Abstract
Catheterization of the intestinal lymph trunk in neonatal pigs is a technique allowing for the long-term collection of large quantities of intestinal (central) efferent lymph. Importantly, the collection of central lymph from the intestine enables researchers to study both the mechanisms and lipid constitutes associated with lipid metabolism, intestinal inflammation and cancer metastasis, as well as cells involved in immune function and immunosurveillance. A ventral mid-line surgical approach permits excellent surgical exposure to the cranial abdomen and relatively easy access to the intestinal lymph trunk vessel that lies near the pancreas and the right ventral segment of the portal vein underneath the visceral aspect of the right liver lobe. The vessel is meticulously dissected and released from the surrounding fascia and then dilated with sutures allowing for insertion and subsequent securing of the catheter into the vessel. The catheter is exteriorized and approximately 1 L/24 hr of lymph is collected over a 7 day period. While this technique enables the collection of large quantities of central lymph over an extended period of time, the success depends on careful surgical dissection, tissue handling and close attention to proper surgical technique. This is particularly important with surgeries in young animals as the lymph vessels can easily tear, potentially leading to surgical and experimental failure. The video demonstrates an excellent surgical technique for the collection of intestinal lymph.
Keywords: Medicine, Issue 109, Intestinal lymph trunk, surgery, efferent lymph, long-term, neonatal pigs, ApoB48 lipoprotein, lipoprotein metabolism, catheterization
Introduction
The lymphatic system is an understudied area of physiology. Preclinical models of lymphatic catheterization occur in different animal species1-8 and are used by pharmaceutical industries and research institutions to investigate mechanisms involved in lipid8-12 and drug metabolism13-15, cancer metastasis16 with experimental treatment17, and immune function18-26. This study explores the use of intestinal lymph trunk catheterization in a domestic pig model to measure components of lipoprotein metabolism. Lipoprotein metabolism is involved in the production and secretion of chylomicrons, as well as changes in associated lipids and total protein. These are important considerations as there are major differences in lipid metabolism between commonly used rodent models and humans and as such, employing swine models to collect intestinal lymph could provide more comparable information for studying lipid metabolism in people27-31.
Several surgical techniques are used to collect the intestinal lymph in large animal species: a cranial shoulder approach (i.e., thoracic duct catheterization)5, a lateral upper flank approach32-34, and a ventral midline or paramedian approach22,35. This video describes in detail the surgical procedure in swine using a ventral midline surgical approach for the catheterization of the intestinal lymph trunk. Careful surgical technique permits this method of lymphatic catheterization to collect large quantities of lymph and its constituents over extended periods of time.
This technique opens a myriad of applications to many disciplines examining various physiologic functions. Applications could include, but are not limited to, whole body lipoprotein and lipid metabolism, immunosurveillance, tumor genesis and metastasis, intestinal function, and the development and progression of intestinal inflammatory disease.
Protocol
All procedures on experimental animals described in both the video and manuscript were approved by the Institutional Animal Care and Use Committee and followed the guidelines set by the Canadian Council of Animal Care.
1. Surgical Anesthesia and Surgical Preparation of the Neonatal Pigs
In a separated anteroom, premedicate 25 kg pigs near the base of the neck with an intramuscular sedative-anesthetic drug cocktail containing: azaperone (0.3 mg/kg), ketamine hydrochloride (10 mg/kg), dexmedetomidine (15 µg/kg). Note: Add buprenorphine (0.005-0.02 mg/kg) in the pre-anesthetic drug cocktail for enhanced intra-operative pain control.
Anesthetize the pigs with inhaled isoflurane inhalation gas (4-5% isoflurane at 500 ml-1,000 ml/min O2) using a face mask. Visualize the vocal cords using a veterinary laryngeal scope (17-25 cm long straight blade), and apply topical 10% lidocaine spay to the vocal cords. Allow the lidocaine spray to contact the vocal cords 30-60 sec prior to intubation to reduce the possibility of vocal cord spasm and airway obstruction.
Intubate the pigs by passing a cuffed endotracheal tube (5.0-7.0 mm Internal Diameter (ID)) between the vocal cords and maintain the anesthesia with isoflurane gas (0.5-2.0% isoflurane at 1,000-2,000 ml/min O2) using a closed circuit rebreathing anesthetic system throughout the surgery. Assess the level of anesthesia by jaw tone, and both pedal and palpebral reflex responses. Expired anesthetic gas is scavenged and vented outside the surgical suite.
