Abstract
We demonstrate a method to fabricate highly sensitive surface-enhanced Raman spectroscopic (SERS) substrates using a filter syringe system that can be applied to the detection of various chemical contaminants. Silver nanoparticles (Ag NPs) are synthesized via reduction of silver nitrate by sodium citrate. Then the NPs are aggregated by sodium chloride to form nanoclusters that could be trapped in the pores of the filter membrane. A syringe is connected to the filter holder, with a filter membrane inside. By loading the nanoclusters into the syringe and passing through the membrane, the liquid goes through the membrane but not the nanoclusters, forming a SERS-active membrane. When testing the analyte, the liquid sample is loaded into the syringe and flowed through the Ag NPs coated membrane. The analyte binds and concentrates on the Ag NPs coated membrane. Then the membrane is detached from the filter holder, air dried and measured by a Raman instrument. Here we present the study of the volume effect of Ag NPs and sample on the detection sensitivity as well as the detection of 10 ppb ferbam and 1 ppm ampicillin using the developed assay.
Keywords: Chemistry, Issue 108, Silver nanoparticles, SERS, filter syringe, ferbam, ampicillin
Introduction
Surface enhanced Raman spectroscopy (SERS) is a technique combining Raman spectroscopy with nanotechnology. The intensity of Raman scattering of analytes at noble metallic nano-surfaces is greatly enhanced by the localized surface plasmon resonance.1 Silver nanoparticles (Ag NPs) are by far the most widely used SERS substrates due to its high enhancement ability.2 Up to now, various synthetic methods of Ag NPs have been developed.3-6 Ag NPs can be used alone as effective SERS substrates, or combined with other materials and structures to enhance its sensitivity and/or functionality.7-11
SERS techniques have demonstrated great capacity for detection of various trace amount contaminants in food and environmental samples.12 Traditionally, there are two common ways for preparing a SERS sample: solution-based and substrate-based methods.13 Thesolution-based method uses NP colloids to mix with samples. Then the NP-analyte complex is collected using centrifugation, and deposited onto a solid support for Raman measurement after drying. The substrate-based method is usually applied by depositing several microliters of liquid sample onto the pre-fabricated solid substrate.14 However, neither of these two methods are effective and applicable for a large amount of sample volume. Several modifications of the SERS assays overcame the volume limits, such as the integration of a filter system15-21 or the incorporation of a microfluidic device.21-24 The modified SERS assays have shown great enhancement in sensitivity and feasibility for monitoring the chemical contaminants in large water samples.
Here we demonstrate the detailed protocol of fabrication and application of a syringe filter based SERS method to detect trace amount of pesticide ferbam and antibiotic ampicillin.
Protocol
1. Silver Nanoparticle Synthesis15
Dissolve 18 mg silver nitrate in 100 ml ultrapure water (18.2 ΩU) and vortex for 5 sec.
Dissolve 27 mg sodium citrate dihydrate in 1 ml water and vortex for 5 sec.
Transfer all of the prepared silver nitrate solution to a conical flask containing a stirring bar and put the flask on a magnetic hot plate. Heat the flask under vigorous stirring with a stirring speed of 700 rpm at ~350 °C (setting temperature on the plate).
When boiling, add all of the prepared sodium citrate solution to the conical flask immediately, and leave the solution to boil for an additional 25 min until the solution turns greenish brown, which indicates the formation of Ag NPs.
Remove the flask from the hot plate and put it on another magnetic plate (do not heat) and stir O/N at the same stirring speed at RT until the mixture reaches a stable state, with a constant color and transparency. Use a UV-vis spectrometer to determine the absorbance of the prepared Ag NPs if necessary.
Dilute the final mixture with ultrapure water to 100 ml.
Use a Zetasizer to measure the size of the Ag NPs if necessary according to manufacturer's protocol.
Transfer the Ag colloid to a sealed container and protect it from light with aluminum foil. The colloid can be stored in a refrigerator at 4-7 °C for 2 months if needed.
2. Fabrication of a SERS Active Filter Membrane
Dissolve 2.92 g sodium chloride (NaCl) in 100 ml water to make a 50 mM NaCl solution.
Add 1 ml of the 5 mM NaCl solution into 1 ml of the prepared Ag NPs and mix them on a nutating mixer for 10 min at 20 rpm. This step is to aggregate the Ag NPs into Ag nanoclusters.
