Abstract
Major depressive disorder (MDD) is a devastating disease affecting over 300 million people worldwide, and costing an estimated 380 billion Euros in lost productivity and health care in the European Union alone. Although a wealth of research has been directed toward understanding and treating MDD, still no therapy has proved to be consistently and reliably effective in interrupting the symptoms of this disease. Recent clinical and preclinical studies, using genetic screening and transgenic rodents, respectively, suggest a major role of the CRF1 gene, and the central expression of CRF1 receptor protein in determining an individual’s risk of developing MDD. This gene is widely expressed in brain tissue, and regulates an organism’s immediate and long-term responses to social and environmental stressors, which are primary contributors to MDD. This review presents the current state of knowledge on CRF physiology, and how it may influence the occurrence of symptoms associated with MDD. Additionally, this review presents findings from multiple laboratories that were presented as part of a symposium on this topic at the annual 2014 meeting of the International Behavioral Neuroscience Society (IBNS). The ideas and data presented in this review demonstrate the great progress that has been made over the past few decades in our understanding of MDD, and provide a pathway forward toward developing novel treatments and detection methods for this disorder.
Keywords: Depression, CRF, CRF1, Stress
1. Major depression
Major depressive disorder (MDD) is a devastating, multifactorial psychiatric disorder, characterized by successive episodic changes in affect, cognition, and neurovegetative function. MDD patients possess overwhelming feelings of sadness, “emptiness” or irritable mood, accompanied by somatic and cognitive changes, which significantly affect an individual’s productivity, and general capacity to function (American Psychiatric Association, 2013). Suicide is also a critical concern in patients diagnosed with MDD, with suicidal thoughts, ideation, and attempts contributing a significant proportion of the reduced productivity, and in some cases premature deaths, that are associated with MDD (Krishnan and Nestler, 2008).
MDD is highly prevalent worldwide, appearing at similar frequencies across cultural, regional, and economic borders. A comprehensive study to determine the global burden of MDD in disability-adjusted life years (DALY), which estimates the future years of disability-free life that an individual loses to a disease, found that MDD contributes an estimated average of 65.6 years of lost productivity to those afflicted worldwide, the highest global burden for any disease. While this is observed in both sexes, the study also confirmed the higher occurrence of depression in women (100% higher than men), and this is reflected in the higher burden of the disease that is observed in women (Kessler, 2003; Kessler et al., 1993; Collins et al., 2011; Marcus et al., 2014).
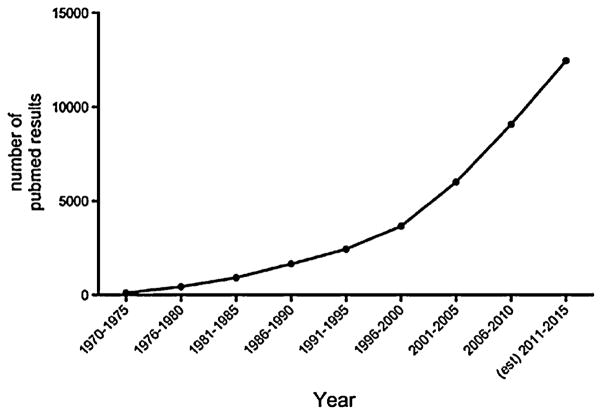
The growing concern over the burden of MDD is apparent in an increase in the number of studies published on the topic of depression over the past decades (PubMed: keyword search [depression] AND [mood] since 1970) (Fig. 1). These studies have advanced our understanding of the etiology of MDD, and have improved our ability to understand and recognize the behavioral and physiological symptoms of this disease. Despite these advances, no universally applicable mechanism has been identified that specifically detects and addresses the physiological and behavioral dysfunctions associated with MDD.
Fig. 1.
Graphical representation of the increase in number of publications returned using a PubMed search from (depression [title/abstract]AND(mood)AND(year range) 1970–2015. Future data (December 2014–December 2015) extrapolated from current numbers.