Clean the exterior surface of the ear with 2.0% chlorhexidine surgical scrub solution followed by a 70% isopropyl alcohol rinse. With a 20 G intravenous catheter, catheterize an ear vein to provide intravenous fluids (Lactated Ringer's Solution; 5-10 ml/kg/hr) during the surgery. Secure a pulse oximeter to the mucosal surface of the tongue with medical tape to monitor heart rate and the saturation of peripheral blood oxygenation (SpO2).
Place the anesthetized pig in a dorsal recumbent position and shave the ventral abdomen from the mid thorax caudally to the ventral aspect of the pubis. Clean this area with two alternating 2.0 % chlorhexidine surgical scrubs and sterile water washes.
Transfer the anaesthetized pig to the surgical suite and apply the final surgical scrub of 70% isopropyl alcohol rinse, allow it to dry, and then drape the animal.
Insert a rectal temperature probe approximately 2-4 cm into the rectum to monitor body temperature. Place the pigs on a water recirculating heating pad to maintain normal body temperature (38-40 °C) during surgical procedure.
Drape the pig with four towels drapes placed in an overlying quadrant pattern around the abdomen. Place the first drape across xiphisternum, the second drape along lateral aspect of the abdomen approximately 5 cm lateral to the abdominal midline. Place the third drape across the ileal crest of the pelvis and the fourth drape, like the second drape (although on the opposite side), is placed along the lateral aspect of the abdomen approximately 5 cm lateral to the abdominal midline.
Place a large table drape, with a slit-opening allowing access to the surgical site, over the underlying towel drapes and cover the pig and entire surgical table. The final drape is a disposable steri-drape placed over the large table drape.
2. Abdominal Surgery and Catheterization of the Intestinal Lymphatic Trunk
Make a 20 cm skin incision with a scalpel blade to expose the underlying abdominal muscles. Incise the abdominal muscle layers with mono-polar electrocautery (20 Watts setting) to expose the parietal peritoneum. Open a 20 cm linear segment of parietal peritoneum with Metzenbaum scissors to access the abdominal viscera and lymphatic vessel.
Place a retractor at the cranial aspect of the surgical incision to keep the abdominal cavity open for the duration of the surgery.
Moisten all tissues with warm (37 °C) sterile saline for the entire surgical procedure. Gently lift a large segment of intestine including the colon, cecum, ileum and jejunum from the abdominal cavity and exteriorize it to the left flank of the pig to access the upper abdomen, liver and lymphatic vessel. Secure the exteriorized intestine in position with additional towel drapes to form a sling to gently support the intestine.
Locate the lymphatic vessel, it lays approximately 4 cm cranial-medial of the right renal vein, 6 cm caudal-medial- ventral of the caval foremen and underneath the visceral aspect of the right liver lobe near the pancreas22,36,37. Identify the lymphatic vessel as a translucent structure juxtaposed to the right ventral segment of the portal vein36,37.
Separate the lymphatic vessel from the surrounding fascia by gently teasing away the attached tissue with Q-tip applicators. Once the lateral aspects of vessel are separated from the surrounding tissue, create a "tunnel" opening underneath the vessel with fine blunt tipped forceps.
Pass three 2-0 silk sutures underneath the lymphatic vessel with fine forceps. Ligate the most caudal suture first to occlude, dilate and fill the vessel with lymph. Purposefully leave the ends of this suture relatively long (4 cm) to secure the catheter to the lymphatic vessel. Place two other sutures separated 1.0 cm from each other and are approximately 1.0-1.5 cm cranial to the secured caudal suture. Leave these two sutures with a 'single loose ligature tie' to allow for faster securing of the catheter into the vessel. Note: The segment of the lymphatic vessel located between the most caudal ligating suture and middle suture (of the two cranial sutures) is the site for catheterization. Regarding suture material, 2-0 polyglactin suture can substitute for 2-0 silk sutures if required.
