Abstract
The autonomic nervous system (ANS) consists of two branches, the parasympathetic and sympathetic nervous systems, and controls the function of internal organs (e.g., heart rate, respiration, digestion) and responds to everyday and adverse experiences 1. ANS measures in children have been found to be related to behavior problems, emotion regulation, and health 2-7. Therefore, understanding the factors that affect ANS development during early childhood is important. Both branches of the ANS affect young children’s cardiovascular responses to stimuli and have been measured noninvasively, via external monitoring equipment, using valid and reliable measures of physiological change 8-11. However, there are few studies of very young children with simultaneous measures of the parasympathetic and sympathetic nervous systems, which limits understanding of the integrated functioning of the two systems. In addition, the majority of existing studies of young children report on infants’ resting ANS measures or their reactivity to commonly used mother-child interaction paradigms, and less is known about ANS reactivity to other challenging conditions. We present a study design and standardized protocol for a non-invasive and rapid assessment of cardiac autonomic control in 18 month old children. We describe methods for continuous monitoring of the parasympathetic and sympathetic branches of the ANS under resting and challenge conditions during a home or laboratory visit and provide descriptive findings from our sample of 140 ethnically diverse toddlers using validated equipment and scoring software. Results revealed that this protocol can produce a range of physiological responses to both resting and developmentally challenging conditions, as indicated by changes in heart rate and indices of parasympathetic and sympathetic activity. Individuals demonstrated variability in resting levels, responses to challenges, and challenge reactivity, which provides additional evidence that this protocol is useful for the examination of ANS individual differences for toddlers.
Keywords: Medicine, Issue 108, physiology, pediatrics, nursing, psychology, cardiology, sympathetic nervous system, parasympathetic nervous system, autonomic nervous system (ANS), stress reactivity, childhood, electrocardiogram, cardiac impedance
Introduction
Individual differences in children’s autonomic nervous system (ANS) reactivity play an important role in the development and maintenance of physical and mental health problems 12-16. A growing body of evidence reveals that individual differences in ANS measures at rest and reactivity are predictive of variations in children’s internalizing and externalizing psychopathology 4,9,17-19 and physical health 7,20. In addition to these direct or main effects of ANS functioning, an accumulating body of studies have found that children’s reactivity can interact with contextual risk factors, such as adverse events or marital conflict, to moderate those effects on children’s wellbeing and health (see reference 21 for a review). Despite this growing evidence that children’s autonomic reactivity plays a role in health across the life course, and the accompanying interest in those processes, there is need for additional research examining the development of ANS functioning in children under three years of age.
The ANS consists of two branches, the parasympathetic and sympathetic nervous systems. It regulates the function of internal organs (e.g., heart rate, respiration, digestion, and sexual arousal), largely through unconscious mechanisms, in response to everyday and adverse experiences 1. The parasympathetic nervous system (PNS) is the ‘rest and restorative’ system that maintains a low resting heart rate and restorative state when sleeping or relaxing The sympathetic nervous system (SNS) is the ‘fight or flight’ system that responds to emergencies or threatening situations by accelerating one’s heart rate 23. Respiratory sinus arrhythmia (RSA) is a reliable index of the PNS influence on cardiac functioning, and pre-ejection period (PEP) is a reliable index of the SNS influence on cardiac functioning. Both RSA and PEP have been found to be valid measures through experimental pharmacological blockade in samples of adults 24,25. More recently, this work has been extended into child and adolescent samples, further establishing RSA and PEP as valid and reliable measures of ANS activity throughout development 8,26,27. Reliable impedance cardiography measurements (PEP) using band and spot electrodes have been used for considerable time in adult samples 28. More recently, parallel measurement and analytic techniques have also been shown to be reliable and valid in samples of children 11,29,30, although collection of PEP measures in young children is rare.
There is an extant large body of literature on PNS functioning in infants and young children, including vagal tone and respiratory sinus arrhythmia measures. There are fewer studies of the SNS using PEP, and very few simultaneously examining both the parasympathetic and sympathetic nervous systems, which is critical for building understanding of their highly integrated functioning. Of the few studies of young children’s PEP, most find that their protocols do not produce significant group level changes in PEP in response to challenges 8,31. Studies have demonstrated that there is stability in resting PNS measures starting in infancy and they have relations with temperament, behavior, and health,13,16,26,32,33 but there is a limited understanding of SNS responding and its stability over time, relations to development, and the factors that shape that its developmental trajectory. PNS responses have been attributed primarily to social engagement 16 and SNS responses proposed to reflect “fight or flight responses” 1 as well as reward sensitivity (see reference 34 for a review). In young children, it is challenging to simulate specific challenges in a laboratory and to engage them due to their short attention span. There are also measurement challenges for toddlers who may experience discomfort with the seven spot electrodes needed to measure PNS and SNS simultaneously. In addition, within the fields of developmental science, there few standards about how to elicit “stress reactivity” sufficiently while also respecting children’s developmental needs and the ethics in working with children. This article presents information on the administration and scoring of a standardized protocol comprised of resting and challenging conditions designed to elicit parasympathetic and sympathetic nervous system responses from 18 month old children.
ANS reactivity is typically conceptualized as the physiological response to a discrete external stimulus relative to a comparison or resting state, which varies across individual organisms 22. Examination of the early life etiology of mental and physical health problems has led scientists to be particularly interested in understanding children’s reactivity to situations that evoke adaptive responding or can be thought of as stressful challenges. The preponderance of literature refers to this phenomenon as “stress reactivity”, although encountering challenging stimuli has the potential to elicit responses across a broad range of domains. Thus, the protocol described below was designed to elicit ANS responses across multiple domains including two forms of rest (while listening to a soothing lullaby and while watching a calm, neutral video) and three developmentally-appropriate challenges for 18-month olds: anticipation/startle (jack-in-the-box), sensory (lemon juice), and socioemotional (listening to a sick infant cry). The protocol was adapted from our existing protocols for 12 month olds 8 and 3-5 year olds 11, in order to make it developmentally-challenging, engaging and tolerable for toddlers (18-21 months).
