Abstract
Purpose of review
It is the current opinion that pathogens, such as viruses, are contributing to the development of type 1 diabetes (T1D) in susceptible individuals. This opinion is based on epidemiological associations, direct isolation of pathogens from the islets of Langerhans, as well as a large amount of data from various experimental animal models. Human enteroviruses have dominated the literature associated with the etiology of T1D. However, virus infections have also been reported to protect from autoimmune disorders.
Recent findings
Here we review the evidence for virus infections to be involved in the pathogenesis of T1D and discuss potential mechanisms of how such infections could accelerate the destruction of insulin-producing β-cells. In addition, we will review evidence from epidemiologic and experimental animal studies showing that virus infections could also have protective properties.
Summary
Virus infections play an important role in the pathogenesis of T1D by inducing or accelerating the autodestructive process, but also by protecting from autoimmunity. Thus, multiple sequential infections might shape the autoreactive immune repertoire and the pathogenesis of T1D in a complex fashion.
Keywords: chemokines, inflammation, molecular mimicry, regulatory T cells, rat insulin promoter-lymphocytic choriomeningitis virus model
Introduction
Recently, a large meta-analysis confirmed that there is a significant association between human enteroviruses (HEV) infection and the development of type 1 diabetes (T1D) [1■■]. The study thereby confirms the hypothesis that besides a genetic predisposition, which is dominated by human leukocyte antigen (HLA) class I and II genes, environmental factors are involved in the cause of T1D [2,3]. Viruses are prime candidates, as they activate the innate and adaptive immune system and thereby cause acute and sometimes more chronic inflammation. In addition, a structural similarity between virus and host might result in cross-reactive activity of antiviral immune responses with host structures and epitopes. This concept has been termed ‘molecular mimicry’ [4–6].
Epidemiological Evidence
Infections with viruses, such as HEV [7], rotavirus [8], mumps virus [9], rubella virus [10] and cytomegalovirus [11], have all been associated with the development of T1D. The strongest evidence for an involvement exists from HEVs [7], which have also been found frequently in pancreata of T1D patients [12–14]. At least some of the pancreatic HEV isolates can also induce T1D in mice [15] and are able to infect and destroy human islet cells in vitro [16]. Further, RNA of the HEV coxsackievirus B (CVB) has been detected in the blood of recent onset T1D patients [17–19] and the presence of HEV RNA in the serum constitutes indeed a risk factor for β-cell autoimmunity and T1D [20]. In other studies, HEV proteins have been detected by immunohistochemistry in the pancreas and even within the islets of Langerhans of recent onset T1D patients [21–23]. Recently, it has been shown in the context of the ‘Diabetes and Autoimmunity Study in the Young’ (DAISY) study that the progression to T1D was increased significantly in children in the time interval following HEV serum conversion [24■]. These findings indicate that in genetically predisposed children carrying antibodies to islet antigens enterovirus infection might push the preexisting autoimmune condition to overt disease. In contrast, the ‘Babydiet’ study [25■], that examines the influence of first gluten exposure on the development of islet-autoimmunity, revealed no significant correlation between the presence of HEVs in stool samples in the first year of life and the development of islet autoantibodies. Similarly, the frequency of HEV RNA in stool samples of Norwegian children with a high genetic risk for T1D was not significantly different before and after serum conversion [26]. Interestingly, the effect of HEV infection on the development of T1D-associated autoimmunity seems to be modified by the exposure to cow's milk based formula [27■]. Namely, an association between HEV-infection and islet-autoantibodies formation has been found in children who have been exposed to cow's milk before the first three months of age but not in children exposed to cow's milk at a later time [27■]. Obviously, the epidemiologic data obtained by many different groups working on a variety of cohorts with variable parameters, such as ethnicity, age and gender distribution, diet, and genetic background are somewhat controversial, which was one of the major reasons for Yeung et al. [1■■] to perform a large meta-analysis. They performed a systematic review of 33 T1D prevalence studies from 1990 to 2010 involving a total of 1931 T1D patients and 2517 control individuals. They found odds ratios of 3.7 between HEV infection and T1D related autoimmunity and 9.8 between HEV infection and clinical T1D [1■■]. Overall the study [1■■] confirmed a significant association between HEV infection and the development of T1D.