Cut a small hole into the vessel with iris scissors and dilate the vessel with fine blunt forceps. Insert approximately 1.0-1.5 cm of specialized catheter tubing (4.06 Outer Diameter (OD) X 2.31 mm ID) with a beveled end into the vessel and tie the two cranial sutures to secure the catheter in place. Use the long suture ends of the caudal suture to secure the catheter to the vessel.
Wash the exteriorized intestine with copious amounts of warm saline and gently return it to the abdominal cavity ensuring correct anatomical positioning of the gut.
Exteriorize the catheter at the left mid flank (5-10 cm ventrally from the paralumbar fossa). Make a skin incision with a scalpel and pass a trocar from the abdominal cavity to the skin surface to create an opening for the exteriorization of the catheter. Use a large Kelly forceps to exteriorize the catheter from the abdominal cavity through the trocar opening.
Close the parietal peritoneum with a simple continuous suture pattern of 2-0 polyglactin suture with a round (tapered) needle. Close the abdominal muscle layers with a simple interrupted suture pattern with a 2-0 polyglactin suture on a round needle.
Close the skin in a subcuticular pattern with 2-0 polyglactin suture on a cutting needle. Secure the exteriorized catheter to the skin with a purse-string suture pattern and a 2-0 nylon suture on a cutting needle.
Place a specialized jacket on the pig while still anesthetized to ease its placement and reduce the stress of the pig during recovery.
3. Post-surgical Recovery and Lymph Collection
Approximately 10 min prior to the discontinuation of inhalation anesthesia, administer bupenorphine (0.1 mg/kg) intramuscularly to provide immediate post-surgery analgesia. Continue bupenorphine (0.1 mg/kg) every 12 hr for 24-48 hr to maintain post-surgery analgesia.
Monitor the pigs for post-surgical complications every 8-12 hr for a 7 day period.
Collect the lymph in 500 ml polypropylene wash bottles, coated with ethylenediamine tetraacetic acid (EDTA), and supplemented with antibiotics; penicillin (6,000 IU), streptomycin (6 mg) and amphotericin B (3 mg) every 12 hr for a 7 day period.
4. Quantification of Lipoprotein ApoB48, Triglyceride, Cholesterol and Total Protein Collected from Lymph
Centrifuge the lymph sample at 1,800 x g for 5 min at 4 °C. Collect the supernatant and use it for the quantification of triglyceride, cholesterol and total protein.
Divide the supernatant into three samples: an undiluted sample, a sample diluted 1:20 distilled water and a final sample diluted 1:100 with distilled water.
Use the undiluted supernatant to measure cholesterol levels, with a commercially available kit.
Use the 1:20 and 1:100 diluted samples to measure triglyceride and total protein levels with a commercially available kit and the bicinchoninic acid-total protein assay respectively.
5. Quantification of Lipoprotein ApoB48, Collected from Lymph38
Determine the concentration of lipoprotein ApoB48 with an adapted immune Western Blotting method38. Separate total lymph with 3-8% tris-acetate- sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Transfer the separated proteins to a polyvinylidene fluoride membrane (0.45 µm) and incubate them with a goat polyclonal antibody to ApoB (1:4,000), then bind it with an anti-goat secondary antibody.
Quantify the lipoprotein ApoB48 with chemiluminescence using a linear densitometric comparison with purified rodent ApoB48 protein standard.
Representative Results
Lymphatic catheterization of the intestinal lymphatic trunk of neonatal pigs allows collection of approximately 1 L/24 hr of central lymph over a 7 day period. The lymph collected in this experiments contained components of lipid metabolism, namely total lymph protein, ApoB48 lipoprotein, triglycerides, total protein, and cholesterol. Table 1 highlights representative amounts of these lipid components from pooled lymph samples of three pigs. Notably, lymph flow and lipid constituents are in-line with values of central lymph reported by other investigators following catheterization of intestinal lymphatic vessels (lymph flow 570 ± 158 to 979 ± 284 ml/24 hr25, 360 ± 120 mLl/24 hr to 1080 ± 720 ml/24 hr26; triglyceride 687 ± 110 mg/dL9; cholesterol 63.1 ± 5.6 mg/dL9); indicating proper placement of the catheter within the lymphatic vessel.