Place a filter membrane (PVDF, 0.1 µm pore size) into a filter holder, which can be attached to a syringe. The smaller pore size membrane was found more effective than the larger pore size membrane (i.e., 0.22 µm) in trapping Ag nanoclusters and producing consistent signals.
Load 2 ml of the prepared Ag nanoclusters into the syringe for filtration. Attach the filter holder to the syringe and manually pass the entire volume of Ag nanoclusters through the membrane at the flow rate of 1 drop/sec. The membrane traps Ag nanoclusters, forming a SERS-active filter membrane.
Detach the filter membrane from the filter holder. Special caution is needed when holding the membrane on the outer rim using a pair of tweezers to ensure no damage to the membrane. Air dry for about 3 min and place membrane on a glass slide.
- Raman detection of the SERS substrate
- Set the Raman instrument to a 780 nm wavelength laser with a laser power of 5 mW, exposure time of 1 sec and exposure number of 2. Set the microscopic objective to 10X. Make sure the objective on the software is set accordingly too.
- Place the glass slide with the membrane on top onto the platform of the Raman instrument and use the microscope to focus on the surface of the membrane.
- Randomly select 8-10 spots from the membrane surface and the instrument will collect them automatically in sequence. Open spectral data in manufacturer's software for analysis.
3. Application of the SERS Active Filter System to Detect Chemical Contaminants
- Prepare a 10 ppb ferbam solution. Caution: Ferbam is highly volatile. Use precautions (respirator and goggles) when weighing the solid.
- Weigh 2 mg ferbam powder and dissolve it in 20 ml 50% acetonitrile (10 ml acetonitrile and 10 ml water) to make a stock solution (100 ppm). Vortex the flask for 30 sec.
- Take 1 ml of the 100 ppm ferbam solution in a test tube and add 9 ml 50% acetonitrile to make a 10 ppm solution. Vortex the tube for 5 sec.
- Take 1 ml of the 10 ppm solution in a test tube and add 9 ml 50% acetonitrile to make a 1 ppm solution. Vortex the tube for 5 sec.
- Take 1 ml of the 1 ppm solution in a test tube and add 9 ml 50% acetonitrile to make a 100 ppb solution. Vortex the tube for 5 sec.
- Take 1 ml of the 100 ppb solution in a test tube and add 9 ml 50% acetonitrile to make a 10 ppb solution. Vortex the tube for 5 sec.
- Prepare a 1 ppm ampicillin solution.
- Weigh 10 mg ampicillin powder and dissolve it in 100 ml water to make a 100 ppm ampicillin solution. Vortex the flask for 30 sec.
- Take 1 ml of the 100 ppm solution in a test tube and add 9 ml water to make a 10 ppm ampicillin solution. Vortex the tube for 5 sec.
- Take 1 ml of the 10 ppm solution in a test tube and add 9 ml water to make a 1 ppm ampicillin solution. Vortex the tube for 5 sec.
Put the filter membrane back to the filter holder, with the NP coated side facing up.
Load 5 ml of one sample into a new syringe, and then attach it to the filter holder with a Ag coated membrane inside.
Manually pass the entire volume of sample through the membrane at the flow rate of 1 drop/sec. Target molecules can be adsorbed and concentrated onto the NPs coated on the filter membrane.
Detach filter membrane from the filter holder, air dry for about 3 min and measure the signals using the Raman instrument using the same method as described in step 2.6.
Repeat step 2.2 to 2.6 to prepare another Ag-coated membrane, and follow from step 3.3 for the detection of the other sample.
Representative Results
The major steps of this experiment were shown in the schematic diagram (Figure 1). Figure 2 demonstrated the importance to use the optimized volume of AgNPs in the membrane coating in order to reach the maximized sensitivity. 1 ml of Ag NPs provides the strongest signal when using ferbam, as compared to 0.5 ml (insufficient coating) or 2 ml (too much coating).
We were able to detect ferbam at 10 ppb level and ampicillin at 1 ppm with great signal intensity by the developed filter-based SERS assay (Figure 1). The SERS spectrum of ferbam exhibits distinct characteristic peaks at 10 ppb. The peak at 1,386 cm-1 is from the mixed vibration of CN and C=S stretching, and symmetric CH3 deformation. The peak at 1,516 cm-1 is associated with CH3 and CN stretching. The peak at 561 cm-1 is generated by SS streching.25-27 The spectrum of 1 ppm ampicillin was also clearly detected. The peak at 1,594 cm-1 and 1,447 cm-1 are from C=C stretching and CH3/CH2 deformation, respectively. The strong peak at 1,001 cm-1 is from the benzene ring vibration. The peak at 852 cm-1 is associated with symmetric CNC stretching.28-29 The experimental time for analyzing one sample is less than 20 min including the fabrication of SERS-active filter membrane with pre-synthesized Ag NPs.