Here we present the Developmental Challenges Protocol (DCP) and ANS data from the 18 month old study visit of the Stress, Eating and Early Development (SEED) Study of pregnant women and their offspring. Maternal participants with healthy pregnancies were recruited during 2013-14 for a study of gestational weight gain and prenatal stress and were overweight, low income, and racially and ethnically diverse. Parents’ informed consent for the study of her offspring was obtained just after birth and again prior to the start of the data collection for the study reported here, when the offspring were 18-21 months of age during 2014-2015.
Protocol
This study was approved by the Committee on Human Research of the University of California, San Francisco.
1. Pre-protocol Set-up (See Materials Spreadsheet for Complete List of Equipment)
Set up the equipment by turning on the computer and connecting the ANS data acquisition unit to a power source. NOTE: Do not turn the acquisition unit on.
Prior to beginning any data collection, set up the ANS data collection software. Choose settings to match Figure 1.
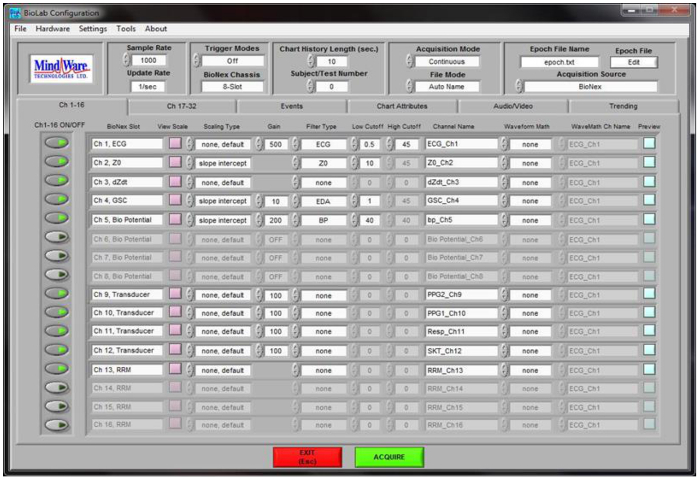 Figure 1. BioLab Software Configuration settings. This figure displays a screenshot of the configuration settings to be used during ANS data collection as described in step 1.2.
Figure 1. BioLab Software Configuration settings. This figure displays a screenshot of the configuration settings to be used during ANS data collection as described in step 1.2.
Keep spot electrodes in a sealed package until they are needed because electrode quality degrades over time with exposure to air. Snap the spot electrodes onto each of the 7 snap leads using a hard surface. Ensure that the leads are untangled and firmly connected to the electrodes.
Turn the tablet on and cue audio and visual applications (see Step 4 below). Set volume to 80% of max volume on the tablet and full volume on the speaker.
Set up tripod and video camera in unobtrusive place.
Set up the lemon juice task items prior to beginning the protocol. Pour some water in a small paper cup for rinsing after the lemon juice task (about 3 ounces). Put a very small amount of lemon juice from a packet into another cup and fill the disposable plastic pipette with 2 drops of lemon juice (this amount will come up to the first narrowing of the pipette and is equal to about 0.1 ml). Rest dropper on a paper towel or in the lemon juice cup and set out of the way.
2. Preparing the Mother and Child for the Developmental Challenges Protocol
Ensure that two individuals, the Child Assessor (CA) and Computer Operator (CO), are prepared to administer the protocol. Introduce staff to the mother and child and make them comfortable. Make eye contact and talk to the child in order to establish a trusting relationship between the mother, child, and staff. Allow the child some space to acclimatize to the CA and CO. Do not overwhelm the toddler with attention.
Ask the mother if the child needs to be fed or have his or her diaper changed before the electrodes are placed on the child to avoid disruptions later. Let the mother attend to the child’s needs before starting the protocol.
Allow the child to play on the floor or an open space during the introduction. Once protocol begins, instruct the mother to keep the child on her lap until it is completed.
Review the protocol with the mother by reading Script A (see supplemental information). Also, speak to the child directly about what is going to happen during the visit.
3. Connecting the ANS Acquisition Equipment to the Child
Instruct the CA to apply the electrodes to the child’s neck and torso (see Figure 2), while the CO distracts the child. Use white circle stickers to demonstrate the electrode placement on a doll to engage the child if necessary. Move quickly and place the electrodes underneath the child’s shirt to minimize likelihood that child will touch or remove the electrodes.
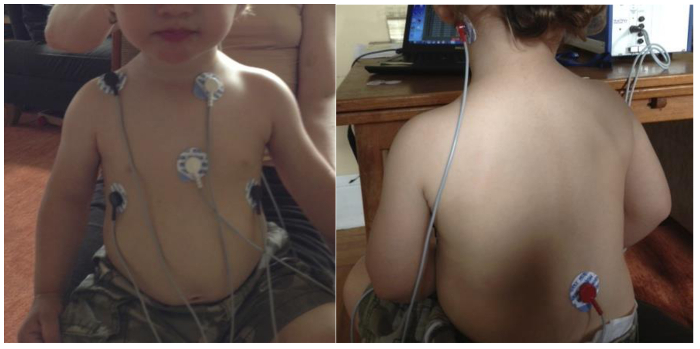 Figure 2. Electrode Configuration for 18 month olds. Note: the 2 ECG electrodes are applied in a Lead II configuration. Also, the alternative electrode placement for the nape of the neck is shown here.
Figure 2. Electrode Configuration for 18 month olds. Note: the 2 ECG electrodes are applied in a Lead II configuration. Also, the alternative electrode placement for the nape of the neck is shown here.
- Apply the electrodes directly to the skin. Press with fingers over the paper tape around the gel to ensure that the spot electrode adhered to the skin. All electrodes must be placed at least 3 cm apart from neighboring electrodes to ensure proper signal conduction. For any distances that are difficult to determine, use a measuring tape to measure and verify this minimum distance by measuring the length between the centers of each electrode. NOTE: The following order of placing electrodes on children has been efficient and well-tolerated by toddlers.
- Place the electrode attached to the red (-) lead on the center of the lower back.
