Abstract
Endothelium-derived nitric oxide (NO) produced from endothelial NO-synthase (eNOS) is one of the most important vasoprotective molecules in cardiovascular physiology. Dysfunctional eNOS such as uncoupling of eNOS leads to decrease in NO bioavailability and increase in superoxide anion (O2.−) production, and in turn promotes cardiovascular diseases. Therefore, appropriate measurement of NO and O2.− levels in the endothelial cells are pivotal for research on cardiovascular diseases and complications. Because of the extremely labile nature of NO and O2.−, it is difficult to measure NO and O2.− directly in a blood vessel. Numerous methods have been developed to measure NO and O2.− production. It is, however, either insensitive, or non-specific, or technically demanding and requires special equipment. Here we describe an adaption of the fluorescence dye method for en face simultaneous detection and visualization of intracellular NO and O2.− using the cell permeable diaminofluorescein-2 diacetate (DAF-2DA) and dihydroethidium (DHE), respectively, in intact aortas of an obesity mouse model induced by high-fat-diet feeding. We could demonstrate decreased intracellular NO and enhanced O2.− levels in the freshly isolated intact aortas of obesity mouse as compared to the control lean mouse. We demonstrate that this method is an easy technique for direct detection and visualization of NO and O2.− in the intact blood vessels and can be widely applied for investigation of endothelial (dys)function under (physio)pathological conditions.
Keywords: Molecular Biology, Issue 108, Arteries, Confocal Microscopy, DHE, DAF-2DA, Endothelium, Nitric Oxide, Superoxide Anion, Vascular Biology
Introduction
The vascular endothelial cells keep vascular functional and structural integrity by releasing vasoactive factors1. Among these factors, endothelium-derived nitric oxide (NO) produced from L-arginine via endothelial NO-synthase (eNOS) is the most important and best characterized factor in cardiovascular physiology2. NO causes smooth muscle relaxation and inhibits the cell proliferation, inhibits platelet aggregation and inflammatory cell adhesion and infiltration into the subendothelial space, therefore protecting against vascular disease development3. Under many physiological and pathological conditions, including aging, hypertension, diabetes, hyperlipidemia, etc., endothelial dysfunction characterized by decreased NO bioavailability and increased O2.- production is present and promotes pathogenesis of atherosclerosis2. Studies from recent years demonstrate that uncoupling of eNOS is an important mechanism for the endothelial dysfunction, in which the eNOS enzyme generates O2.- instead of NO, under the various aforementioned conditions1,4. Therefore, analysis of endothelial function, in particular, endothelial NO production and O2.- generation is pivotal for experimental research on cardiovascular diseases and complications.
There are numerous methodological approaches that have been developed to analyze and measure NO production in biological samples. Due to the extremely labile nature of NO which is readily oxidized to NO2- and NO3- with a half-life of 3 to 6 sec, it is difficult to measure NO directly. Therefore determination of NO2-/NO3- in the fluid samples was used as an index of NO released from cells or tissues5. Although the procedure is relatively easy, the method is, however, easily affected by high background of the stable NO2-/NO3- contained in the solution. Because NO stimulates soluble guanylate cyclase to produce cyclic guanosine monophosphate (cGMP)6, the cellular cGMP level has also been determined to estimate NO release7. Again, this is an indirect estimation and may not be specific, since some endothelium-derived factors such as C-type natriuretic peptide (CNP) could also enhance cGMP levels through activation of particulate guanylate cyclase8. NO is produced from L-arginine with generation of L-citrulline as a by-product9, measurement of L-citrulline production is therefore also used as an indirect method to estimate NO production. The major drawbacks of this method are that it is radioactive and it does not measure bioactive NO levels, since released NO could be rapidly inactivated by O2.−; Moreover, L-citrulline can be recycled to L-arginine10. Other chemical methods such as chemiluminescence detection11, electron spin resonance12, or electrochemical porphyrinic NO sensor13 are used by several investigators. These methods are usually not easy in operating, detecting procedures and require special equipment. It is also to mention that many studies apply organ bath experiments with isolated blood vessels with or without the endothelium to assess endothelial function and indirectly measure endothelium-derived NO mediated vascular relaxations. However, this method, although it is mostly close to physiological situation, but strictly to say, does not measure NO function, it rather assesses endothelium-mediated vasomotor responses in general that reflect net effects of eNOS function, production of other endothelium-derived relaxing factors and endothelium-derived contracting factors, production of O2.−, and also the responses of smooth muscle cells to these factors. A specific analysis of eNOS function or NO production is usually required3.
