Abstract
Several new methods have been developed in the field of biotechnology to obtain autologous cellular suspensions during surgery, in order to provide one step treatments for acute and chronic skin lesions. Moreover, the management of chronic but also acute wounds resulting from trauma, diabetes, infections and other causes, remains challenging. In this study we describe a new method to create autologous micro-grafts from cutaneous tissue of a single patient and their clinical application. Moreover, in vitro biological characterization of cutaneous tissue derived from skin, de-epidermized dermis (Ded) and dermis of multi-organ and/or multi-tissue donors was also performed. All tissues were disaggregated by this new protocol, allowing us to obtain viable micro-grafts. In particular, we reported that this innovative protocol is able to create bio-complexes composed by autologous micro-grafts and collagen sponges ready to be applied on skin lesions. The clinical application of autologous bio-complexes on a leg lesion was also reported, showing an improvement of both re-epitalization process and softness of the lesion. Additionally, our in vitro model showed that cell viability after mechanical disaggregation with this system is maintained over time for up to seven (7) days of culture. We also observed, by flow cytometry analysis, that the pool of cells obtained from disaggregation is composed of several cell types, including mesenchymal stem cells, that exert a key role in the processes of tissue regeneration and repair, for their high regenerative potential. Finally, we demonstrated in vitro that this procedure maintains the sterility of micro-grafts when cultured in Agar dishes. In summary, we conclude that this new regenerative approach can be a promising tool for clinicians to obtain in one step viable, sterile and ready to use micro-grafts that can be applied alone or in combination with most common biological scaffolds.
Keywords: Medicine, Issue 109, Micro-grafts, Rigenera protocol, skin, cell viability, stem cells, bio-complexes
Introduction
In the last years, several new methods have been developed in the field of biotechnology to obtain autologous cellular suspensions during surgery in order to provide one-step treatments for acute and chronic skin lesions. Moreover, the management of acute but mainly chronic wounds resulting from trauma, diabetes, infections, and other causes, remains challenging. There is mounting evidence that chronic wounds have become a serious global health issue, causing an enormous financial burden on healthcare systems worldwide1.
To increase the rate of success in the treatment of skin lesions, the absence of extensive manipulation (including cellular enhancement) and the maintenance of sterile conditions are essential, in order to create a cellular suspension that can be immediately applied on the damaged area of the patients, thereby avoiding a longer processing in cleanrooms such as Cell Factories. Starting from small skin biopsies, grinding, centrifugation and other separation methods (e.g., enzymatic or mechanical), are frequently used to obtain a cellular suspension, which can be cultured in a growth medium. All of these methods generally require a long time of execution, stressing the cell structures, and leading to a reduction of cell viability. Another significant aspect is to obtain an autologous cellular suspension ready to be used by clinicians, for example, to repair damaged areas. Furthermore, it is well established that autologous tissue grafts survive the transfer procedures to eventually survive in the recipient site by the principles of induction and conduction 2, 3. The ideal graft tissue should be readily available and have low antigenicity and donor site morbidity 4.
On the basis of these evidences, the first aim of this study was to create autologous bio-complexes suitable for clinical application in the tissue repair. For this purpose, we describe a new method to obtain autologous micro-grafts starting from cutaneous tissues which were disaggregated by this protocol. A case presentation is also herein described as a clinical application of autologous micro-grafts obtained by this protocol in combination with collagen sponges. This approach has already been reported to be efficient in the mechanical disaggregation of human tissues5 and it has been used clinically for grafts and regeneration of dermal tissues 6,7 as well as for regenerative therapies of connective tissues in oral-maxillofacial surgery 8-10.
In addition, the second aim of this study was the biological characterization of the cutaneous tissues after their disaggregation by this protocol. To this purpose, different homologous samples of cutaneous tissue derived from the trunk area of different multi-organ and/or multi-tissue donors were processed following National Rules on harvesting, processing and distributing tissues for transplantation (CNT 2013) at Emilia Romagna Regional Skin Bank.
CASE PRESENTATION:
A 35-year-old female patient showing a complex trauma due to car accident was admitted to the Intensive Care Unit of Ancona Hospital. The patient showed an infection on the leg due to an open wound and a compound fracture stabilized with external fixation. Two radical debridement were performed and when the wound became clean after negative pressure therapy (V.A.C. therapy) and the periosteum appeared healthy, we applied the protocol after two months from recovery. After disaggregation with this system, the micro-grafts obtained were used to create bio-complexes with a collagen sponge which were subsequently implanted in order to investigate their efficacy on the lesion repair.