Evidence from experimental models
The mechanisms by which HEVs or other pathogens might be involved in the etiology of T1D include a release of sequestered antigens, the generation of a ‘fertile’ inflammatory field, and a cross-reactive immune response due to molecular ‘mimicry’ between pathogen and host structures. Such cross-reactivity indeed exists [28] and has been detected between pathogens and autoantigens recognized by antibodies or T cells of patients with a broad variety of autoimmune diseases [6,29]. One of the best-characterized cases of molecular mimicry is involved in the Guillain Barré syndrome (GBS), in which, an association with Campylobacter jejuni infection sharing a structural homology with the lipooligosaccharide of the peripheral nerve GM1 ganglioside, could also be reproduced convincingly in an animal model of the disease [30]. However, proof that cross-reactivities between pathogen and self-determinants would actually cause or accelerate human diseases has been hard to establish. One of the best examples of postinfectious autoimmunity due to molecular mimicry has been established for Streptococcus pyogenes-induced acute rheumatic fever (ARF), in which the lysoganglioside of the host shares a structural similarity to N-acetyl-β-D-glucosamine, the dominant epitope of the group A streptococcal carbohydrate [31].
In T1D, molecular mimicry has been reported for the P2-C protein of CVB that shares sequence homology with the islet autoantigen glutamic acid decarboylase (GAD)65 [32]. However, although a similar sequence homology has been found in the nonobese diabetic (NOD) mouse, infection of mice with CVB did not influence the GAD65-specific T cell response and the development of T1D [33]. More recently, Honeyman et al. [34] reported that molecular mimicry between human T cell epitopes of the rotavirus protein VP7 and GAD65 as well as tyrosine phosphatase-like insulinoma Ag 2 (IA2) might be involved in T1D. Interestingly, cross-reactive epitopes present on RV-VP7, GAD65 and IA2 also bound strongly to the T1D susceptibility HLA allele DRB1*04 and furthermore, RV-VP7-specific T cell clones cross-reacted to IA2 [34].
The rat insulin promoter-lymphocytic choriomeningitis virus (RIP-LCMV) model offers a unique opportunity to study the influence of single of multiple virus infections on the pathogenesis of T1D. RIP-LCMV mice express the glycoprotein or nucleoprotein of the lymphocytic choriomeningitis virus under control of the rat insulin promoter specifically in the β-cells of the islets of Langerhans [6,35]. In contrast to NOD mice, RIP-LCMV mice do not develop T1D spontaneously. Only upon infection with LCMV an antiviral immune response is initiated that also targets the β-cells that express identical viral proteins (glycoprotein or nucleoprotein) transgenically [35,36]. Thus, the RIP-LCMV model is based on an initiation mechanism of molecular identity rather than molecular mimicry [6]. Induction of autoimmunity is most efficient, if the host (viral protein) structure is not expressed in the thymus. Thus, RIP-LCMV-glycoprotein mice lacking thymic expression of the target protein (LCMV-glycoprotein) develop rapid T1D independent of CD4 T cell help, whereas RIP-LCMV-nucleoprotein mice that express nucleoprotein in the thymus require CD4 T cell help and develop T1D much more slowly [36].
The first demonstration that molecular mimicry between self and viral antigens can accelerate (albeit not precipitate) autoimmune diabetes development was obtained in the RIP-LCMV model. Infection of naïve RIP-LCMV-nucleoprotein mice with Pichinde virus, which shares a subdominant cross-reactive epitope of its nucleoprotein (Pichinde virus-nucle-oprotein) with LCMV-nucleoprotein, elicits only a marginal anti-NP CD8 T cell response and does not cause T1D [37]. In contrast, when RIP-LCMVnucleoprotein mice are infected with LCMV followed by Pichinde virus, T1D is accelerated significantly [37]. The mechanism for this acceleration was the expansion of autoreactive CD8 T cells with reactivity to the subdominant Pichinde virus/LCMV-nucleoprotein epitope that confers molecular mimicry. The findings suggest that an experienced, ‘memory’ immune repertoire is activated more easily by cross-reactive viruses than a naïve repertoire.