With increasing sample volume, we can further increase the detection limit, as shown in Figure 4. We observed an increase of peak intensity when increasing the sample volume. This is the advantage of the filter based method as the volume is adjustable and the limit of detection is also adjustable.
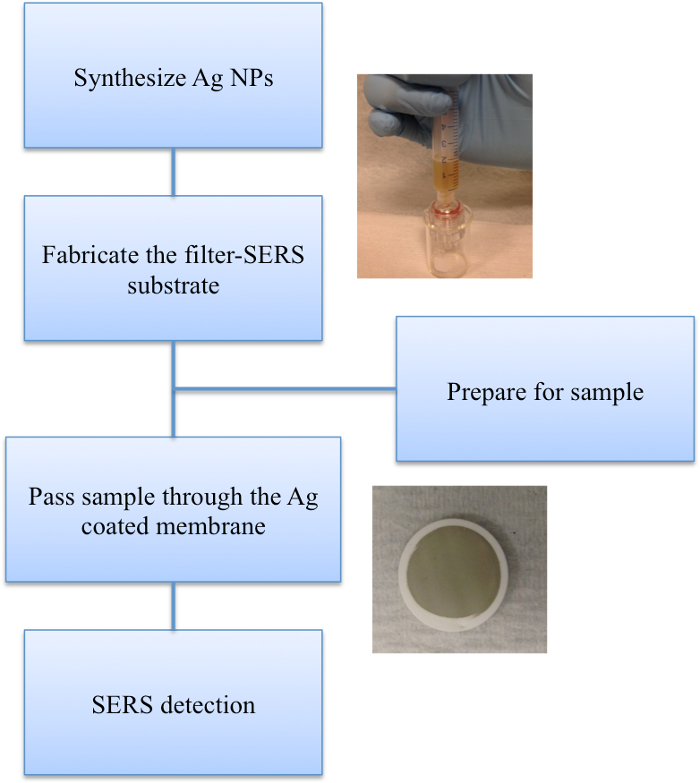 Figure 1.
A schematic diagram of filter SERS assay.
Please click here to view a larger version of this figure.
Figure 1.
A schematic diagram of filter SERS assay.
Please click here to view a larger version of this figure.
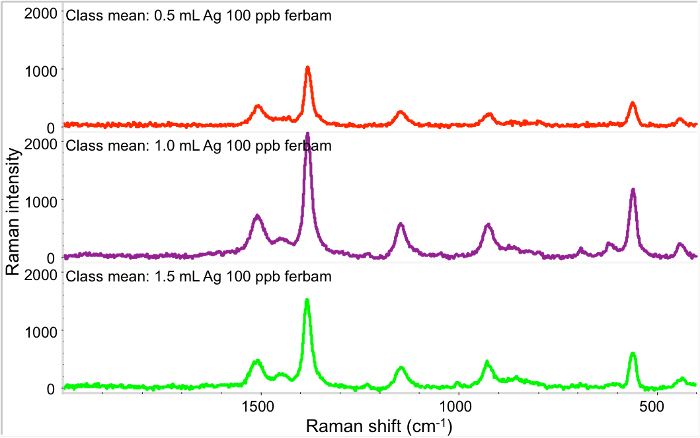 Figure 2.SERS spectra of 5 ml 100 ppb ferbam passing through the membranes coated by different amount of Ag NPs. From top to bottom: 0.5 ml Ag colloid with 0.5 ml NaCl, 1.0 ml Ag with 1.0 ml NaCl, 1.5 ml Ag with 1.5 ml NaCl, respectively. Please click here to view a larger version of this figure.
Figure 2.SERS spectra of 5 ml 100 ppb ferbam passing through the membranes coated by different amount of Ag NPs. From top to bottom: 0.5 ml Ag colloid with 0.5 ml NaCl, 1.0 ml Ag with 1.0 ml NaCl, 1.5 ml Ag with 1.5 ml NaCl, respectively. Please click here to view a larger version of this figure.