- Place the electrode attached to the white (-) lead just above the xiphoid process, which is located on the xiphosternal junction (bottom tip of the sternum; where the left and right rib cage meet). Ensure that this electrode is at least 3 cm above the red (-) spot electrode.
- Place the electrode attached to the white (+) lead just left of suprasternal notch (assessor’s right) on the edge of the clavicle.
- Use the measuring tape to measure, verify, and record the distance in cm between the 2 front electrodes (white (+) and white (-)) for later use in the impedance scoring program.
- Place the first ECG electrode attached to the brown (-) lead on the right clavicle where the clavicle bone meets the shoulder bone. Press on the foam around the electrode and feel the clavicle bone. Place electrode lengthwise along bone.
- Place the second ECG electrode attached to the brown (+) lead on the left lower rib. Press on the foam around the electrode and feel the rib bone.
- Place the grounding electrode attached to the black (-) lead on the child’s lower right rib in line with horizontal plane of the lower left rib electrode.
Bundle and clip lower end of leads to the child’s clothing to minimize movement of leads. Instruct the CO to monitor the computer. Instruct the CA to engage the child and review the activities involved in the protocol with the mother (see Script B in supplemental information).
- Turn on the ANS data acquisition unit in order to acquire impedance and electrocardiogram (ECG) signals. Press “ Redetect” to begin EKG analysis, then click once on “Start” located next to the green light in the lower right hand corner of the program window.
- Wait 5 min after the electrodes have been placed to allow for the gel to adhere fully to the child’s skin for optimal conductance of the ECG and impedance signals. After 5 min, check the computer screen to view the signals and makes sure they look normal (CO). Turn on the video camera (CO).
- Avoid small talk with the child during this time. Offer child a standardized “optional” soft toy to hold if he or she seems uncomfortable (plan to remove toy from hand just prior to protocol beginning). NOTE: Explain to the child and mother that it is necessary to wait a bit to make sure the equipment is working correctly.
- Show computer image of child’s heart activity to mother and explain what each signal represents. Remind mother that this activity is not used to diagnose any health problems. Assure her that if the research team notices something concerning, she will be notified. Follow Script B (see supplemental information).
4. Administering the Tasks in the Developmental Challenges Protocol
Open the media player in the tablet. Play the first 1-min lullaby. Keep the tablet out of reach of the child.
Hold jack-in-the-box in front of the child. Slowly rotate the arm and play the music until the sock monkey pops up, making it take 15 sec to finish one round. Repeat four times.
- Start lemon juice task with Script C (see supplemental information). Place 2 drops of concentrated lemon juice on the center of the child’s tongue, slightly back from the front, so juice does not roll off the tongue. NOTE: If the pipette was resting in the juice cup, shake off extra drops from the outside of the pipette prior to dropping it on the tongue.
- Wait 10 sec quietly with a friendly, neutral expression. Say “What did it taste like?” if the child needs reassurance. NOTE: The lemon juice task must take at least 20 sec, starting from telling the child about the liquid through waiting until after the drops are in their mouth and then watching for a reaction.
- At least 10 sec after dropping lemon juice say, “Now it is time to drink some water,” and give the child a sip of water. If child refuses, wait a few seconds and try again by saying, “Now you can take a drink of water.” Press the F4 key as the assessor starts offering the water (CO).
Play the 30 sec recording of the sick infant crying on the tablet using a media player. Keep the tablet out of reach of the child.
Play the second 1 min lullaby on the tablet using a media player. Keep the tablet out of reach of the child.
Play the 2 min neutral video on the tablet using a media player. Hold the tablet roughly 12 inches from the child’s face but do not let them grab and hold the tablet.
5. Marking Protocol Segments During the Developmental Challenges Administration (CO)
Instruct the CO to push function (F) keys (set a priori by the user to identify the tasks or potential problems during data scoring) to mark important protocol steps in the ANS data (see Table 1). Push F11 to indicate that a task ended early (type “ended task early” and explanation into text box) or for major protocol problems or violations, like an electrode falling off or someone walking in the room and distracting child (type explanation into text box).
| Marker Key | Event | Duration after Start |
| F1 | Lullaby 1 start | 60 sec |
| F2 | Jack in the box start | 60 sec |
| F3 | Lemon juice script start | At least 20 sec |
| F4 | Lemon juice end (water drink offered) | At least 10 ses |
| F5 | Infant cry start | 30 sec |
| F6 | Lullaby 2 start | 60 sec |
| F7 | Video start | 120 sec |
| F10 | End of DCP (video end) | |
| F11 | Serious irregular events |
Table 1: Marker-Keys for Protocol Event Marking During Challenge Tasks. Marker keys as described in step 5.1 are shown, used to indicate important protocol events and rare occurrences in the ANS data file.
6. Post Developmental Challenges Protocol
Have the CA say to the mother:“So how was that for you? (pause to hear response) It’s interesting to see how children respond to these various activities. Now it is time to gently remove the electrodes from your child’s skin.”
Turn off the ANS data acquisition before touching/removing electrodes. NOTE: The equipment will not conduct or transmit electricity when it is turned off.
Remove electrodes from the child by using one finger to hold the skin tight while the other hand removes the electrode. Pull up on the tab on the electrode and away from the direction the tab is pointing. Remove the most sensitive electrodes last (front and back of the neck). Allow the mother to remove electrodes if the child is particularly upset or if she desires to. Use a wet wipe to remove any excess residue remaining on the skin from the electrodes and a dry gauze pad if the skin is wet or there is gel on the skin.
Turn off the video camera. Save the ANS data file, exit the program, and turn off the computer. NOTE: The ANS data acquisition software creates two data files, the “.mw” file (raw ANS data) and an “.event” file, which is a text file of function keys pressed.
7. Respiratory Sinus Arrythmia Scoring
Open the Heart Rate Variability (HRV) Analysis program to clean ECG data and score heart rate (HR), RSA, and respiratory rate (RR).