Protocol
Ethics statement: since the clinical application of the protocol requires the use of cutaneous autologous tissue of the patient, its characterization in vitro was performed before clinical use on homologous cutaneous tissue at Emilia Romagna Regional Skin Bank following the guidelines of National Rules on harvesting, processing and distributing tissues for transplantation (CNT 2013).
1. Bio-complex Building for Clinical Application
NOTE: This protocol is clinically based on the use of Rigeneracons (tissue disruptor) and the Rigenera Machine (tissue disruptor system) (Figure 1A). The tissue disruptor is a biological medical disruptor of human tissues able to disrupt small pieces of tissues using a grid provided by hexagonal blades and filtering cells and components of extracellular matrix with a cut-off of about 50 microns.
Collect skinsamples of the patient through a biopsy punch (Figure 1B) and disaggregate adding 1 ml of saline solution for each piece to obtain autologous micro-grafts 6,7,9 (or see step 2.1).
Place 1 ml of micro-grafts on collagen sponges (Figure 1C) toform bio-complexes to use for clinical application.
Culture another 1 ml of micro-grafts on collagen sponges in the presence of 6 ml of DMEM medium supplemented with 10% Fetal Bovine Serum at 37 °C in a 5% CO2 humidified atmosphere.
Following 3 days of culture, fix the bio-complexes with 0.3% Paraformaldehyde for 10 min at RT. Pour the paraffin with a specific dispenser directly onto the sample. Obtain slices with a microtome with a thickness of 5 µm and put directly in a glass-slide.
Immerse paraffin slices of 5 µm in a glass for histological analyses, containing 15 - 20 ml Xylene (commercially available - a mix of m-xylene (40 - 65%), p-xylene (20%), o-xylene (20%) and ethyl benzene (6 - 20%) and traces of toluene, trimethyl benzene, phenol, thiophene, pyridine and hydrogen sulfide) for 3 min each.
Immerse the slices in decreasing grades (100% to 70%) of ethanol (100% ethanol for 1hr, 95% ethanol for 1 hr, 80 % ethanol for 1hr, 70% ethanol for 1 hr) and then deionized water for de-paraffinizing and rehydrating the sections.
Stain the sections with 1 - 2 ml of 1g/L Ematoxylin for 1 - 2 min and subsequently rinse in water to remove any Ematoxylin surplus.
Stain the sections for 4 - 5 min with Eosin Y alcoholic solution at 1% of concentration mixed with ethanol 70% and diluted in water.
Use 1 - 2 ml of Eosin Y for each slide section and rinse under running tap water.
Immerse sections in increasing grades of ethanol (see step 1.6) and finally, after a passage in Xylene for 1h, coverslip with a based-mounting mediumand observe under a light microscope at 100X magnification (Figure 1D).
2. Collection, Disaggregation and In Vitro Analysis of the Tissues
- Using a dermatome, take independent skin tissue, papillary de-epidermized dermis (Ded) or reticular dermis (Dermis) respectively of 0.6 mm, 1 mm or 2 mm in thickness from the trunk area of 4 different multi-organ and/or multi-tissue donors in a range from 40 to 55 years, following National Rules on harvesting, processing and distributing tissues for transplantation (CNT 2013).
- Gently rinse all samples in 0.9% NaCl solution putting them in a dish on an orbital shaker for 5 min.
- Using the 5-mm biopsy punch, create samples which are uniform in diameter from the skin tissue, Ded and Dermis and weigh all tissue specimens before the disaggregation.
- Insert eight, three or four uniform samples of skin tissue, Ded or Dermis respectively, in the tissue disruptor, adding 1.5 ml of saline solution for the disaggregation.
- Perform different times of disaggregation for all tissue samples as indicated in Table 1.
- Use a correspondent number of punch biopsies derived from intact tissue samples as controls.
- After mechanical disaggregation, aspirate the saline solution containing micro-grafts and place separately each sample in a single well of a 12-well plate. Perform the same protocol for intact control punch biopsies.
- Add 1ml of RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and antibiotics to each sample.
