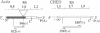Abstract
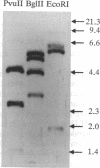
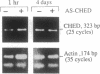
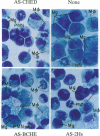
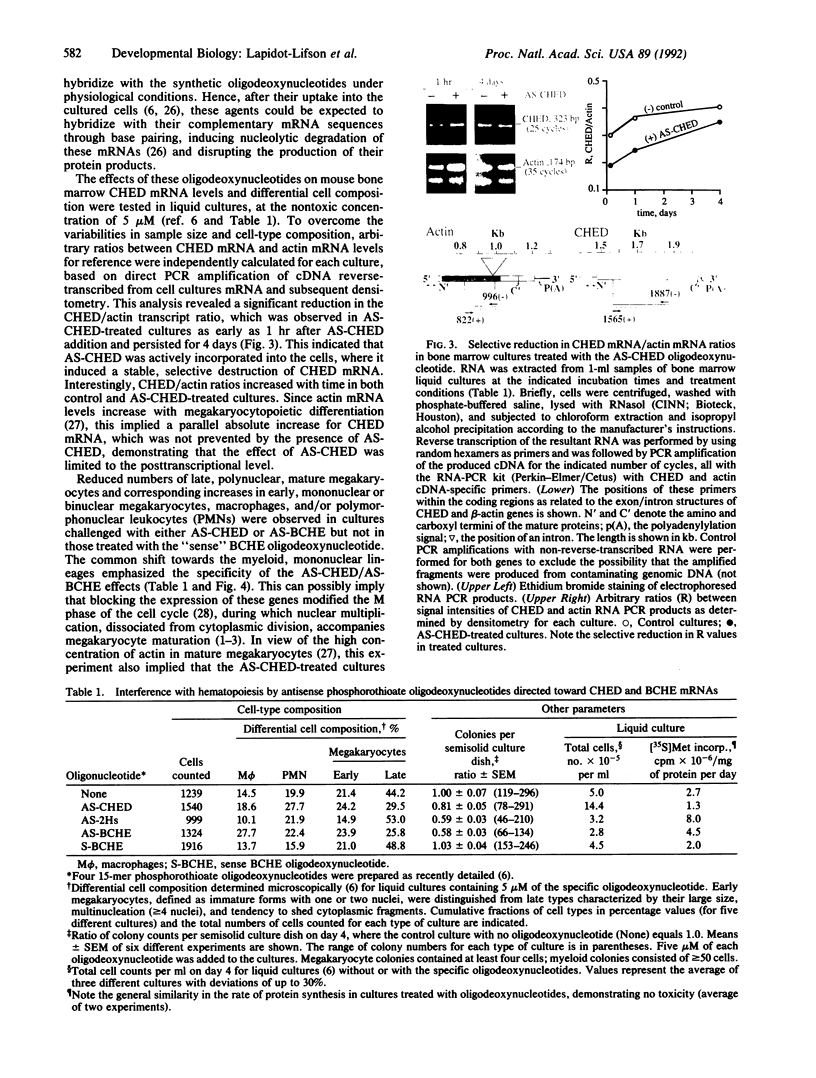
Mechanisms triggering the commitment of pluripotent bone marrow stem cells to differentiated lineages such as mononuclear macrophages or multinucleated megakaryocytes are still unknown, although several lines of evidence suggested correlation between cholinergic signaling and hematopoietic differentiation. We now present cloning of a cDNA coding for CHED (cholinesterase-related cell division controller), a human homolog of the Schizosaccharomyces pombe cell division cycle 2 (cdc2)-like kinases, universal controllers of the mitotic cell cycle. Library screening, RNA blot hybridization, and direct PCR amplification of cDNA reverse-transcribed from cellular mRNA revealed that CHED mRNA is expressed in multiple tissues, including bone marrow. The CHED protein includes the consensus ATP binding and phosphorylation domains characteristic of kinases, displays 34-42% identically aligned amino acid residues with other cdc2-related kinases, and is considerably longer at its amino and carboxyl termini. An antisense oligodeoxynucleotide designed to interrupt CHED's expression (AS-CHED) significantly reduced the ratio between CHED mRNA and actin mRNA within 1 hr of its addition to cultures, a reduction that persisted for 4 days. AS-CHED treatment selectively inhibited megakaryocyte development in murine bone marrow cultures but did not prevent other hematopoietic pathways, as evidenced by increasing numbers of mononuclear cells. An oligodeoxynucleotide blocking production of the acetylcholine-hydrolyzing enzyme, butyrylcholinesterase, displayed a similar inhibition of megakaryocytopoiesis. In contrast, an oligodeoxynucleotide blocking production of the human 2Hs cdc2 homolog interfered with production of the human 2Hs cdc2 homolog interfered with cellular proliferation without altering the cell-type composition of these cultures. Therefore, these findings strengthen the link between cholinergic signaling and cell division control in hematopoiesis and implicate both CHED and cholinesterases in this differentiation process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balduini W., Murphy S. D., Costa L. G. Developmental changes in muscarinic receptor-stimulated phosphoinositide metabolism in rat brain. J Pharmacol Exp Ther. 1987 May;241(2):421–427. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Courchesne W. E., Kunisawa R., Thorner J. A putative protein kinase overcomes pheromone-induced arrest of cell cycling in S. cerevisiae. Cell. 1989 Sep 22;58(6):1107–1119. doi: 10.1016/0092-8674(89)90509-6. [DOI] [PubMed] [Google Scholar]
- Courtney M. A., Stoler M. H., Marder V. J., Haidaris P. J. Developmental expression of mRNAs encoding platelet proteins in rat megakaryocytes. Blood. 1991 Feb 1;77(3):560–568. [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Elion E. A., Grisafi P. L., Fink G. R. FUS3 encodes a cdc2+/CDC28-related kinase required for the transition from mitosis into conjugation. Cell. 1990 Feb 23;60(4):649–664. doi: 10.1016/0092-8674(90)90668-5. [DOI] [PubMed] [Google Scholar]
