Abstract
In vivo animal model systems, and in particular mouse models, have evolved into powerful and versatile scientific tools indispensable to basic and translational research in the field of transplantation medicine. A vast array of reagents is available exclusively in this setting, including mono- and polyclonal antibodies for both diagnostic and interventional applications. In addition, a vast number of genotyped, inbred, transgenic, and knock out strains allow detailed investigation of the individual contributions of humoral and cellular components to the complex interplay of an immune response and make the mouse the gold standard for immunological research.
Vascularized Composite Allotransplantation (VCA) delineates a novel field of transplantation using allografts to replace "like with like" in patients suffering traumatic or congenital tissue loss. This surgical methodological protocol shows the use of a non-suture cuff technique for super-microvascular anastomosis in an orthotopic mouse hind limb transplantation model. The model specifically allows for comparison between established paradigms in solid organ transplantation with a novel form of transplants consisting of various different tissue components. Uniquely, this model allows for the transplantation of a viable vascularized bone marrow compartment and niche that have the potential to exert a beneficial effect on the balance of immune acceptance and rejection. This technique provides a tool to investigate alloantigen recognition and allograft rejection and acceptance, as well as enables the pursuit of functional nerve regeneration studies to further advance this novel field of transplantation.
Keywords: Medicine, Issue 108, Mouse, Vascularized Composite Allotransplantation, Hind Limb Transplantation, Immunology, Non-suture Cuff Technique
Introduction
The late nineties heralded the pioneering days of reconstructive transplantation with the first successful hand transplant performed in France in 1998. Since then, the use of VCAs for reconstruction of devastating tissue defects has been successfully employed in a wide spectrum of patients. To date, the world counts 76 recipients of 112 upper extremities as well as 31 faces 1-3. In addition, several other types of VCAs such as abdominal wall 4, larynx 5, trachea 6, vascularized joints 7, and even penis 8 have been performed. Furthermore, the live birth of a baby was recently reported after uterus transplantation 9. This growing world experience is indicative for how reconstructive transplantation has become a valid therapeutic option for patients suffering of significant functional tissue defects not amendable to conventional reconstructive and restorative surgery and treatment.
While the idea of replacing "like with like" sparked clinical enthusiasm, initial skepticism still prevails with regards to side effects of conventional high-dose immunosuppression required to maintain allografts and their function 10,11. However, as shown by seminal work of Lee et al., these composite grafts are less likely to reject than its individual components, and furthermore, some of the tissue components such as the vascularized bone compartment have fueled optimism as they might exert unique immunological effects onto the balance of immune acceptance and rejection 12.
Our group pioneered several microsurgical animal models for solid organ transplantation, as well as vascularized composite allotransplantation 13-19. Here we describe a novel surgical procedure using a non-suture cuff technique to perform super micro-vascular anastomosis in an orthotopic mouse hind limb transplantation model. This transplant model provides a useful tool for investigating immune acceptance and rejection mechanisms, as well as the role of individual tissue components, such as the vascularized bone marrow compartment, towards tolerance induction in the immunologically versatile setting of the mouse species. Additionally, the orthotopic placement of the limb opens the possibilities for nerve regeneration and functional outcome studies, which are critically important to the setting of VCA.
Protocol
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institute of Health (NIH) and were approved by the Johns Hopkins University Animal Care and Use Committee (JHUACUC). The specific procedures were performed under the approved ACUC protocol MO13M108.
1. Donor Operation
Administer analgesia at the appropriate time point for each pharmacological formulation prior to surgery. As per the approved animal care and use protocol use 0.1 mg/kg BW of buprenorphine subcutaneously 1 hr prior to skin incision.
Sedate the donor with isoflurane applied through a chamber attached to an isoflurane vaporizer at 4%; maintain sedation and anesthesia at 2% through a nose cone. Perform toe pinch withdrawal reflection to monitor the depth of anesthesia prior to the initiation of the procedure.
Wear masks, disposable isolation gowns and gloves.