A similar effect of mimicry on disease progression has been observed in the CYP2D6 mouse model for autoimmune hepatitis (AIH) [38]. Infection of CYP2D6 transgenic mice expressing the human autoantigen cytochrome P450 2D6 (CYP2D6), with an adenovirus expressing the indentical human CYP2D6 was less effective in triggering AIH than infection of wildtype mice that exclusively express the similar mouse Cyp homologues (Ehser & Christen, unpublished observations). Interestingly, the CYP2D6-specific T cell response targeted CYP2D6-epitopes located within a cross-reactive ‘hot spot’ region exhibiting intermediate homology between trigger and target molecule (Ehser & Christen, unpublished observations). In contrast to infection of RIP-LCMV mice with Pichinde virus that resulted only in the acceleration of an ongoing autoimmune process but failed to initiate autoimmune disease, molecular mimicry in the CYP2D6 model targets an immunodominant autoantigen and elicits a sufficient number of autoaggressive, CYP2D6-specific T cells to actually induce disease. As an additional example, molecular mimicry has been suggested to be involved in the etiology of primary biliary cirrhosis (PBC). Antimitochondrial antibodies directed against the E2 subunit of pyruvate dehydrogenase complex (PDC-E2) are the hallmarks of PBC [39]. Interestingly, several potential environmental inducers for PBC, including bacteria, such as Novosphingobium aromaticivorans, and chemical xenobiotics show cross-reactivity to the immunodominant structure in PDC-E2 containing the prosthetic group lipoic acid [40,41]. Among the chemical xenobiotics the cosmetic and food additive 2-octoynoic acid (2-OA) shows a high structural similarity to lipoic acid and injection of 2-OA coupled to bovine serum albumin (BSA) resulted in the generation of PBC like disease in wildtype C57BL/6 mice [42]. Mice treated with 2-OA-BSA manifested autoimmune cholangitis, antimitochondrial antibodies and infiltration of the liver by activated CD8 T cells [42].
Collectively, molecular mimicry has been demonstrated in a variety of experimental models to be involved in the initiation and/or acceleration of autoimmune processes that might subsequently result in autoimmune disease.
Virus infection as protector from type 1 diabetes
Besides the role of viruses as inducers or accelerators of T1D, it is important to acknowledge the evidence that viruses can also reduce the incidence of T1D in animal models [43,44,45■], thus supporting the so-called ‘hygiene hypothesis’ [46–48]. LCMV has been demonstrated to block the development of T1D in the NOD mouse already in the late 1980s [49]. Similarly, secondary infection of RIP-LCMV mice with a LCMV strain that predominantly replicates outside of the pancreas abrogated the destructive process [43]. Such a secondary infection resulted in increased inflammation at lymphoid sites and thus the redirection of autoreactive CD8 T cells from the islets of Langerhans leading to their apoptosis [43]. Thus, viral inflammation could function as an ‘immune-tuning’ event by leading to the bystander demise of aggressive T cells [50]. Another mechanism of how viruses might block an ongoing auto-destructive process is the induction of counteracting regulatory mechanisms. Infection of prediabetic NOD mice with either LCMV or CVB3 reduced the frequency of T1D and delayed the onset of disease [44] by increasing the number of CD4+ CD25+ regulatory T cells producing transforming growth factor (TGF)-β and maintaining long-term protection [44]. Recently, it has been also demonstrated that upon virus infection invariant natural killer cells (iNKT cells) induce plasmacytoid dendritic cells (pDC) to generate TGF-β, which in turn convert naïve anti-islet T cells to FoxP3+ CD4+ regulatory T cells [45■]. Thus, besides their disease promoting property some HEV strains have also been associated with a reduction of T1D. In particular, infection of young (4–6-week-old) NOD mice with CVB3 failed to accelerate T1D, but provided long-term protection from disease [51]. Interestingly, the replication properties and the dose of CVB3 strains critically influenced the outcome of T1D in the NOD mouse. Whereas administration of a low dose of the poorly virulent and slowly replicating strain CVB3/GA delayed T1D in prediabetic NOD mice, a higher dose accelerated T1D [52].