One emerging candidate for this purpose is corticotropin-releasing factor (CRF), the hypothalamic secretogogue, identified three decades ago by Wylie Vale (Vale et al., 1981, and reviewed within). Initially discovered as an initiator of the hypothalamic–pituitary–adrenal (HPA) axis, this peptide also exhibits high levels of expression across multiple brain areas, and modulates the activity of multiple neurotransmitter systems (Bale and Vale, 2004; Hauger et al., 2009; Sahuque et al., 2006, and many others cited within this article). This article reviews the current state of research on CRF physiology and the role that it plays in the etiology of MDD. This report also presents data from a panel of scientists who are actively investigating this neuropeptide as a source, a therapeutic pathway, and a biomarker for MDD.
2. Corticotropin releasing factor peptide and receptors (Table 1 for summary)
Table 1.
Summary list of CRF family peptides, splice variants of each receptor subtype, and the affinity of each ligand for receptors.
| Receptor | Splice variant | Ligand affinity
|
|||
|---|---|---|---|---|---|
| CRF | Urocortin 1 | Urocortin 2 | Urocortin 3 | ||
| CRF1 | CRF1A (archetype) | High | Equal | Low | Low |
| CRF1B (intracellular domain) | |||||
| CRF1C (N-terminus) | |||||
| CRF1D (transmembrane domain) | |||||
| CRF1E (N-terminus) | |||||
| CRF1F (intracellular domain) | |||||
| CRF1G (transmembrane domain) | |||||
| CRF1H (N-terminus) | |||||
| CRF2 | CRF2A (archetype) | Low | Equal | High | High |
| CRF2B (N-terminus) | |||||
| CRF2C (N-terminus) | |||||
2.1. CRF family ligands
The 41 amino acid peptide referred to as corticotropin releasing factor (CRF), hormone (CRH), or corticoliberin (Vale et al., 1981) is the eponymous member of a family of peptides, which also includes three urocortins: Urocortin 1 [sharing roughly 45% homology with CRF in humans (Donaldson et al., 1996)], Urocortin 2 or stresscopin-related peptide, and Urocortin 3 or stresscopin, three urotensins (urotensin 1, urotensin 2, and urotensins 3), and sauvagine. The CRF family of peptides transduces neural and endocrine signals by binding to two major CRF receptor types, CRF1 and CRF2, the actions of which can be modified for all peptides in the family by CRF binding protein (CRFBP; Hauger et al., 2003).
2.2. CRF receptors
The CRF1 and CRF2 receptors are similar in composition, constructed of seven transmembrane α-helical proteins (class II or B receptor super-family) with binding in the interior of the helical protein cone stimulating signal transduction via G proteins (Hillhouse and Grammatopoulos, 2006; Perrin and Vale, 1999). Distinct genes encode for CRF1 (human chromosome 17q21.31, mouse 11E1) and CRF2 receptors (human chromosome 7p4.3; mouse 6B3), retaining a 70% peptide sequence homology. The CRF1 receptor protein and its variants are constructed from 415–446 amino acids. The protein variants of the CRF2 receptors have from 397–438 amino acids.
2.3. CRF receptors variants
Receptor variability is increased by alternative splicing, resulting in isoforms that are archetypal (CRF1(a), CRF2(a)), N-terminal variants (CRF1(c), CRF1(e), CRF1(h), CRF2(b), CRF2(c)), and have altered intracellular (CRF1(b), CRF1(f)) or transmembrane (CRF1(d), CRF1(g)) domains (Lovenberg et al., 1995; Zmijewski and Slominski, 2010). CRF binds with high affinity to the CRF1 receptor, and exhibits a much lower (15 times) affinity for the CRF2 receptor (Hauger et al., 2003; Perrin and Vale, 1999). Urocortins, urotensins, and sauvagine also bind CRF receptors, although the three urotensins and sauvagine have not been identified in mammals, and will not be discussed further. Among the urocortins, Urocortin 1 binds CRF1 receptors with similar affinity to CRF, and binds CRF1 and CRF2 with similar affinities. In contrast, Urocortin 2 and Urocortin 3 bind with much greater affinity to the CRF2 receptor, with little or no effect on CRF1 receptor. Urocortin 1, Urocortin 2, and Urocortin 3 also bind the CRF2 receptor isoforms with approximately 100 times greater affinity than does CRF (Hauger et al., 2003). Therefore CRF and Urocortin 1 can be considered the endogenous ligands for the CRF1 receptor, and while the entire family of CRF peptides generally binds CRF2 receptors, Urocortin 2 and Urocortin 3 are CRF2 specific (Hauger et al., 2003). The CRF binding protein (CRFBP) is a 37KDa, 322 amino acid glycoprotein that binds CRF and urocortins (except Urocortin 3) with a high affinity, which is similar to or greater than that of CRF receptors (Behan et al., 1995a; Boorse and Denver, 2006; Huising and Flik, 2005; Potter et al., 1991; Seasholtz et al., 2002).
