Abstract
The kidney normally functions to maintain hemodynamic homeostasis and is a major site of damage caused by drug toxicity. Drug-induced nephrotoxicity is estimated to contribute to 19- 25% of all clinical cases of acute kidney injury (AKI) in critically ill patients. AKI detection has historically relied on metrics such as serum creatinine (sCr) or blood urea nitrogen (BUN) which are demonstrably inadequate in full assessment of nephrotoxicity in the early phase of renal dysfunction. Currently, there is no robust diagnostic method to accurately detect hemodynamic alteration in the early phase of AKI while such alterations might actually precede the rise in serum biomarker levels. Such early detection can help clinicians make an accurate diagnosis and help in in decision making for therapeutic strategy. Rats were treated with Cisplatin to induce AKI. Nephrotoxicity was assessed for six days using high-frequency sonography, sCr measurement and upon histopathology of kidney. Hemodynamic evaluation using 2D and Color-Doppler images were used to serially study nephrotoxicity in rats, using the sonography. Our data showed successful drug-induced kidney injury in adult rats by histological examination. Color-Doppler based sonographic assessment of AKI indicated that resistive-index (RI) and pulsatile-index (PI) were increased in the treatment group; and peak-systolic velocity (mm/s), end-diastolic velocity (mm/s) and velocity-time integral (VTI, mm) were decreased in renal arteries in the same group. Importantly, these hemodynamic changes evaluated by sonography preceded the rise of sCr levels. Sonography-based indices such as RI or PI can thus be useful predictive markers of declining renal function in rodents. From our sonography-based observations in the kidneys of rats that underwent AKI, we showed that these noninvasive hemodynamic measurements may consider as an accurate, sensitive and robust method in detecting early stage kidney dysfunction. This study also underscores the importance of ethical issues associated with animal use in research.
Keywords: Medicine, Issue 109, Sonography, real-time imaging, non-invasive methodology, renal toxicity assessment, ethical use of animals in research, drug-induced nephrotoxicity, hemodynamics
Introduction
Serum creatinine (sCr) has been the gold standard metric to assess kidney function for more than two decades. Recently, many studies have reported that renal injury occurs much earlier than the changes in sCr1. However, there are no robust methods for detection of hemodynamic changes that occur early in the course of renal injury including drug-induced nephrotoxicity.
Drug-induced acute renal hemodynamic dysfunction leads to renal tissue damage and further progression to renal failure2,3. In the past couple decades, studies indicate that imaging tools such as computed tomography (CAT), Functional magnetic resonance imaging (fMRI) and sonography play a role in hemodynamic assessment4. In the current imaging tools, gray scale sonography coupled with Color-Doppler techniques, are the most commonly used to establish and assess anatomical status of kidney3,5,6. Sullivan et. al. and Bonnin et. al. recently reported that sonography is an effective, powerful and non-invasive tool in analysis hemodynamic changes in vasoconstriction and hypoxia stress animal models7,8. This technique is also commonly used to detect arterial stenosis9,10.
Latest technical advances in the field of high-resolution ultrasound imaging have allowed investigators to address cardiovascular toxicity using high-frequency (25-80 MHz) and high-resolution (< 0.03 mm resolution) probes, in vivo11. We hypothesize that using this high-resolution sonography to study kidney will provide an unprecedented opportunity for a non-invasive and sensitive method for early detection of nephrotoxicity.
Cisplatin is used to treat testicular, ovarian, bladder, head, lung, and neck cancers in combination with other drugs12-14. Cisplatin has had well-documented nephrotoxicity due to cell necrosis of proximal tubules (PT) and collecting ducts resulted in rising blood urea nitrogen (BUN) and sCr15. Herein, we provide a detailed step-by-step methodology of using non-invasive renal sonography to characterize kidney dysfunction using the rat model of Drug (Cisplatin)-induced nephrotoxicity.
Protocol
Perform all procedures in male Sprague Dawley rats purchased from Charles River Laboratories in accordance with American Veterinary Medical Association (AVMA) guidelines and using approved Institutional Animal Care and Use Committee (IACUC) protocols.