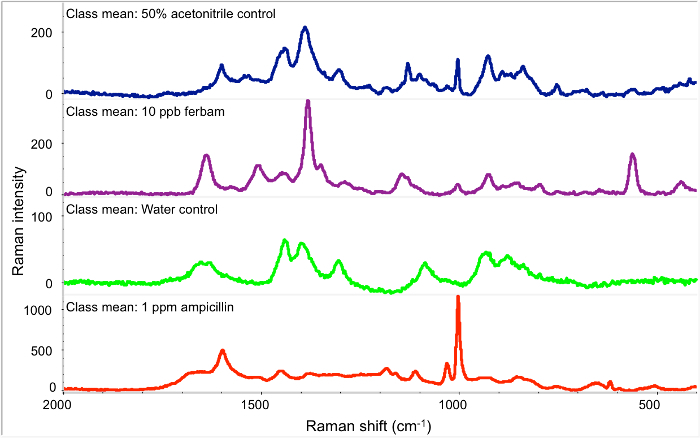 Figure 3.SERS spectra of ferbam and ampicillin on Ag NPs coated filter membrane. From top to bottom: control of 50% acetonitrile, 10 ppb ferbam, control of water, 1 ppm Ampicillin, respectively. Please click here to view a larger version of this figure.
Figure 3.SERS spectra of ferbam and ampicillin on Ag NPs coated filter membrane. From top to bottom: control of 50% acetonitrile, 10 ppb ferbam, control of water, 1 ppm Ampicillin, respectively. Please click here to view a larger version of this figure.
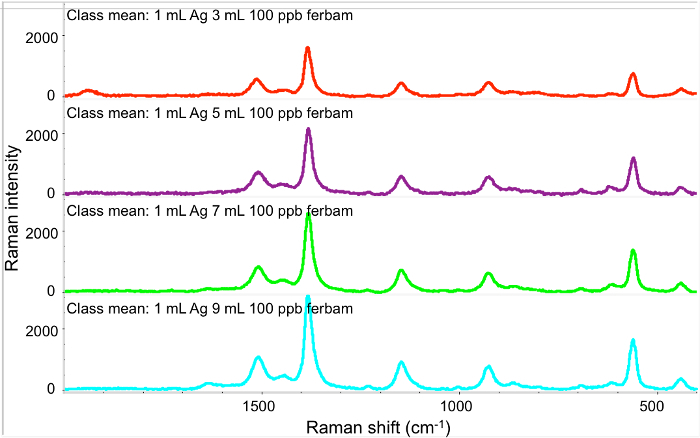 Figure 4.SERS spectra of different volumes of 100 ppb ferbam on Ag NPs coated filter membrane. From top to bottom: 3 ml ferbam, 5 ml ferbam, 7 ml ferbam, 9 ml ferbam, respectively. Please click here to view a larger version of this figure.
Figure 4.SERS spectra of different volumes of 100 ppb ferbam on Ag NPs coated filter membrane. From top to bottom: 3 ml ferbam, 5 ml ferbam, 7 ml ferbam, 9 ml ferbam, respectively. Please click here to view a larger version of this figure.
Discussion
One of the critical steps in this protocol is the Ag NPs synthesis, where uniform Ag NPs are the key for consistent results. The heating time and the concentrations of precursors must be precisely controlled. The average size of this AgNPs preparation is 80 nm, which was measured by the Zetasizer (data not shown). Another critical step is the salt aggregation where the salt concentration and aggregation time must be precisely controlled. In addition, the choice of membrane is also critical as the membrane with a smaller pore size was found more effective to trap Ag nanoclusters. For the particular membrane used in this study, there is a front and back side where the front side must be placed up in the holder to connect the syringe. If it was placed down, the coating was much less effective. Avoiding bubbles when passing through the membrane is another key to a successful coating.
For troubleshooting of this assay, the following steps are recommended. If no or little signal is detected, check for the following causes. The main cause could be the Ag NPs are not aggregated enough to be trapped in the pores of the filter membrane. Increasing the salt concentration and/or incubation time can enhance the aggregation. Otherwise, check that the backside of the filter membrane is facing up and that the volume or concentration of sample loaded on to the membrane is not too low. If the signal of the target molecule is not consistent, check for the following causes: the size distribution of the Ag NPs may be too broad or the NPs are not evenly distributed on the membrane, probably due to too much aggregation of NPs or too fast passing through the membrane.