2.4. CRF binding proteins
While CRFBP in plasma reduces bioavailability of CRF-like peptides by binding CRF and dimerizing, perhaps preventing CRF from binding to receptors (Behan et al., 1995a), more broadly the potential for the CRFBP is for buffering, inhibiting, or enhancing the effects of the CRF family peptides binding to CRF1 or 2 receptors (Seasholtz et al., 2002). The type of effect produced by binding protein relies on localization and concentration, based on the Law of Mass Action; and in that way they are similar to receptors. The effect is almost assuredly different depending on the tissue (Seasholtz et al., 2002), and CRFBP is highly expressed in brain, plasma, heart, lungs, intestines and placenta (Boorse and Denver, 2006; Potter et al., 1992; Vitoratos et al., 2006). Neurons and glia of all CRF related pathways in the brain express membranal CRFBP, sometimes directly colocalized with CRF or CRF receptors (Behan et al., 1995b; Potter et al., 1992).
2.5. CRF receptor intracellular signaling
Following CRF receptor binding, which results in G protein phosphorylation and dissociation of α subunits (from βγ) to stimulate 2nd messenger cascades (see below), G protein related kinases (GRKs) rapidly desensitize the receptor via phosphorylation of serines and threonines in the third intracellular loop or C-terminus (Dautzenberg et al., 2002; Kelly et al., 2008; Kohout and Lefkowitz, 2003; Krasel et al., 2005; Moore et al., 2007). Thus, the affinity of the receptor for β-arrestin is rapidly enhanced. Termination of receptor signaling for CRF1 and CRF2 can be accomplished by β-arrestin1 and β-arrestin2 which bind to clathrin and β-adaptin from vesicles which are internalized containing the complex of CRF-CRF1 or 2-arrestin (Markovic et al., 2008; Oakley et al., 2007). Internalized and desensitized receptors are dephosphorylated by specific phosphatases, and resensitized receptors are recycled to the plasma membrane.
Although amino acid sequences and affinities of CRF1 and CRF2 receptors are distinct, both stimulate the Gsα → ACIC3 (3rd intracellular loop of adenylate cyclase) → cAMP → PKA second messenger cascade (Dautzenberg and Hauger, 2002; Hauger et al., 2003, 2006, 2009; Hillhouse and Grammatopoulos, 2006). The CRF1 receptor interacts preferentially with Gsα, but also binds with lower affinity to Goα, Gq/11α, Giα and Gzα (Grammatopoulos et al., 1999). Protein kinase A and inositol trisphosphate (IP3) stimulated by CRF can trigger Ca2+ influx from cell membrane and intracellular ion channels, thereby activating calcium–calmodulin kinase II (CamK2). Binding to CRF1 or CRF2 receptors, CRF and Urocortin 1 triggering PKA and CamK2 stimulate the mitogen-activating protein kinases (MAPK) cascade through Rap1 or Rap2 → B-Raf → MEK1 and MEK2 → extracellular signal-regulated kinase (ERK) both ERK1 and ERK2. The CRF receptors can activate ERK, p38, or Jun N-terminal kinase (JNK) MAPK cascades. The CRF1 enhanced production of cAMP also directly stimulates this cascade via guanine nucleotide exchange factor (RAPGEF3 or Epac) stimulation of RAP GTPases. The CRF1 mediated PKA cascade also upregulates serine-threonine protein kinase (SGK1) (Sheng et al., 2008). Intracellular Ca2+ mobilization is enhanced by CRF1 and CRF2 receptors via AC → Epac2, which stimulates the epsilon isoforms of phospholipase C (PLCε) and protein kinase C (PKC). The 2nd messenger cascades stimulated by CRF1 and/or CRF2 receptor binding may be influenced by the altered amino acid sequences in splice variant isoforms. In certain cells Urocortin 2 and Urocortin 3, but not CRF, transduce ERK signaling via CRF2 receptors. While it is unclear whether splice variation influences specific pathways of signal transduction, regional expression of CRF1 and CRF2 receptor isoforms is specifically correlated. Not every cell stimulated by CRF or Urocortin activates this full complement of 2nd messenger cascades; ERK systems may not be elicited.