Many research groups including ours have in recent years used the fluorescence dye method to detect intracellular production of NO14-19. In this method the cell permeable fluorescence indicator diaminofluorescein-2 diacetate (DAF-2DA) was used to measure free NO and NOS function in living cells and tissues in vitro or ex vivo. The principle is that in the living cells, DAF-2DA is deacetylated by intracellular esterase to non-fluorescent 4,5-diaminofluorescein (DAF-2) which was then converted to fluorescent DAF-2 triazole (DAF-2T) by reacting with NO. The fluorescence from DAF-2T can be observed under a fluorescence microscope or a fluorescence confocal microscope 14. The intracellular fluorescence intensity therefore reflects the intracellular NO production in the cells or the endothelium of an in intact blood vessel. Combined with a specific fluorescence dye such as dihydroethidium (DHE), one can simultaneously assess intracellular NO and O2.− generation in the cells or in blood vessels14. Similarly, DHE is also a cell-permeable compound that is oxidized by O2.− inside the cells, and the oxidative product then intercalates with nucleic acids to emit a bright red color detectable quantitatively by fluorescent microscope or fluorescence confocal microscope. DHE is a very specific dye for detection of O2.− from biological samples, as it detects essentially superoxide radicals, is retained well by cells, and may even tolerate mild fixation20. One of the advantages of this fluorescence dye method is that it detects and visualizes NO and/or O2.− en face directly on the intact endothelial layer of a living blood vessel.
In this paper, we describe this fluorescence dye method to detect NO and O2.− which we have adapted for en face detection of NO and O2.− in intact aortas of an obesity mouse model induced by high-fat-diet (HFD) feeding. We demonstrate that this method could successfully and reliably measure NO and O2.− levels and evaluate eNOS (dys)function in the endothelial layer of freshly isolated intact mouse aortas in obesity.
Protocol
Animal work was approved by the Ethical Committee of Veterinary Office of Fribourg, Switzerland. The protocol follows the guidelines on animal care and experimentation at our institution.
1. Preparation of a Set-up for Incubation of Isolated Arteries
Construct an organ bath system which can be heated to 37 °C and aerated with 95% O2 and 5% CO2 from a carbon gas tank.
Prepare as much Krebs-Ringer bicarbonate buffer as needed with the concentration of the following composition: (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 0.026 mM EDTA, and 11.1 mM D-glucose).
Keep the stock buffer on ice and aerate the buffer with 95% O2 and 5% CO2.
Switch on the organ bath system, set the temperature at 37 °C, add 5 ml of the ready-to-use buffer to each chamber and keep aerating the buffer with 95% O2 and 5% CO2.
Wash the chambers once with Krebs-Ringer buffer for 30 min.
Add 5 ml Krebs-Ringer buffer to each chamber and cover the chamber to avoid evaporation.
2. Isolation of Mouse Aortas
Inject pentobarbital which is dissolved in 0.9% NaCl intraperitoneally at the concentration of 150 mg/kg to sacrifice the mice.
Lay the mouse supinely on a surgical board.
Spray the fur of the chest region with 70% ethanol for the purposes of sanitization and moisture.
Open chest cavity by cutting around the rib to expose the heart and lungs, and remove the lungs.
Remove the blood in the chest cavity gently with a paper tissue.
Grasp the heart gently with a forceps and detach the thoracic aorta by cutting the perivascular fat tissue between the aorta and spine with surgical scissors.
Immediately immerse the whole tissues in ice cold (4 °C) Krebs-Ringer buffer.
Remove the heart and aortic arch under a dissection microscope and keep the thoracic aortic segment in the buffer.
Dissect the aorta free from adhering perivascular tissue under a microscope with surgical scissors and forceps.
Grasp the end edge of aorta with a forceps, and flush away the blood in the vascular lumen by gently flushing Krebs-Ringer buffer with a 26 G × 1/2ʺ syringe.
Cut the cleaned aortic rings into 3 mm long segments. Note: Pay attention at this step to not damage endothelial layer.
3. DHE and DAF-2DA Staining
Transfer the cleaned aortic segments with a forceps to the organ bath chambers filled with the Krebs-Ringer Buffer at 37 °C aerated with 95% O2 and 5% CO2. Note: Touch only the adventitial side of the aortic rings with forceps and don't clamp the blood vessels.
Equilibrate the arteries in the organ bath chamber for 30 min.
Add acetylcholine (ACh) to the organ bath to the final concentration 1 µM, and incubate the arteries for 10 min.