Compared with our previous data on using Ag dendrites as SERS substrate,30-31 the sensitivity of this filter-based SERS assay is much higher on ferbam detection. This is due to the advantage of the filter-based system, which can flow large amount of sample, so that more analyte molecules are concentrated onto the SERS substrate. Another advantage of using the filter-based system over the solution-based method is the ease of operation and fieldable measurement, as no centrifugation is needed to collect the NP-analyte complex. The limitation of this method is it cannot be used for complex liquid matrices such as milk directly, as the complex components can block the membrane pores. Pretreatment is needed to remove interfering components before passing the membrane.
In summary, we demonstrate a simple and sensitive filter-based SERS assay, which could be applied to the detection of contaminants or adulterations in liquid food matrix and environmental samples. To further push the limit of detection, optimization of the parameters, such as NP sizes and amount, salt concentration, sample volume and instrument parameters is needed.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This material is based upon work supported by the U.S. Department of Homeland Security under Grant Award Number 2010-ST-061-FD0001 through a grant awarded by the National Center for Food Protection and Defense at the University of Minnesota. Disclaimer: The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the U.S. Department of Homeland Security or the National Center for Food Protection and Defense.
References
- Albrecht MG, Creighton JA. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977;99(15):5215–5217. [Google Scholar]
- Schatz GC, Young MA, Van Duyne RP. Electromagnetic mechanism of SERS. Surface-enhanced Raman scattering. Springer Berlin Heidelberg; 2006. pp. 19–45. [Google Scholar]
- Matijevic E. Preparation and properties of uniform size colloids. Chem. Mater. 1993;5(4):412–426. [Google Scholar]
- Nickel U, zu Castell A, Pöppl K, Schneider S. A silver colloid produced by reduction with hydrazine as support for highly sensitive surface-enhanced Raman spectroscopy. Langmuir. 2000;16(23):9087–9091. [Google Scholar]
- Khanna PK, Subbarao VVVS. Nanosized silver powder via reduction of silver nitrate by sodium formaldehydesulfoxylate in acidic pH medium. Mater. Lett. 2003;57(15):2242–2245. [Google Scholar]
- Henglein A, Giersig M. Formation of colloidal silver nanoparticles: capping action of citrate. J. Phys. Chem. B. 1999;103(44):9533–9539. [Google Scholar]
- Sun X, Li Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Edit. 2004;43(5):597–601. doi: 10.1002/anie.200352386. [DOI] [PubMed] [Google Scholar]
- Lu L, et al. Seed-mediated growth of large, monodisperse core-shell gold-silver nanoparticles with Ag-like optical properties. Chem. Commun. 2002. pp. 144–145. [DOI] [PubMed]
- Aslan K, Wu M, Lakowicz JR, Geddes CD. Fluorescent core-shell Ag@SiO2 nanocomposites for metal-enhanced fluorescence and single nanoparticle sensing platforms. J. Am. Chem. Soc. 2007;129(6):1524–1525. doi: 10.1021/ja0680820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Yin Y, Li ZY, Xia Y. Synthesis and self-assembly of Au@ SiO2 core-shell colloids. Nano. Lett. 2002;2(7):785–788. [Google Scholar]
- Link S, Wang ZL, El-Sayed MA. Alloy formation of gold-silver nanoparticles and the dependence of the plasmon absorption on their composition. J. Phys. Chem. B. 1999;103(18):3529–3533. [Google Scholar]
- He L, et al. Rapid Detection of Ricin in Milk Using Immunomagnetic Separation Combined with Surface Enhanced Raman Spectroscopy. J. Food. Sci. 2011;76(5):N49–N53. doi: 10.1111/j.1750-3841.2011.02196.x. [DOI] [PubMed] [Google Scholar]
- Zheng J, He L. Surface-Enhanced Raman Spectroscopy for the Chemical Analysis of Food. Compr. Rev. Food. Sci. F. 2014;13(3):317–328. doi: 10.1111/1541-4337.12062. [DOI] [PubMed] [Google Scholar]