2.6. CRF receptor variant modification of intracellular signaling
The functional expression of the CRF1 signaling cascades may be modulated by expression or co-expression of CRF receptor isoforms (Zmijewski and Slominski, 2010). The classic CRF1(a) pathway stimulates 2nd messenger cascades, as does the CRF1(b) alternative pathway, and the intracellular CRF1(h) receptors. Classical signaling is inhibited by dimerization of CRF1(a) with CRF1(d), CRF1(f) or CRF1(g) receptors. This inhibition of classical signaling is extended by intracellular retention and degradation of CRF1(a) dimerized with CRF1(d), CRF1(f), or CRF1(g) receptors. Alternative signaling is limited in CRF1(b) receptors during their fast recycling. The CRF1(c) receptors exhibit impaired agonist binding, and CRF1(d) impaired G protein binding. Additional limitations on 2nd messenger cascade signaling may be due to mRNA decay caused by CRF1(e, f, g, k) receptors (due to premature stop codons introduced by alternative splicing) and secreted CRF1(e) and CRF1(h) receptors acting as decoy receptors (Zmijewski and Slominski, 2010). Translational science and clinical therapies must necessarily consider these receptor interactions to ensure positive therapeutic effects.
2.7. Functional anatomy of CRF expression
As CRF and the CRF family of peptides are likely to be the heart of a coordinated neural and endocrine stress response and coping system, the circuitry linking brain regions involved with behavioral, physiological and endocrine stress responses are critical. While it is likely that the CRF family of peptides produce a coordinated result, activating behavioral, neuroendocrine, and physiological stress response systems, the specific regional distribution of the peptides and receptors, along with different binding affinities, suggest more specific functions for each in the larger synchronized scheme. The peptide CRF has its highest expression in hypothalamus and median eminence, suggesting its potent hypothalamic–pituitary–adrenal (HPA) axis endocrine function, but it is also expressed in extrahypothalamic brain regions like amygdala, and in peripheral tissues like heart and blood vessels, skin, lung, spleen, pancreas, kidney, liver, adipose tissue, digestive tract, testes, ovaries and placenta (Boorse and Denver, 2006; Potter et al., 1994). Urocortins also appear to be widely distributed in brain, pituitary, heart and vasculature, skeletal muscle, kidney, adipose tissue, digestive tract and gonads (Boorse and Denver, 2006; Kageyama et al., 1999).
Regions of intensive CRF production exist in the extended amygdala, brainstem and several nuclei of the hypothalamus (Cummings et al., 1983; Olschowka et al., 1982; Swanson et al., 1983). The extended amygdala is implicated in the etiology of anxiety and depression, and contains CRF cells in central (CeA), basolateral (BLA), medial (MeA), lateral (LA), and posterior cortical amygdala, as well as the bed nucleus of the stria terminalis (BNST) and amygdalohippocampal area (Sakanaka et al., 1986). Of these, the greatest CRF fiber output projects from dense pyramidal CRF neurons in the CeA to lateral hypothalamus, mesencephalic reticular formation, locus ceruleus (LC), raphé, ventral tegmental area (VTA), dorsal and ventral parabrachial nuclei, mesencephalic nucleus of the trigeminal nerve, core and shell ventromedial hypothalamus, ventral subiculum, corticomedial amygdala, and the lateral BNST (Cummings et al., 1983; Sakanaka et al., 1986). The MeA produces Urocortin 3 (Hsu and Hsueh, 2001; Lewis et al., 2001; Deussing et al., 2010), and a variety of amygdalar nuclei, including CeA and MeA have Urocortin 1 fibers (Weitemier et al., 2005; Wong et al., 1996). Neurons of the dorsomedial and fusiform subdivisions of the anterior BNST contain glutamate (Glu) and CRF (including expression of the CRF gene); (Ju and Swanson, 1989; Ju and Han, 1989; Moga et al., 1989; Choi et al., 2007). These regions of the BNST are also sites for CRF terminals, receiving Glu and CRF fibers from the CeA (Choi et al., 2007; Sakanaka et al., 1986, 1987). In addition, the posterior part of the BNST expresses Urocortin 3 (Hsu and Hsueh, 2001; Lewis et al., 2001; Deussing et al., 2010). While the BNST contains both CRF1 and CRF2 receptors, CRF1 are more abundant (Chalmers et al., 1995; Rybnikova et al., 2003). The BNST also plays an important role in regulating endocrine output of CRF from the hypothalamus. In addition to inhibitory GABAergic control (Herman and Cullinan, 1997), the dorsomedial and fusiform regions of the BNST have excitatory CRF and Glu projections to the medial parvocellular PVN (Choi et al., 2007, 2008). The BNST therefore, both produces and receives CRF; together these sources of CRF influence endocrine physiology (Choi et al., 2008) and behavior (Sahuque et al., 2006).
