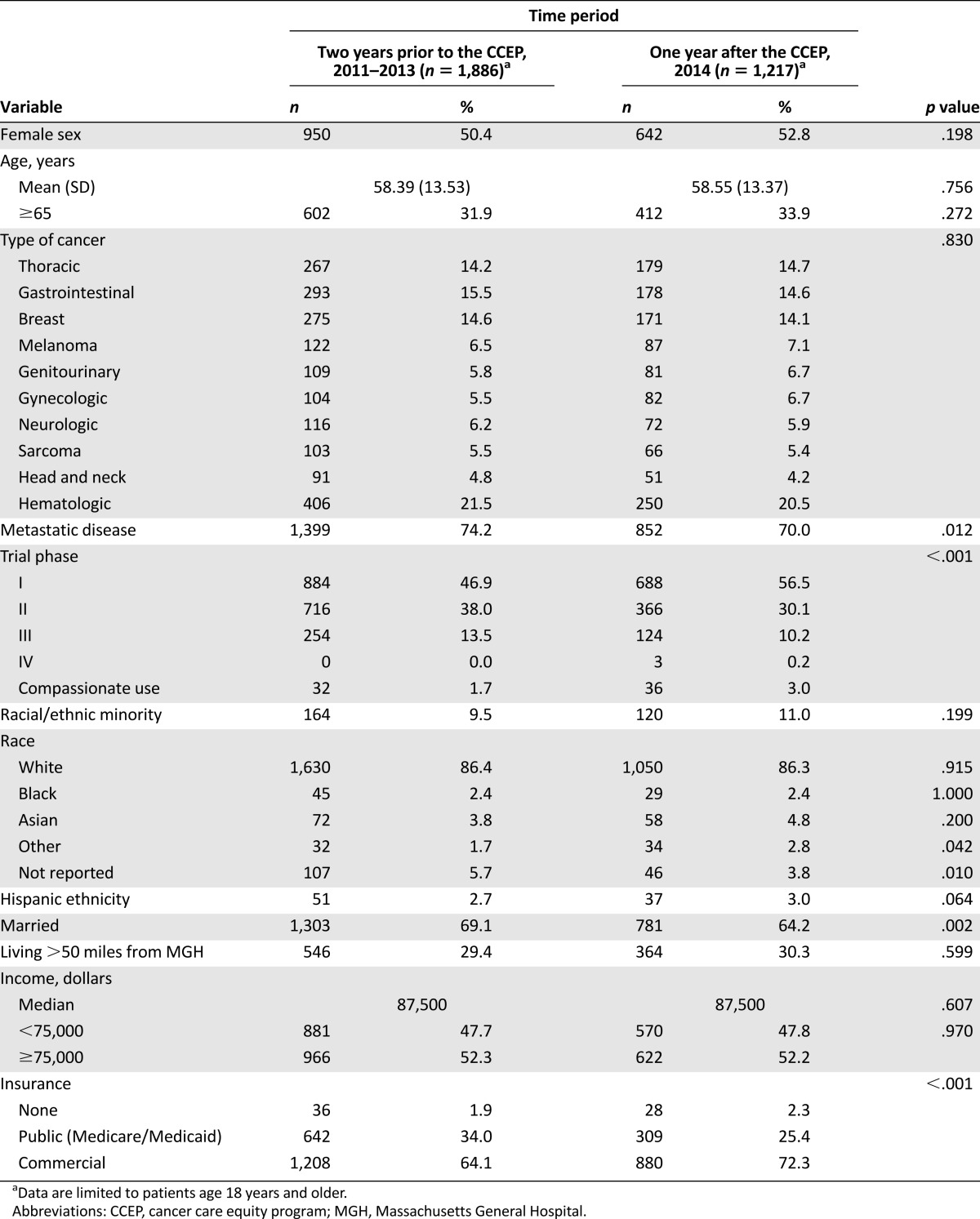
Table 1 summarizes participant demographic and clinical characteristics for participants in the 2 years prior to the CCEP and for the year after implementation of the CCEP. Following implementation of the CCEP, the proportion of commercially insured (72.3% vs. 64.1%; p < .001) and phase I clinical trial participants (56.5% vs. 46.9%; p < .001) increased. Conversely, the proportion of married participants (64.2% vs. 69.1%; p = .002) and those with metastatic disease (70.0% vs. 74.2%; p = .012) decreased. Using logistic regression and controlling for patient demographics, the differences in insurance, phase I trial participation, marital status, and presence of metastatic disease remained significantly different between the year after the CCEP and the prior 2 years.