- Evaluate the cell viability immediately. To each well containing a micro-graft (obtained by the simultaneous disaggregation of eight, three or four uniform samples of skin tissue, Ded or Dermis respectively) add 1ml of medium containing 0.5 mg/ml of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide) solution and incubate for 3 hr at 37 °C in an atmosphere of 5% CO2/air. Perform the same protocol for intact control punch biopsies.
- After incubation, remove all medium containing MTT and add to each sample 1 ml dimethyl sulfoxide (DMSO) for 10 min.
- Transfer each sample and DMSO in a cuvette and read at optical density (OD) at 570 nm using a spectrophotometer. Calculate cell viability as the ratio of absorbance at 570 nm and the weight in grams (gr) of tissue used before disaggregation. Perform the same protocol for intact control punch biopsies.
- After mechanical disaggregation, aspirate the saline solution containing micro-graft derived from skin tissue, Ded and Dermis samples of a single donor.
- Place separately each sample in a single well of a 12-well plate or in a culture flask for cell viability test and morphological analysis respectively.
- Culture micro-grafts adding 1 ml (12-well plate) or 5 ml (culture flask) of RPMI 1640 medium supplemented with 10% FBS and antibiotics at 37 °C in an atmosphere of 5% CO2/air for 24 hr or 7 days.
- Evaluate the cell viability after 24 hr or 7 days (repeat steps 2.1.8-2.1.10).
- Perform morphological analysis evaluating the presence of cell suspension by light microscopy after 24 hr and 7 days of culture in flask.
- Analyze the samples of Dermis for positivity to the mesenchymal and hematopoietic cell markers, including CD146, CD34 and CD45 antigens by FACS analysis 6.
- Under laminar flow hood seed each micro-graft (obtained by the simultaneous disaggregation of eight, three or four uniform samples of skin tissue, Ded or Dermis respectively) and a correspondent small fragment of each tissue sample not totally disaggregated (>50 micron in size after disaggregation process) on Columbia agar plate containing 5% sheep blood broth 100 µl.
- Incubate the plate at 37 °C for three days and perform microbiological analysis on Columbia agar plate in order to assess the sterility11.
Representative Results
In this preliminary study, the first aim was to investigate the ability of human autologous micro-grafts combined with a biological support, such as collagen, to produce bio-complexes ready to use. These bio-complexes were implanted in a patientwith a leg lesion caused by a car accident (Figure 2A) and a complete re-epithelialization associated with tissue repair after 30 days (Figure 2B) was observed. Moreover, the clinical follow-up showed a good texture and softness of the damaged area after 5 months (Figure 2C). In parallel to clinical application, in vitro studies were also performed to evaluate the cell viability of different cutaneous tissues such as skin tissue, Ded and Dermis before and after disaggregation by this system. In particular, taking into account the different thickness of each type of tissue, the threshold of eight, four and three biopsies that can be processed in a single step for skin tissue, Ded and Dermis, respectively, was established. The established number of biopsies were then disaggregated at four different times as indicated in the Table 1, according to their biological characteristics in order to identify the optimal condition of disaggregation to maintain a good cell viability.
Although the tissue processing itself inevitably induced an impairment of cell viability compared to intact tissue, the mechanical disaggregation performed with this system seems to maintain a mean value of cell viability of 30% in all samples of skin, Ded and dermisevaluated at different times (Figure 3A), with respect to intact tissue (Figure 3B) (mean of 92 OD/gr for intact skin tissue and 29 OD/gr after disaggregation; from 22 OD/gr to 7 OD/gr for intact Ded with respect to disaggregation and finally from 16 OD/gr to 2.7 OD/gr for intact dermis with respect to disaggregation).Thus, these preliminary results show that this system is able to maintain appreciable levels of cell viability after immediate disaggregation. The effect of disaggregation on cell viability was also investigated on tissue samples cultured for 24 hr or 7 days and variable results were observed. In particular, no substantial variation of cell viability in skin tissue samples was observed independently by time of homogenization and culture compared to starting time (T0) (Figure 4A). On the other hand, a reduced viability of Ded samples after 24 hr of culture compared to starting time (T0) was observed but surprisingly the cell viability was restored after 7 days of culture (Figure 4B). Similar results were also observed for samples of Dermis (Figure 4C). Each sample was evaluated in duplicate and values reported in the Table 2.