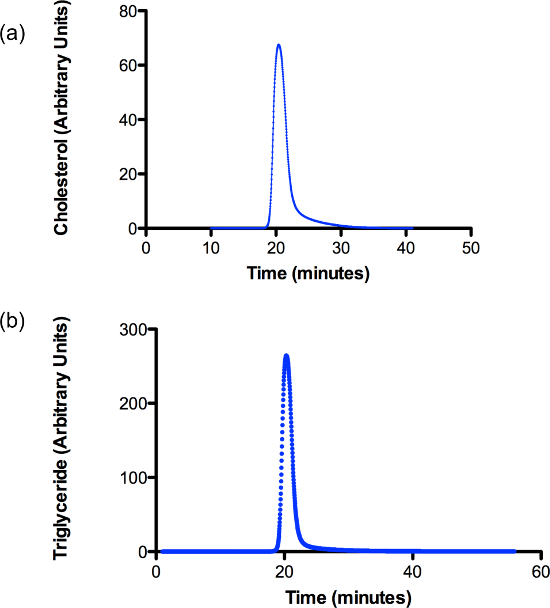 Figure 2. A Fast Protein Liquid Chromatography (FPLC) profile demonstrating the purity of the chylomicron preparation in neonatal pig intestinal lymph. Lymph collected from three pigs is separated using a 1.006 g/ml density gradient ultracentrifugation protocol. The profile shows a single peak for (A) cholesterol and (B) triglyceride in the lymph sample. Please click here to view a larger version of this figure.
Figure 2. A Fast Protein Liquid Chromatography (FPLC) profile demonstrating the purity of the chylomicron preparation in neonatal pig intestinal lymph. Lymph collected from three pigs is separated using a 1.006 g/ml density gradient ultracentrifugation protocol. The profile shows a single peak for (A) cholesterol and (B) triglyceride in the lymph sample. Please click here to view a larger version of this figure.
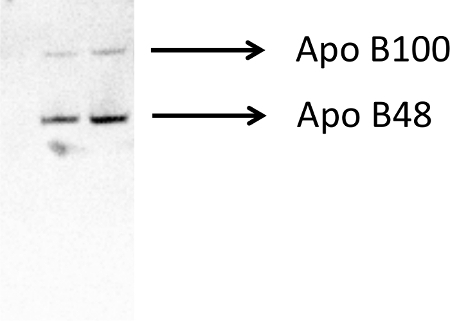 Figure 1. A representative Western blot analysis demonstrating the contribution of ApoB48 and ApoB100 lipoproteins in neonatal pig intestinal lymph. Lymph collected from three pigs is separated using a 1.006 g/ml density gradient ultracentrifugation protocol. The figure demonstrates a small amount of contamination with approximately 15% of plasma derived ApoB100. Please click here to view a larger version of this figure.
Figure 1. A representative Western blot analysis demonstrating the contribution of ApoB48 and ApoB100 lipoproteins in neonatal pig intestinal lymph. Lymph collected from three pigs is separated using a 1.006 g/ml density gradient ultracentrifugation protocol. The figure demonstrates a small amount of contamination with approximately 15% of plasma derived ApoB100. Please click here to view a larger version of this figure.
| Lymph Characteristics | |
| ApoB48 (mg/ml) | 3022.4 ± 440.4 |
| Triglyceride (mg/dL) | 607 ± 203.6 |
| Cholesterol (mg/dL) | 58.5 ± 9.6 |
| Results are expressed as mean ± SD (n=3) |
Table 1. Components of intestinal lymph collected from neonatal pigs 7 days post-surgery. Results are repeated measures of lymph and expressed as mean SD (n=3)
Discussion
Collecting intestinal lymph is an excellent method to investigate mechanisms involved in lipid8-12 and drug13-15 metabolism, cancer metastasis16,17, cell trafficking and immune function18-26, in various experimental animal models. Indeed, the ability to harvest large quantities of either peripheral (afferent) and central (efferent and large trunk vessels) lymph over an extended period has been particularly important for understanding temporal changes that occur in cell populations following challenges with immune modulating agents18-22,25,26. Similarly, collection of central lymph has been helpful in delineating processes involved in lipoprotein metabolism8-12. The utility of lymph collection is dependent on the success of the catheterization procedure and the patency of the catheter within the lymphatic vessel.