Table 1.
Clinical trial participant demographics and clinical characteristics
Clinical Trial Enrollees (non-CCEP vs. CCEP) for the Period Following the CCEP
For the period following implementation of the CCEP, 171 patients were referred to the CCEP; 15 declined applying for financial assistance, stating that they felt others would benefit more from the program, and 3 patients were deemed ineligible for financial assistance based on the Lazarex Cancer Foundation financial screen.
We enrolled 153 participants in the CCEP. More than one-third enrolled while still being screened for a clinical trial and before starting their clinical trial treatments, and 90% enrolled either before or within 1 month of starting their trial. On average, the program reimbursed participants from Massachusetts approximately $185 per month, regional participants (New England, excluding Massachusetts) were reimbursed $300 per month, and out-of-region participants, $900 per month.
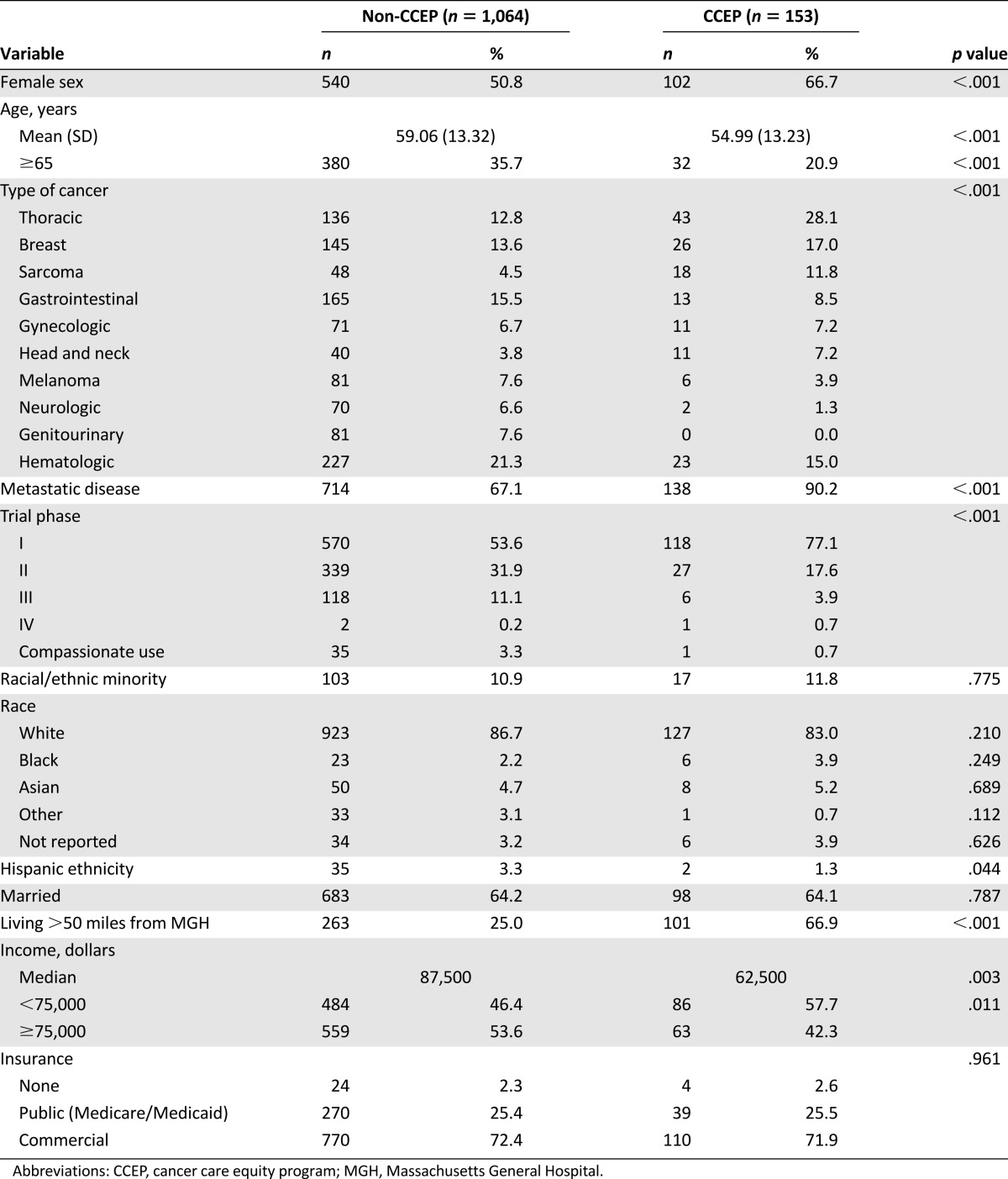
Compared with non-CCEP trial participants (n = 1,064), those enrolled in the CCEP were younger and more likely to be female, reside farther (>50 miles) from MGH, be enrolled in phase I clinical trials, and have metastatic disease and lower incomes (Table 2). Additionally, the types of cancer represented in the CCEP and non-CCEP groups differed. A higher proportion of CCEP enrollees had breast cancer, thoracic cancer, and sarcoma. Using multivariable logistic models, we found that the differences in age, sex, distance from MGH, phase I trial enrollment, and presence of metastatic disease remained significant.
