The incidences of human papillomavirus (HPV)-related anal cancer and its precursor lesion, anal intraepithelial neoplasia, are rising globally. Treatment outcomes with systemic therapy in patients with advanced anal cancer are poor; therefore, prevention may be the best approach for reducing disease burden. The data regarding potential use of the HPV vaccine in anal cancer prevention and treatment are reviewed.
Keywords: Anus neoplasm; Papillomavirus vaccines; Papillomavirus infection; Anal intraepithelial neoplasia; Carcinoma, squamous cell
Abstract
The incidences of human papillomavirus (HPV)-related anal cancer and its precursor lesion, anal intraepithelial neoplasia, are rising in the U.S. and globally. Five-year survival rates with current modalities of treatment for anal cancer are generally favorable for localized and regional disease. For metastatic disease, the relative survival rate is poor. Major contributing factors for the increase in anal cancer incidence include increasing receptive anal intercourse (hetero- and homosexual), increasing HPV infections, and longer life expectancy of treated people who are seropositive for human immunodeficiency virus. Because treatment outcomes with systemic therapy in patients with advanced disease are so poor, prevention may be the best approach for reducing disease burden. The association of a major causative agent with anal cancer provides an excellent opportunity for prevention and treatment. The advent of the HPV vaccine for anal cancer prevention and treatment is a significant milestone and has the potential to greatly impact these cancers. The data regarding potential use of the HPV vaccine in anal cancer prevention and treatment are reviewed.
Implications for Practice:
The incidences of human papillomavirus (HPV)-related anal cancer and its precursor lesion, anal intraepithelial neoplasia, are on the rise in the U.S. and globally. Based on recent studies, the HPV vaccine is approved for prevention of the infection and development of HPV-related anal cancer. In addition, several small studies have shown that the vaccine may be useful as adjuvant therapy for anal cancer. There is a need for public health strategies aimed at education of both patients and practitioners to improve the use of the vaccine for prevention of HPV-related anal cancer. The development of a therapeutic vaccine is a work in progress.
Introduction
Over the last three decades, there has been evidence implicating human papillomavirus (HPV) in anal cancer and the various grades of anal intraepithelial neoplasia (AIN) (the precursor lesions to anal cancer) as opposed to the previously held view that it was due to perianal inflammation [1]. In 2007, HPV was classified as a biologic carcinogen [2] and the U.S. Centers for Disease Control and Prevention (CDC) currently reports that 91% of anal cancers in the U.S. are attributable to this virus [3]. Depending on the stage at presentation, treatment options include surgical resection, combined chemoradiation, or radiation alone. According to the National Cancer Institute Surveillance Epidemiology and End Results (SEER) program database, the 5-year survival rates for patients with localized and regional disease are 79.7% and 58.1%, respectively. For patients with distant disease, the relative survival rate is only 32.0% [4]. Male sex, tumor size (T stage), and nodal involvement (N stage) are predictors of higher relapse rates (both local and distant), higher colostomy rates, and, in general, poorer survival following chemoradiation treatment [5]. As systemic treatment outcomes for metastatic disease remain disappointing, prevention of anal cancer holds the best potential for disease burden reduction [6].
Given HPVs role in cervical, anal, and cancers of other anatomic sites, the advent of the preventive HPV vaccines is a significant public health milestone. This review discusses the role of the HPV vaccines in the prevention of precancerous and cancerous anal lesions and also discusses future therapeutic approaches to HPV-related anal lesions.
Epidemiology and Risk Factors for Anal Cancer
Anal carcinoma is a rare malignancy with global age-standardized incidences ranging from 0.2 per 100,000 among women in Osaka, Japan, to 1.4 per 100,000 among white non-Hispanic men in San Francisco, California. The female-to-male ratios vary geographically, but generally there is a slight female preponderance [7].
The incidence of anal cancer, however, has been increasing over the last three decades in several developed countries around the world, including the U.S. [8]. Although anal cancer currently makes up only 2.5% of all gastrointestinal malignancies in the U.S., there are, on average, 7,210 new cases reported annually [9]. Approximately 95% of these are diagnosed in patients 35 years old or younger [10]. According to the SEER database, rates for new cases of anal cancer have been rising an average of 2.2% per year over the past decade, with death rates resulting from the disease rising on the average by 1.7% over the same time period. Additionally, the incidence has increased 3 times in men (from 1.0 to 3.0 per 100,000 person-years) and 1.7 times in women (from 1.4 to 2.4 per 100,000 person-years) when data from 1973 through 1996 was compared with 1997–2009. In terms of racial variation, anal cancer rates were higher among white women, whereas in blacks, rates were higher in men [4].
The incidence of anal cancer is disproportionately higher in certain high-risk populations, especially men who have sex with men (MSM) and patients seropositive for HIV. An incidence of 37 cases of anal cancer per 100,000 was reported before the HIV epidemic [11], two times more than the incidence in HIV-negative MSM [12]. These rates are comparable to rates of cervical cancer before the introduction of Papanicolaou smear screening around the world [13]. Unlike other HIV/AIDS-associated malignancies, like non-Hodgkin’s lymphoma and Kaposi’s sarcoma, which have been on the decline in this era of antiretroviral therapy (ART), the incidence of anal cancer has remained stable or increased [14, 15]. Rates of 174 cases per 100,000 person-years among people positive for HIV have recently been reported in an analysis from Kaiser Permanente in California [16], with even higher rates in HIV-positive individuals with lower CD4+ counts [17]. Other high-risk groups compared with the general population include women who are positive for HIV, women with cervical or vulvar cancer [18, 19], and people in chronic immunosuppressive states not due to HIV [20]. The estimated national expenditures for HPV-associated malignancies range from $160 million to $1.6 billion; the average is $418 million per year [21].
Risk Factors
Currently known risk factors for the development of HPV-associated AIN and anal cancer include women with HPV-related vulvar and/or cervical disease [18, 19], high-risk sexual practices including receptive anal intercourse, anal HPV infection, HIV infection, cigarette smoking [22–24], and chronic immunosuppressive states such as those of organ transplant recipients on immunosuppression [20]. In this review, sexual practices, HIV infection, and anal HPV infection are discussed in detail.
Sexual Practices
Earlier studies looking at the increased incidence of anal cancer in MSM suggested a correlation between anal intercourse and anal cancer [11, 22], with subsequent studies confirming this association, which exposes the anal mucosa to HPV infection [23, 24]. The long-held perception that receptive anal intercourse is limited to homosexual practices is rapidly becoming obsolete as increasing global rates of heterosexual anal intercourse have been reported [25, 26]. In terms of absolute numbers, women in the U.S. are more likely to engage in unprotected anal intercourse than homosexual men [27]. In a population-based case-control study in Denmark and Sweden, Frisch et al. reported an increased risk of anal cancer in women with first receptive anal intercourse before the age of 30 and/or who have had 2 or more anal intercourse partners [28]. However, the majority of men and women in the study with anal cancer reported that they did not practice anal intercourse [28]. The implication is that either modes of anal transmission for HPV other than anal intercourse exist, or there needs to be a critical look at the role stigmatization plays in participant responses regarding anal intercourse. Regardless, it is evident that heterosexual anal intercourse can no longer be ignored when it comes to HPV and anal cancer.