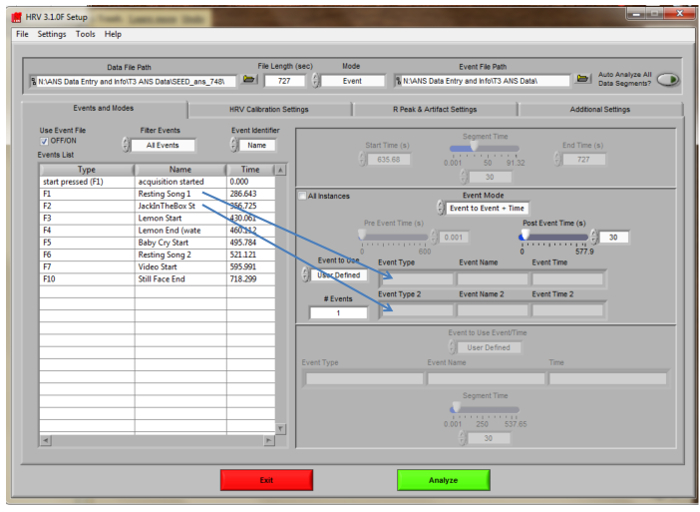 Figure 3. HRV Analysis Main Navigation Screen. Settings for scoring the toddler ANS data using the HRV Analysis scoring software are displayed. To score segments, drag events to the ‘Event Type” boxes.
Figure 3. HRV Analysis Main Navigation Screen. Settings for scoring the toddler ANS data using the HRV Analysis scoring software are displayed. To score segments, drag events to the ‘Event Type” boxes.
- Review any available field notes from data collection to be aware of unusual circumstances or errors that may have occurred. Locate and double-click the “.mw” file to be scored from the pop-up window (“Choose or Enter Path of File”). NOTE: The file name for the participant data being scored should appear in the white box on the top of the screen.
- Set the “Channel Labels” as follows: Channel 1- ECG, Channel 2- Zo, Channel 3- DZ/dt. Verify that the Sampling Frequency is set to 1,000. Click “ok” at the bottom of the screen to open the main navigation screen (Figure 3). NOTE: These channels should match those set during data collection.
- Choose settings to match those in the figure. Set Mode to “Event.” Set Event Identifier to “Name” to view the name of the task (i.e., “jack-in-the-box”). Set the “Post Event Time(s)” to 30 sec. NOTE: File Length will vary for each child’s file. “Type” refers to the Function (F) key that was pressed during the protocol by the CO. If the Mode is not Event but is Time, the “Segment Time” can be set to different lengths of time in seconds (i.e., 30 or 60).
- Click the “HRV Calibration Settings” tab. Set calculation method to “Entire”. Set respiratory signal to “Zo” (not dZ/dt). Set windowing Function to “Hamming”. Set VLF Band range to 0.003 to 0.04 Hertz (Hz), low frequency or LF Band range to 0.04 to 0.15 Hz, and high frequency HF Band range to 0.15-1.04 Hz for infants and toddlers.
- Click the next tab, “R Peak & Artifact Settings.” Set IBI and MAD MED to “on.” Set the Maximum Heart Rate (beats per minute (BPM)) to 230 and Minimum Heart Rate to 40 BPM. Set the slider bar for the R Peak Detection Sensitivity to 1. Set manual override to OFF. Turn Baseline and Muscle Noise Filter on (bright green) and notch filter off (dark gray). Set the noise filter to 60 Hz.
- Click the next tab, “Additional Settings.” Click the yes/no box in the output settings section (“Use default output path”) to choose the default location to save the output file. Check the “Show report” button (bright green). NOTE: Synchronous video can be viewed by clicking the “Yes/No” box under “use default video path.” A previously edited data file can be viewed by clicking the “use edited data” Yes/No box.
- Return to the “Events and Modes” tab. Click the “analyze” button.
- Choose “yes” or “no” when asked, “Is the ECG inverted? No/Yes.” NOTE: The majority of ECGs are not inverted. The analysis window will open, showing 30 sec of data. The HRV Analysis program marks each R peak with a light blue dot and marks a yellow star when there is a potential ‘outlier’ or irregular pattern. Outliers may be marked when the distance between two R peaks is different that the regular R-R interval for this individual.
- Review all outliers (yellow stars) to identify the reason for the yellow star (see editing options in 7.1.1.9). Record variables of interest in the data log after making any necessary edits. NOTE: The bottom right box of the analysis window shows the calculated values from this segment, including HR, RR and RSA.
- Click “Edit R’s” (the green button in the Controls window) to examine outliers. Click the magnifying glass icon to zoom in further.
- Use the red “delete” button to remove yellow stars that are incorrectly placed and the white “insert” button to mark the R peaks. NOTE: It is usually clear where R peaks should be deleted or manually marked. Extremely messy sections can be removed using the yellow “Remove Data Portion” button, after sliding the light blue x cursors around the section to be deleted. This should only be done at the beginning or end of a segment; there must be 20 consecutive sec of data to calculate valid scores for a 30 sec segment.
- Click “OK’ at the bottom of the screen to save edits, return to the previous screen, and display edited data. Click “Write” (the white button in the top toolbar) to output scored data to an Excel file. Repeat this process for each 30 sec interval or task to be scored. NOTE: “Cancel” exits without saving changes.
- Click “Done” on the “Controls” panel after editing and writing all 30 sec segments of data. NOTE: A pop-up window will request a filename for the edited data. All of the edited data and scored data for each child will be saved to one .edh file.
8. Pre-ejection Period Scoring
NOTE: The Impedance (IMP) Analysis program allows one to clean the impedance data and obtain HR and PEP values.
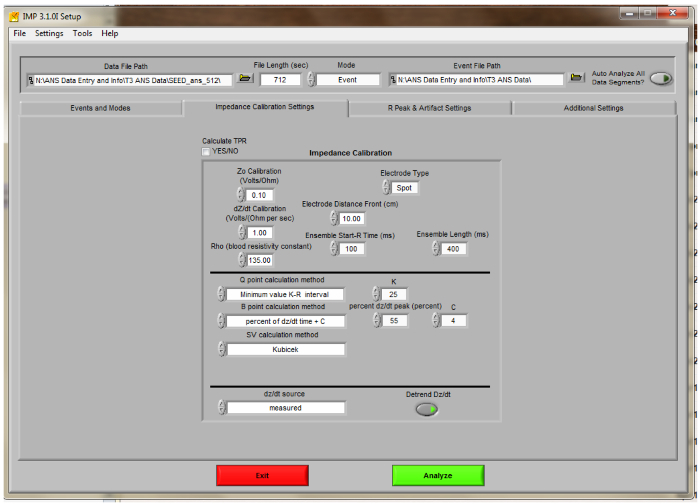 Figure 4. IMP Analysis Impedance Calibration Settings Screen. Settings for scoring the toddler ANS data using the Impedance Analysis scoring software are displayed.