Table 2.
Clinical trial enrollees (non-CCEP vs. CCEP) for period following implementation of the CCEP
Survey Data
Of 87 participants who completed the financial barriers survey (63% overall response rate), we received responses from 49 of 77 CCEP enrollees (64% response rate) and 38 of 61 non-CCEP trial participants (62% response rate). We found no significant differences in demographic or clinical characteristics between surveyed and nonsurveyed trial participants for both groups.
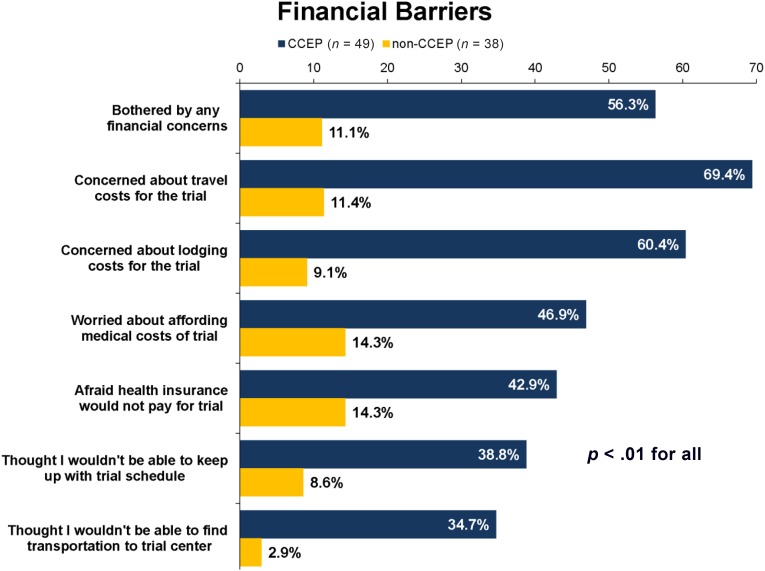
Compared with non-CCEP trial participants, those enrolled in the CCEP were more likely to report any financial concerns (56% vs. 11%; p < .01) (Fig. 3). Additionally, CCEP participants were more likely to report concerns with medical costs (47% vs. 14%; p < .01), travel costs (69% vs. 11%; p < .01), finding transportation (35% vs. 3%; p < .01), lodging (60% vs. 9%; p < .01), treatment schedule (39% vs. 9%; p < .01), and insurance coverage (43% vs. 14%; p < .01) related to clinical trial participation.
Figure 3.
Financial barriers survey.
Abbreviation: CCEP, cancer care equity program.
Discussion
To our knowledge, this is the first study suggesting a positive impact of a financial assistance intervention on cancer clinical trial enrollment. After implementation of the CCEP, cancer clinical trial enrollment increased compared with the previous years. Although our study does not definitively prove that the CCEP directly led to this increase, the enrollment trends and financial burden expressed by CCEP enrollees suggest a positive association between increased clinical trial enrollment and the implementation of the CCEP. For example, participants who were able to enroll in trials with assistance of the CCEP reported experiencing increased financial barriers before trial enrollment, lived farther from MGH, and had lower incomes than non-CCEP trial participants. Additionally, most patients enrolled in the CCEP within 1 month of starting their clinical trial, while more than one-third actually enrolled while being screened for their trial. Thus, the CCEP targeted a patient population particularly in need of financial assistance and provided a means to address the financial barriers that may prevent clinical trial participation.