Infection of human islets by CVB3 induces a strong inflammatory response resulting in the activation of dendritic cells. Interestingly, upon phagocytosis of infected islets the dendritic cells induce the expression of interferon-stimulated genes (ISG), including the RIG-I-like helicases RIG-I and melanoma differentiation associated gene-5 (MDA5), and thus induce an antiviral state that protects the DCs from further infection [53■]. Interestingly, genome-wide association studies have identified human polymorphisms in both the Rig-I and Mda5 genes that are linked to a resistance to develop T1D [54,55]. It was further demonstrated that the production of type I interferons (IFNs) (IFNα and IFNβ) through TLR3 and MDA5 by plasmacytoid dendritic cells (pDCs) was indeed critical for the prevention of virus-induced diabetes [56■■]. McCartney et al. [56■■] used the β-cell-tropic encephalomyocarditis virus strain D (EMCV-D) and found that wildtype C57BL/6 mice, in contrast to Tlr-/- and Mda5-/- mice, were protected from EMCV-D-induced T1D. Genome-wide association studies in the rat link an entire network of IFN response genes, extending beyond MDA5, to the development of T1D [57,58]. Thus, viral infection causing IFN-I production might protect the islets of Langerhans from a subsequent infection with a pancreas-tropic pathogen, such as HEV, that otherwise would induce or accelerate T1D. A similar scenario has been recently reported in the Kilham rat virus (KRV) model. Viral precipitation of T1D has been previously demonstrated in multiple rat strains infected with KRV [59]. More recently, this model has been investigated in more detail and LEW.1WR1 rats have been infected with either KRV or rat cytomegalovirus (RCMV) resulting in diabetes in up 40–60% of mice [60]. Simultaneous infection with KRV and RCMV induced T1D even in up to 100% of rats [60]. Interestingly, infection of dams with either KRV or RCMV before pregnancy prevented the development of T1D in the offspring in a virus dependent manner [60]. Thus, parental virus-infection might generate a preexisting immunity protecting the offspring from a subsequent diabetes-inducing infection of the offspring with the same virus. It has been speculated that such an inherited protection might be also mediated by antiviral responses evoked by the production of IFN-I [60].
Conclusion
HEVs seem to play an important role in the pathogenesis of T1D. Evidence from epidemiological studies, isolation of virus particles and RNA from the blood, pancreas and gut of T1D patients, and from animal studies demonstrate that HEVs can accelerate the pathogenesis of T1D in susceptible individuals. However, viruses and other pathogens have also been associated with the protection from autoimmunity. Here, epidemiological studies suggest that individuals who live in an environment with a low degree of hygiene associated with a higher risk of infection have a lower risk of acquiring autoimmune diseases, such as T1D or MS [61,62]. The ‘hygiene theory’ is further supported by experimental evidence from animal models. In particular, virus infections can deplete autoaggressive lymphocytes and induce a variety of regulatory ‘immune-tuning’ mechanisms. In this context, it is important to note that hygiene as well as diet influence gut microbiota, which subsequently would influence the nature of immune responses and thus the pathogenesis of autoimmune diseases [63].
Other environmental factors such as sunlight exposure that strongly influences vitamin D levels might as well be involved in the pathogenesis of T1D [64]. High vitamin D levels could protect from autoimmunity by establishing a more regulatory milieu. Indeed, vitamin D treatment protects NOD mice from T1D most likely by activation of dendritic cell-induced apoptosis of autoaggressive T cells [65,66]. A recent study [67■] with a large cohort of T1D patients and healthy controls clearly associated vitamin D deficiency and polymorphisms in three vitamin D metabolizing enzymes with a higher risk for T1D. This link is however still controversial, as some studies failed to demonstrate an association [68] and the risk for T1D is much lower than reported for other polymorphism, such as HLA or insulin-related genes [2]. Nevertheless, vitamin D supplementation has been tested in several studies with the overall finding that the most promising potential for vitamin D supplementation may be in prevention rather than treatment [64].