Prepare 1 ml of DHE/DAF-2DA solution (5 µM of each dye, diluted from 1,000× stock) with pre-warmed Krebs-Ringer buffer in 1.5 ml microcentrifuge tube. Wrap the microcentrifuge tube with aluminum foil to avoid light exposure.
After stimulation, transfer the aortic rings from organ bath to the DHE/DAF-2DA solution in a microcentrifuge tube at 37 °C aerated with 95% O2 and 5% CO2 and incubate the aortic rings for 30 min. Note: From this step, keep the aortas always in dark.
Transfer the aortic rings to a new microcentrifuge tube with Krebs-Ringer buffer for washing and repeat the washing three times within 1 min.
Transfer the aortic rings to 4% paraformaldehyde solution for fixation for 30 min.
Prepare 1 ml 4',6-diamidino-2-phenylindole (DAPI) solution (300 nM, diluted from 1,000× stock) with Phosphate Buffered Saline (PBS) buffer in a 1.5 ml microcentrifuge tube. Counterstain the aortas for 3 min.
Transfer the aortas to new microcentrifuge tube with PBS buffer for washing for 1 min. Repeat the washing three times. Note: Pay attention not to clamp the aortic rings or damage the endothelial layer.
4. En Face Mounting
Add a drop of mounting medium to the slide.
Cut the aortic rings longitudinally with microsurgical scissors under microscope, make the rolled-up aortas flat and mount it en face on the mounting medium with the endothelium facing down on the glass slide. Do not move the aortas back and forth.
Cover the slide with cover slip and seal the slide with nail polish.
After air drying under light protection, use the slides for imaging directly within hours or store them at -20 °C for next few days.
5. Confocal Microscopic Imaging
- Use a confocal microscope to detect the fluorescence signals. Switch on the machine and optimize the imaging settings such as magnification, scanning speed, resolution, Z-stack.
- For this protocol, use a 200 Hz scanning speed, resolution of 1,024 × 1,024 pixels and Z-step size of 0.25 µm. Excite fluorescence from DAF-2DA with 488 nm argon laser and detect at 515 nm, whereas excite fluorescence from DHE at 514 nm and detect at 605 nm emission.
Use 10X magnifications to focus on the sample until the DAPI signal is clear with ultraviolet rays, and then adjust the objective to 40X magnifications.
Define the range of endothelial layer by adjusting Z position on the control panel. The signals of DAPI-stained nuclei are oval or round dots in the endothelial layer.
Scan from the top (endothelial layer on the lumen border) of the sample through the full thickness of endothelial signal and record images. Choose at least 3 different fields for scanning each sample.
6. Analysis of Images
Use software to open the data scanned by confocal microscope. Choose three consecutive images per field for analysis and evaluate at least 3 different fields per sample. Export the chosen images and save them as JPEG files.
- Quantify the images from DAF-2DA, DHE and DAPI staining with image processing software. For the image from DAPI staining, choose 'Plugins'→'Analyze'→'Cell Counter' to count the number of DAPI positive nucleus.
- For the images from DAF-2DA and DHE staining, choose 'Analyze'→'Measure' to analyze the intensity of the signals, and take the 'Mean' value as the relative signal intensity. Present then the results as the ratio of DAF-2DA to DAPI positive nuclei or ratio of DHE to DAPI. For each sample, take the average value of every image and field.
Divide the result of every sample by the average of the control group to get the fold change. Statistical analysis was performed with unpaired Student t test or ANOVA with Dunnett or Bonferroni post-test. Give data as mean±SEM. Consider differences in mean values as significant at p<0.0514.
Representative Results
Obesity is an important risk factor of ischemic coronary heart disease and is associated with decreased endothelial NO bioavailability, a hallmark of atherosclerotic vascular disease21. eNOS-uncoupling has been shown to be an important mechanism of endothelial dysfunction under numerous physiological and pathological conditions including aging22, atherosclerosis, and obesity14. Therefore, here we compare the lean and obese mice to show the representative result of NO and O2.− levels in the aortas.