Previous efforts to improve clinical trial enrollment have included interventions involving informational booklets and videos aimed at educating patients about the benefits and risks of clinical trials [38–40]. Others have tried to increase trial enrollment by improving the informed consent process [41]. While these efforts improve patients’ understanding of clinical trials, they have been largely unsuccessful at increasing clinical trial participation [39–41]. Studies involving minority-based community clinical oncology programs have shown promise as a way to facilitate clinical trial participation among racial minorities and other underserved populations [42]. The African American Men randomized trial (the AAMEN Project) demonstrated that more intensive recruitment methods resulted in higher recruitment rates [43]. Specifically, the arm that provided transportation for participants proved most successful. Despite the promising results with these programs, investigators have highlighted the challenges involved, such as provider communication and trust issues among patients, as well as the lack of adequate funding and infrastructure within institutions to sustain these programs [44].
Our findings support prior studies suggesting that financial concerns represent a major barrier to patient participation in cancer clinical trials [5, 36, 45]. In a study of 1,256 cancer patients assessing barriers to cancer clinical trial participation, worries about health insurance coverage of clinical care costs represented one of the strongest barriers [36]. A second study, which sought to evaluate why patients decline cancer clinical participation, demonstrated that distance from the cancer center and insurance denial were common reasons for refusal to participate [5]. A third study of black patients found that economic stress played an important role in their willingness to participate in trials [45]. Therefore, efforts to improve clinical trial participation must include interventions targeting the financial barriers that often influence patients’ decisions to participate in trials.
Additionally, whether a patient enrolls in a cancer clinical trial may depend on the knowledge and perceptions of their treating clinician [5, 11, 46, 47]. An oncologist may choose not to offer a clinical trial to their patient because of concerns that the patient may struggle with the financial or logistical demands of the trial [5, 11, 46, 47]. Thus, efforts to increase cancer clinical trial accrual will need to target both oncologists as well as patients. Our CCEP intervention represents one such strategy, as this program sought to remove some of the financial and logistical barriers to trial participation. Consequently, the CCEP may have encouraged oncologists to offer trial participation to patients they otherwise may not have approached.
In addition to an overall increase in cancer clinical trial participation following implementation of the CCEP, the enrollment patterns and survey data suggest that we enrolled a population particularly in need of financial assistance. Compared with non-CCEP participants, those enrolled in the CCEP were younger and had lower incomes. Studies have shown that, in general, younger patients and those with lower incomes experience considerable cancer-related financial problems [24, 48–51]. We also found that a higher proportion of CCEP participants were enrolled in phase I clinical trials and had metastatic disease. Both of these groups are at greater risk for financial burden, considering that patients with metastatic disease often receive multiple lines of treatment [52], and phase I trials are often recommended only after patients have received and experienced disease progression on previous lines of therapy. Early phase studies represent an increasingly larger proportion of clinical trials in recent years, yet they are complex and often require frequent clinic visits and additional tests [53]. Importantly, the CCEP reimburses trial participants for travel and lodging expenses, and CCEP patients were more likely to live farther from MGH.
Finally, our survey data also suggest that the CCEP served a population in need of assistance. Compared with those not enrolled in the program, patients enrolled in the CCEP reported more financial concerns and barriers to clinical trial participation when asked to think back to when they were considering trial enrollment. Notably, the amount reimbursed to CCEP participants in our study represents only a fraction of the amount sponsors and institutions spend to conduct these trials [27, 54].
Several limitations of our study warrant discussion. First, the interrupted time-series design evaluated monthly trial enrollment during the time leading up to the CCEP and following its implementation. Our conclusions that CCEP increased trial participation is based on regression models that accounted for trial availability and prior trends for increasing enrollment. Thus, we cannot definitively conclude that our intervention was responsible for the increase in clinical trial enrollment. Changes in the patient population enrolling in trials (e.g., younger patients, those with metastatic disease, those seeking phase I studies) may have contributed to, rather than resulted from, the increase associated with CCEP. Other factors, including increased awareness about the importance of clinical trials, the emergence of novel drug targets, and improved infrastructure for pursuing clinical trials in our cancer center, likely also contributed to the increase.