HIV
The role of HIV in the development of HPV-associated preinvasive and invasive anal lesions, whether direct or interdependent, is not clearly understood. It is apparent that the higher incidence of AIN in people seropositive for HIV cannot be explained by sexual practices alone [29, 30]. Several studies, including the large multicohort study in the North American AIDS Cohort Collaboration on Research and Design, showed a higher incidence of preinvasive and invasive anal lesions in heterosexual men and women seropositive for HIV as well as MSM seropositive for HIV when compared with HIV seronegative individuals [31]. Persistent infection with one or more subtypes of HPV increases the chances of developing high-grade AIN (HG-AIN) in men seropositive for HIV [32], with a relatively faster rate of progression from low-grade AIN to HG-AIN over a 2-year period compared with men who were seronegative for HIV. The rate of progression was further increased in HIV seropositive men with CD4 counts of less than 200 (relative risk, 3.1) [33]. As such, the development of HPV-related neoplastic lesions is probably a function of increased viral persistence from repeated exposure and/or immunosuppression.
Anal HPV Infection
According to a global systematic literature review conducted by Hoots et al., the prevalence of invasive anal carcinoma was highest in Europe (80%), followed by North America (77%), and lowest in Asia (57%) [34]. It also estimated the prevalence of HPV in all causes of invasive anal carcinoma was 71% and squamous cell carcinoma of the anus was 78%. Among those positive for HPV, 85% were positive for HPV-16, 7% were positive for HPV-18, and the remainder was accounted for by other HPV subtypes such as HPV-33, -31, and -45 [34]. The CDC reports that the oncogenic variants HPV-16 and -18 (also high-risk types) are associated with 91% of anal squamous cell carcinomas [3].
HPV Virology and Immunology
HPV is a capsid-enclosed, double-stranded DNA virus with a genome that encodes for eight genes identified as E or L (early or late) depending on the timing of their expression during epithelial differentiation. Early proteins include E1–E7; L1 and L2 are the late proteins [35] and are present on the capsid shell. E6 and E7 are the main HPV oncoproteins [36]. E6 prevents apoptosis by binding to host p53, and E7 causes cell cycle arrest by binding to host retinoblastoma protein [35]. Independently, both E6 and E7 can prevent or defer senescence, but when they are expressed at the same time, they can lead to cell immortalization [37]. E6 and E7 oncoproteins are imperative, but insufficient, for malignant transformation on their own. In most cases, after HPV transmission, infection is cleared in 1–2 years. In a few cases, there is persistent infection, premalignant changes, and then malignancy, all of which may take place over as few as 1–5 years [35].
A cell-mediated immune (CMI) response usually follows HPV infection with resultant lesion regression and future protection against infection with the same type of HPV [38]. The type of T cells involved in lesion regression is currently unknown [39], as are the mechanisms behind persistent infection or reinfection with the same HPV type. It is currently considered that CMI is of limited value in protection from HPV [40].
Despite being inconsistently generated following natural HPV infection, humoral immunity depends on the recognition of distinct conformational epitopes and, even with activation, antibody peak levels are relatively low. The possible factors involved in this phenomenon include the intraepithelial replication of HPV, absence of viremia needed to invoke high-level antibody production and local antigen-presenting cells, as well as paucity of macrophages to generate a more rugged response [38]. Demonstration in animal models that minimal levels of neutralizing antibody against HPV are protective for long periods is the driving focus of the development of a prophylactic and potentially therapeutic vaccine that will be dependent on the more efficient antibody-mediated immunity [38].
Pathogenesis of HPV-Related Anal Cancer
AIN or anal squamous intraepithelial lesions usually precede anal cancer. AIN can be classified as low grade (AIN grade 1) or high grade (AIN grade 2 or 3). AIN may follow one of two paths: It may regress on its own, or become a high-grade lesion [41]. Progression to high-grade lesions is facilitated by the risk factors already discussed and may ultimately lead to anal cancer in 9% to 13% of patients—rates comparable to cervical intraepithelial neoplasia (CIN). Regression of high-grade anal lesions is very rare [42].
The squamocolumnar junction of the anal canal transition zone where the columnar epithelium of the rectum transitions to squamous epithelium of the anus is the vulnerable region within the anal canal where the histologic manifestations of HPV are most evident [43].
Anal-canal HPV infection can be latent (normal tissue appearance detected through HPV DNA testing), subclinical (abnormal tissues detected with cytology or high-resolution anoscopy), or clinically overt (changes such as condylomata or anal cancer) [44].
The HPV Vaccine and AIN/Anal Cancer
Currently, there are two licensed prophylactic HPV vaccines: the bivalent vaccine against HPV-16 and -18 (Cervarix; GlaxoSmithKline Biologicals, Rixensart, Belgium, https://www.gsksource.com) and the quadrivalent vaccine (qHPV) against HPV types 6, 11, 16, and 18 (Gardasil; Merck and Co., Kenilworth, NJ, http://www.merck.com). The vaccines are made up of DNA-free, virus-like particles, both produced by expression of the major structural L1 gene of the HPV types [45–47]. When initially licensed, the quadrivalent vaccine was approved for a 3-dose schedule based on a 99% seroconversion rate, with participants aged 9–13 years having the highest response [48]. The high cost of the vaccine sparked interest in considering a two-dose schedule. This was supported by a post-trial analysis of participants who received fewer than three doses [49]. Dobson et al. went on to conduct a randomized clinical trial comparing HPV titers between a 2-dose and 3-dose schedule in girls aged 9–13 years [48]. They found the 2-dose schedule was noninferior for HPV-16/18 titers 1 month after the last dose. However, after 24 months, the noninferiority to HPV-18 was lost and, after 36 months, it was lost for HPV-16 [48]. The results of this study played a role in the recent switch to the 2-dose schedule in the European Union, Switzerland, The Netherlands, and Mexico for girls aged 9–14 years [50]. However, the study created several questions of immunity, especially in girls aged 11–12 years and the need to study efficacy in girls older than 13 years [51]. In the U.S., a 2-dose schedule could be problematic because the uptake rates are higher in girls older than 13 years [51].