- He L, Haynes CL, Diez-Gonzalez F, Labuza TP. Rapid detection of a foreign protein in milk using IMS-SERS. J. Raman. Spectrosc. 2011;42(6):1428–1434. [Google Scholar]
- Wei WY, White IM. A simple filter-based approach to surface enhanced Raman spectroscopy for trace chemical detection. Analyst. 2012;137(5):1168–1173. doi: 10.1039/c2an15947c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ML, Tsai BC, Yang J. Silver nanoparticle-treated filter paper as a highly sensitive surface-enhanced Raman scattering (SERS) substrate for detection of tyrosine in aqueous solution. Anal. Chim. Acta. 2011;708(1):89–96. doi: 10.1016/j.aca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Fierro-Mercado PM, Hernández-Rivera SP. Highly sensitive filter paper substrate for SERS trace explosives detection. Int. J. Spectrosc. 2012;2012:716527. [Google Scholar]
- Tran CD. Subnanogram detection of dyes on filter paper by surface-enhanced Raman scattering spectrometry. Anal. Chem. 1984;56(4):824–826. [Google Scholar]
- Wu D, Fang Y. The adsorption behavior of p-hydroxybenzoic acid on a silver-coated filter paper by surface enhanced Raman scattering. J. Colloid Interface Sci. 2003;265(2):234–238. doi: 10.1016/s0021-9797(03)00348-5. [DOI] [PubMed] [Google Scholar]
- Wigginton KR, Vikesland PJ. Gold-coated polycarbonate membrane filter for pathogen concentration and SERS-based detection. Analyst. 2010;135(6):1320–1326. doi: 10.1039/b919270k. [DOI] [PubMed] [Google Scholar]
- Berthod A, Laserna JJ, Winefordner JD. Analysis by surface enhanced Raman spectroscopy on silver hydrosols and silver coated filter papers. J Pharm Biomed Anal. 1988;6(6):599–608. doi: 10.1016/0731-7085(88)80073-6. [DOI] [PubMed] [Google Scholar]
- Ackermann KR, Henkel T, Popp J. Quantitative Online Detection of Low-Concentrated Drugs via a SERS Microfluidic System. ChemPhysChem. 2007;8(18):2665–2670. doi: 10.1002/cphc.200700554. [DOI] [PubMed] [Google Scholar]
- Walter A, März A, Schumacher W, Rösch P, Popp J. Towards a fast, high specific and reliable discrimination of bacteria on strain level by means of SERS in a microfluidic device. Lab. Chip. 2011;11(6):1013–1021. doi: 10.1039/c0lc00536c. [DOI] [PubMed] [Google Scholar]
- Lee S, et al. Fast and sensitive trace analysis of malachite green using a surface-enhanced Raman microfluidic sensor. Anal. Chim. Acta. 2007;590(2):139–144. doi: 10.1016/j.aca.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Guo H, et al. Analysis of Silver Nanoparticles in Antimicrobial Products Using Surface-Enhanced Raman Spectroscopy (SERS) Environ. Sci. Technol. 2015;49(7):4317–4324. doi: 10.1021/acs.est.5b00370. [DOI] [PubMed] [Google Scholar]
- Narayanan VA, Begun GM, Stokes DL, Sutherland WS, Vo-Dinh T. Normal Raman and surface enhanced Raman scattering (SERS) spectra of some fungicides and related chemical compounds. J. Raman. Spectrosc. 1992;23(5):281–286. [Google Scholar]
- Kang JS, Hwang SY, Lee CJ, Lee MS. SERS of dithiocarbamate pesticides adsorbed on silver surface; thiram. Bull. Korean. Chem. Soc. 2002;23(11):1604–1610. [Google Scholar]
- Li YT, et al. Rapid and sensitive in-situ detection of polar antibiotics in water using a disposable Ag-graphene sensor based on electrophoretic preconcentration and surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2013;43:94–100. doi: 10.1016/j.bios.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Clarke SJ, Littleford RE, Smith WE, Goodacre R. Rapid monitoring of antibiotics using Raman and surface enhanced Raman spectroscopy. Analyst. 2005;130(7):1019–1026. doi: 10.1039/b502540k. [DOI] [PubMed] [Google Scholar]
- Zheng J, Pang S, Labuza TP, He L. Semi-quantification of surface-enhanced Raman scattering using a handheld Raman spectrometer: a feasibility study. Analyst. 2013;138(23):7075–7078. doi: 10.1039/c3an01450a. [DOI] [PubMed] [Google Scholar]
- Zheng J, Pang S, Labuza TP, He L. Evaluation of surface-enhanced Raman scattering detection using a handheld and a bench-top Raman spectrometer: A comparative study. Talanta. 2014;129:79–85. doi: 10.1016/j.talanta.2014.05.015. [DOI] [PubMed] [Google Scholar]