Currently, we cannot explain the exact mechanism by which the CCEP might have produced an increase in clinical trial enrollment. We did not survey members of the cancer care team to determine whether the CCEP may have influenced their decision to recommend clinical trials to certain patients, but this will be the focus of future investigation. Additionally, although our survey data highlight that CCEP participants experienced considerable financial concerns and barriers to trial enrollment, we have not yet determined whether the program reduced their financial distress. Finally, CCEP participants were those referred by their care team at a single academic institution with a distinct patient population. Therefore, these results may not apply to a more general cancer clinical trial population. Despite these limitations, our study suggests a positive impact of a novel intervention that proved feasible, targeted a vulnerable population, and likely helped improve cancer clinical trial participation.
Conclusion
Our use of a financial assistance program to improve cancer clinical trial participation is, to our knowledge, first-in-kind, and holds promise for finding novel and feasible ways to increase trial accrual. Clinical trials represent an increasingly important option for patients with cancer, and, thus, future efforts to increase clinical trial participation need to address the financial barriers. Future strategies should include efforts to refine our ability to assess trial participants’ financial burden while also seeking to identify the specific patient populations most in need of financial assistance interventions. Our current efforts include longitudinal assessments of trial participants’ financial distress and other patient-reported outcomes to help us better understand the trajectory of this distress and to further determine the impacts of the CCEP. Collectively, our work will provide important information for key stakeholders, such as study sponsors and payers, to help inform widespread policy change focused on alleviating the financial burden related to cancer clinical trial participation.
Acknowledgments
This study was supported by the Lazarex-MGH Cancer Care Equity Program.
This study was presented in part as an oral abstract at the 2015 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL.
Author Contributions
Conception/Design: Ryan D. Nipp, Hang Lee, Elizabeth Powell, Nicole E. Birrer, Emily Poles, Daniel Finkelstein, Karen Winkfield, Sanja Percac-Lima, Bruce Chabner, Beverly Moy
Provision of study material or patients: Elizabeth Powell, Emily Poles, Beverly Moy
Collection and/or assembly of data: Ryan D. Nipp, Hang Lee, Elizabeth Powell, Nicole E. Birrer, Emily Poles, Daniel Finkelstein, Karen Winkfield, Sanja Percac-Lima, Bruce Chabner, Beverly Moy
Data analysis and interpretation: Ryan D. Nipp, Hang Lee, Elizabeth Powell, Nicole E. Birrer, Emily Poles, Daniel Finkelstein, Karen Winkfield, Sanja Percac-Lima, Bruce Chabner, Beverly Moy
Manuscript writing: Ryan D. Nipp, Hang Lee, Elizabeth Powell, Nicole E. Birrer, Emily Poles, Daniel Finkelstein, Karen Winkfield, Sanja Percac-Lima, Bruce Chabner, Beverly Moy
Final approval of manuscript: Ryan D. Nipp, Hang Lee, Elizabeth Powell, Nicole E. Birrer, Emily Poles, Daniel Finkelstein, Karen Winkfield, Sanja Percac-Lima, Bruce Chabner, Beverly Moy
Disclosures
Karen Winkfield: Novartis (C/A); Bruce Chabner: Epizyme, PharmaMar, EMD Serono, Synta, Midatech (C/A), Agios, Gilead, PharmaMar, Regeneron, TG Therapeutics, Biomarin, Epizyme (OI), Eli Lilly (ET). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 2.Scoggins JF, Ramsey SD. A national cancer clinical trials system for the 21st century: Reinvigorating the NCI Cooperative Group Program. J Natl Cancer Inst. 2010;102:1371. doi: 10.1093/jnci/djq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nass SJ, Moses HL, Mendelsohn J, editors. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: National Academics Press; 2010. [PubMed] [Google Scholar]