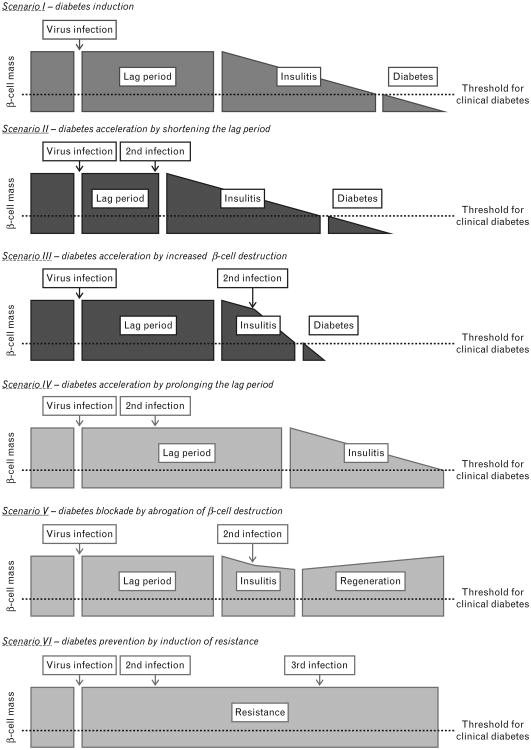
Connecting virus infections with the development of T1D the following scenarios should be considered (Fig. 1). First, virus infections can interfere with self-tolerance to islet antigens by mechanisms such as molecular mimicry or inflammation, resulting in accelerated β-cell destruction and precipitation of T1D. Second, one or more infections might shorten the duration of the prediabetic phase even more. Third, and in contrast, a secondary virus infection might decelerate T1D by prolonging the prediabetic phase of by the abrogating the autodestructive process by inducing apoptosis of autoaggressive lymphocytes or by enhancing counter-regulatory processes. Very likely a combination of such scenarios is involved in human T1D, as most T1D patients as well as non-diabetic individuals suffer from several infections during their lifetime. Therefore, it is of upmost importance that large prospective studies, such as the TEDDY study (website: teddy.epi.usf.edu), are conducted that target the identification of environmental factors, such as diet, pathogen infection and exposure to chemicals.
Figure 1.
Induction acceleration or abrogation of type 1 diabetes (T1D) by virus infection(s) – several scenarios of how environmental factors, such as viruses, influence T1D pathogenesis can be considered. First, virus infections can interfere with self-tolerance to islet antigens and, after a variable lag period, insulitis ensues and β-cells are continuously destroyed. Once the β-cell mass is decreased beyond a certain threshold clinical diabetes manifests. Second, an additional virus infection might cause a shortening of the lag phase by causing an earlier islet infiltration and thereby accelerate disease onset more. Third, alternatively a secondary virus infection, occurring at a time when insulitis has already started, might enhance the β-cell destruction process itself and hence accelerate the reduction in the β-cell mass. Fourth, the lag period might alternatively be extended by additional virus infections that temporarily deviate autoaggressive cells from the pancreas. Fifth, secondary virus infections could permanently remove autoaggressive cells from the system and/or induce specific regulatory cells that would allow β-cell mass preservation or even regeneration. Last, a primary viral infection might induce a protective antiviral state that decreases the degree of further infections and thereby prevents virally mediated acceleration of β-cell destruction and possibly the development of diabetes.
Key Points.
Substantial epidemiological evidence indicates a contribution of human enteroviruses (HEV) to the pathogenesis of type 1 diabetes (T1D).
HEVs have been isolated from the pancreas, the gut and the blood of T1D patients.
Animal models revealed potential mechanisms of how pathogens can induce or accelerate T1D.
In addition to viral inflammation, molecular mimicry could be an important driving force for the breakdown of self-tolerance to islet auto-antigens.
Virus infections can also protect from T1D by reducing the number of aggressive lymphocytes, improving regulatory mechanisms or inducing a β-cell protective environment in the islets of Langerhans.
Acknowledgments
We would like to thank Edith Hintermann for critically reading the manuscript.
Conflicts of interest: U.C. is supported by grants of the German Research Foundation (DFG). M.G.v.H. is supported by a scholar award of the Juvenile Diabetes Research Foundation (JDRF) and a Program Project Grant (PPG) of the National Institute of Health (NIH) to the La Jolla Institute for Allergy and Immunology.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 438–439).