Starting at the age of 7 weeks, the male mice (C57BL/6J) were given free access during 14 weeks to either a normal chow (NC; energy content: 10.6% fat, 27.6% protein, and 57% carbohydrate, fiber 4.8%) or a high fat diet (HFD, energy content: 55% fat, 21% protein, and 24% carbohydrate). After 14 weeks of HFD mice were sacrificed and thoracic aortas were dissected, and cleaned of adhering tissues. Before DAF-2DA and DHE staining step, the eNOS inhibitor L-NG-Nitroarginine Methyl Ester (L-NAME) (1 mM) was added to the bath chamber for 1 hr to block eNOS activity14. The representative confocal fluorescence results were shown in Figure 1. The blue color in the upper panel represents the nucleus of endothelial cells stained by DAPI. In the middle panel, the green color is the fluorescence signal from DAF-2T converted from the non-fluorescent DAF-2 by NO, which means if the intensity of green signal is higher, there are more NO in the cells. Similarly, the red color in the lower panel is the fluorescence signal from DHE oxidized by O2.−, so the sample showing more red color has more O2.− in the cells.
Confocal fluorescence microscopy revealed decreased NO production (DAF-2DA staining) and increased L-NAME-sensitive O2.− generation (DHE staining) in the aortic endothelial layer of the mice fed HFD as compared to that of the mice fed NC (Figure 1)14, suggesting eNOS-uncoupling in obesity. The signals were quantified and presented in the bar graphs 14.
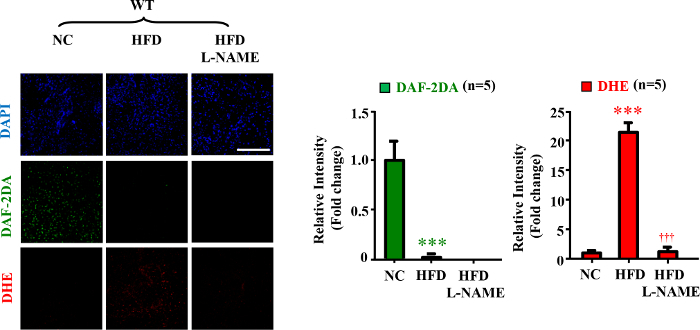 Figure 1. eNOS-uncoupling in obesity. Confocal microscopic en face detection of NO and O2.− by DAF-2DA and DHE staining, respectively. n=5; ***p<0.005 vs NC; †††p < 0.005 vs HFD group. Scale bar = 0.1 mm. (Data were from reference14) Please click here to view a larger version of this figure.
Figure 1. eNOS-uncoupling in obesity. Confocal microscopic en face detection of NO and O2.− by DAF-2DA and DHE staining, respectively. n=5; ***p<0.005 vs NC; †††p < 0.005 vs HFD group. Scale bar = 0.1 mm. (Data were from reference14) Please click here to view a larger version of this figure.
Discussion
Detection of NO or O2.− with fluorescent dyes was frequently used in many studies in cultured endothelial cells and also in tissue cryosections23. Here we extended this method to intact living blood vessels, i.e., en face detection of NO and O2.− levels in the endothelial layer with DAF-2DA and DHE, respectively, which is effective, relatively simple, and intuitional. In comparison with the method in vascular cryosections, this method shows lower background and is more quantitative, since elastic fibers in the media of an artery particularly the aorta in the cryosections give very strong autofluorescence signals which could interfere with the specific NO or O2.− signals generated in endothelial cells. Moreover, specific O2.− generation by NADPH oxidase or other enzymes in the medial smooth muscle cells could also interfere with the signals in endothelial cells in the vascular section, which represents the disadvantage of the method with vascular cryosections. In contrast, the en face staining method detects signals specifically from the endothelial layer of an intact blood vessel segment and therefore gives more precise analysis. A cryostat machine for cryosection preparation is also an expensive investment. Comparing to other biochemical methods, the main limitation of this method is less quantitative, but it is a good choice for relative comparison between samples. Moreover, the requirement of a confocal microscope which is expensive may also be a limitation of this method. The multi-organ chamber system is convenient and if this is not available in the lab, one can easily establish a system with water bath and tubes which are connected to a carbon gas tank with regulatory system, so that the blood vessels in the tubes are kept at 37 °C and aerated with 95% O2 and 5% CO2.
There are several critical steps that one has to pay attention. Be sure that the perivascular tissue is cleaned for easy mounting. The blood clots in the vascular lumen must be flushed away, because they may cause artificial signals and interfere with the fluorescence signals. During the whole procedure of blood vessel preparation, incubation, washing, cutting and mounting, one has to be extremely cautious not to damage the endothelial layer of the blood vessels. Do not let the blood vessel dry during the whole procedure of preparation. DHE and DAF-2DA are both fluorescent probes, so starting from the staining step the aortas should be always protected from light exposure. Before the fixation step the endothelial cells of aortas must be alive, so the aortas should be always kept in the Krebs-Ringer buffer aerated with 95% O2 and 5% CO2. Images of the samples should be taken as soon as possible after preparation. The fluorescence signal will become weaker in a few days even with the protection of mounting medium, which may affect the accuracy of the results. The method may not apply for blood vessels with too thick vascular wall.