Shave the surgical area, in particular the hind limb and groin, and prep with 10% Povidone - Iodine.
Use a sterile field drape, autoclaved instruments and a high magnification microscope (40X).
Make groin skin incision using scissors proximally to the mid-thigh area and circumferentially connect the incision to demarcate the hind limb from the rest of the mouse body.
Identify and dissect the femoral artery, vein and nerve. Separate all three structures using forceps and micro-scissors.
Once the vascular pedicle is dissected divide the vessels at the level of the inguinal ligament using micro scissors.
Next, continue to divide the individual ventral (gracilis and medial thigh muscles) and dorsal muscle groups 20 proximally at the level of the mid-thigh to separate the graft from the donor animal using scissors.
Transect the femur and cut at the mid of the femoral shaft using scissors.
Euthanize animal by isoflurane overdose followed by cervical dislocation. Confirm cessation of heart beat and respiration.
Flush the limb with 2 ml heparinized (30 I.E.) cold (4 °C) saline by using a 33 G flushing needle mounted on a syringe (see Materials Table).
Place one polyimide cuff on the femoral vein and artery, respectively.
Wrap graft into wet cotton gauze, place in petri dish and store at 4 °C until inset.
2. Recipient Operation
- Removal of the Hind Limb
- Administer analgesia at the appropriate time point for each pharmacological formulation prior to surgery. As per the approved animal care and use protocol use 0.1 mg/kg BW of Buprenorphine SC 1 hr prior to skin incision.
- Sedate the donor with isoflurane applied through a chamber attached to an isoflurane vaporizer at 4%; maintain sedation and anesthesia at 2% through a nose cone. Perform toe pinch withdrawal reflection to monitor the depth of anesthesia prior to the initiation of the procedure.
- Use veterinary ointment on the eyes of the mouse to prevent dryness while under anesthesia.
- Shave the surgical area, in particular the hind limb and groin and prep with 10% Povidone - Iodine.
- Make groin skin incision using scissors proximally to the mid-thigh area and circumferentially connect the incision to demarcate the hind limb from the rest of the mouse body.
- Identify and dissect the femoral artery, vein and nerve and separate all three structures using forceps and micro-scissors.
- Once the vascular pedicle is dissected, clamp the femoral vessels at the level of the inguinal ligament.
- Cut the vessels distal at the level of the superficial epigastric artery.
- Next, continue to divide the individual ventral (gracilis and medial thigh muscles) and dorsal muscle groups 20 proximally at the level of the mid-thigh to separate the native hind limb from the recipient animals using scissors.
- Transect the femur in the middle of the femoral shaft using scissors.
- Cauterize previously transected thigh muscles to prevent bleeding of the dissection site and thus recipient blood loss.
- Implantation
- Minimize fluid loss by irrigating the operative field with warm saline (37 °C) and injecting 0.3 ml warm saline before and after the operation.
- Place the graft in a way that reflects the accurate anatomical position of the native hind limb by aligning the femur bone of the recipient and the graft and connect them using a 20 G spinal needle as an intramedullary rod.
- Coapt the ventral and dorsal muscle groups using absorbable suture material (6-0 Polysorb).
- Connect the femoral vessels using the non-suture cuff technique; in detail, pull the recipient side of the vessel over the cuffs previously mounted on the vessel ends of the graft. Use a 10-0 Nylon suture and perform a circumferential tie to fix the recipient vessel onto the cuff.
- Next release the clamps. At this stage visually verify cuff rotation and optimal positioning to prevent mis-rotation and kinking of the vessels.
- Perform meticulous hemostasis using electro cautery with a particular focus on the muscle recipient donor interface and the bone ends.
- Close the skin using non-absorbable Nylon sutures (6-0 Ethilon).
- Establish normothermic conditions by allowing the animal to recover in its cage under a heating lamp. Continue regular monitoring for at least 4 hr prior to returning it to the housing facility.
- Provide post-operative analgesia with Buprenorphine at a dose of 0.1mg/kg SC every 6-8 hr for 3 days.