Figure 4. IMP Analysis Impedance Calibration Settings Screen. Settings for scoring the toddler ANS data using the Impedance Analysis scoring software are displayed.
- Open the IMP Analysis Program. Double click on the IMP Analysis program and choose the same .mw file just scored in HRV Analysis. Choose the same channels for each “Channel Label:” Channel 1- ECG, Channel 2- Zo, Channel 3- dZ/dt. Press “OK.” On the Events and Modes tab, choose the same settings as for the HRV Analysis program.
- Click the “Impedance Calibration Settings” tab (Figure 4). Choose settings to match those in the Figure. Set “Electrode Type” to “spot”. Enter the electrode distance (distance between white electrodes as measured and recorded during data collection). Set “Ensemble Length (ms)” to 400 for toddlers.
- Set “Q point calculation method” to “Minimum value K-R interval.” Set the K value to 25 for 18 month olds (the K value can be adjusted on a per-child basis if needed). Set “B point calculation method” to “percent of dZ/dt time + C.” Set the percent dZ/dt peak to “55” and the constant to “4”. Set the “dZ/dt source” to “measured”. Verify that the “Detrend dZ/dt” light is ON for the non-mobile acquisition unit.
- Click the “R Peak & Artifact Settings” tab. Verify that the IBI min/max and MAD MED checks are “on.” Set the Maximum Heart Rate (BPM) to 230 for toddlers and the Minimum Heart Rate (BPM) is 40. Set the Peak Detection Sensitivity to 1. Set Manual Override to off. Turn on the “Baseline and Muscle Noise Filter” (bright green). Turn off the “notch filter” (dark green). Turn off the 60 Hz setting.
- Click the “Additional Settings” tab. Do not check the “Auto Analyze All Data Segments” button. Choose “YES” in the “Use Edited Data” yes/no box if any edits were made in the HRV program. Double-click the edited (.edh) file from the pop-up box. Choose “Excel” for the output mode. Click the bright green “Analyze (Enter)” button. NOTE: If no edits were made while scoring HRV, it is not necessary (or possible) to use edited data here.
- Click “Ensemble Editor” to see a full-screen view of the ensemble wave, which is an image that averages all the waveforms from the segment.
- Check the placement of the Q point, which is determined by the K value entered during set-up. Scroll through all of the segments to check the Q placement. NOTE: The Q point should be the lowest point just before the red (EKG) line starts to rise to the R peak. If there is a gradually sloping “pre-Q” area, the Q should be marked at the point where the slope to R starts to rise more steeply.
- Return to the Impedance Calibration Settings tab and increase the K value by 5 if the Q point marking is not correct when K=25. Review segments again. Continue making changes to K until the Q marking is optimal. NOTE: The K value needs to be consistent for each child; do not choose different values for different segments and do not move any marks by hand.
- Record the PEP value for each segment scored. NOTE: PEP is fairly stable within each individual and typically fluctuates by 4-6 ms throughout the assessment for each child. However, PEP values may be very different across children.
- Click the “Write” button in order to output score data to an Excel file. Repeat this process for each section to be scored.
Representative Results
The SEED study enrolled 162 mother-child dyads (37% African American; 30% Latina; 16% white; 17% other or multiracial). For the 18 month visit, we completed the in-person assessment with 140 children (87% of the enrolled sample); 6 participants moved away, and the remaining participants were unreachable or unavailable for this visit. The refusal rate for ANS data collection component of the visit was 3/140 (2%) for mothers available for this visit. Three visits were conducted after 21 months (more than 3 months beyond the target age), resulting in 135 children with ANS data collected at the target time period. Poor signal quality prevented us from scoring two children’s ANS data. Therefore, ANS data were available for 133 children. The mean age of the children with ANS data presented here was 18.89 months (range 17.59-21.60; SD = .80) and 53% of the children are female. Mean, sex- and age-adjusted weight-for-length percentile was 68.40 (SD = 27.47).
Of the 133 target-aged children with scoreable ANS data, 100% tolerated the application of the electrodes and also began the developmental challenges protocol. The protocol was discontinued mid-way for three subjects and after the baby cry task for five subjects. Two subjects were not shown the video due to equipment problems. All available data from the 133 children were scored. Of these, three children had no scoreable PEP data due to noise/movement artifact. Four more children had no scorable first resting lullaby data for PEP. Seven other children had unscorable PEP data for one or more segments after the baseline lullaby.
Descriptive statistics for the HR, RSA, and PEP measures by task are presented in Table 2. Table 2 also presents mean changes between tasks calculated in two ways: challenge task reactivity calculations (relative to resting level during the first lullaby) and task-to-task change calculations (relative to preceding task) for each ANS measure. The mean changes that were significantly different from zero are noted. Figure 5 presents boxplots for the three domains of challenge reactivity for HR, RSA, and PEP. Although the mean reactivity values presented in Table 2 and Figure 5 are small, the vast majority are significantly different from zero, and the boxplots reveal consistent evidence across all tasks and all ANS indices that children demonstrated variability in their responses to the challenges. Figure 6 presents mean RSA and PEP responses to each of the tasks throughout the protocol, and indicates when they significantly changed from task-to-task. RSA significantly changed between most of the adjacent tasks, indicating that, on average, children’s parasympathetic nervous system responses changed in response to each new task. The number of significant mean changes between tasks for PEP were fewer than for RSA, although significant change was identified from the lullaby to both the first and second challenge tasks, and from the last lullaby to the neutral video, indicating that, on average, children’s SNS responses were different from resting conditions to subsequent tasks. Child age was not significantly correlated with any challenge reactivity scores. These patterns suggest that this series of tasks elicited sample-level variability in responding throughout the protocol and across both branches of the ANS, and that there were different patterns between the PNS and SNS branches for some tasks.