On the basis of these results, we identified the suitable time of disaggregation to maintain cell viability for three types of cutaneous tissue as reported in Table 3. In addition to cell viability, the morphological aspect of cultured cellular suspension was also evaluated, identifying single cells after 24 hr from tissue disaggregation, while after 7 days there residues of fibers in both skin tissue and Ded/Dermis samples were observed (Figure 5 A-B). In addition, a cell characterization by flow cytometry analysis was performed in a sample of dermis after its homogenization: a heterogeneous pool of cells composed by several cellular types, including endothelial cells and mesenchymal stem cells was identified (Figure 6). Furthermore, the absence of bacterial growth was identified in all disaggregated samples opportunely seeded on Agar dishes, evidencing the sterility of experimental procedure (Figure 7).
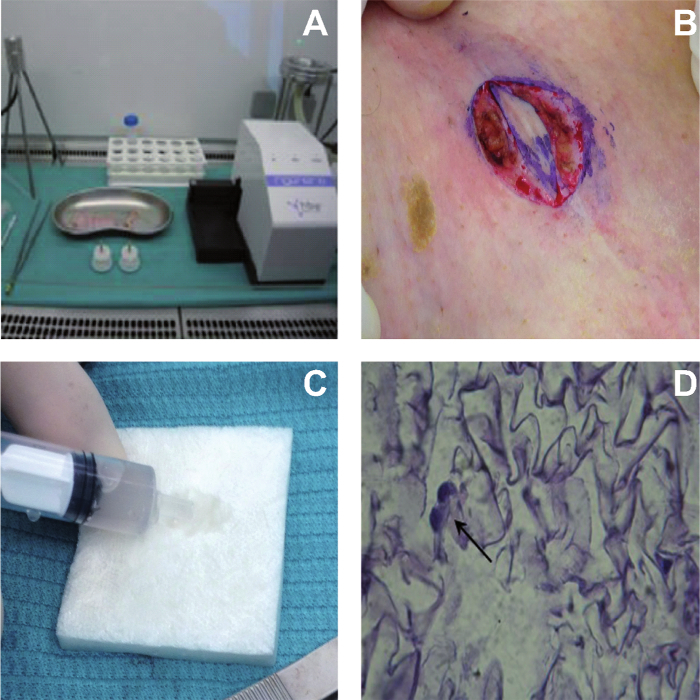 Figure 1. Bio-complexes Building Tissue Disrupting System (A) and Collection of a Small Piece of Derma from the Lesion Area of the Patient that was Subsequently Disaggregated with Tissue Disruptors. The bio-complexes were obtained combining the micro-grafts on collagen sponges to obtain human patches ready to use for lesion repair (C). (D) Hematoxylin & eosin (H&E) staining of a representative bio-complex cultured for three days in DMEM medium and observed to microscopy Please click here to view a larger version of this figure.
Figure 1. Bio-complexes Building Tissue Disrupting System (A) and Collection of a Small Piece of Derma from the Lesion Area of the Patient that was Subsequently Disaggregated with Tissue Disruptors. The bio-complexes were obtained combining the micro-grafts on collagen sponges to obtain human patches ready to use for lesion repair (C). (D) Hematoxylin & eosin (H&E) staining of a representative bio-complex cultured for three days in DMEM medium and observed to microscopy Please click here to view a larger version of this figure.
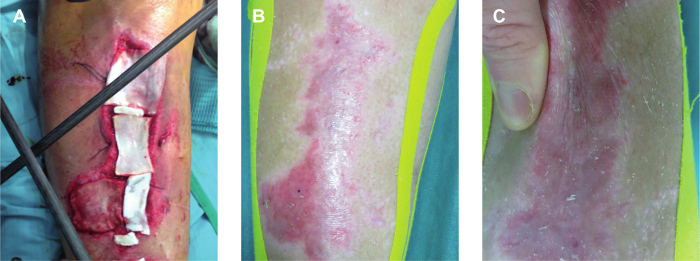 Figure 2. Bio-complexes Application on Leg Lesion The bio-complexes composed by collagen and micro-grafts obtained from the patient were applied on the leg lesion (A) and the wound was evaluated after 30 days (B) and 5 months (C). Please click here to view a larger version of this figure.