Representative Results
Performing vascularized composite allotransplantation in a mouse model using a non-suture cuff technique allows to achieve excellent and long term graft and animal survival as shown in Figure 1. Furthermore, it represents a reliable method of obtaining reproducible outcomes of gradual allograft rejection in vascularized composite allotransplantation as documented by the images shown in Figure 2. H&E histology of tissues obtained from animals undergoing rejection further underlines the reproducible dynamics of allograft rejection in this murine model (Figure 3).
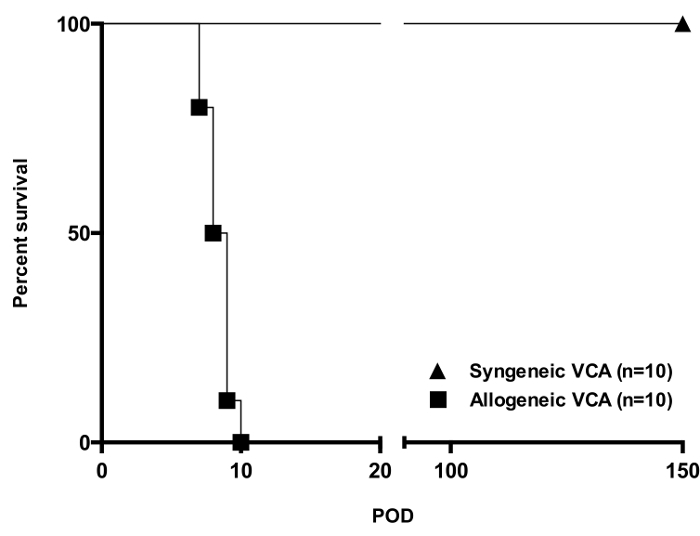 Figure 1. Allograft Survival in a Fully H2-mismatched Mouse Strain Combination [Balb/c (H2Kd) into C57BL6 (H2Kb)]. While all syngeneic transplants (n = 10) were accepted long term, untreated allografts (n = 10) were acutely rejected within and average of 8 - 9 days. Skin rejection Grade 3 according to Banff criteria was considered full rejection in this study. Please click here to view a larger version of this figure.
Figure 1. Allograft Survival in a Fully H2-mismatched Mouse Strain Combination [Balb/c (H2Kd) into C57BL6 (H2Kb)]. While all syngeneic transplants (n = 10) were accepted long term, untreated allografts (n = 10) were acutely rejected within and average of 8 - 9 days. Skin rejection Grade 3 according to Banff criteria was considered full rejection in this study. Please click here to view a larger version of this figure.
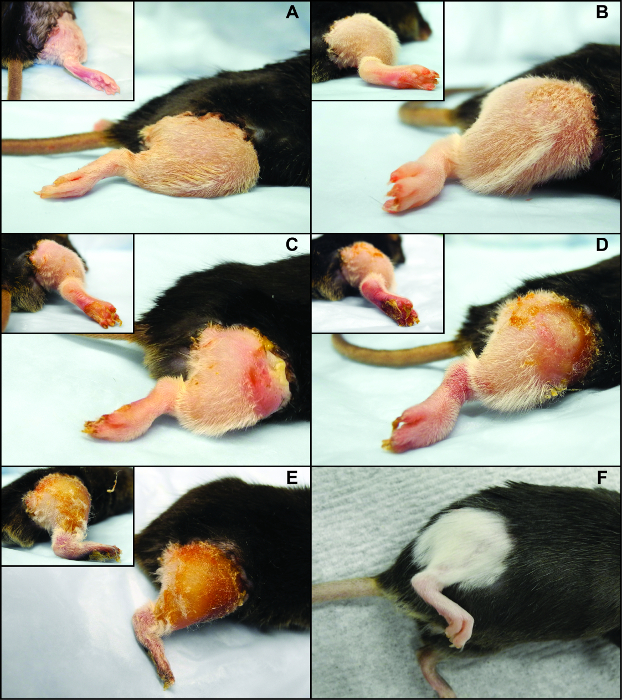 Figure 2. Clinical Rejection Features and Dynamics of a Fully H2-mismatched [Balb/c (H2Kd) into C57BL6 (H2Kb)] Murine Orthotopic Hind Limb Transplant. (A) Clinical Grade 0, (B) Clinical Grade 1, (C) Clinical Grade 2, (D) Clinical Grade 3, and (E) Clinical Grade 4 rejection, (F) long term surviving allograft (POD 100) treated with a costimulation blockade (antiCD40 mAb + CTLA4Ig) based regimen. Please click here to view a larger version of this figure.