1. Animal Preparation and Surgical Procedures
Acclimate all animals for one week before any experimental procedure.
Anesthetize animal using isoflurane (2-3% to induce, and 1.0% to maintain) and apply eye ointment to both eyes to prevent desiccation, irritation or ulceration.
Remove hair from the animal’s chest using #40 blade and depilatory cream as necessary. It may have to remove hair form the animal’s back if we cannot have good image data obtained from ventral side imaging.
2. Nephrotoxicity Rat Model
For Cisplatin-induced nephrotoxicity model, administer Cisplatin, using protocol as described previously. 15
Perform sonography at baseline, 24 hr prior to Cisplatin administration (Day 0). (See step 3, Imaging Protocol)
Randomize rats (n=6) into two groups. At Day 1, administer Cisplatin (10 mg/ml) (10 mg/Kg body weight, single dose nephrotoxicity induction), injection volume (1ml/Kg body weight) calculated by animal’s body weight), intraperitoneally in study group and normal saline (NS) in control group.
Anesthetize animal as step 1.2 at 24, 48, 72, 96, 120, 144 hr after Cisplatin administration.
Take image using high resolution ultrasound system (See Materials and Equipment Table) under stable anesthesia stage of animal. Continue to monitor the animal’s basic physiological function during imaging from anesthesia induction through full recovery.
Monitor animal’s vital signs during imaging procedures: rat-temperature: 35.9-37.5, respiratory rate: 66-144/min, heart rate: 250-600/min. The optimal vital sign reading in our proposed study is: temperature: 36.5-37.0, respiratory rate: 80-100/min, heart rate: 450-550/min. NOTE: Use Intravenous fluid infusion, and heating lamp to maintain animal’s normal physiological condition to minimize the effects of surgery and anesthesia. Assist respiration with mechanical ventilator during the procedure if necessary. However, mechanical ventilation is rarely needed in this experiment.
3. Imaging Protocol
Note: The ultrasound machine provider provides the heated platform for long imaging procedure. However, we do not use the heated platform in our demonstrated experiment because it only takes 5 to 15 min. Its controlled for body temperature which is monitored with a rectal thermometer connected to the physiology control switch.
- Transverse image of Kidney (B Mode):
- Using MS 250 ultrasound with center frequency of 21 MHz connected to the active-port, set the application preset to “General Imaging”.
- With the animal supine on the platform, position the 21 MHz ultrasound probe using the rail system, midline on animal and isolate the aorta. In this position the probe angle is 90 degrees to the left parasternal line (transverse axis) (Figure 1A,B).
- From this position slide the platform with the animal such that the probe is now at the level of the renal artery (either left or right, can image one at a time).
- By using the micromanipulators, view either the right or the left renal artery.
- Adjust the probe angle by tilting slightly along the y axis of the probe to obtain a full kidney view in the center of the screen.
- Once the proper landmarks (renal pelvis, renal artery) are identified as illustrated in Figure 1C and D, cine store the image using the highest frame-rate allowed with the probe used.
- Transverse image of Kidney (Color-Doppler view):
- Using the Color-Doppler key on the keyboard, turn on Color Doppler acoustic window. This helps to isolate renal artery and renal vein (Figure 1D). (Blue color indicates arterial flow; and red color indicates venous flow).
- Ensure that the focus depth (indicated by and yellow arrowhead on the Y axis) lies in the center of kidney. Record the data with cine store.
- Make sure record the data at the highest possible frame rate possible (>200 frames/sec).
- Transverse image of Kidney (Pulsed-wave or PW view):
- Click on the PW key, while in Color-Doppler mode, to bring up a yellow indicator line (Pulsed-wave Doppler sample volume) on the screen (Figure 1F).
- Place the yellow line in the renal artery, at an angle that parallels the directionality of the flow through the vessel by using the PW angle key.