In December 2010, the advisory committee on immunization practices (ACIP) recommended the routine use of the quadrivalent vaccine for prevention of AIN in women and men [52]. Emerging studies appear to show that vaccination may be the most realistic long-term approach to prevention [53] and possibly treatment of AIN and anal cancer [54].
Whereas the efficacy of the vaccine in prevention of anogenital precursor malignant lesions has been adequately proven in trials [45, 46], there is a paucity of data looking directly at efficacy of the vaccine against anal HPV infection and anal cancer.
Efficacy of Preventive Role of HPV Vaccines in HPV-Related AIN/Anal Cancer
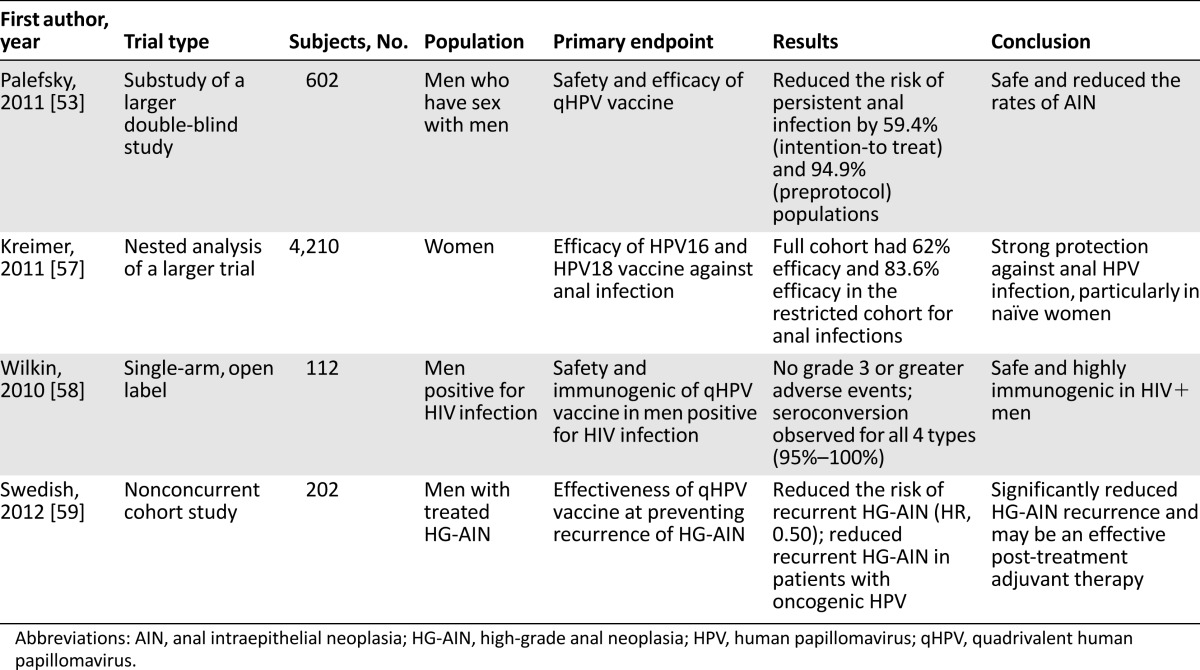
Unlike CIN/cervical cancer, there are currently no standardized practices for routine screening and treatment for AIN/anal cancer. The lack of screening makes the need for a preventive vaccine even more imperative. Given that persistent infection with high-risk HPV types is associated with increased risk of developing dysplasia or cancer, prevention is especially important in patients who are seropositive for HIV, who are particularly susceptible to persistent HPV infection [55, 56]. Several studies have evaluated the potential role the HPV vaccine can play in preventing HPV-related anal cancer (Table 1).
Table 1.
Clinical trials evaluating preventive role of HPV vaccine in HPV-related anal intraepithelial neoplasia and anal cancer
Given that persistent infection with high-risk HPV types is associated with increased risk of developing dysplasia or cancer, prevention is especially important in patients who are seropositive for HIV, who are particularly susceptible to persistent HPV infection.
In a substudy of a phase III, efficacy, multicenter, double-blind study, Palefsky et al. [53] showed that the use of the qHPV vaccine decreased the rate of AIN/HG-AIN among 602 MSM aged 16–26 years, of whom 299 were vaccinated. Participants who were seronegative for HPV, completed all 3 vaccine doses, and were followed for 2.2 years were analyzed in a per-protocol group. This group had a vaccine efficacy rate of 77.5% against AIN associated with HPV-6, -11, -16, or -18, whereas efficacy against HPV of any type was 54.9% [53]. The risk of persistent anal infection with covered types was reduced by 94.9%. In the intention-to-treat group (only 1 dose required with follow-up), the efficacy was 50.3% against AIN from HPV-6, -11, -16, or -18 [53]. The authors concluded that despite the narrow age range, limited sexual activity, and short follow-up, the qHPV showed efficacy against HPV-6, -11, -16, or -18 AIN or HG-AIN [53]. Although it was recognized that targeted vaccination programs toward a specific group based on sexual practices (i.e., MSM) have the potential to fail, they concluded that early vaccination of this group will likely yield significant reductions in vaccine-covered HPV neoplasia [53]. In addition, based on the biological similarity between anal cancer in heterosexual men and women [34], they projected similar per-protocol group results.
In the Costa Rica vaccine trial, a randomized double-blind controlled trial, Kreimer et al. [57] evaluated the efficacy of the bivalent vaccine against cervical and anal HPV-16/18 infection as well as premalignant lesions. Anal swab samples were obtained from consenting women at the year 4 exit visit and the HPV DNA status was assessed. A substantial vaccination efficiency of 62.0% against anal HPV-16/18 DNA was observed, but was still less than the 76.4% vaccination efficiency for the cervix. For the restricted cohort of women who were negative for either cervical HPV-16/18 DNA and/or antibodies at the time they entered the study, protection for the cervix and anus was similar (87.9% and 83.6%, respectively). In the full cohort, vaccination efficiency was noted to be higher in women who reported anal intercourse (73.9%) compared with women who had no reports of anal intercourse (55.3%). The authors concluded that the bivalent vaccine protects young women, especially those who are HPV naïve and who practiced anal intercourse, against anal HPV infection [57]. The biggest limitation was the absence of anal HPV DNA samples at enrollment.
Wilkin et al. conducted a multicenter clinical trial to assess the safety and immunogenicity of qHPV vaccine in men infected with HIV [58]. The overall outcome was that the qHPV appeared to be safe and highly immunogenic in this group. The implication is that a significant proportion of men will likely benefit from qHPV despite being older than the current age limit for which the vaccine is approved, and having significant prior anal HPV exposure may not be an exclusion criterion [58].