Figure 2. Bio-complexes Application on Leg Lesion The bio-complexes composed by collagen and micro-grafts obtained from the patient were applied on the leg lesion (A) and the wound was evaluated after 30 days (B) and 5 months (C). Please click here to view a larger version of this figure.
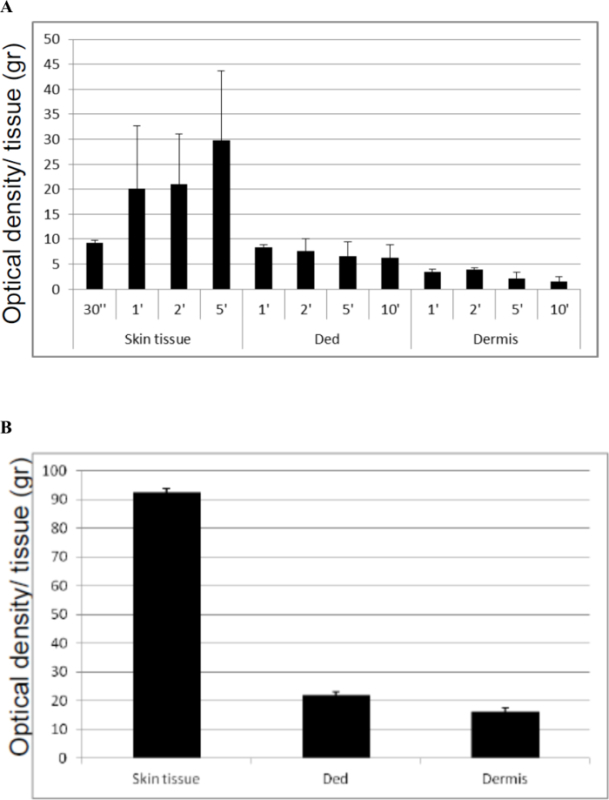 Figure 3. Cell Viability Immediately after Disaggregation at Different Times of Three Type of Cutaneous Tissues (A) with respect to Intact Cutaneous Tissues (B). The Skin, Ded and Dermis tissues derived from four different donors were disaggregated with tissue disruptors as indicate in the appropriate section in the text. Cell viability was assessed by MTT test performed in duplicate for each tissue sample. The graph is representative of four different experiments performed in duplicate for each sample. The results are expressed as a ratio between Optical Density (OD) and grams (gr) of tissue; the standard deviation was calculated on the ratio between Optical Density (OD) and grams (gr) of tissue. Please click here to view a larger version of this figure.
Figure 3. Cell Viability Immediately after Disaggregation at Different Times of Three Type of Cutaneous Tissues (A) with respect to Intact Cutaneous Tissues (B). The Skin, Ded and Dermis tissues derived from four different donors were disaggregated with tissue disruptors as indicate in the appropriate section in the text. Cell viability was assessed by MTT test performed in duplicate for each tissue sample. The graph is representative of four different experiments performed in duplicate for each sample. The results are expressed as a ratio between Optical Density (OD) and grams (gr) of tissue; the standard deviation was calculated on the ratio between Optical Density (OD) and grams (gr) of tissue. Please click here to view a larger version of this figure.
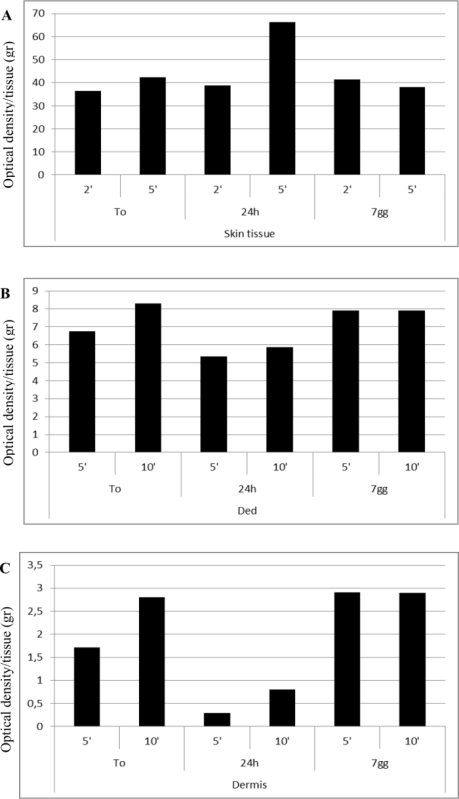 Figure 4. Cell Viability Levels of a Disaggregated Sample of Skin Tissue (A), Ded (B) and Dermis (C) to Starting Time (T0) and after Subculture for 24 hr and 7 Days. The tissues were homogenized with tissue disruptors as indicate in the appropriate section in the text. The disaggregated samples were subsequently cultured for 24 hr and 7 days after the disaggregation and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics at 37 °C in an atmosphere of 5% CO2/air. Cell viability was assessed by MTT as previously described. The graph is representative of an experiment performed in duplicate for each sample of skin tissue, Ded and Dermis derived from a single donor. The results are expressed as ratio between Optical Density (OD) and grams (gr) of tissue. Please click here to view a larger version of this figure.