- 1■■.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35. doi: 10.1136/bmj.d35. This study compares the data obtained from 33 independent T1D prevalence studies and confirmed a significant association between HEV infection and the development of T1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457–467. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity. 2010;32:468–478. doi: 10.1016/j.immuni.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damian RT. Molecular mimicry: antigen sharing by parasite and host and its consequences. Am Natur. 1964;98:129–149. [Google Scholar]
- 5.Oldstone MBA. Molecular mimicry as a mechanism for the cause and as a probe uncovering etiologic agent(s) of autoimmune disease. Curr Top Microbiol Immunol. 1989;145:127–136. doi: 10.1007/978-3-642-74594-2_11. [DOI] [PubMed] [Google Scholar]
- 6.Christen U, Hintermann E, Holdener M, et al. Viral triggers for autoimmunity: is the ‘glass of molecular mimicry’ half full or half empty? J Autoimmun. 2010;34:38–44. doi: 10.1016/j.jaut.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracy S, Drescher KM, Chapman NM. Enteroviruses and type 1 diabetes. Diabetes Metab Res Rev. 2011;27:820–823. doi: 10.1002/dmrr.1255. [DOI] [PubMed] [Google Scholar]
- 8.Honeyman MC, Coulson BS, Stone NL, et al. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes. 2000;49:1319–1324. doi: 10.2337/diabetes.49.8.1319. [DOI] [PubMed] [Google Scholar]
- 9.Hyoty H, Leinikki P, Reunanen A, et al. Mumps infections in the etiology of type 1 (insulin-dependent) diabetes. Diab Res. 1988;9:111–116. [PubMed] [Google Scholar]
- 10.Gale EA. Congenital rubella: citation virus or viral cause of type 1 diabetes? Diabetologia. 2008;51:1559–1566. doi: 10.1007/s00125-008-1099-4. [DOI] [PubMed] [Google Scholar]
- 11.Pak CY, Eun HM, McArthur RG, et al. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet. 1988;2:1–4. doi: 10.1016/s0140-6736(88)92941-8. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hello H, Paananen A, Eskelinen M, et al. An enterovirus strain isolated from diabetic child belongs to a genetic subcluster of echovirus 11, but is also neutralised with monotypic antisera to coxsackievirus A9. J Gen Virol. 2008;89:1949–1959. doi: 10.1099/vir.0.83474-0. [DOI] [PubMed] [Google Scholar]
- 13.Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci USA. 2007;104:5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon JW, Austin M, Onodera T, et al. Virus-induced diabetes mellitus: isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee NK, Nejman C, Gerling I. Purification and characterization of a strain of coxsackievirus B4 of human origin that induces diabetes in mice. J Med Virol. 1988;26:57–69. doi: 10.1002/jmv.1890260109. [DOI] [PubMed] [Google Scholar]
- 16.Elshebani A, Olsson A, Westman J, et al. Effects on isolated human pancreatic islet cells after infection with strains of enterovirus isolated at clinical presentation of type 1 diabetes. Virus Res. 2007;124:193–203. doi: 10.1016/j.virusres.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Andreoletti L, Hober D, Hober-Vandenberghe C, et al. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. J Med Virol. 1997;52:121–127. doi: 10.1002/(sici)1096-9071(199706)52:2<121::aid-jmv1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Schulte BM, Bakkers J, Lanke KH, et al. Detection of enterovirus RNA in peripheral blood mononuclear cells of type 1 diabetic patients beyond the stage of acute infection. Viral Immunol. 2010;23:99–104. doi: 10.1089/vim.2009.0072. [DOI] [PubMed] [Google Scholar]
- 19.Clements GB, Galbraith DN, Taylor KW. Coxsackie B virus infection and onset of childhood diabetes. Lancet. 1995;346:221–223. doi: 10.1016/s0140-6736(95)91270-3. [DOI] [PubMed] [Google Scholar]
- 20.Lonnrot M, Salminen K, Knip M, et al. Enterovirus RNA in serum is a risk factor for beta-cell autoimmunity and clinical type 1 diabetes: a prospective study. Childhood Diabetes in Finland (DiMe) Study Group. J Med Virol. 2000;61:214–220. [PubMed] [Google Scholar]
- 21.Yoon JW, Austin M, Onodera T, et al. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 22.Richardson SJ, Willcox A, Bone AJ, et al. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 23.Richardson SJ, Willcox A, Hilton DA, et al. Use of antisera directed against dsRNA to detect viral infections in formalin-fixed paraffin-embedded tissue. J Clin Virol. 2010;49:180–185. doi: 10.1016/j.jcv.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 24■.Stene LC, Oikarinen S, Hyoty H, et al. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY) Diabetes. 2010;59:3174–3180. doi: 10.2337/db10-0866. This study demonstrates a significant association of HEV infection and the development of T1D and summarizes the settings of the DAISY program. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25■.Simonen-Tikka ML, Pflueger M, Klemola P, et al. Human enterovirus infections in children at increased risk for type 1 diabetes: the Babydiet study. Diabetologia. 2011;54:2995–3002. doi: 10.1007/s00125-011-2305-3. This study stands in contrast to Ref. [24■]. The authors found no association between the presence of HEVs in stool samples and the development of islet autoantibodies. [DOI] [PubMed] [Google Scholar]