Figure 2. Clinical Rejection Features and Dynamics of a Fully H2-mismatched [Balb/c (H2Kd) into C57BL6 (H2Kb)] Murine Orthotopic Hind Limb Transplant. (A) Clinical Grade 0, (B) Clinical Grade 1, (C) Clinical Grade 2, (D) Clinical Grade 3, and (E) Clinical Grade 4 rejection, (F) long term surviving allograft (POD 100) treated with a costimulation blockade (antiCD40 mAb + CTLA4Ig) based regimen. Please click here to view a larger version of this figure.
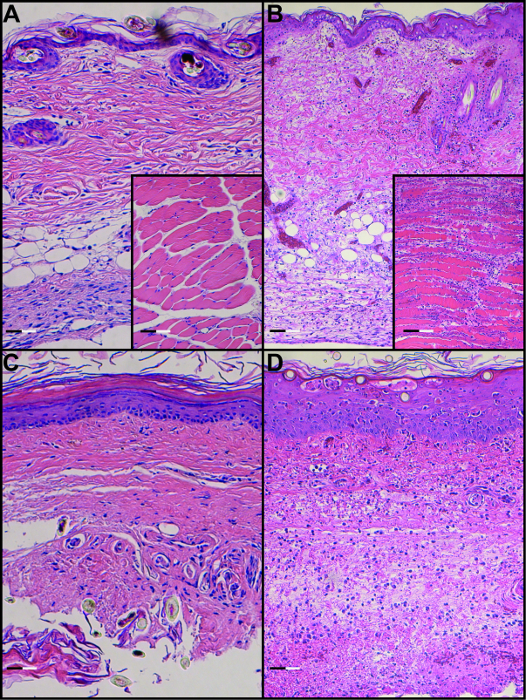 Figure 3. H&E Staining of Skin and Muscle in a Syngeneic Transplant (A) and Allogeneic Transplant (B) on POD 8 as well as H&E Staining of Footpad Skin and Muscle in a Syngeneic Transplant (C) and Allogeneic Transplant on POD 8 (D) (Scale bar: 0.1 µm).
Please click here to view a larger version of this figure.
Figure 3. H&E Staining of Skin and Muscle in a Syngeneic Transplant (A) and Allogeneic Transplant (B) on POD 8 as well as H&E Staining of Footpad Skin and Muscle in a Syngeneic Transplant (C) and Allogeneic Transplant on POD 8 (D) (Scale bar: 0.1 µm).
Please click here to view a larger version of this figure.