In a nonconcurrent cohort study involving 202 patients with a history of HG-AIN, Swedish et al. evaluated the prevention of recurrent HG-AIN with qHPV in MSM and concluded that the vaccine maybe an effective post-treatment adjuvant tool [59]. A 3-dose series of qHPV was offered off-label to all patients who were MSM, negative for HIV, and 18 years or older with a biopsy-proven and treated HG-AIN at each clinical visit. Twelve vaccinated patients (13.6%) and 35 (30.7%) unvaccinated patients developed recurrence of HG-AIN during the 340.4 person-years follow-up. Analysis by multivariable hazard ratio (HR) showed the qHPV was associated with decreased risk of HG-AIN recurrence (HR: 0.50; p = .04). The vaccine was again associated with a decreased risk of HG-AIN recurrence at 2 years after study entry in patients infected with oncogenic HPV (HR: 0.47; p = .05) [59]. This study was unique in several aspects. It looked at HG-AIN in older MSM who were negative for HIV and showed an association between the qHPV and decreased risk of disease recurrence that appears to persist for at least 2 years.
There is a need to conduct randomized control trials to answer two other major questions: Is the vaccine beneficial in older patients who practice receptive anal intercourse? Should it be used as adjuvant therapy in HG-AIN, given the high recurrence rate after treatment with surgery, topical, and pharmacologic therapy [60–62]?
Prospects of Therapeutic Vaccines
Currently, the treatment recommendation for HPV-related squamous cell carcinoma of the anus involves screening at-risk patients and observing precancerous lesions with the intention of early detection and eradication. Treatment options include laser ablation, surgical resection, or chemoradiation therapy. Successful eradication has been difficult and recurrence is high, particularly among patients seropositive for HIV [63, 64]. Current cancer treatment consensus is that a multimodality treatment approach that combines immunotherapy with radiation and chemotherapy is clearly needed to have the best effect on tumor cell reduction and eradication [65].
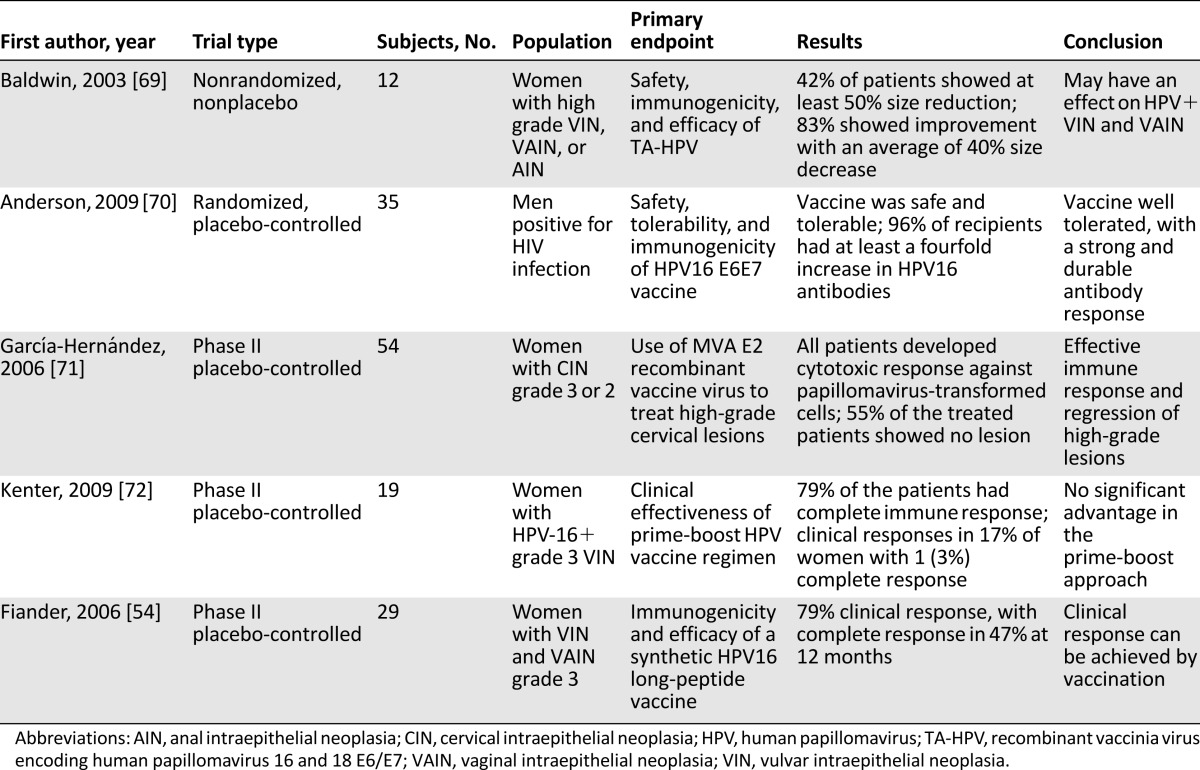
While the full extent and potential of prophylactic vaccination is a work in progress, there is a rapidly evolving interest in the prospect of therapeutic vaccinations. These are likely to be based on the platform of immunotherapy targeting E6 and E7 oncoproteins including T-helper 1 and CD8+ T cells specific to the virus [66]. The oncogenic pathophysiology of HPV-related anal squamous cell carcinoma makes this approach a reasonable one. Several early trials have shown immense promise, but there have been inconsistent clinical responses [67, 68] (Table 2).
Table 2.
Summary of clinical trials evaluating therapeutic use of HPV vaccine for HPV-related carcinoma
In a nonrandomized, phase II, prime-boost vaccine trial using heterologous HPV vaccines to determine their clinical effectiveness in the management of noncervical anogenital intraepithelial neoplasia (AGIN), Fiander et al. selected 29 women with biopsy-proven AGIN 3 and vaccinated them with 3 doses of a recombinant fusion protein made up of HPV-16 E6/E7/L2 (TA-CIN) followed by a dose of recombinant vaccinia virus encoding HPV-16 and -18 E6/E7 (TA-HPV) [54]. Clinical responses (defined as total disappearance of the lesion with no evidence on histological examination of biopsy, or partial disappearance if the lesion’s area was reduced by at least 50%) were evaluated by pre- and postvaccination symptoms, photographs, and biopsies. Five women (17%) showed clinical response: 1 complete and 4 partial responses, with 15 women (62%) showing improvement in symptoms [54], with no benefits observed by Baldwin et al. [69] when compared with unboosted strategies.