- 4.Fouad MN, Lee JY, Catalano PJ, et al. Enrollment of patients with lung and colorectal cancers onto clinical trials. J Oncol Pract. 2013;9:e40–e47. doi: 10.1200/JOP.2012.000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 6.Somkin CP, Altschuler A, Ackerson L, et al. Organizational barriers to physician participation in cancer clinical trials. Am J Manag Care. 2005;11:413–421. [PubMed] [Google Scholar]
- 7.Fu S, McQuinn L, Naing A, et al. Barriers to study enrollment in patients with advanced cancer referred to a phase I clinical trials unit. The Oncologist. 2013;18:1315–1320. doi: 10.1634/theoncologist.2013-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young RC. Cancer clinical trials--a chronic but curable crisis. N Engl J Med. 2010;363:306–309. doi: 10.1056/NEJMp1005843. [DOI] [PubMed] [Google Scholar]
- 9.Umutyan A, Chiechi C, Beckett LA, et al. Overcoming barriers to cancer clinical trial accrual: Impact of a mass media campaign. Cancer. 2008;112:212–219. doi: 10.1002/cncr.23170. [DOI] [PubMed] [Google Scholar]
- 10.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 11.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 12.Unger JM, Gralow JR, Albain KS, et al. Patient income level and cancer clinical trial participation: A prospective survey study. 2016;2:137–139. doi: 10.1001/jamaoncol.2015.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 14.Baquet CR, Ellison GL, Mishra SI. Analysis of Maryland cancer patient participation in National Cancer Institute-supported cancer treatment clinical trials. J Clin Oncol. 2008;26:3380–3386. doi: 10.1200/JCO.2007.14.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 16.Gross CP, Steiner CA, Bass EB, et al. Relation between prepublication release of clinical trial results and the practice of carotid endarterectomy. JAMA. 2000;284:2886–2893. doi: 10.1001/jama.284.22.2886. [DOI] [PubMed] [Google Scholar]
- 17.Bernard DS, Farr SL, Fang Z. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. J Clin Oncol. 2011;29:2821–2826. doi: 10.1200/JCO.2010.33.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covinsky KE, Goldman L, Cook EF, et al. The impact of serious illness on patients’ families. JAMA. 1994;272:1839–1844. doi: 10.1001/jama.272.23.1839. [DOI] [PubMed] [Google Scholar]
- 19.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. The Oncologist. 2013;18:381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stump TK, Eghan N, Egleston BL, et al. Cost concerns of patients with cancer. J Oncol Pract. 2013;9:251–257. doi: 10.1200/JOP.2013.000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534–2542. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nipp R, Zullig L, Peppercorn J, et al. Coping with cancer treatment-related financial burden. J Clin Oncol. 2014;32(suppl 31):161a. [Google Scholar]
- 25.Goldman DP, Berry SH, McCabe MS, et al. Incremental treatment costs in national cancer institute-sponsored clinical trials. JAMA. 2003;289:2970–2977. doi: 10.1001/jama.289.22.2970. [DOI] [PubMed] [Google Scholar]
- 26.Zafar SY, Abernethy AP. Financial toxicity, part I: A new name for a growing problem. Oncology (Williston Park) 2013;27:80–81, 149. [PMC free article] [PubMed] [Google Scholar]
- 27.Emanuel EJ, Schnipper LE, Kamin DY, et al. The costs of conducting clinical research. J Clin Oncol. 2003;21:4145–4150. doi: 10.1200/JCO.2003.08.156. [DOI] [PubMed] [Google Scholar]
- 28.Goldman DP, Schoenbaum ML, Potosky AL, et al. Measuring the incremental cost of clinical cancer research. J Clin Oncol. 2001;19:105–110. doi: 10.1200/JCO.2001.19.1.105. [DOI] [PubMed] [Google Scholar]
- 29.Martin PJ, Davenport-Ennis N, Petrelli NJ, et al. Responsibility for costs associated with clinical trials. J Clin Oncol. 2014;32:3357–3359. doi: 10.1200/JCO.2014.57.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nipp RD, Powell E, Chabner B, et al. Recognizing the financial burden of cancer patients in clinical trials. The Oncologist. 2015;20:572–575. doi: 10.1634/theoncologist.2015-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Department of Commerce. United States Census Bureau. http://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml. Accessed November 20, 2015.
- 32.CAHPS Clinician and Group Surveys. Supplemental Items for the Adult Surveys. https://cahps.ahrq.gov/surveys-guidance/item-sets/2357a_Adult_Supp_Eng_2.pdf. Accessed September 20, 2015.