Discussion
Vascularized Composite Allotransplantation, such as upper extremity and face transplantation for reconstruction of devastating tissue defects, has evolved as a valid treatment option for patients not amendable to conventional reconstructive procedures. Technical advances in the field of reconstructive microsurgery as well as a vast experience with potent immunosuppressive and immune modulatory therapies in solid organ transplantation, now enables long-term allograft survival in this unique patient population 3,21. However, significant side effects of long-term immunosuppression required for allograft maintenance and survival still limit the broader application of these life enhancing but not life-saving reconstructive modalities 3,22,23. Furthermore, the success of VCAs in contrast to solid organ transplants also depends on timely regeneration of recipient nerves into the allograft to re-innervate both muscles for motor function, as well as sensory components for touch and temperature sensation to regain function. From an immunologic perspective, as in all of transplantation medicine, the overarching goal in reconstructive transplantation is to achieve a state of operational tolerance allowing for acceptance of an allograft without the need for long-term maintenance immunosuppression 23. In this regard, the mouse has evolved to represent the main in vivo model system in transplant immunology research to investigate ways of achieving alloantigen specific tolerance in an intact immune system. Furthermore, the mouse H2 complex closely resembles the human MHC complex. Thus, inbred and genotyped mouse strains allow to model clinical scenarios of related and non-related living and cadaveric donor settings by employing various degrees of alloantigen mismatching from a syngeneic to a fully allogeneic strain combination. The availability of transgenic animals and specific knockout animals additionally allows investigation of the roles and effects of individual molecular pathways and regulatory mechanisms on immune acceptance and rejection by providing the possibility of selective activation or depletion of cell or protein components. This is paralleled by a broad availability of diagnostic and therapeutic agents (e.g., antibodies) uniquely developed for the mouse system for in vitro and in vivo studies 24. Overall, these aspects make the murine system the "gold standard" for basic transplant immunology research.
Although there have been various small 13,16,18,20,25,26 and large animal 14,27-29 models described for VCA as recently reviewed by Brandacher et al. 30 only very few are actually applicable for basic mechanistic immunological research 16,24,30. Due to the small vessel diameter of the mouse femoral artery and vein, it requires advanced surgical and super-microsurgical training and skills to perform successful anastomosis as the key component of orthotopic hind limb transplantation. While meticulous and careful dissection of the anatomical structures is as critically relevant in this model as in previously published models 20,31,32, the cuff technique has shown a less steep learning curve compared to suture anastomosis of sub-millimeter vessels 16,25 and can be achieved in a couple of months training with rigorous daily practice. Based on our experience, an untrained microsurgeon will need 30 -50 procedural attempts to acquire sufficient and reliable skills to perform this model with high success rates. For the experienced and highly trained microsurgeon 15 - 30 attempts should suffice to master this cuff-based mouse hind limb transplant model.The reduced complexity of the procedure presented here is further reflected by the fact that this approach requires limited vessel length and thus dissection. We found taking the donor vessels at the inguinal ligament and the recipient vessels at the level of the superficial epigastric vessels provides sufficient length, despite the common notion that the cuff technique requires extensive additional length to be applicable. For example, one previously published approach describes the necessity to harvest donor vessels at the level of the external iliac vessels and recipient vessels at the level of the popliteal vessels 32. Furthermore, with the cuff technique, performing the anastomosis requires less time compared to the suture technique and leads to an overall reduced operating time. In experienced hands, both donor and recipient procedures can be completed in an average of 90 min. This is a significant improvement compared with previously reported methods in which extended duration of recipient anesthesia was a key determinant for success and perioperative survival; making the necessity of a two-surgeon approach a prerequisite 31-33. Moreover, minimal to no bleeding from the anastomosis further contributes to significantly reduced blood loss and thereby reduces the need for fluid resuscitation of the recipients, as described previously as another determinant for success in this model 33. Finally, the cuff technique represents a method with a significant cost advantage compared to the high costs of 11-0 micro-suture material. Thus, the non-suture cuff technique represents the most critical step of the underlying protocol. In an elegant study by Tung et al., various different myo- and osteomyocutaneous flaps including a vascularized groin skin flap have been described based off of the mouse femoral vessel pedicle 32. While the method presented here focuses exclusively on the transplantation of the entire mouse hind limb, the presented principles may be easily translated and employed in various other anatomical flap designs. Noteworthy is the fact that while previous authors report automutilation or autophagy of the graft as a significant complication during post-operative survival 31,32 this has not been observed in our experience. Additionally, rapid recovery and limited impairment of the animal's capability of feeding and grooming underlines that the orthotopic inset of the transplanted leg inflicts only minimal recipient morbidity.