Catheterization of the major lymphatic vessels involved in intestinal lymph drainage in large animals generally uses one of three surgical approaches. The first technique uses a cranial shoulder approach to enter the thoracic duct overlying the common jugular vein (cow thoracic duct)5. The second uses an upper flank approach over the mid-lateral aspect of the right kidney32,33. The third technique as described in this video employs either a ventral midline or paramedian22,35 laparotomy to expose the intestinal lymphatic trunk near the pancreas and juxtaposed to the right ventral aspect of the portal vein22,36,37. Although both the upper flank and ventral midline surgical techniques enable catheterization of the intestinal lymphatic trunk, we believe the ventral midline approach allows for better exposure of the vessel with adequate space within the upper abdomen to handle and manipulate the vessel.
Importantly, as in any experimental surgery there are steps that are particularly crucial for the successful outcome of the experimental procedure. There are four key areas that require careful attention using the ventral midline approach to access the intestinal lymphatic vessel. First, excellent blood supply to the exteriorized intestine is required, as prolonged compromised circulation to the intestine induces ischemic intestinal mucosal necrosis and surgical failure. In this procedure a 'towel sling' reduces the tension on the mesenteric blood vessels by supporting the weight of the intestines ensuring excellent tissue perfusion. Second, careful manipulations of the vessel including: the release of the vessel from surrounding fascia and adipose tissue, inserting and firmly securing the catheter within the vessel for long-term lymph collection is critical for successful catheterization. Indeed, it is suggested that the structural integrity of the vessels in neonatal pigs is not as robust as in mature animals and as such can easily tear39,40. Using Q-tip applicators to slowly and gently separate fascial layers and fine blunt-ended instruments to pass the suture underneath the vessel greatly reduces the inadvertent puncture or tearing of the lymphatic vessel. Rough handling of the vessel often creates a friable vessel with weak structure making catheterization very challenging. Third, a common cause of catheterization failure is the unintentional 'pulling-out of the catheter' from the lymphatic vessel. Securing the catheter directly to the vessel reduces the incidence of catheter loss. This is accomplished by tying the catheter directly to the vessel with the caudal suture employed to dilate the vessel. Finally, it is important to ensure good lymph flow is present following catheterization and prior to abdomen closure. Indeed, if lymph flow is sluggish during the procedure, over time this induces clot formation within the catheter and markedly reduces the lymph collection period. In the investigator's experience, with long term lymph collection of non-intestinal lymph in sheep41, it is very difficult to remove the clot from the catheter as the clot develops within the catheter and is deep-seated within the lymphatic vessel. Consequently, this will greatly reduce (approximately 40%) the numbers of animals successfully catheterized for long-term experimentation (unpublished data). Therefore, if lymph flow is slowed or has discontinued during the procedure, the lymphatic catheter should be reinserted cranially 0.5-1 cm from the initial catheterization site.
All models used to investigate scientific questions have inherant limitations and catheterization of the intestinal lymphatic trunk is no exception. The most pronounced limitation for the procedure is the inability to effectively isolate, catheterize the lymphatic vessel, and stabilize the catheter within the vessel. As such, this procedure requires people with excellent technical and surgical skills. Another significant limitation is that prolonged surgical times during major abdominal surgery increases the risk and post-surgical morbidity and mortality. Notably, however, the pigs in our experiment recovered quickly from surgery and there were no complications such as infections, intestinal ileus, excessive tissue injury, or inappetence following the procedure. Most certainly, pigs were observed to be bright, responsive, ambulating and eating normally 24 hr post-surgery and this continued over the 7 day lymph collection period. Notably, although serum chemistries or complete blood counts were not assessed in these pigs following the 7 day experiment, previous work by the investigators in long-term lymph collection in sheep41 demonstrated that catheterized sheep were never leukopenic, lymphopenic, hypoproteinemic with electrolyte imbalances or clinically portraying poor health (unpublished data). Importantly and as stated above, this observation correlates to catheterized pigs also displaying clinical characteristics of good health.
Interestingly, investigators noted variations in the volume of lymph flow. The flow was greatest during the day when pigs were feeding and active with approximately 70% of the daily collected lymph occurring at this time. The remaining 30% of lymph flow happens in the evening during rest and sleep.