The novel therapeutic HPV-16 E6E7 ISCOMATRIX vaccine for HPV-related AIN was evaluated for safety, tolerability, and immunogenicity in MSM with HIV who had moderate immunosuppression and harbored high-risk HPV types, abnormal anal cytology, AIN 1, or HG-AIN by Anderson et al. in a randomized, multicenter, blinded, placebo-controlled, dose-escalating study [70]. They found the therapeutic vaccine to be safe and tolerable with an ability to induce a strong and durable antibody response and moderate interferon-γ levels. The study was not designed to evaluate clearance of infection or to measure changes in disease severity, because no biopsies were taken. Hence, inferences cannot be made on clinical response. Garcia-Hernandez et al. conducted a phase II clinical trial using a vaccinia virus MVA E2 recombinant vaccine that showed successful regression of high-grade cervical lesions in female patients [71]. Hopefully, further studies can be done and replicated for AIN/HG-AIN.
Kenter et al., observed encouraging clinical responses when they studied the immunogenicity and efficacy of the synthetic long-peptide HPV vaccine against oncoproteins E6 and E7 in women with HPV-16-positive high-grade vulvar intraepithelial neoplasia (a condition whose pathogenesis is comparable to AIN) [72]. This success is the driving force behind a future designated phase III trial for the vaccine, as well as a combined phase I/II study designed to evaluate the safety and efficacy of this vaccine in MSM who are positive for HIV and have HPV-16 positive AIN /HG-AIN that has not responded to previous treatment [72].
Discussion
Several encouraging conclusions and inferences can be made from these studies. Persistent infection with HPV is known to increase the chances of developing AIN/HG-AIN [32]. The qHPV is effective in reducing persistent anal infection with HPV-6, -11, -16, or -18 [53], and this is supported by findings in the Costa Rica vaccine trials, albeit with some limitations of the latter [57]. The study by Palefsky et al. also showed that the vaccine decreases the incidence of AIN/ HG-AIN in MSM who are eligible for the vaccine [53], and this finding was instrumental in the ACIP recommendation of the vaccine for this purpose [52]. With the increasing rates of heterosexual anal intercourse [25–27] and increased risk of anal cancer with receptive anal intercourse [28], the benefits of the vaccine observed by Palefsky et al. in MSM [53] can be extrapolated to heterosexuals if at-risk individuals are identified.
Individuals older than 26 years may benefit from the qHPV vaccine. The significant number of older participants who were seronegative and anal-canal HPV-DNA negative and yet showed a high immunogenic response and safety profile in the trial conducted by Wilkin et al. [58] supports this contention. The trial by Swedish et al. [59], which enrolled older HIV-negative MSM, can also be cited in a call for further studies to demonstrate benefits in older individuals.
There is a possible role of the qHPV as an adjuvant to improve treatment outcomes for individuals with HG-AIN, as shown by Swedish et al. in a small study with off-label use of the vaccine [59]. Randomized trials are required to confirm this and also determine, if possible, predictors for responders and nonresponders.
The unique opportunity provided by the presence of a pathogen directly related to AIN/HG-AIN and HPV-related anal squamous cell carcinoma is being exploited in several trials that continue to show promise but inconsistent clinical results [67, 68]. The results obtained by Baldwin et al. [68] and successfully reproduced by Fiander et al. [54] using the recombinant fusion protein of HPV-16 E6/E7/L2, as well as those reported for the vaccinia virus MVA E2 vaccine by Garcia-Hernandez et al. [71] should be evaluated on a larger scale because this can be extended to HPV-related AIN/ anal cancer. The results of a combined phase I/II study using the synthetic long-peptide HPV vaccine specifically for AIN/HG-AIN will be of therapeutic interest [73].
Conclusion
The incidences of AIN and anal cancer are on the rise in the U.S. and globally [4, 8, 9, 74]. Although it has a female preponderance [7], the incidence is rapidly increasing in men [4]. Compared with the general population, the rate is disproportionately higher in certain high-risk groups, namely, MSM [11, 12]; people seropositive for HIV, especially in the era of ART [14–17]; women with cervical or vulvar cancer [18, 19]; and people with chronic immunosuppressive states other than HIV [20]. The perception that receptive anal intercourse is limited to MSM is obsolete because data are emerging of increasing heterosexual receptive anal intercourse globally [25–28], and this is very significant from the public health perspective. The association of HPV with 91% of cases of anal cancer [3] provides an excellent opportunity for prevention and treatment strategies.
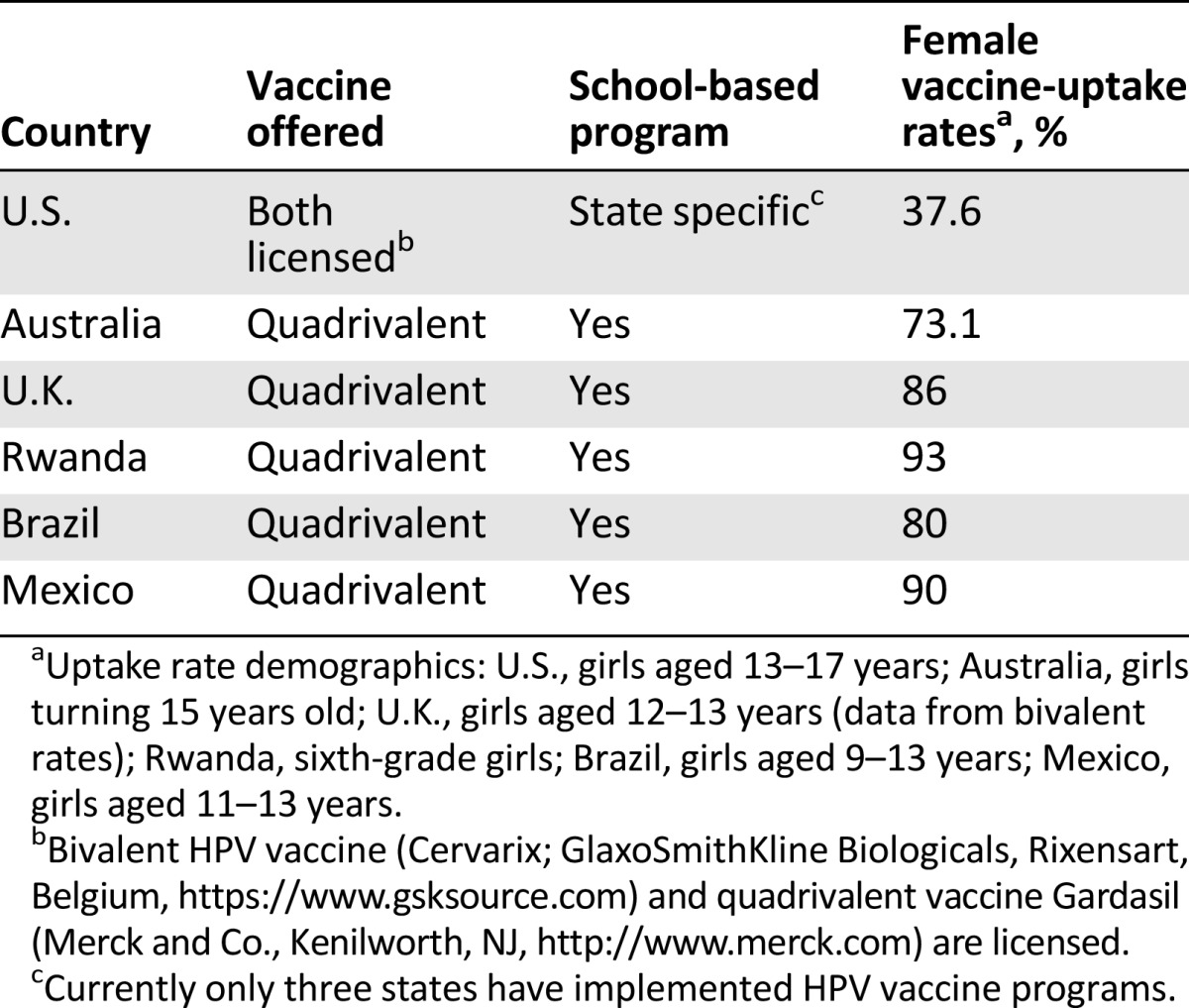