- 26.Tapia G, Cinek O, Rasmussen T, et al. Human enterovirus RNA in monthly fecal samples and islet autoimmunity in Norwegian children with high genetic risk for type 1 diabetes: the MIDIA study. Diabetes Care. 2011;34:151–155. doi: 10.2337/dc10-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27■.Lempainen J, Tauriainen S, Vaarala O, et al. Interaction of enterovirus infection and cow's milk-based formula nutrition in type 1 diabetes-associated autoimmunity. Diab Metab Res Rev. 2011 doi: 10.1002/dmrr.1294. In this study the authors found that the influence of a HEV infection on the development of T1D seems to be dependent on the diet. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasappa J, Saegusa J, Prabhakar BS, et al. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol. 1986;57:397–401. doi: 10.1128/jvi.57.1.397-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christen U, von Herrath MG. Induction, acceleration or prevention of autoimmunity by molecular mimicry. Mol Immunol. 2004;40:1113–1120. doi: 10.1016/j.molimm.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Ang CW, Jacobs BC, Laman JD. The Guillain-Barre syndrome: a true case of molecular mimicry. Trends Immunol. 2004;25:61–66. doi: 10.1016/j.it.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Kirvan CA, Swedo SE, Heuser JS, et al. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9:914–920. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson MA, Bowman MA, Campbell L, et al. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest. 1994;94:2125–2129. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horwitz MS, Bradley LM, Harbertson J, et al. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 34.Honeyman MC, Stone NL, Falk BA, et al. Evidence for molecular mimicry between human T cell epitopes in rotavirus and pancreatic islet autoantigens. J Immunol. 2010;184:2204–2210. doi: 10.4049/jimmunol.0900709. [DOI] [PubMed] [Google Scholar]
- 35.Oldstone MBA, Nerenberg M, Southern P, et al. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: Role of antiself (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 36.von Herrath MG, Dockter J, Oldstone MBA. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 37.Christen U, Edelmann KH, McGavern DB, et al. A viral epitope that mimics a self-antigen can accelerate but not initiate autoimmune diabetes. J Clin Invest. 2004;114:1290–1298. doi: 10.1172/JCI22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holdener M, Hintermann E, Bayer M, et al. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205:1409–1422. doi: 10.1084/jem.20071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 40.Selmi C, Balkwill DL, Invernizzi P, et al. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–1257. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 41.Amano K, Leung PS, Rieger R, et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–5883. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 42.Wakabayashi K, Lian ZX, Leung PS, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–540. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christen U, Benke D, Wolfe T, et al. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest. 2004;113:74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filippi CM, Estes EA, Oldham JE, et al. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J Clin Invest. 2009;119:1515–1523. doi: 10.1172/JCI38503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45■.Diana J, Brezar V, Beaudoin L, et al. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. J Exp Med. 2011;208:729–745. doi: 10.1084/jem.20101692. This recent study demonstrates how virus infection potentially can protect from rather than induce/accelerate T1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehlers S, Kaufmann SH. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: lifestyle changes affecting the host-environment interface. Clin Exp Immunol. 2010;160:10–14. doi: 10.1111/j.1365-2249.2010.04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filippi CM, von Herrath MG. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: viruses, autoimmunity and immunoregulation. Clin Exp Immunol. 2010;160:113–119. doi: 10.1111/j.1365-2249.2010.04128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatenoud L, You S, Okada H, et al. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: immune therapies of type 1 diabetes: new opportunities based on the hygiene hypothesis. Clin Exp Immunol. 2010;160:106–112. doi: 10.1111/j.1365-2249.2010.04125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oldstone MB. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science. 1988;239:500–502. doi: 10.1126/science.3277269. [DOI] [PubMed] [Google Scholar]