Figure 4. Cell Viability Levels of a Disaggregated Sample of Skin Tissue (A), Ded (B) and Dermis (C) to Starting Time (T0) and after Subculture for 24 hr and 7 Days. The tissues were homogenized with tissue disruptors as indicate in the appropriate section in the text. The disaggregated samples were subsequently cultured for 24 hr and 7 days after the disaggregation and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics at 37 °C in an atmosphere of 5% CO2/air. Cell viability was assessed by MTT as previously described. The graph is representative of an experiment performed in duplicate for each sample of skin tissue, Ded and Dermis derived from a single donor. The results are expressed as ratio between Optical Density (OD) and grams (gr) of tissue. Please click here to view a larger version of this figure.
 Figure 5. Morphological Analysis of Disaggregated Skin Tissue (A) and Ded/Dermis (B) after 24 hr and 7 Days of Culture. Morphological analysis was performed using light microscopy after 24 hr and 7 days of culture in the presence of RPMI medium on skin tissue and Ded/dermis tissues. Please click here to view a larger version of this figure.
Figure 5. Morphological Analysis of Disaggregated Skin Tissue (A) and Ded/Dermis (B) after 24 hr and 7 Days of Culture. Morphological analysis was performed using light microscopy after 24 hr and 7 days of culture in the presence of RPMI medium on skin tissue and Ded/dermis tissues. Please click here to view a larger version of this figure.
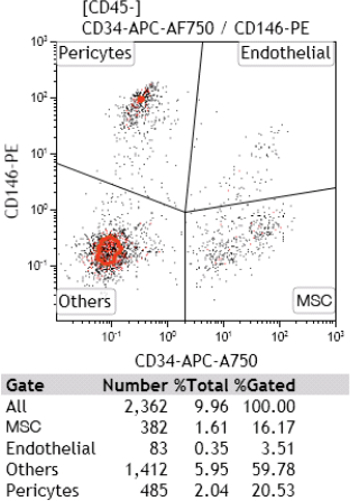 Figure 6. Cell Characterization by Flow Cytometry Analysis. After mechanical disaggregation, micro-grafts obtained by dermis were put in culture and after 7 days were analyzed for positivity to mesenchymal and hematopoietic cell marker, including CD146, CD34 and CD45 antigens. Please click here to view a larger version of this figure.
Figure 6. Cell Characterization by Flow Cytometry Analysis. After mechanical disaggregation, micro-grafts obtained by dermis were put in culture and after 7 days were analyzed for positivity to mesenchymal and hematopoietic cell marker, including CD146, CD34 and CD45 antigens. Please click here to view a larger version of this figure.
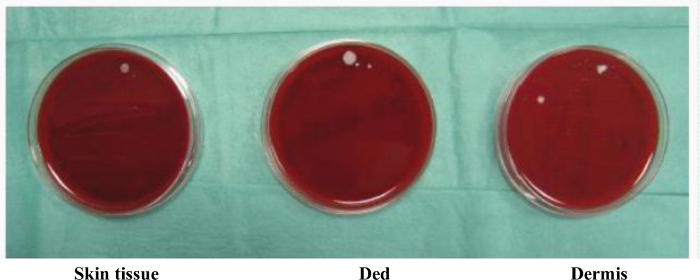 Figure 7. Microbiological Analysis of Disaggregated Skin Tissue, Ded and Dermis Samples. Small fragments of disaggregated tissue samples were seeded on Columbia agar added of 5% sheep blood (BioMerieux Company) under laminar flow hood and incubated at 37 °C for three days to perform the microbiological analysis and assess the sterility of procedure. Please click here to view a larger version of this figure.