- For flow assessment in the right renal artery, place the PW yellow indicator line along the renal artery in the direction of the flow (this is shown in blue in Figure 1D and E) ensuring the Doppler angle is 60 degrees or less.
- In this mode, the accoustic window splits up into upper and lower sections.
- Use Cine store to capture the image of the wave forms that indicate the velocity of the arterial flow at peak systole and diastole.
4. Animal Handling After Imaging
From Day 0 to Day 5, place animal into a clean recovery area (with clean paper towel on bedding) in sternal recumbency position after imaging. Note that we handle all animals with extreme care with “Tail Holding” method for aggressive animals such as animals recover from anesthesia.
During anesthetic recovery, keep animal’s body temperature with an external heat source and monitor animal’s vital sign with electrophysiological probes until animal fully recovers from anesthesia.
Return recovered animals to the facility housing room when they are alert and active.
Euthanize all rats according to institutional guidelines on Day 6 and harvest kidneys (see step 4.7) for histological assessment as well as step 4.5.
Collect animal’s urine from collection tubes attached in the metabolic cage for creatinine test to check kidney function.
Perform paraffin section of animal kidney, and carry out HE (Hematoxylin and Eosin) staining to check nephrotoxicity (see step 4.7 for detail).
- Sacrifice animals and exsanguinate with 0.9% NaCl solution, followed by 10% buffered formalin fixation through the left ventricle. After exsanguination with 0.9% NaCl solution, remove the rat kidneys for histological assessment16.
- Paraffin embed 6-mm sections to observe the kidney morphology and nephrotoxicity. Dehydrate kidney tissue in 30% sucrose in phosphate-buffered saline (PBS) for 48 hr at 4 ºC. Then fix the sections in 10% buffered formalin for 24–48 hr at 4 ºC.
- Next, embed the kidney tissue in paraffin, and store the tissue paraffin blocks at RT until sectioning. Further section the tissue blocks using a paraffin section machine and place the sections on a coated glass slide.
- Deparrafinize the section and rehydrated and stained with Hematoxylin for 10 min followed by Eosin for 3 min. Mount the sections on a slide and have them evaluated by a rodent pathologist.
5. Data Calculation and Analysis
Calculate renal arterial peak velocities from the Color Doppler images obtained from step 3.2. Select Velocity Time Integral (VTI) tool to trace the peaks of systolic and diastolic velocity.
Calculate Resistive Index (RI) and Pulsatile Index (PI) using the equations below. RI= (peak systolic velocity-end diastolic velocity)/peak systolic velocity PI= (peak systolic velocity-end diastolic velocity)/mean velocity.
Perform statistical analysis of RI and PI results with standard deviations from the average of three cycle measurements. For other standard parameters, please refer to the manuals from the manufacturer to perform data analysis using proprietary software (see Materials and Equipment Table).
Representative Results
The images presented in this study were taken by a single operator. All images were collected using a high frequency ultrasound machine (please see Materials and Equipment Table). All imaging data was analyzed by a single investigator. The results showed that Cisplatin-treated animals had sCr ranging from 0.5 to 2.1 (normal range <1.1) at day 6 (Figure 2A). However, the histological studies demonstrated consistent patterns of acute tubule interstitial injuries when compared to normal saline treated animals.
Using high-resolution ultrasound imaging to measure hemodynamic changes of kidney, data showed that there was no change of morphology in animals treated with NS between day 0 and day 6, while pulsus parvus morphology was detected in animals at day six after Cisplain treatment. The upper limit of normal RI and PI are 0.7 and 1.15, respectively, in rats 17. Using the above indices to assess hemodynamic changes of kidney, which demonstrated that there is significant increase of RI and PI in Cisplatin-treated animals at day 6.