- 50.Christen U, von Herrath MG. Infections and autoimmunity—good or bad? J Immunol. 2005;174:7481–7486. doi: 10.4049/jimmunol.174.12.7481. [DOI] [PubMed] [Google Scholar]
- 51.Tracy S, Drescher KM, Chapman NM, et al. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J Virol. 2002;76:12097–12111. doi: 10.1128/JVI.76.23.12097-12111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanno T, Kim K, Kono K, et al. Group B coxsackievirus diabetogenic phenotype correlates with replication efficiency. J Virol. 2006;80:5637–5643. doi: 10.1128/JVI.02361-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53■.Schulte BM, Kramer M, Ansems M, et al. Phagocytosis of enterovirus-infected pancreatic beta-cells triggers innate immune responses in human dendritic cells. Diabetes. 2010;59:1182–1191. doi: 10.2337/db09-1071. This study describes that infection by certain HEV-strains induces an antiviral state to islet cells and thereby protects from further infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smyth DJ, Cooper JD, Bailey R, et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 55.Shigemoto T, Kageyama M, Hirai R, et al. Identification of loss of function mutations in human genes encoding RIG-I and MDA5: implications for resistance to type I diabetes. J Biol Chem. 2009;284:13348–13354. doi: 10.1074/jbc.M809449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56■■.McCartney SA, Vermi W, Lonardi S, et al. RNA sensor-induced type I IFN prevents diabetes caused by a beta cell-tropic virus in mice. J Clin Invest. 2011;121:1497–1507. doi: 10.1172/JCI44005. This elegant study demonstrates that virus induced protection from T1D is dependent on TLR3- and MDA5-mediated production of IFN-I by plasmacytoid dendritic cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinig M, Petretto E, Wallace C, et al. A trans-acting locus regulates an antiviral expression network and type 1 diabetes risk. Nature. 2010;467:460–464. doi: 10.1038/nature09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foxman EF, Iwasaki A. Genome-virome interactions: examining the role of common viral infections in complex disease. Nat Rev Microbiol. 2011;9:254–264. doi: 10.1038/nrmicro2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellerman KE, Richards CA, Guberski DL, et al. Kilham rat triggers T-cell-dependent autoimmune diabetes in multiple strains of rat. Diabetes. 1996;45:557–562. doi: 10.2337/diab.45.5.557. [DOI] [PubMed] [Google Scholar]
- 60.Tirabassi RS, Guberski DL, Blankenhorn EP, et al. Infection with viruses from several families triggers autoimmune diabetes in LEW * 1WR1 rats: prevention of diabetes by maternal immunization. Diabetes. 2010;59:110–118. doi: 10.2337/db09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurtzke JF. Epidemiologic evidence for multiple sclerosis as an infection. Clin Microbiol Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tracy S, Drescher KM, Jackson JD, et al. Enteroviruses, type 1 diabetes and hygiene: a complex relationship. Rev Med Virol. 2010;20:106–116. doi: 10.1002/rmv.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 64.Wolden-Kirk H, Overbergh L, Christesen HT, et al. Vitamin D and diabetes: its importance for beta cell and immune function. Mol Cell Endocrinol. 2011;347:106–120. doi: 10.1016/j.mce.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 65.Gregori S, Giarratana N, Smiroldo S, et al. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 66.Decallonne B, van Etten E, Overbergh L, et al. 1Alpha, 25-dihydroxyvitamin D3 restores thymocyte apoptosis sensitivity in nonobese diabetic (NOD) mice through dendritic cells. J Autoimmun. 2005;24:281–289. doi: 10.1016/j.jaut.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 67■.Cooper JD, Smyth DJ, Walker NM, et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. 2011;60:1624–1631. doi: 10.2337/db10-1656. This study demonstrates an association between polymorphisms in vitamin D metabolism associated genes and the risk for T1D. Thus, the study shows that environmental factors other than pathogen infection might be involved in the cause of T1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nejentsev S, Cooper JD, Godfrey L, et al. Analysis of the vitamin D receptor gene sequence variants in type 1 diabetes. Diabetes. 2004;53:2709–2712. doi: 10.2337/diabetes.53.10.2709. [DOI] [PubMed] [Google Scholar]