The best approach for addressing the rising rate of anal cancer [4, 8–10] will be the institution of primary and secondary prevention strategies. Primary preventive strategies should begin with improvement in the vaccination coverage rates, which are, at best, average for the current targeted population [75]. Currently, the ACIP and CDC recommend vaccination of male and female young people between the ages of 9 and 26 years [52]. National statistics on U.S. vaccine rates among girls aged 13–17 years in 2013 showed a rate of only 37.6% [75]. The rates among U.S. boys in the same age group are even lower at 13.6% [76]. In comparison, other developed countries such as Australia and the U.K. have much higher rates among girls. Australia boasts a 73.1% rate among girls turning 15 [77], whereas the U.K. has the highest rate among girls between the ages of 12 and 13 receiving the bivalent vaccine: 86% [78]. Australia has adopted vaccine programs for boys and currently has a rate of 60% in boys turning 15 [77]. Currently, the U.K. does not provide the vaccine to boys under the National Health Service. In developing countries, programs are being created to improve HPV vaccine uptake. Through low-cost vaccine programs, countries in Sub-Saharan Africa have seen success in providing coverage. For example, Rwanda was able to achieve a 93% coverage rate among sixth-grade girls. South Africa recently introduced a vaccination program in 2014, and several other countries are conducting school-based pilot programs [79]. In Latin America, Mexico introduced a vaccination program in 2008 for girls aged 11–13 years with a 67% coverage rate in 2010 [80]. Brazil introduced a vaccination program in 2014 for girls aged 9–13 years and surpassed the target of 80% coverage [81].
In developing countries, programs are being created to improve HPV vaccine uptake. Through low-cost vaccine programs, countries in Sub-Saharan Africa have seen success in providing coverage. For example, Rwanda was able to achieve a 93% coverage rate among sixth-grade girls. South Africa recently introduced a vaccination program in 2014, and several other countries are conducting school-based pilot programs.
The success rates in these countries are from implementation of school-based programs and national health coverage of the vaccine (Table 3). Currently, in the U.S., many states have school vaccination requirements for hepatitis B virus and varicella, which have translated to high uptake rates [76]. However, only three states have implemented HPV vaccination requirements, which may be due to the political social controversy surrounding the HPV vaccine [76].
Table 3.
Summary of vaccination programs and uptake rates in select countries
Education of policy makers, health care professionals, and targeted populations should be pursued diligently to improve vaccination coverage in the U.S. Research should be conducted to evaluate the benefits of vaccination for older individuals, especially if they are identified as being at risk. The value of the vaccine in decreasing persistent anal HPV infection [53] should be evaluated further.
Secondary preventive strategies should be aimed at screening and treating individuals for AIN/HG-AIN. This calls for standardized screening recommendation for high-risk groups, taking into consideration changing sexual practices and other risk factors. To do this, the question of which determinant predicts AIN/ HG-AIN progression to anal cancer and whether HG-AIN treatment reduces the incidence of anal cancer should be answered through focused studies of biomarkers and in clinical trials [82]. The search for a therapeutic HPV vaccine is underway and improvements in our knowledge of viral tumorigenesis and immunology will hasten discovery.
Acknowledgment
Felix A. Mensah is currently affiliated with the Section of Hematology/Oncology, Georgia Regents University, Augusta, Georgia, USA.
Author Contributions
Conception/Design: Felix A. Mensah, James S. Lewis Jr., A. Craig Lockhart
Collection and/or assembly of data: Felix A. Mensah, Mudresh R. Mehta
Data analysis and interpretation: Mudresh R. Mehta
Manuscript writing: Felix A. Mensah, Mudresh R. Mehta, A. Craig Lockhart
Final approval of manuscript: James S. Lewis Jr., A. Craig Lockhart
Disclosures
The authors indicated no financial relationships.
References
- 1. International Agency for Research on Cancer. Monographs on the Evalaution of carcinogenic risks to Humans : Human Papillomaviruses. vol 90. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Genital HPV infection - fact sheet. 2009; http://www.cdc.gov/std/HPV/STDFact-HPV.htm. Accessed May 12, 2014.
- 3.Centers for Disease Control and Prevention. Human papillomavirus (HPV)-associated cancers. Available at http://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed May 12, 2014.
- 4.National Cancer Institute. SEER Stat Fact Sheets: Anal cancer. http://seer.cancer.gov/statfacts/html/anus.html. Accessed May 9, 2014.
- 5.Abbas A, Yang G, Fakih M. Management of anal cancer in 2010. Part 1: Overview, screening, and diagnosis. Oncology (Williston Park) 2010;24:364–369. [PubMed] [Google Scholar]
- 6.Abbas A, Yang G, Fakih M. Management of anal cancer in 2010. Part 2: Current treatment standards and future directions. Oncology (Williston Park) 2010;24:417–424. [PubMed] [Google Scholar]
- 7.Curado MP EB, Shin HR, Storm H, et al (eds). Cancer incidence in five continents vol IX. International Agency for Research on Cancer Scientific Publications. Available at http://www.iarc.fr/en/publications/pdfs-online/epi/sp160/
- 8.Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer. 2004;101:281–288. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 9.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute- Surveillance, Epidemiology, and End Results Program: Anal cancer by race and ethnicity. 2014. http://seer.cancer.gov/faststats/selections.php?series=race. Accessed May 5, 2014.