The model described in this video publication additionally introduces distinct advantages to the mouse setting compared to previously published techniques. First and foremost, as reported previously by our group 16, the cuff technique can be employed in vessels with a lumen diameter smaller than 1 millimeter, allowing for orthotopic transplantation of a hind limb allograft. This approach provides the basis for employing this model combined in both immunological as well as functional studies of nerve regeneration related to VCA 16,25. Nerve regeneration is of key importance to the field of reconstructive transplantation, as these are non-life saving procedures whose success is primarily determined by the restoration of functional and aesthetic defects.
The heterotopic inset of an osteomyocutaneous flap originating from the hind limb is an additional model available to the surgeon15. In this approach however, surgical removal of structures of potential interest, such as the nails and the glabrous skin of the footpad, limits the versatility of this method compared with the orthotopic transplantation of an anatomically unaltered hind limb allograft. This is of particular interest in reconstructive transplantation as these structures have been described as the target of atypical rejection processes in patients inflicting mechanical stress to their hand allografts 34. Inherently unique to a vascularized composite tissue allograft is the bone component, as vascularized bone contains viable bone marrow and is a constantly renewing source of components and precursors of the donor immune system. While this was cautiously regarded as a possible source for graft versus host disease (GvHD), this has not been observed in both animal models 35,36 as well as humans 37. Much to the contrary actually, as shown in preclinical 36 and clinical studies 38-40, the combination of organ transplantation with donor bone marrow transplantation or the transfusion of selected bone marrow-derived cell products actually shows advantageous effects regarding the amount of immunosuppression needed 21; some protocols have even shown operational tolerance without the need for drug-based immunosuppression 41.
The model and methodology shown by this publication yielded 100% long-term animal survival in syngeneic donor - recipient combinations (Figure 1) as well as demonstrated a well characterized patterns of graft acceptance and rejection in the allogeneic strain combinations as outlined in Figures 2 & 3. Moreover, the skin as a visible primary target of rejection in VCA, follows a reproducible pattern of 4 distinct clinical scenarios of rejection correlating with those for human VCA as outlined by the Banff 2007 working classification of skin-containing VCA 42, making this model particularly translatable. Thus, this highly reliable mouse model for reconstructive transplantation above all, introduces the availability of a versatile immunological model system of genetically defined inbred and transgenic mouse strains which opens the possibilities for diagnostic and interventional studies with translational impact on clinical VCA.
The need for basic microsurgical skills prior to embarking on mastering the described technique may be regarded as a limitation to this model, however, it is the same main obstacle to any mouse microsurgical model. Thus, this video protocol is primarily intended to provide the seasoned microsurgeon with an alternative approach to microvascular anastomosis in a murine in vivo model for vascularized composite allotransplantation. Furthermore, the model allows for transplantation of an intact vascularized bone marrow component, as well as the use of a functional model in nerve regeneration research.
In conclusion, we established a novel, versatile, and reliable mouse model for orthotopic hind limb transplantation using a non-suture cuff technique that opens the door to basic mechanistic, as well as translational research related to any aspect of VCA.
Disclosures
The authors declare that they have no competing financial interest.
Acknowledgments
This work was supported by the Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort, under Award No. W81XWH-13-2-0053. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
The authors would like to thank Jessica Izzi, D.V.M, Caroline Garrett, D.V.M. and Julie Watson, D.V.M. for their excellent veterinary support during this study.