Figure 7. Microbiological Analysis of Disaggregated Skin Tissue, Ded and Dermis Samples. Small fragments of disaggregated tissue samples were seeded on Columbia agar added of 5% sheep blood (BioMerieux Company) under laminar flow hood and incubated at 37 °C for three days to perform the microbiological analysis and assess the sterility of procedure. Please click here to view a larger version of this figure.
| TISSUE SAMPLES | DISAGGREGATION TIMES (sec/min) |
| Skin | 30 sec |
| 1 min | |
| 2 min | |
| 5 min | |
| Ded | 1 min |
| 2 min | |
| 5 min | |
| 10 min | |
| Dermis | 1 min |
| 2 min | |
| 5 min | |
| 10 min |
Table 1. Schematic Representation of the Four Different Times used for Disaggregation of Skin, Ded and Dermis. Eight, four and three punch biopsies of Skin, Ded and Dermis respectively were disaggregated at four different times, according to their biological characteristics.
| Skin tissue | |||||
| To | 24 hr | 7 days | |||
| 2 ’ | 5 ’ | 2 ’ | 5 ’ | 2 ’ | 5 ’ |
| 37.1 | 41.07143 | 41.07143 | 65.78571 | 41.14286 | 37.42857 |
| 35.8 | 43.82143 | 36.60714 | 66.5 | 41.46429 | 38.67857 |
| Ded | |||||
| T0 | 24 hr | 7 days | |||
| 5' | 10' | 5' | 10' | 5' | 10' |
| 6,649485 | 8.237113 | 5.463918 | 5.731959 | 7.835052 | 7.85567 |
| 6,845361 | 8,360825 | 5.257732 | 5.989691 | 8 | 7.938144 |
| Dermis | |||||
| To | 24 hr | 7 days | |||
| 10' | 5' | 10' | 5' | 10' | |
| 1.690909 | 2.77193 | 0.293898 | 0.786704 | 2.880952 | 2.869048 |
| 1.736364 | 2.830409 | 0.306351 | 0.814404 | 2.940476 | 2.928571 |
Table 2. Range of Values Expressed in OD/gr for One Biological Replicate. Values of cell viability from skin tissue, Ded and dermis evaluated to starting time and after mechanical disaggregation to indicated times. The results are representative of one experiment performed in duplicate and expressed as ratio between Optical Density (OD) and grams (gr) of tissue.
| Specimen | Disaggregation Time |
| Skin tissue | 5 min |
| Ded | 1 min |
| Derma | 2 min |
Table 3. Schematic Representation of the Best Time of Disaggregation to Maintain a Good Cell Viability in Different Tissue Samples. Skin, Ded and Dermis tissue samples show the optimal cell viability after 5, 1 and 2 min of disaggregation, respectively.
Discussion
This preliminary study showed that micro-grafts obtained by this protocol can be combined with collagen sponges, as already reported in other clinical applications, to optimize the efficacy of micro-grafts implants9, 10. In particular, this study reported the capacity of bio-complexes, constituted by micro-grafts and collagen sponges, to adjuvant the wound healing of a leg lesion after 30 days from clinical application. Furthermore, in vitro results provide evidence about the effectiveness of this protocol to disaggregate three different cutaneous tissues, maintaining an appreciable cell viability both immediately after the mechanical disaggregation and also after 24 hr and 7 days of culture.
The maintenance of cell viability after this kind of homogenization allows to obtain in only one step sterile autologous micro-grafts, avoiding extensive manipulation. This evidence is in agreement with other recent papers in which the efficacy of this protocol in vitro was tested also in other type of tissues, including periosteum, biopsy of cardiac atria and lateral rectus muscle of eyeball5. In particular, we observed in Ded and dermis samples an increased cell viability after 7 days of culture, probably due to the use of a medium supplemented with 10% fetal bovine serum.