- 11.Daling JR, Weiss NS, Klopfenstein LL, et al. Correlates of homosexual behavior and the incidence of anal cancer. JAMA. 1982;247:1988–1990. [PubMed] [Google Scholar]
- 12.Melbye M, Coté TR, Kessler L, et al. High incidence of anal cancer among AIDS patients. Lancet. 1994;343:636–639. doi: 10.1016/s0140-6736(94)92636-0. [DOI] [PubMed] [Google Scholar]
- 13.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 14.Bower M, Powles T, Newsom-Davis T, et al. HIV-associated anal cancer: Has highly active antiretroviral therapy reduced the incidence or improved the outcome? J Acquir Immune Defic Syndr. 2004;37:1563–1565. doi: 10.1097/00126334-200412150-00004. [DOI] [PubMed] [Google Scholar]
- 15.Diamond C, Taylor TH, Aboumrad T, et al. Increased incidence of squamous cell anal cancer among men with AIDS in the era of highly active antiretroviral therapy. Sex Transm Dis. 2005;32:314–320. doi: 10.1097/01.olq.0000162366.60245.02. [DOI] [PubMed] [Google Scholar]
- 16.Siverberg M, Xu L, Chao C et al. Immunodeficiency, HIV RNA levels, and risk of non-AIDS-defining cancers. Paper presented at: 17th Conference on Retroviruses and Opportunistic Infections, Abstract 28 February 16-19, 2010; San Francisco, California. [Google Scholar]
- 17.Chaturvedi AK, Madeleine MM, Biggar RJ, et al. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melbye M, Sprøgel P. Aetiological parallel between anal cancer and cervical cancer. Lancet. 1991;338:657–659. doi: 10.1016/0140-6736(91)91233-k. [DOI] [PubMed] [Google Scholar]
- 19.Ogunbiyi OA, Scholefield JH, Robertson G, et al. Anal human papillomavirus infection and squamous neoplasia in patients with invasive vulvar cancer. Obstet Gynecol. 1994;83:212–216. [PubMed] [Google Scholar]
- 20.Patel HS, Silver AR, Northover JM. Anal cancer in renal transplant patients. Int J Colorectal Dis. 2007;22:1–5. doi: 10.1007/s00384-005-0023-3. [DOI] [PubMed] [Google Scholar]
- 21.Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;198:500.e1–500.e7. doi: 10.1016/j.ajog.2008.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters RK, Mack TM. Patterns of anal carcinoma by gender and marital status in Los Angeles County. Br J Cancer. 1983;48:629–636. doi: 10.1038/bjc.1983.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyitray AG, Smith D, Villa L, et al. Prevalence of and risk factors for anal human papillomavirus infection in men who have sex with women: A cross-national study. J Infect Dis. 2010;201:1498–1508. doi: 10.1086/652187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widdice LE, Breland DJ, Jonte J, et al. Human papillomavirus concordance in heterosexual couples. J Adolesc Health. 2010;47:151–159. doi: 10.1016/j.jadohealth.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miguez-Burbano MJ, Pineda-Medina L, Lecusay R, et al. Continued high risk behaviors in HIV infected drug abusers. J Addict Dis. 2002;21:67–80. doi: 10.1300/J069v21n04_07. [DOI] [PubMed] [Google Scholar]
- 26.Tian LH, Peterman TA, Tao G, et al. Heterosexual anal sex activity in the year after an STD clinic visit. Sex Transm Dis. 2008;35:905–909. doi: 10.1097/OLQ.0b013e318181294b. [DOI] [PubMed] [Google Scholar]
- 27.McBride KR, Fortenberry JD. Heterosexual anal sexuality and anal sex behaviors: A review. J Sex Res. 2010;47:123–136. doi: 10.1080/00224490903402538. [DOI] [PubMed] [Google Scholar]
- 28.Frisch M, Glimelius B, van den Brule AI, et al. [Sexually transmitted infection as a cause of anal cancer] Ugeskr Laeger. 1998;160:7109–7117. [PubMed] [Google Scholar]
- 29.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 30.Critchlow CW, Hawes SE, Kuypers JM, et al. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998;12:1177–1184. doi: 10.1097/00002030-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Silverberg MJ, Lau B, Justice AC, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54:1026–1034. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palefsky JM, Holly EA, Ralston ML, et al. High incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men. AIDS. 1998;12:495–503. doi: 10.1097/00002030-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Palefsky JM, Holly EA, Hogeboom CJ, et al. Virologic, immunologic, and clinical parameters in the incidence and progression of anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual men. J Acquir Immune Defic Syndr Hum Retrovir. 1998;17:314–319. doi: 10.1097/00042560-199804010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124:2375–2383. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 35.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 36.Münger K, Baldwin A, Edwards KM, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duensing S, Munger K. Mechanisms of genomic instability in human cancer: Insights from studies with human papillomavirus oncoproteins. Int J Cancer. 2004;109:157–162. doi: 10.1002/ijc.11691. [DOI] [PubMed] [Google Scholar]
- 38.Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24(suppl 3):S3/106–113. doi: 10.1016/j.vaccine.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 39.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl 1):S16–S22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Schiffman M, Kjaer SK. Chapter 2: Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003;(31):14–19. doi: 10.1093/oxfordjournals.jncimonographs.a003476. [DOI] [PubMed] [Google Scholar]
- 41.Arends MJ, Buckley CH, Wells M. Aetiology, pathogenesis, and pathology of cervical neoplasia. J Clin Pathol. 1998;51:96–103. doi: 10.1136/jcp.51.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaki SR, Judd R, Coffield LM, et al. Human papillomavirus infection and anal carcinoma. Retrospective analysis by in situ hybridization and the polymerase chain reaction. Am J Pathol. 1992;140:1345–1355. [PMC free article] [PubMed] [Google Scholar]
- 43.Palefsky JM. Anal human papillomavirus infection and anal cancer in HIV-positive individuals: An emerging problem. AIDS. 1994;8:283–295. doi: 10.1097/00002030-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Handsfield HH. Clinical presentation and natural course of anogenital warts. Am J Med. 1997;102(5a):16–20. doi: 10.1016/s0002-9343(97)00179-4. [DOI] [PubMed] [Google Scholar]
- 45.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 46.FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 47.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 48.Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: A randomized clinical trial. JAMA. 2013;309:1793–1802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 49.Kreimer AR, Rodriguez AC, Hildesheim A, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–1451. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jit M, Brisson M, Laprise JF, et al. Comparison of two dose and three dose human papillomavirus vaccine schedules: Cost effectiveness analysis based on transmission model. BMJ. 2015;350:g7584. doi: 10.1136/bmj.g7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahn JA, Bernstein DI. HPV vaccination: Too soon for 2 doses? JAMA. 2013;309:1832–1834. doi: 10.1001/jama.2013.4147. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention (CDC) Recommendations on the use of quadrivalent human papillomavirus vaccine in males–Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–1708. [PubMed] [Google Scholar]