References
- Khalifian S, et al. Facial transplantation: the first 9 years. Lancet. 2014. [DOI] [PubMed]
- Petruzzo P, Dubernard JM. The International Registry on Hand and Composite Tissue allotransplantation. Clin. Transpl. 2011. pp. 247–253. [PubMed]
- Shores JT, Brandacher G, Lee WA. Hand and Upper Extremity Transplantation: An Update of Outcomes in the Worldwide Experience. Plast. Reconstr. Surg. 2014. [DOI] [PubMed]
- Levi DM, et al. Transplantation of the abdominal wall. Lancet. 2003;361:2173–2176. doi: 10.1016/S0140-6736(03)13769-5. [DOI] [PubMed] [Google Scholar]
- Strome M, et al. Laryngeal transplantation and 40-month follow-up. N.Engl.J. Med. 2001;344:1676–1679. doi: 10.1056/NEJM200105313442204. [DOI] [PubMed] [Google Scholar]
- Rose KG, Sesterhenn K, Wustrow F. Tracheal allotransplantation in man. Lancet. 1979;1:433. doi: 10.1016/s0140-6736(79)90902-4. [DOI] [PubMed] [Google Scholar]
- Hofmann GO, et al. Allogeneic vascularized transplantation of human femoral diaphyses and total knee joints--first clinical experiences. Transplant. Proc. 1998;30:2754–2761. doi: 10.1016/s0041-1345(98)00803-3. [DOI] [PubMed] [Google Scholar]
- Hu W, et al. A preliminary report of penile transplantation. Eur. Urol. 2006;50:851–853. doi: 10.1016/j.eururo.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, et al. Livebirth after uterus transplantation. Lancet. 2014. [DOI] [PubMed]
- Sarhane KA, et al. Diagnosing skin rejection in vascularized composite allotransplantation: advances and challenges. Clin. Transplant. 2014;28:277–285. doi: 10.1111/ctr.12316. [DOI] [PubMed] [Google Scholar]
- Schneeberger S, Khalifian S, Brandacher G. Immunosuppression and monitoring of rejection in hand transplantation. Tech. Hand Up. Extrem. Surg. 2013;17:208–214. doi: 10.1097/BTH.0000000000000019. [DOI] [PubMed] [Google Scholar]
- Lee WP, et al. Relative antigenicity of components of a vascularized limb allograft. Plast. Reconstr. Surg. 1991;87:401–411. doi: 10.1097/00006534-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Sucher R, et al. Hemiface allotransplantation in the mouse. Plast. Reconstr. Surg. 2012;129:867–870. doi: 10.1097/PRS.0b013e3182450aff. [DOI] [PubMed] [Google Scholar]
- Ibrahim Z, et al. A modified heterotopic swine hind limb transplant model for translational vascularized composite allotransplantation (VCA) research. J Vis Exp. 2013. [DOI] [PMC free article] [PubMed]
- Oberhuber R, et al. Murine cervical heart transplantation model using a modified cuff technique. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed]
- Sucher R, et al. Mouse hind limb transplantation: a new composite tissue allotransplantation model using nonsuture supermicrosurgery. Transplantation. 2010;90:1374–1380. doi: 10.1097/TP.0b013e3181ff4fc3. [DOI] [PubMed] [Google Scholar]
- Maglione M, et al. A novel technique for heterotopic vascularized pancreas transplantation in mice to assess ischemia reperfusion injury and graft pancreatitis. Surgery. 2007;141:682–689. doi: 10.1016/j.surg.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Sucher R, et al. Orthotopic hind-limb transplantation in rats. J Vis Exp. 2010. [DOI] [PMC free article] [PubMed]
- Zou Y, Brandacher G, Margreiter R, Steurer W. Cervical heterotopic arterialized liver transplantation in the mouse. J. Surg. Res. 2000;93:97–100. doi: 10.1006/jsre.2000.5964. [DOI] [PubMed] [Google Scholar]
- Zhang F, et al. Development of a mouse limb transplantation model. Microsurgery. 1999;19:209–213. doi: 10.1002/(sici)1098-2752(1999)19:5<209::aid-micr1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Schneeberger S, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann. Surg. 2013;257:345–351. doi: 10.1097/SLA.0b013e31826d90bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari K, Brandacher G. Vascularized composite allotransplantation. Curr Opin Organ Transplant. 2013;18:631–632. doi: 10.1097/MOT.0000000000000028. [DOI] [PubMed] [Google Scholar]