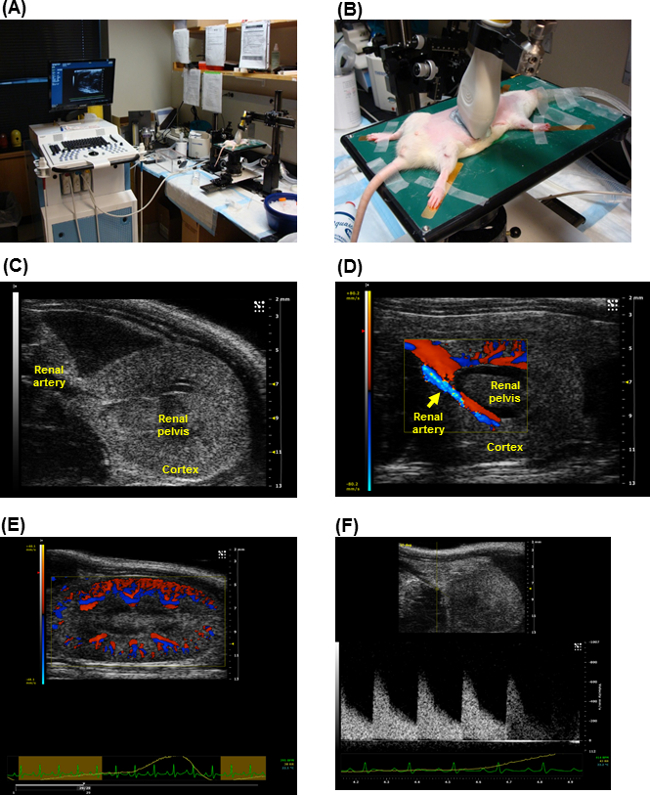 Figure 1. Ultrasound apparatus settings for detecting kidney images in rats. Graphical illustrations of imaging system with the setting of animal stage (A) and imaging probe position (B) during the operation of rat kidney sonographic imaging. The sample sonographic images obtained from rat kidney using the high-frequency, high-resolution ultrasound systems (see Material andand Equipment Table). (C-F). The data demonstrate clear kidney anatomic structure and blood flow in the renal vessels with sufficient information for further hemodynamic parameter measurement and analysis. Please click here to view a larger version of this figure.
Figure 1. Ultrasound apparatus settings for detecting kidney images in rats. Graphical illustrations of imaging system with the setting of animal stage (A) and imaging probe position (B) during the operation of rat kidney sonographic imaging. The sample sonographic images obtained from rat kidney using the high-frequency, high-resolution ultrasound systems (see Material andand Equipment Table). (C-F). The data demonstrate clear kidney anatomic structure and blood flow in the renal vessels with sufficient information for further hemodynamic parameter measurement and analysis. Please click here to view a larger version of this figure.
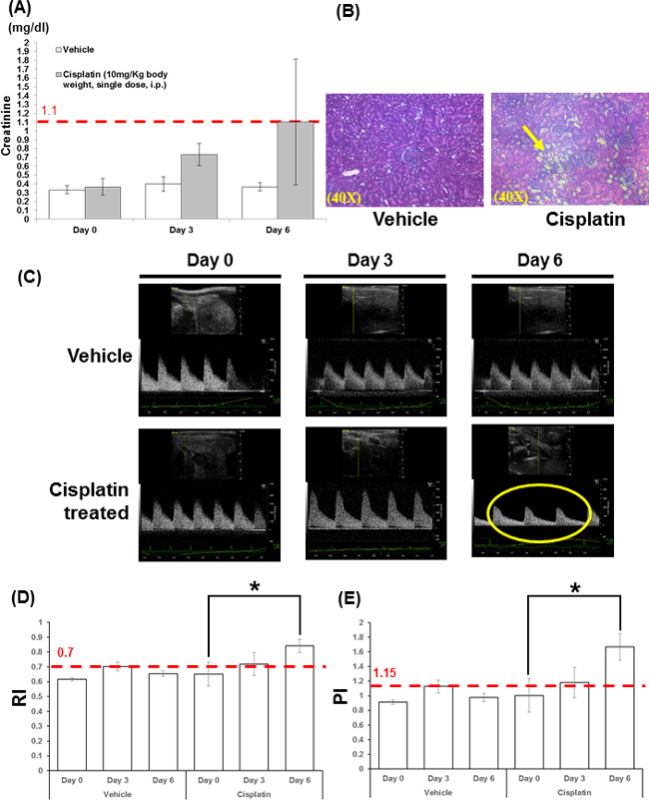 Figure 2. Histology and Kidney sonographic images of rats under Cisplatin treatment. Serum creatinine (sCr) and histology study presents normal kidney tissue in vehicle treated rat and severe proximal tubular kidney injury (yellow arrow) in Cisplatin treated rat (A, B). sCr increased marginally after Cisplatin treatment, but remained within the normal range (<1.1). Sonographic images of right kidney of rats in Color-Doppler Mode at day 0, 3, and 6 on vehicle and Cisplatin treated rats (C); hemodynamic parameters, RI and PI, were significant increased, assessed by Color-Doppler ultrasound (D, E). The upper limit of normal RI is 0.7 and 1.15 for PI. Importantly, the above data show those hemodynamic changes preceded the rising of sCr. Pulse wave velocity measurement show a slow and weak pulse (pulsus parvus sign, yellow circle) after Cisplatin treatment which correlate with histology study results. This phenomenon indicates renal artery stenosis, obstruction and further kidney dysfunction. Histological data showed successful drug-induced proximal tubular kidney injury and sonographic assessment showed significant changes in RI, PI and pulse wave velocity using Color-Doppler technology. N=3, *, p<0.05. Please click here to view a larger version of this figure.