In addition to the maintenance of cell viability, this system is safe and easy to use and in a few minutes is able to mechanically disaggregate different types of tissues preventing the disruption of cell structures, with respect to traditional methods of cell isolation, such as enzymatic digestion that require longer time of execution. Furthermore, this system is able to select cells with a cut-off of 50 microns and this aspect is very important in consideration of the success of injectable micro-grafts, because this range includes progenitor cells able to differentiate in many cells types and then ameliorate the lesion repair. This protocol allows us not only to obtain viable but also autologous micro-grafts, given that the donor subject is also the acceptor of these micro-grafts. The capacity of this system to obtain autologous micro-grafts was especially reported in the field of dentistry where it has been shown that transplantation of autologous Dental Pulp Stem Cells (DPSCs) in a non- contained infrabony defect contributed to periodontal repair and regeneration of atrophic maxilla5,6. The ability of these micro-grafts to improve the wound healing process was also previously reported in the management of complex wounds after surgical interventions or complications4.
Another advantage on the use of autologous and ready to use micro-grafts is certainly the keeping of sterile conditions in view of their subsequent implant on the skin lesions.
In conclusion, the protocol described in this paper showed the capacity to create human micro-graft tissue ready to use in clinical intervention to improve healing of acute/chronic skin wounds, such as those indicated in this study. In vitro results on the possibility to create viable micro-grafts have also been reported and this parameter is certainly significant to increase the percentage of success in the tissue repair. Taken together, the data showed that this new system could be considered a promising tool for clinicians who are in need of a rapid and safe instrument in the management of skin wounds.
At the moment, there are not particular modifications or limitations for the protocol in this specific application, given that the skin represents a tissue easy to use and in other studies we reported the efficacy of the same protocol in different skin lesions 6,7. We hope in the future to use this system on a large number of subjects to validate its clinical use in other clinical areas, (for example the orthopedics for bone regeneration or in vascular medicine for the ulcers treatment).
Disclosures
The author Antonio Graziano is the Scientific Director of Human Brain Wave s.r.l. that produces and markets the Rigenera system. The author Letizia Trovato is a collaborator of Scientific Division of Human Brain Wave s.r.l.
Acknowledgments
The authors wish to thanks Dr. Federica Zanzottera for contributing to the study performing flow cytometry analysis.
References
- Sen CK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis E, III, Sinn D. Use of homologous bone grafts in maxillofacial surgery. J Oral Maxillofac Surg. 1993;51:1181–1193. doi: 10.1016/s0278-2391(10)80286-1. [DOI] [PubMed] [Google Scholar]
- Nguyen A, Pasyk KA, Bouvier TN, Hassett CA, Argenta LC. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Sur. 1990;85:378–389. [PubMed] [Google Scholar]
- Abuzeni PZ, Alexander RW. Enhancement of Autologous Fat Transplantation With Platelet Rich Plasma. AJCS. 2001;18(2):59–70. [Google Scholar]
- Trovato L, et al. A New Medical Device Rigeneracons Allows to Obtain Viable Micro-Grafts from Mechanical Disaggregation of Human Tissues. J Cell Physiol. 2015. [DOI] [PubMed]
- Zanzottera F, Lavezzari E, Trovato L, Icardi A, Graziano A. Adipose Derived Stem Cells and Growth Factors Applied on Hair Transplantation Follow-Up of Clinical Outcome. JCDSA. 2014;4(4):268–274. [Google Scholar]
- Giaccone M, Brunetti M, Camandona M, Trovato L, Graziano A. A New Medical Device, Based on Rigenera Protocol in the Management of Complex Wounds. J Stem Cells Res, Rev & Rep. 2014;1(3):3. [Google Scholar]
- Aimetti M, Ferrarotti F, Cricenti L, Mariani GM, Romano F. Autologous dental pulp stem cells in periodontal regeneration: a case report. Int J Periodontics Restorative Dent. 2014;34(3):s27–s33. doi: 10.11607/prd.1635. [DOI] [PubMed] [Google Scholar]
- Graziano A, Carinci F, Scolaro S, D'Aquino R. Periodontal tissue generation using autologous dental ligament micro-grafts: case report with 6 months follow-up. AOMS. 2013;1(2):20. [Google Scholar]
- Brunelli G, Motroni A, Graziano A, D'Aquino R, et al. Sinus lift tissue engineering using autologous pulp micro-grafts: A case report of bone density evaluation. J Indian Soc Periodontol. 2013;17(5):644–647. doi: 10.4103/0972-124X.119284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner PD, Stoessel CJ, Drakeford E, Vasi F. A new culture medium for medical bacteriology. Am J Clin Pathol. 1966;45:502–504. doi: 10.1093/ajcp/45.4_ts.502. [DOI] [PubMed] [Google Scholar]