- Pomahac B, Gobble RM, Schneeberger S. Facial and hand allotransplantation. Cold Spring Harb. Perspect. Med. 2014;4 doi: 10.1101/cshperspect.a015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong AS, Alegre ML, Miller ML, Fairchild RL. Lessons and limits of mouse models. Cold Spring Harb. Perspect. Med. 2013;3:a015495. doi: 10.1101/cshperspect.a015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, et al. The neck as a preferred recipient site for vascularized composite allotransplantation in the mouse. Plast. Reconstr. Surg. 2014;133:133e–141e. doi: 10.1097/01.prs.0000437229.69811.3a. [DOI] [PubMed] [Google Scholar]
- Shapiro RI, Cerra FB. A model for reimplantation and transplantation of a complex organ: the rat hind limb. J. Surg. Res. 1978;24:501–506. doi: 10.1016/0022-4804(78)90048-3. [DOI] [PubMed] [Google Scholar]
- Leto Barone AA, et al. The gracilis myocutaneous free flap in swine: an advantageous preclinical model for vascularized composite allograft transplantation research. Microsurgery. 2013;33:51–55. doi: 10.1002/micr.21997. [DOI] [PubMed] [Google Scholar]
- Mathes DW, et al. A preclinical canine model for composite tissue transplantation. J. Reconstr. Microsurg. 2010;26:201–207. doi: 10.1055/s-0030-1247717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth RN, et al. Prolonged survival of composite facial allografts in non-human primates associated with posttransplant lymphoproliferative disorder. Transplantation. 2009;88:1242–1250. doi: 10.1097/TP.0b013e3181c1b6d0. [DOI] [PubMed] [Google Scholar]
- Brandacher G, Grahammer J, Sucher R, Lee WP. Animal models for basic and translational research in reconstructive transplantation. Birth Defects Res C Embryo Today. 2012;96:39–50. doi: 10.1002/bdrc.21002. [DOI] [PubMed] [Google Scholar]
- Foster RD, Liu T. Orthotopic hindlimb transplantation in the mouse. J. Reconstr. Microsurg. 2002;19:49. doi: 10.1055/s-2003-37191. [DOI] [PubMed] [Google Scholar]
- Tung TH, Mohanakumar T, Mackinnon SE. Development of a Mouse Model for Heterotopic Limb and Composite-Tissue Transplantation. J. Reconstr. Microsurg. 2001;17:267–274. doi: 10.1055/s-2001-14520. [DOI] [PubMed] [Google Scholar]
- Zhang F, Shi DY, Kryger Z, Moon W. Development of a mouse limb transplantation model. Microsurgery. 1999;19(5):209–213. doi: 10.1002/(sici)1098-2752(1999)19:5<209::aid-micr1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Schneeberger S, et al. Atypical acute rejection after hand transplantation. Am. J. Transplant. 2008;8:688–696. doi: 10.1111/j.1600-6143.2007.02105.x. [DOI] [PubMed] [Google Scholar]
- Mathes DW, et al. Stable mixed hematopoietic chimerism permits tolerance of vascularized composite allografts across a full major histocompatibility mismatch in swine. Transpl. Int. 2014;27:1086–1096. doi: 10.1111/tri.12380. [DOI] [PubMed] [Google Scholar]
- Yamada Y, et al. Use of CTLA4Ig for induction of mixed chimerism and renal allograft tolerance in nonhuman primates. Am. J. Transplant. 2014;14:2704–2712. doi: 10.1111/ajt.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism. Cold Spring Harb. Perspect. Med. 2014;4:a015529. doi: 10.1101/cshperspect.a015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Sachs DH. Tolerance induction: hematopoietic chimerism. Curr Opin Organ Transplant. 2013;18:402–407. doi: 10.1097/MOT.0b013e328363621d. [DOI] [PubMed] [Google Scholar]
- Kawai T, Sachs DH, Sykes M, Cosimi A.B. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2013;368:1850–1852. doi: 10.1056/NEJMc1213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci. Transl. Med. 2012;4:124–128. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendales LC, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am. J. Transplant. 2008;8:1396–1400. doi: 10.1111/j.1600-6143.2008.02243.x. [DOI] [PubMed] [Google Scholar]