Figure 2. Histology and Kidney sonographic images of rats under Cisplatin treatment. Serum creatinine (sCr) and histology study presents normal kidney tissue in vehicle treated rat and severe proximal tubular kidney injury (yellow arrow) in Cisplatin treated rat (A, B). sCr increased marginally after Cisplatin treatment, but remained within the normal range (<1.1). Sonographic images of right kidney of rats in Color-Doppler Mode at day 0, 3, and 6 on vehicle and Cisplatin treated rats (C); hemodynamic parameters, RI and PI, were significant increased, assessed by Color-Doppler ultrasound (D, E). The upper limit of normal RI is 0.7 and 1.15 for PI. Importantly, the above data show those hemodynamic changes preceded the rising of sCr. Pulse wave velocity measurement show a slow and weak pulse (pulsus parvus sign, yellow circle) after Cisplatin treatment which correlate with histology study results. This phenomenon indicates renal artery stenosis, obstruction and further kidney dysfunction. Histological data showed successful drug-induced proximal tubular kidney injury and sonographic assessment showed significant changes in RI, PI and pulse wave velocity using Color-Doppler technology. N=3, *, p<0.05. Please click here to view a larger version of this figure.
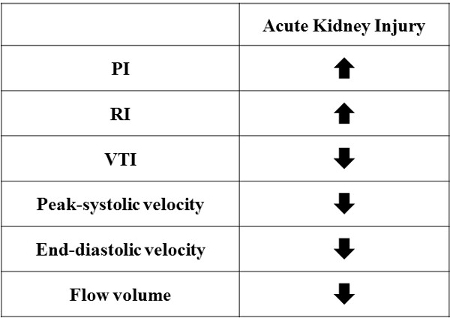 Table 1.
Renal hemodynamic parameters for Drug-induced AKI
Table 1.
Renal hemodynamic parameters for Drug-induced AKI
Discussion
In the past decade, many advancements have occurred in sonographic technology including the development of high-frequency mechanical probes, which offer sonographic data with high quality, sensitivity, and accuracy. These probes can provide approximately 50 µm axial resolution at a penetration depth of 5 to 12 mm and high frame rates (greater than 200 frame/sec), thus it can serve as a robust tool to study small animals such as rats and mice18,19. Furthermore, it also allows to collect sonographic images on lightly sedated or conscious animals with vital signs at physiological levels. In addition, this non-invasive modality also provides opportunity to perform longitudinal assessment of structural and functional changes during disease progression without sacrificing animals19.