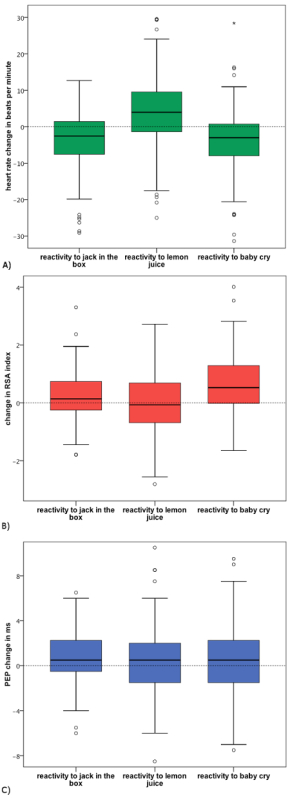 Figure 5. Boxplots Representing Heart Rate, Parasympathetic, and Sympathetic Activity by Developmental Challenge Task. (A) represents heart rate reactivity, (B) represents RSA reactivity, and (C) represents PEP reactivity. Note: the boxes represent the interquartile range containing the middle 50% of values, with the line across the box representing the median. The whiskers extend from the highest and lowest values, excluding outliers which lie at least 1.5 box lengths outside the box. Please click here to view a larger version of this figure.
Figure 5. Boxplots Representing Heart Rate, Parasympathetic, and Sympathetic Activity by Developmental Challenge Task. (A) represents heart rate reactivity, (B) represents RSA reactivity, and (C) represents PEP reactivity. Note: the boxes represent the interquartile range containing the middle 50% of values, with the line across the box representing the median. The whiskers extend from the highest and lowest values, excluding outliers which lie at least 1.5 box lengths outside the box. Please click here to view a larger version of this figure.
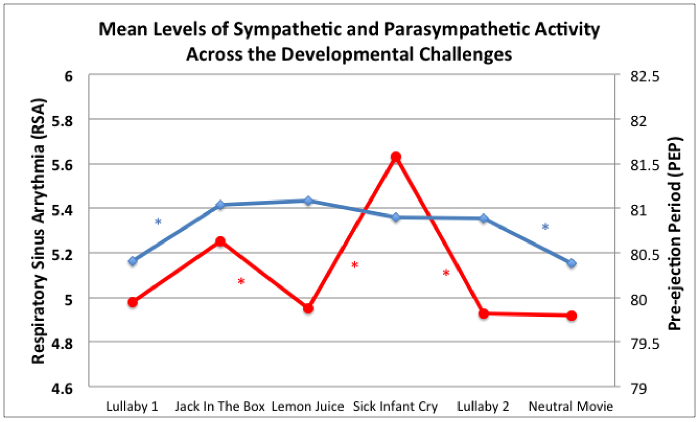 Figure 6. Mean Levels of Sympathetic and Parasympathetic Activity Across the Developmental Challenge Protocol. Decreases in RSA (red line) and PEP (blue line) reflect mean-level autonomic reactivity via PNS withdrawal and SNS activation, respectively. * indicates that the sample average score for that ANS measure changed significantly between those adjacent tasks (p <.05).
Figure 6. Mean Levels of Sympathetic and Parasympathetic Activity Across the Developmental Challenge Protocol. Decreases in RSA (red line) and PEP (blue line) reflect mean-level autonomic reactivity via PNS withdrawal and SNS activation, respectively. * indicates that the sample average score for that ANS measure changed significantly between those adjacent tasks (p <.05).
Supplemental File 1: Script A, B, C Please click here to download this file.
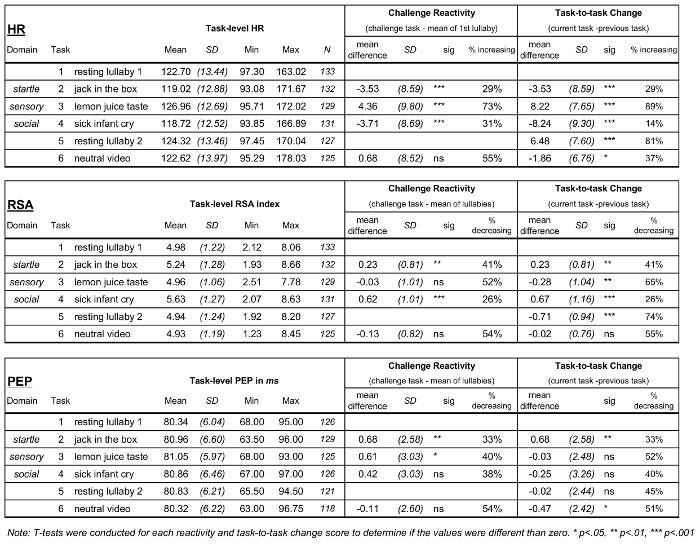
Table 2: Descriptives for ANS Measures: Task-level Arousal and Two Difference Scores of ANS Responding. Table 2 presents descriptive statistics for HR, RSA, and PEP. The left column displays task specific statistics, the middle column displays results for challenge tasks relative to the resting measure (mean of the 2 lullabies), and the right column presents the change for each task from the previous task. Please click here to view a larger version of this figure.
Discussion
This study revealed that, within a sample of 18 month-old children, standardized resting and challenge tasks designed to elicit responses from a range of domains (startle, sensory, social, resting) led to a range of ANS responses. The mean ANS responses found under these resting and challenge conditions were similar to ANS values reported by others who have measured ANS responsiveness with young children using similar tasks 8,11,27,29. The mean levels of the ANS measures found in our sample are between those typically found in young infants and those found in older children, which is in line with evidence that heart rate decreases and RSA and PEP increase with age during childhood 10. We also found there was a range of reactivity scores (the difference in physiological response from resting state to the challenge conditions) across the three physiological indices, with several statistically significant meaningful differences between many of the challenge and resting scores for both RSA and PEP. Collectively, these findings show that the protocol was successful at capturing ANS change during transitions between calming activities and developmentally challenging tasks and eliciting a measurable stress response within both the sympathetic and parasympathetic branches of the ANS in an understudied age group. In addition to the evidence for sample-level mean changes in ANS, individuals demonstrated significant variability in resting ANS levels, task ANS responses, and challenge reactivity scores, which provides further evidence that this protocol is useful for the examination of ANS individual differences in toddlers 35.