Further characterization of lymph samples are provided in Figures 1, 2 and Table 1. Figure 1 demonstrates a small amount of contamination with approximately 15% of plasma derived ApoB100. The presence of plasma lipoprotein within intestinal lymph is an observation that often occurs in pigs42. In suckling pigs, the majority of the lipoprotein is produced by the liver and released in plasma. From the plasma, ApoB100 likely seeps into collecting lacteals42 and then through the lymphatic vascular network drains into the intestinal lymphatic trunk. Various methods of chromatography are used to assess cholesterol, triglycerides and other phospholipid contents in collected lymph samples42-45. The single peak in Figure 2 clearly demonstrates cholesterol and triglycerides are present in the chylomicron fraction. This method is a convenient and accurate technique of profiling lymph lipoproteins and should be considered when assessing the purity of chylomicron samples from intestinal lymph45. Finally, Table 1 provides the biochemical components of intestinal lymph collected from neonatal pigs 7 days post-surgery.
In conclusion, long-term catheterization of the intestinal lymph trunk is successful in young pigs. With this technique, lymphatic catheterization can be used to collect and investigate components of lipid metabolism. The purity of lymph, amount of lymph produced and the quantities of constituents of lipid metabolism are similar to amounts present in pig central lymph in other experiments9,25,26,42. The results indicate that employing a ventral midline surgical approach with careful tissue dissection and vessel handling to catheterize the intestinal lymphatic trunk in young pigs is an excellent method to collect large quantities of central lymph.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The work was supported in part by funding from Alberta Livestock and Meat Agency and Natural Science and Research Council Discovery grant to S. D. Proctor.
References
- Lindsay FEF. The cisterna chyli as a source of lymph samples in the cat and dog. Res. Vet. Sci. 1974;17:256–258. [PubMed] [Google Scholar]
- Kohan AB, Howles PN, Tso P. Methods for studying rodent intestinal lipoprotein production and metabolism. Curr. Protoc. Mouse Biol. 2012;2:219–230. doi: 10.1002/9780470942390.mo120049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, et al. Intestinal uptake and lymphatic absorption of beta-carotene in ferrets: a model for human beta-carotene metabolism. Am. J. Physiol. 1992;263(4 Pt 1):G480–G486. doi: 10.1152/ajpgi.1992.263.4.G480. [DOI] [PubMed] [Google Scholar]
- Hein WR, Barber T, Cole SA, Morrison L, Pernthaner A. Long-term collection and characterization of afferent lymph from the ovine small intestine. J.Immunol. Methods. 2004;293(1-2):153–168. doi: 10.1016/j.jim.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Hartmann PE, Lascelles AK. The flow and lipid composition of thoracic duct lymph in the grazing cow. J. Physiol. 1966;184(1):193–202. doi: 10.1113/jphysiol.1966.sp007910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave TG, Dunne KB. Chylomicron formation and composition in unanaesthetised rabbits. Atherosclerosis. 1975;22(3):389–400. doi: 10.1016/0021-9150(75)90019-2. [DOI] [PubMed] [Google Scholar]
- Binns RM, Hall JG. The paucity of lymphocytes in the lymph of unanaesthetised pigs. Br. J. Exp. Pathol. 1966;47(3):275–280. [Google Scholar]
- Ohlsson L, Kohan AB, Tso P, Ahren B. GLP-1 released to the mesenteric lymph duct in mice: Effects of glucose and fat. Regul. Pept. 2014;189:40–45. doi: 10.1016/j.regpep.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HT, Kim DN, Lee KT. Intestinal apolipoprotein B-48 synthesis and lymphatic cholesterol transport are lower in swine fed high fat, high cholesterol diet with soy protein than with casein. Atherosclerosis. 1989;77(1):15–23. doi: 10.1016/0021-9150(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Arnold M, Dai Y, Tso P, Langhans W. Meal-contingent intestinal lymph sampling from awake, unrestrained rats. Am. J. Physiol. Integr. Comp. Physiol. 2012;302(12):R1365–R1371. doi: 10.1152/ajpregu.00497.2011. [DOI] [PubMed] [Google Scholar]