In 1959, Drs. Rusell and Brush first described the three “R” rules (Replacement, Reduction, and Refinement) to raise awareness of ethical issues in animal use in research. The proposed protocol shows for the first time that non-invasive small animal sonography can utilizes minimal number of animals under least pain, suffering or distress in Nephorotoxicity study. Therefore, it is a potential effective modality to meet the three “R’ rules for experimental animals.
Many sonographic studies have focused in cardiac applications; the kidney function assessments were often derived from measurements of cardiac status rather than a direct study of kidney 20-25. We have established an imaging methodology to visualize anatomical and functional changes in kidney in real time. We used a pre-selected set of complementary acoustic windows, gray-scale/B Mode and Color-Doppler, specific for kidney view. We used the RI and PI indices to evaluate the relationship between these indices and the changes of renal function in the Cisplatin induced toxicity model.
However, there are few challenges and limitations to the proposed imaging methods as follows: 1) Appropriate choice of anesthetic agent and the degree of anesthesia are crucial for cardiac and respiratory stability. Inconsistent physiological phenomenon (including respiratory and heart rate fluctuations) affect renal artery flow, quality of imaging and kidney function assessment. We will use IACUC approved injectable anesthetic agent, Pentobarbital (50 mg/kg body weight, i.p.), as our backup anesthetic agent to ensure proper normal physiological function during the imaging and kidney function assessment; 2) Depilation is a critical step, as presence of chest hair affects the quality of the sonographic images; 3) While sonography of kidneys is relatively straightforward for a trained operator; for the average operator, it is critical to adapt technique to individual animal’s unique anatomy and make minor manipulations; 4) If the size of rats is rather large (above 350 grams), a lower frequency probe (less than used in this study 21 MHz) may be required for optimal imaging. It might be prudent to take a training course before the proposed operation of the imaging system.
The novelty in detecting drug-induced nephrotoxicity using the proposed sonographic methodology and derived protocol is its early robust detection of hemodynamic changes in the event of kidney injury. The results indicate that the intra-renal vascular hemodynamic changes in fact precede the rising sCr. These data is benchmarked against the conventional gold standard using histological analysis and demonstrate that small animal sonography is a noninvasive, sensitive, and reproducible modality, which has minimal requirement of animal use. It is thus an effective tool for early detection of drug-induced nephrotoxicity using rat model.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank Fred Roberts for exemplary technical support. We thank Brigham Women’s Hospital Cardiovascular Physiology Core for providing with the instrumentation and the funds for this work. This work was supported in part by NHLBI grants HL093148, HL086967, and Departmental Funds from (SUNDRY).
References
- Bonventre JV. Diagnosis of acute kidney injury: from classic parameters to new biomarkers. Contrib Nephrol. 2007;156:213–219. doi: 10.1159/000102086. [DOI] [PubMed] [Google Scholar]
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Progress in cardiovascular diseases. 2009;52(3):196–203. doi: 10.1016/j.pcad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas MG, Kizer JR. Echocardiographic assessment of the right ventricle and associated hemodynamics. Progress in cardiovascular diseases. 2012;55(2):144–160. doi: 10.1016/j.pcad.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Milman Z, et al. Hemodynamic response magnetic resonance imaging: application for renal hemodynamic characterization. Nephrol Dial Transplant. 2013;28:1150–1156. doi: 10.1093/ndt/gfs541. [DOI] [PubMed] [Google Scholar]
- Anavekar NS, et al. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study) Am J Cardiol. 2008;101(5):607–612. doi: 10.1016/j.amjcard.2007.09.115. [DOI] [PubMed] [Google Scholar]