This method enables simultaneous imagination of NO and O2.− production in the endothelial layer of intact blood vessels, if the two dyes were added to the blood vessels together. One can also use this method to evaluate pharmacological effects of drugs on NO and O2.− production in vitro in isolated blood vessels. For this purpose, the cleaned aorta is usually cut into two parts, one is for control and another is for drug treatment, and the drug should be added to the incubation buffer after equilibration before DAF-2DA and DHE staining step. It is particularly useful, if this method is used together with another method, e.g., with analysis of vasomotor responses of isolated blood vessels, a physiological function of endothelial cells can be confirmed. This method shall be also suitable for analysis of any endothelial (dys)function in intact blood vessels of disease models and the underlying mechanisms.
In summary, we presented a simple protocol to simultaneously detect NO and O2.− production in endothelial layer of intact blood vessels with a fluorescence confocal microscope. We show how this method successfully worked in obesity mouse model. This method is a useful technique in investigation of endothelial NO and O2.− production under many vascular disease conditions.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by the Swiss National Science Foundation (310030_141070/1), Swiss Heart Foundation, and National Center of Competence in Research (NCCR-Kidney.CH) Switzerland. Yu Yi is supported by the Chinese Scholarship Council.
References
- Yang Z, Ming XF. Arginase: the emerging therapeutic target for vascular oxidative stress and inflammation. Front Immunol. 2013;4:149. doi: 10.3389/fimmu.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4(1):53–65. doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab. 2012;302(5):481–495. doi: 10.1152/ajpendo.00540.2011. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA. 1989;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Sheng H, Warner TD, Forstermann U, Murad F. A simple and sensitive bioassay method for detection of EDRF with RFL-6 rat lung fibroblasts. Am. J. Physiol. 1991;261(2):598–603. doi: 10.1152/ajpheart.1991.261.2.H598. [DOI] [PubMed] [Google Scholar]
- Guo HS, et al. Inhibitory effect of C-type natriuretic peptide on spontaneous contraction in gastric antral circular smooth muscle of rat. Acta Pharmacol Sin. 2003;24(10):1021–1026. [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc. Natl. Acad. Sci. USA. 1990;87(21):8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Nagano T, Hayakawa H, Hirata Y, Hirobe M. Detection of nitric oxide production from a perfused organ by a luminol-H2O2 system. Anal. Chem. 1993;65(13):1794–1799. doi: 10.1021/ac00061a025. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Wang P, Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J. Biol. Chem. 1995;270(1):304–307. doi: 10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]
- Malinski T, Mesaros S, Tomboulian P. Nitric oxide measurement using electrochemical methods. Methods Enzymol. 1996;268:58–69. doi: 10.1016/s0076-6879(96)68009-4. [DOI] [PubMed] [Google Scholar]
- Yu Y, Rajapakse AG, Montani JP, Yang Z, Ming XF. p38 mitogen-activated protein kinase is involved in arginase-II-mediated eNOS-Uncoupling in Obesity. Cardiovasc Diabetol. 2014;13(1):113. doi: 10.1186/s12933-014-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsubo N, et al. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427(2):263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- Hink U, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88(2):14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- Okuda M, et al. Expression of glutaredoxin in human coronary arteries: its potential role in antioxidant protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(9):1483–1487. doi: 10.1161/hq0901.095550. [DOI] [PubMed] [Google Scholar]
- Cortese-Krott MM, et al. Human red blood cells at work: identification and visualization of erythrocytic eNOS activity in health and disease. Blood. 2012;120(20):4229–4237. doi: 10.1182/blood-2012-07-442277. [DOI] [PubMed] [Google Scholar]
- Nunez C, et al. Discrepancies between nitroglycerin and NO-releasing drugs on mitochondrial oxygen consumption, vasoactivity, and the release of NO. Circ Res. 2005;97(10):1063–1069. doi: 10.1161/01.RES.0000190588.84680.34. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105(14):1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- Yang Z, Ming XF. mTOR signalling: the molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obes Rev. 2012;13(Suppl 2):58–68. doi: 10.1111/j.1467-789X.2012.01038.x. [DOI] [PubMed] [Google Scholar]
- Yepuri G, et al. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell. 2012;11(6):1005–1016. doi: 10.1111/acel.12001. [DOI] [PubMed] [Google Scholar]
- Matsuno K, et al. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112(17):2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]