- Nguyen TM, Sawyer JK, Kelley KL, Davis MA, Kent CR, Rudel LL. ACAT2 and ABCG5/G8 are both required for efficient cholesterol absorption in mice: evidence from thoracic lymph duct cannulation. J. Lipid Res. 2012;53(8):1598–1609. doi: 10.1194/jlr.M026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Kawata Y, Erami K, Ikeda I, Imaizumi K. LXR agonist increases the lymph HDL transport in rats by promoting reciprocally intestinal ABCA1 and apo A-I mRNA levels. Lipids. 2008;43(2):125–131. doi: 10.1007/s11745-007-3131-8. [DOI] [PubMed] [Google Scholar]
- Boyd M, Risovic V, Jull P, Choo E, Wasan KM. A stepwise surgical procedure to investigate the lymphatic transport of lipid-based oral drug formulations: Cannulation of the mesenteric and thoracic lymph ducts within the rat. J. Pharmacol. Toxicol. Methods. 2004;49(2):115–120. doi: 10.1016/j.vascn.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Sugawara T, et al. Intestinal absorption of dietary maize glucosylceramide in lymphatic duct cannulated rats. J. Lipid Res. 2010;51(7):1761–1769. doi: 10.1194/jlr.M002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleford DM, et al. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. J. Pharmacol. Exp. Ther. 2003;306(3):925–933. doi: 10.1124/jpet.103.052522. [DOI] [PubMed] [Google Scholar]
- Lespine A, et al. Contribution of lymphatic transport to the systemic exposure of orally administered moxidectin in conscious lymph duct-cannulated dogs. Eur. J. Pharm. Sci. 2006;27(1):37–43. doi: 10.1016/j.ejps.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Carr J, Carr I, Dreher B, Betts K. Lymphatic metastasis: invasion of lymphatic vessels and efflux of tumour cells in the afferent popliteal lymph as seen in the Walker rat carcinoma. J. Pathol. 1980;132(4):287–305. doi: 10.1002/path.1711320402. [DOI] [PubMed] [Google Scholar]
- Bennell MA, Husband AJ. Route of lymphocyte migration in pigs. I. Lymphocyte circulation in gut-associated lymphoid tissue. Immunology. 1981;42(3):469–474. [PMC free article] [PubMed] [Google Scholar]
- Knight JS, Baird DB, Hein WR, Pernthaner A. The gastrointestinal nematode Trichostrongylus colubriformis down-regulates immune gene expression in migratory cells in afferent lymph. BMC Immunol. 2010;11:51. doi: 10.1186/1471-2172-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milling SW, Jenkins C, MacPherson G. Collection of lymph-borne dendritic cells in the rat. Nat. Protoc. 2006;1(5):2263–2270. doi: 10.1038/nprot.2006.315. [DOI] [PubMed] [Google Scholar]
- Pernthaner A, Cole SA, Gatehouse T, Hein WR. Phenotypic diversity of antigen-presenting cells in ovine-afferent intestinal lymph. Arch. Med. Res. 2002;33(4):405–412. doi: 10.1016/s0188-4409(02)00375-2. [DOI] [PubMed] [Google Scholar]
- Thielke KH, Pabst R, Rothkotter HJ. Quantification of proliferating lymphocyte subsets appearing in the intestinal lymph and the blood. Clin. Exp. Immunol. 1999;117(2):277–284. doi: 10.1046/j.1365-2249.1999.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G, Fisher R. IgA-containing plasma cells in the lamina propria of the gut: failure of a thoracic duct fistula to deplete the numbers in rat small intestine. Eur. J. Immunol. 1979;9(1):85–91. doi: 10.1002/eji.1830090118. [DOI] [PubMed] [Google Scholar]
- Beh KJ. The origin of IgA-containing cells in intestinal lymph of sheep. Aust. J. Exp. Biol. Med. Sci. 1977;55(3):263–274. doi: 10.1038/icb.1977.21. [DOI] [PubMed] [Google Scholar]
- Bennell MA, Husband AJ. Route of lymphocyte migration in pigs. II. Migration to the intestinal lamina propria of antigen-specific cells generated in response to intestinal immunization in the pig. Immunology. 1981;42(3):475–479. [PMC free article] [PubMed] [Google Scholar]