- 53.Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 54.Fiander AN, Tristram AJ, Davidson EJ, et al. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: Clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006;16:1075–1081. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 55.Palefsky JM. Human papillomavirus infection and anogenital neoplasia in human immunodeficiency virus-positive men and women. J Natl Cancer Inst Monogr. 1998;(23):15–20. doi: 10.1093/oxfordjournals.jncimonographs.a024166. [DOI] [PubMed] [Google Scholar]
- 56.Fruchter RG, Maiman M, Arrastia CD, et al. Is HIV infection a risk factor for advanced cervical cancer? Acquir Immune Defic Syndr Hum Retrovir. 1998;18:241–245. doi: 10.1097/00042560-199807010-00007. [DOI] [PubMed] [Google Scholar]
- 57.Kreimer AR, González P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: A nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011;12:862–870. doi: 10.1016/S1470-2045(11)70213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202:1246–1253. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: A nonconcurrent cohort study. Clin Infect Dis. 2012;54:891–898. doi: 10.1093/cid/cir1036. [DOI] [PubMed] [Google Scholar]
- 60.Kreuter A, Potthoff A, Brockmeyer NH, et al. Imiquimod leads to a decrease of human papillomavirus DNA and to a sustained clearance of anal intraepithelial neoplasia in HIV-infected men. J Invest Dermatol. 2008;128:2078–2083. doi: 10.1038/jid.2008.24. [DOI] [PubMed] [Google Scholar]
- 61.Goldstone RN, Goldstone AB, Russ J, et al. Long-term follow-up of infrared coagulator ablation of anal high-grade dysplasia in men who have sex with men. Dis Colon Rectum. 2011;54:1284–1292. doi: 10.1097/DCR.0b013e318227833e. [DOI] [PubMed] [Google Scholar]
- 62.Goldstone SE, Hundert JS, Huyett JW. Infrared coagulator ablation of high-grade anal squamous intraepithelial lesions in HIV-negative males who have sex with males. Dis Colon Rectum. 2007;50:565–575. doi: 10.1007/s10350-006-0874-x. [DOI] [PubMed] [Google Scholar]
- 63.Klencke B, Matijevic M, Urban RG, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: A phase I study of ZYC101. Clin Cancer Res. 2002;8:1028–1037. [PubMed] [Google Scholar]
- 64.Devaraj B, Cosman BC. Expectant management of anal squamous dysplasia in patients with HIV. Dis Colon Rectum. 2006;49:36–40. doi: 10.1007/s10350-005-0229-z. [DOI] [PubMed] [Google Scholar]
- 65.Cubas R, Li M, Chen C, et al. Colorectal cancer: New advances in immunotherapy. Cancer Biol Ther. 2007;6:11–17. doi: 10.4161/cbt.6.1.3672. [DOI] [PubMed] [Google Scholar]
- 66.Nurkkala M, Wassén L, Nordström I, et al. Conjugation of HPV16 E7 to cholera toxin enhances the HPV-specific T-cell recall responses to pulsed dendritic cells in vitro in women with cervical dysplasia. Vaccine. 2010;28:5828–5836. doi: 10.1016/j.vaccine.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 67.Ma B, Maraj B, Tran NP, et al. Emerging human papillomavirus vaccines. Expert Opin Emerg Drugs. 2012;17:469–492. doi: 10.1517/14728214.2012.744393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morrow MP, Yan J, Sardesai NY. Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev Vaccines. 2013;12:271–283. doi: 10.1586/erv.13.23. [DOI] [PubMed] [Google Scholar]
- 69.Baldwin PJ, van der Burg SH, Boswell CM, et al. Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin Cancer Res. 2003;9:5205–5213. [PubMed] [Google Scholar]
- 70.Anderson JS, Hoy J, Hillman R, et al. A randomized, placebo-controlled, dose-escalation study to determine the safety, tolerability, and immunogenicity of an HPV-16 therapeutic vaccine in HIV-positive participants with oncogenic HPV infection of the anus. J Acquir Immune Defic Syndr Hum Retrovir. 2009;52:371–381. doi: 10.1097/QAI.0b013e3181b7354c. [DOI] [PubMed] [Google Scholar]
- 71.García-Hernández E, González-Sánchez JL, Andrade-Manzano A, et al. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Ther. 2006;13:592–597. doi: 10.1038/sj.cgt.7700937. [DOI] [PubMed] [Google Scholar]
- 72.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 73.Prins J. Therapeutic HPV-16 Vaccination for the Treatment of Anal Dysplasia (VACCAIN-T). [Clinical trial]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01923116?term=SLP-HPV&rank=1. Accessed May 12, 2014.
- 74.Centers for Disease Control and Prevention (CDC) Human papillomavirus-associated cancers - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2012;61:258–261. [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention. 2011 Archived teen vaccination coverage. http://www.cdc.gov/vaccines/who/teens/vac-coverage/vac-coverage-2011.html. Accessed May 12, 2014.
- 76.Schwartz JL, Easterling LA. State vaccination requirements for HPV and other vaccines for adolescents, 1990-2015. JAMA. 2015;314:185–186. doi: 10.1001/jama.2015.6041. [DOI] [PubMed] [Google Scholar]
- 77.Brosi C, Bicknell L, Winch K et al. Human papillomavirus control. How are we going with vaccination coverage seven years in? Poster presented at Communicable Disease Control Conference 2015. June 1-2, 2015 Brisbane, Australia [Google Scholar]
- 78.Public Health England. Human papillomavirus immunisation programme review: 2008 to 2014. https://http://www.gov.uk/government/uploads/system/uploads/attachment_data/file/412264/HPV_Vaccine_Coverage_in_England_200809_to_201314.pdf Accessed July 3, 2015
- 79.Perlman S, Wamai RG, Bain PA, et al. Knowledge and awareness of HPV vaccine and acceptability to vaccinate in sub-Saharan Africa: A systematic review. PLoS One. 2014;9:e90912. doi: 10.1371/journal.pone.0090912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bruni L, Barrionuevo-Rosas L, Albero G et al. Human papillomavirus and related diseases in Mexico. Summary Report. March 20 2015. Available at http://www.hpvcentre.net/statistics/reports/MEX.pdf
- 81.Baker ML, Figueroa-Downing D, Chiang ED, et al. Paving pathways: Brazil’s implementation of a national human papillomavirus immunization campaign. Rev Panam Salud Publica. 2015;38:163–166. [PubMed] [Google Scholar]
- 82.Wentzensen N. Screening for anal cancer: Endpoints needed. Lancet Oncol. 2012;13:438–440. doi: 10.1016/S1470-2045(12)70101-8. [DOI] [PubMed] [Google Scholar]