- Gnatt A., Prody C. A., Zamir R., Lieman-Hurwitz J., Zakut H., Soreq H. Expression of alternatively terminated unusual human butyrylcholinesterase messenger RNA transcripts, mapping to chromosome 3q26-ter, in nervous system tumors. Cancer Res. 1990 Apr 1;50(7):1983–1987. [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hayles J., Beach D., Durkacz B., Nurse P. The fission yeast cell cycle control gene cdc2: isolation of a sequence suc1 that suppresses cdc2 mutant function. Mol Gen Genet. 1986 Feb;202(2):291–293. doi: 10.1007/BF00331653. [DOI] [PubMed] [Google Scholar]
- Hindley J., Phear G. A. Sequence of the cell division gene CDC2 from Schizosaccharomyces pombe; patterns of splicing and homology to protein kinases. Gene. 1984 Nov;31(1-3):129–134. doi: 10.1016/0378-1119(84)90203-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Lapidot-Lifson Y., Prody C. A., Ginzberg D., Meytes D., Zakut H., Soreq H. Coamplification of human acetylcholinesterase and butyrylcholinesterase genes in blood cells: correlation with various leukemias and abnormal megakaryocytopoiesis. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4715–4719. doi: 10.1073/pnas.86.12.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Long M. W., Hutchinson R. J., Gragowski L. L., Heffner C. H., Emerson S. G. Synergistic regulation of human megakaryocyte development. J Clin Invest. 1988 Nov;82(5):1779–1786. doi: 10.1172/JCI113791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Jinno A., Takagi N., Shibuya M. A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol Cell Biol. 1990 May;10(5):2261–2268. doi: 10.1128/mcb.10.5.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990 May 18;61(4):549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Kaplan J. M., Bishop J. M., Varmus H. E. Mitosis-specific phosphorylation of p60c-src by p34cdc2-associated protein kinase. Cell. 1989 Jun 2;57(5):775–786. doi: 10.1016/0092-8674(89)90792-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Patinkin D., Seidman S., Eckstein F., Benseler F., Zakut H., Soreq H. Manipulations of cholinesterase gene expression modulate murine megakaryocytopoiesis in vitro. Mol Cell Biol. 1990 Nov;10(11):6046–6050. doi: 10.1128/mcb.10.11.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce B., Cambray-Deakin M., Morrow C., Grimble J., Murphy S. Activation of muscarinic and of alpha 1-adrenergic receptors on astrocytes results in the accumulation of inositol phosphates. J Neurochem. 1985 Nov;45(5):1534–1540. doi: 10.1111/j.1471-4159.1985.tb07224.x. [DOI] [PubMed] [Google Scholar]
- Prody C. A., Zevin-Sonkin D., Gnatt A., Goldberg O., Soreq H. Isolation and characterization of full-length cDNA clones coding for cholinesterase from fetal human tissues. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3555–3559. doi: 10.1073/pnas.84.11.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Sachs L. The control of growth and differentiation in normal and leukemic blood cells. Cancer. 1990 May 15;65(10):2196–2206. doi: 10.1002/1097-0142(19900515)65:10<2196::aid-cncr2820651006>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Soreq H., Ben-Aziz R., Prody C. A., Seidman S., Gnatt A., Neville L., Lieman-Hurwitz J., Lev-Lehman E., Ginzberg D., Lipidot-Lifson Y. Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9688–9692. doi: 10.1073/pnas.87.24.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]