- Rothkotter HJ, Huber T, Barman NN, Pabst R. Lymphoid cells in afferent and efferent intestinal lymph: lymphocyte subpopulations and cell migration. Clin. Exp. Immunol. 1993;92(2):317–322. doi: 10.1111/j.1365-2249.1993.tb03398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilahur G, Padro T, Badimon L. Atherosclerosis and thrombosis: insights from large animal models. J. Biomed. Biotechnol. 2011;1 doi: 10.1155/2011/907575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32(5):1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skold BH, Getty R, Ramsey FK. Spontaneous atherosclerosis in the arterial system of aging swine. Am. J. Vet. Res. 1966;27(116):257–273. [PubMed] [Google Scholar]
- Reiser R, Sorrels MF, Williams MC. Influence of high levels of dietary fats and cholesterol on atherosclerosis and lipid distribution in swine. Circ. Res. 1959;7:833–846. doi: 10.1161/01.res.7.6.833. [DOI] [PubMed] [Google Scholar]
- Casani L, Sanchez-Gomez S, Vilahur G, Badimon L. Pravastatin reduces thrombogenicity by mechanisms beyond plasma cholesterol lowering. Thromb. Haemost. 2005;94(5):1035–1041. doi: 10.1160/TH05-04-0245. [DOI] [PubMed] [Google Scholar]
- Romosos DR, McGilliard AD. Preparation of thoracic and intestinal lymph duct shunts in calves. J. Dairy Sci. 1970;53(9):1275–1278. doi: 10.3168/jds.S0022-0302(70)86379-2. [DOI] [PubMed] [Google Scholar]
- Shannon AD, Lascelles AK. The intestinal and hepatic contributions to the flow and composition of thoracic duct lymph in young milk-fed calves. Q.J. Exp. Physiol. Cogn. Med. Sci. 1968;5(2):194–205. doi: 10.1113/expphysiol.1968.sp001959. [DOI] [PubMed] [Google Scholar]
- Aliev AA. Intestinal lymph of ruminants. I. Operative techniques for collecting intestinal lymph from ruminants. Acta Vet.Hung. 1990;38(1-2):105–120. [PubMed] [Google Scholar]
- Butterfield AB, Lumb WV, Litwak P. Surgical preparation of miniature swine for atherosclerosis research. Am. J. Vet. Res. 1976;37(12):1519–1523. [PubMed] [Google Scholar]
- Saar LI, Getty R. Sisson and Grossman's: The anatomy of domestic animals. Vol. 2. Philadelphia PA: W. B. Saunders Company; 1975. Lymphatic system; pp. 1343–1358. [Google Scholar]
- Zanchet DJ, de Souza Montero EF. Pig liver sectorization and segmentation and virtual reality depiction. Acta. Cirurgica. Basilera. 2002;17(6):382–387. [Google Scholar]
- Vine DF, Takechi R, Russell JC, Proctor SD. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis. 2007;190(2):282–290. doi: 10.1016/j.atherosclerosis.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Li WC, et al. Biomechanical properties of ascending aorta and pulmonary trunk in pigs and humans. Xenotransplantation. 2008;15(6):384–389. doi: 10.1111/j.1399-3089.2008.00498.x. [DOI] [PubMed] [Google Scholar]
- Arkill KP, Moger J, Winlove CP. The structure and mechanical properties of collecting lymphatic vessels: an investigation using multimodal nonlinear microscopy. J. Anat. 2010;216(5):547–555. doi: 10.1111/j.1469-7580.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwiera RRE, et al. Plasmid DNA induces increased lymphocyte trafficking: a specific role for CpG motifs. Cell. Immunol. 2001;214(2):155–164. doi: 10.1006/cimm.2001.1899. [DOI] [PubMed] [Google Scholar]
- Black DD, Davidson NO. Intestinal apolipoprotein synthesis and secretion in the suckling pig. J. Lipid Res. 1989;30(2):207–218. [PubMed] [Google Scholar]
- Heider JG, Pickens CE, Lawrence KA. Role of acyl CoA:cholesterol acyltransferase in cholesterol absorption and its inhibition by 57-118 in the rabbit. J. Lipid Res. 1983;24:1127–1134. [PubMed] [Google Scholar]
- Noh SK, Koo SI. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J. Nutr. 2004;134:2611–2616. doi: 10.1093/jn/134.10.2611. [DOI] [PubMed] [Google Scholar]
- Brunham LR, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 2006;116(4):1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]