- Lindqvist P, Calcutteea A, Henein M. Echocardiography in the assessment of right heart function. Eur J Echocardiogr. 2008;9(2):225–234. doi: 10.1016/j.euje.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Sullivan JC, et al. Novel use of ultrasound to examine regional blood flow in the mouse kidney. American journal of physiology. Renal physiology. 2009;297:F228–F235. doi: 10.1152/ajprenal.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin P, Sabaa N, Flamant M, Debbabi H, Tharaux PL. Ultrasound imaging of renal vaso-occlusive events in transgenic sickle mice exposed to hypoxic stress. Ultrasound Med Biol. 2008;34(7):1076–1084. doi: 10.1016/j.ultrasmedbio.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Desberg AL, et al. Renal artery stenosis: evaluation with color Doppler flow imaging. Radiology. 1990;177(3):749–753. doi: 10.1148/radiology.177.3.2243982. [DOI] [PubMed] [Google Scholar]
- Ciccone MM, et al. The clinical role of contrast-enhanced ultrasound in the evaluation of renal artery stenosis and diagnostic superiority as compared to traditional echo-color-Doppler flow imaging. International angiology : a journal of the International Union of Angiology. 2011;30(2):135–139. [PubMed] [Google Scholar]
- Bauer M, et al. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res. 2011;108(8):908–916. doi: 10.1161/CIRCRESAHA.110.239574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T, Vougiouka M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs (review) Oncology reports. 2004;11(3):559–595. [PubMed] [Google Scholar]
- Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hye Khan MA, Abdul Sattar M, Abdullah NA, Johns EJ. Cisplatin-induced nephrotoxicity causes altered renal hemodynamics in Wistar Kyoto and spontaneously hypertensive rats: role of augmented renal alpha-adrenergic responsiveness. Exp Toxicol Pathol. 2007;59:253–260. doi: 10.1016/j.etp.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Bonventre JV. Mechanistic biomarkers for cytotoxic acute kidney injury. Expert Opin Drug Metab Toxicol. 2006;2(5):697–713. doi: 10.1517/17425255.2.5.697. [DOI] [PubMed] [Google Scholar]
- Lu TS, Chen HW, Huang MH, Wang SJ, Yang RC. Heat shock treatment protects osmotic stress-induced dysfunction of the blood-brain barrier through preservation of tight junction proteins. Cell stress, & chaperones. 2004;9(4):369–377. doi: 10.1379/CSC-45R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya M. Hemodynamics - New Diagnostic and Therapeutic Approaches. InTech; 2012. pp. 1–30. [Google Scholar]
- Bjornerheim R, Grogaard HK, Kjekshus H, Attramadal H, Smiseth OA. High frame rate Doppler echocardiography in the rat: an evaluation of the method. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2001;2(2):78–87. doi: 10.1053/euje.2000.0050. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. A high-frequency, high frame rate duplex ultrasound linear array imaging system for small animal imaging. IEEE transactions on ultrasonics, ferroelectrics, and frequency. 2010;57:1548–1557. doi: 10.1109/TUFFC.2010.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frea S, et al. Echocardiographic evaluation of right ventricular stroke work index in advanced heart failure: a new index? J Card Fail. 2012;18(12):886–893. doi: 10.1016/j.cardfail.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Marwick TH, Raman SV, Carrio I, Bax JJ. Recent developments in heart failure imaging. JACC Cardiovasc Imaging. 2010;3(4):429–439. doi: 10.1016/j.jcmg.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Pokreisz P. Pressure overload-induced right ventricular dysfunction and remodelling in experimental pulmonary hypertension: the right heart revisited. Eur Heart J supplements. 2007;9(Supplement H):H75–H84. [Google Scholar]
- Senechal M, et al. A simple Doppler echocardiography method to evaluate pulmonary capillary wedge pressure in patients with atrial fibrillation. Echocardiography. 2008;25(1):57–63. doi: 10.1111/j.1540-8175.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- Souders CA, Borg TK, Banerjee I, Baudino TA. Pressure overload induces early morphological changes in the heart. Am J Pathol. 2012;181(4):1226–1235. doi: 10.1016/j.ajpath.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, et al. Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation. 1996;94(5):1109–1117. doi: 10.1161/01.cir.94.5.1109. [DOI] [PubMed] [Google Scholar]