The majority of ANS studies with infants and toddlers include only RSA, the parasympathetic branch of the ANS, due to the difficulty in assessing sympathetic measures in this very young age group. Thus, little is known about the development of sympathetic branch of the ANS at rest or in response to challenge within this period of development or what factors shape its development and related trajectories of health. Additionally, we can learn more about the dynamic or synchronous or asynchronous relationship between the SNS and PNS by measuring them simultaneously, so dual measures are needed. Results from this protocol suggest that measurement of both SNS and PNS activity and change is possible in this age group.
Although reactivity protocols with adults typically involve segments of time with “rest” during which the adults sit or lie still for 5 min without stimuli, resting measures for young children are challenging. Engaging young children in some minimal manner is necessary to limit movement artifact in ANS measures of rest 29,36. In previous studies, “resting or baseline’’ in young children has been assessed as infants listened to a lullaby 8, or as preschool-aged children were read a relaxing story 11, shown a neutral movie clip 4 or a video screen with changing shapes 37. The consensus is that these may constitute reasonable reference points for assessing reactivity depending on the comparison condition. Previous experiments from our lab within a sample of 5 year olds 38 suggest that the psychomotor activity elicited by the movement-inhibiting baseline condition (e.g., talking, social engagement, gesturing) is an important consideration; findings suggested that the use of social engagement to keep children calm and focused, such as reading a story, is an appropriate baseline comparison for social challenges whereas neutral videos may be appropriate as comparison values for reactivity to arousing videos for children. Attending to a video is associated with increased attention and it has a calming response in both branches of the ANS. In this study, for toddlers, the neutral video did not elicit differences in HR, RSA or PEP measures, relative to the first resting lullaby, suggesting that either could have been used as resting values from which to calculate reactivity, or that its inclusion in the protocol is unnecessary. In addition, this protocol does not include rest periods between tasks, which may lead to “carryover” reactivity effects from task to task. This choice was made because of the difficulty in maintaining toddler’s attention for long periods. However, the significant task-to-task change scores, most commonly in opposing directions suggest that carryover effects may have been limited in this protocol.
The setting for the assessment data described here presents a possible limitation; roughly half of assessments were completed in the participant homes and the others were completed in our laboratory. This potential limitation is balanced by the flexibility of completing assessments in participant homes, which allows us to maintain data collection with participants who were unable or unwilling to travel to our lab. Further, although all children had a 5 min waiting period between electrode placement and the start of the Developmental Challenges Protocol, another potential limitation is that some children became upset by the placement of the electrodes and may have been stressed at the start of the protocol 8. Thus, we may not have obtained a valid resting measure at the start of the protocol. This protocol includes a second lullaby in order to offset this possibility, although its occurrence immediately following challenges may also lead to carryover effects. Limited ability to assess “true” resting conditions is a long-standing challenge for ANS researchers working with young children 38. More generally, assessing toddlers is challenging at times and requires occasional modification of procedures (such as stopping to change a diaper or allowing a child to hold their small soft toy to prevent a tantrum that would prohibit continuation of the assessment). In this protocol, we provided details on engaging and distracting young children to reduce the likelihood of need for these modifications. Conducting research with toddlers always requires flexibility and troubleshooting by developmentally-sensitive staff. Overall, it is important to develop a collaborative relationship with participating mothers.
In sum, the results of this study suggest that the Developmental Challenges protocol and ANS data collection procedures presented here may provide researchers with a set of structured challenges that elicit a broad range of responses across both branches of the ANS for children aged 18-21 months. As the children’s ages were not correlated with reactivity scores, these tasks may be appropriate with slightly older children, although this was not tested here; if attempted, ANS scoring settings will need to be adjusted. This protocol provides one option for studying stress physiology and its development early in life within a difficult-to-assess age group. The reactivity calculated for each domain can be examined separately as has been done with older children 6, or can be combined to create an “overall reactivity” score as has been done with infants 8, depending upon the associations among the reactivity variables in a given sample and the aims of the research. Results from the data collected during this protocol can be compared to similar age-appropriate tasks completed by younger 8 and older children 10,38. Assessing ANS functioning across different ages allows for the examination of developmental trajectories across time and the pursuit of related questions about both their ontogeny and their ability to predict mental and physical health outcomes.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This research was supported by NIH 1 U01 HL097973, NHLBI 5 R01 HL116511-02, UCSF-CTSI Grant Number UL1 TR000004, the Robert Wood Johnson Health and Society Scholars Program, and the Lisa and John Pritzker Family Foundation. The authors also wish to acknowledge Michelle Stephens for her assistance with scoring the ANS data as well as Vanessa Tearnan, Marialma Gonzales-Cruz, Yurivia Cervantes, Amy Engler, Stephanie Grover and Karen Jones-Mason for their assistance in collecting the ANS data, and Michael Coccia for his help with the data. We are also thankful to the families for their generous participation in this research and to the volunteers who helped us to test and refine this protocol, including the first author’s two children. We want to acknowledge our mentors who taught us about measuring ANS and why measuring ANS responsivity is meaningful in young children: W. Thomas Boyce, Gary Bernston, and Dave Lozano.