- 33.Miller SM, Hudson SV, Egleston BL, et al. The relationships among knowledge, self-efficacy, preparedness, decisional conflict, and decisions to participate in a cancer clinical trial. Psychooncology. 2013;22:481–489. doi: 10.1002/pon.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meropol NJ, Albrecht TL, Wong YN, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2013;31:6500a. doi: 10.1200/JCO.2015.63.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manne S, Kashy D, Albrecht T, et al. Knowledge, attitudes, and self-efficacy as predictors of preparedness for oncology clinical trials: A mediational model. Med Decis Making. 2014;34:454–463. doi: 10.1177/0272989X13511704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: Factor analysis and correlates of barriers. Eur J Cancer Care (Engl) 2015;24:28–38. doi: 10.1111/ecc.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleisher L, Ruggieri DG, Miller SM, et al. Application of best practice approaches for designing decision support tools: The preparatory education about clinical trials (PRE-ACT) study. Patient Educ Couns. 2014;96:63–71. doi: 10.1016/j.pec.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis PM, Butow PN, Tattersall MH. Informing breast cancer patients about clinical trials: A randomized clinical trial of an educational booklet. Ann Oncol. 2002;13:1414–1423. doi: 10.1093/annonc/mdf255. [DOI] [PubMed] [Google Scholar]
- 39.Du W, Mood D, Gadgeel S, et al. An educational video to increase clinical trials enrollment among lung cancer patients. J Thorac Oncol. 2008;3:23–29. doi: 10.1097/JTO.0b013e31815e8bb2. [DOI] [PubMed] [Google Scholar]
- 40.Du W, Mood D, Gadgeel S, et al. An educational video to increase clinical trials enrollment among breast cancer patients. Breast Cancer Res Treat. 2009;117:339–347. doi: 10.1007/s10549-009-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aaronson NK, Visser-Pol E, Leenhouts GH, et al. Telephone-based nursing intervention improves the effectiveness of the informed consent process in cancer clinical trials. J Clin Oncol. 1996;14:984–996. doi: 10.1200/JCO.1996.14.3.984. [DOI] [PubMed] [Google Scholar]
- 42.McCaskill-Stevens W, McKinney MM, Whitman CG, et al. Increasing minority participation in cancer clinical trials: The minority-based community clinical oncology program experience. J Clin Oncol. 2005;23:5247–5254. doi: 10.1200/JCO.2005.22.236. [DOI] [PubMed] [Google Scholar]
- 43.Ford ME, Havstad SL, Davis SD. A randomized trial of recruitment methods for older African American men in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Clin Trials. 2004;1:343–351. doi: 10.1191/1740774504cn029oa. [DOI] [PubMed] [Google Scholar]
- 44.Wieder R, Teal R, Saunders T, et al. Establishing a minority-based community clinical oncology program: The University of Medicine and Dentistry of New Jersey, New Jersey Medical School-University Hospital Cancer Center experience. J Oncol Pract. 2013;9:e48–e54. doi: 10.1200/JOP.2012.000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Advani AS, Atkeson B, Brown CL, et al. Barriers to the participation of African-American patients with cancer in clinical trials: A pilot study. Cancer. 2003;97:1499–1506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 46.Virani S, Burke L, Remick SC, et al. Barriers to recruitment of rural patients in cancer clinical trials. J Oncol Pract. 2011;7:172–177. doi: 10.1200/JOP.2010.000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somkin CP, Ackerson L, Husson G, et al. Effect of medical oncologists’ attitudes on accrual to clinical trials in a community setting. J Oncol Pract. 2013;9:e275–e283. doi: 10.1200/JOP.2013.001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710–3717. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banthin JS, Bernard DM. Changes in financial burdens for health care: National estimates for the population younger than 65 years, 1996 to 2003. JAMA. 2006;296:2712–2719. doi: 10.1001/jama.296.22.2712. [DOI] [PubMed] [Google Scholar]
- 50.Shankaran V, Jolly S, Blough D, et al. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: A population-based exploratory analysis. J Clin Oncol. 2012;30:1608–1614. doi: 10.1200/JCO.2011.37.9511. [DOI] [PubMed] [Google Scholar]
- 51.Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332–338. doi: 10.1200/JOP.2013.001322. [DOI] [PubMed] [Google Scholar]
- 52.Meropol NJ, Schulman KA. Cost of cancer care: Issues and implications. J Clin Oncol. 2007;25:180–186. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]
- 53.Gerber DE, Lakoduk AM, Priddy LL, et al. Temporal trends and predictors for cancer clinical trial availability for medically underserved populations. The Oncologist. 2015;20:674–682. doi: 10.1634/theoncologist.2015-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cutting Edge Information. Clinical Trials and Operations (PH192). http://www.cuttingedgeinfo.com/research/clinical-development/trial-operations/. Accessed September 20, 2015.