References
- Berntson G, Quigley K, Lozano D. Chapter 8, Cardiovascular Psychophysiology. In: Cacioppo JT, Tassinary LG, Bernston GG, editors. Handbook of Psychophysiology. Cambridge University Press; 2007. [Google Scholar]
- Boyce WT, et al. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Loman M, Gunnar M. Early experience and the development of stress reactivity and regulation in children. Neurosci. Behav. Physiol. 2010;34:867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavioral problems. Biol. Psychol. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, et al. Autonomic and Adrenocortical Reactivity and Buccal Cell Telomere Length in Kindergarten Children. Psychosom. Med. 2011;73:533–540. doi: 10.1097/PSY.0b013e318229acfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon A, et al. Latino children's body mass index at 2-3.5 years predicts sympathetic nervous system activity at 5 years. Child Obes. 2014;10:214–224. doi: 10.1089/chi.2013.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon A, et al. The ontogeny of autonomic measures in 6- and 12-month-old infants. Dev. Psychobiol. 2006;48:197–208. doi: 10.1002/dev.20129. [DOI] [PubMed] [Google Scholar]
- Conradt E, Measelle J, Ablow JC. Poverty, Problem Behavior, and Promise: Differential Susceptibility Among Infants Reared in Poverty. Psychol. Sci. 2013;24:235–242. doi: 10.1177/0956797612457381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon A, Boyce WT, Davis NV, Eskenazi B. Developmental Changes in Autonomic Nervous System Resting and Reactivity Measures in Latino Children from 6 to 60 Months of Age. J. Dev. Behav. 2011;32:668–677. doi: 10.1097/DBP.0b013e3182331fa6. [DOI] [PubMed] [Google Scholar]
- Alkon A, et al. Developmental and contextual influences on autonomic reactivity in young children. Dev. Psychobiol. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Boyce WT. In: Developmental Psychopathology: Developmental Neuroscience. Ciccheti D, Cohen DJ, editors. II. Wiley & Sons; 2006. pp. 797–817. [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biol. Psychol. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray's motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev. Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Conradt E, Measelle J, Poverty Ablow JC. Problem Behavior, and Promise: Differential Suceptibility Among Infants Reared in Poverty. Psychol. Sci. 2013;24:235–242. doi: 10.1177/0956797612457381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol. Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, et al. Autonomic reactivity and psychopathology in middle childhood. Br. J. Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Crowell S, et al. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. J. Abnorm. Psychol. 2006;115:174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Raine A. Autonomic nervous system factors underlying disinhibited, antisocial, and violent behavior. Biosocial perspectives and treatment implications. Ann. N.Y. Acad. Sci. 1996;794:46–59. doi: 10.1111/j.1749-6632.1996.tb32508.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Alkon A, Tschann JM, Chesney MA, Alpert BS. Dimensions of psychobiologic reactivity: Cardiovascular responses to laboratory stressors in preschool children. Ann. Behav. Med. 1995;17:315–323. doi: 10.1007/BF02888596. [DOI] [PubMed] [Google Scholar]
- Bush NR, Boyce WT. The Contributions of Early Experience to Biological Development and Sensitivity to Context. In: Lewis M, Rudolph KD, editors. Handbook of Developmental Psychopathology. Springer; 2014. pp. 287–309. [Google Scholar]
- Matthews KA, et al. Handbook of Stress, Reactivity, and Cardiovascular Disease. John Wiley and Sons; 1986. [Google Scholar]
- Alkon A, Wolff B, Boyce W. In: The Oxford Handbook of Poverty and Child Development. Maholmes V, King R, editors. New York: Oxford University Press; 2012. [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic cardiac control. I. Estimation and validation from pharmacological blockades. Psychophysiology. 1994;31:572–585. doi: 10.1111/j.1469-8986.1994.tb02350.x. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Obrist PA, Langer AW. Evaluation of beta-adrenergic influences on cardiovascular and metabolic adjustments to physical and psychological stress. Psychophysiology. 1986;23:89–104. doi: 10.1111/j.1469-8986.1986.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Dev. Psychobiol. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Quigley K, Stifter C. A comparative validation of sympathetic reactivity in children and adults. Psychophysiology. 2006;43:116–143. doi: 10.1111/j.1469-8986.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Royal S, Hutcheson J, Turner J. Comparison of impedance cardiographic measurements using band and spot electrodes. Psychophysiology. 1992;29:734–741. doi: 10.1111/j.1469-8986.1992.tb02051.x. [DOI] [PubMed] [Google Scholar]
- Allen MT, Matthews KA. Hemodynamic responses to laboratory stressors in children and adolescents: the influences of age, race, and gender. Psychophysiology. 1997;34:329–339. doi: 10.1111/j.1469-8986.1997.tb02403.x. [DOI] [PubMed] [Google Scholar]
- van Dijk AE, et al. Measuring cardiac autonomic nervous system (ANS) activity in children. J. Vis. Exp. 2013. [DOI] [PMC free article] [PubMed]
- Treadwell MJ, Alkon A, Styles L, Boyce WT. Autonomic nervous system reactivity: children with and without sickle cell disease. Nurs. Res. 2011;60:197–207. doi: 10.1097/NNR.0b013e3182186a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter CA, Dollar JM, Cipriano EA. Temperament and emotion regulation: the role of autonomic nervous system reactivity. Dev. Psychobiol. 2011;53:266–279. doi: 10.1002/dev.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Dev. Psychobiol. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Physiological Markers of Emotional and Behavioral Dysregulation in Externalizing Psychopathology. Monogr. Soc. Res. 2012;77:79–86. doi: 10.1111/j.1540-5834.2011.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challege: Conceptual and measurement considerations. Psychosom. Med. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Henderson HA, Marshall PJ. Chapter 20, Developmental Psychophysiology: Conceptual and Methodological Issues. In: Cacioppo JT, Tassinary LG, Bernston GG, editors. Handbook of Psychophysiology. Cambridge University Press; 2007. pp. 453–481. [Google Scholar]
- Gilissen R, Koolstra C, van Ijzendoorn M, Bakermans-Kranenburg M, van der Veer R. Physiological reactions of preschoolers to fear-inducing film clips: Effects of temperamental fearfulness and quality of the parent-child relationship. Dev. Psychobiol. 2007;49:187–195. doi: 10.1002/dev.20188. [DOI] [PubMed] [Google Scholar]
- Bush NR, Alkon A, Stamperdahl J, Obradović J, Boyce WT. Differentiating Challenge Reactivity from Psychomotor Activity in Studies of Children’s Psychophysiology: Considerations for Theory and Measurement. J. Exp. Child Psychol. 2011;110:62–79. doi: 10.1016/j.jecp.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