The site of greatest expression for Urocortin 1 is the Edinger-Westphal nucleus (E-WN, or accessory oculomotor nucleus; Bittencourt et al., 1999; Ryabinin et al., 2005). In the rostral midbrain, its paired nuclei are posterior to the oculomotor nucleus and anterolateral to the aqueduct, sending innervation to and receiving CRF innervation from the dorsal raphé (Cummings et al., 1983). Early immunohistochemistry suggested PVN CRF cells projected to the midbrain adjacent to the aqueduct, in addition to the median eminence (Paull et al., 1982, 1984). The PVN also expresses Urocortin 2 in its magnocellular region (Hashimoto et al., 2004; Hsu and Hsueh, 2001; Reyes et al., 2001). Cells producing CRF are also found in the arcuate (ARC), dorsomedial (DMN), ventromedial (VMN), periventricular (PVa) and supraoptic (SON) of the hypothalamus and the mammillary bodies (Cummings et al., 1983; Krieger et al., 1977). Small SON and PVN rostral projections of CRF terminate in the SCN and septum (Paull et al., 1984). Lesions of the mammillothalamic tract induced CRF production in the mammillary body (Kovacs et al., 1985). In addition to CRF, the SON also expresses Urocortin 1 and Urocortin 2 (Bittencourt et al., 1999; Wong et al., 1996). The ARC produces both CRF and Urocortin 2 (Hashimoto et al., 2004; Hsu and Hsueh, 2001; Reyes et al., 2001), and the rostral perifornical area of the hypothalamus expresses Urocortin 3 (Hsu and Hsueh, 2001; Lewis et al., 2001).
In the cerebral cortex bipolar CRF interneurons are found, most commonly in limbic regions like prefrontal cortex and cingulate gyrus (Swanson et al., 1983). The hippocampus also has a small number of CRF producing cells that remain after a dramatic decline in hippocampal CRF from early postnatal life (Chen et al., 2001). Bipolar CRF cells are found in the dorsal and ventral regions of the LC (Cummings et al., 1983). The reticular nuclei also have a few multipolar CRF cells. A small group of serotonergic neurons in the dorsomedial portion of the dorsal raphé nucleus also produces CRF (Commons et al., 2003). This colocalization suggests CRF and 5-HT are cotransmitters. Interestingly, the projection of these CRF/5-HT cells in the dorsomedial portion of the dorsal raphé is the CeA, describing a prominent reciprocal innervation. The extra-hypothalamic sites of Urocortin 1 expression outside of E-WN and amygdala include neocortex, hippocampus, lateral septum, nucleus of the solitary tract, and lateral superior olivary nucleus (Bittencourt et al., 1999; Wong et al., 1996). Extra-hypothalamic sites for Urocortin 2 include the LC and motor neurons of the brainstem and spinal cord (Hashimoto et al., 2004; Hsu and Hsueh, 2001; Reyes et al., 2001). The lateral septum expresses Urocortin 3 as well as Urocortin 1 and CRF (Hsu and Hsueh, 2001; Lewis et al., 2001).
Receptors for CRF and urocortins are widely distributed throughout the cerebral cortex (prefrontal, frontal, orbital, cingulate, insular, and temporal cortices; also cerebellum), limbic system (hippocampal dentate gyrus, amygdala, BNST, nucleus accumbens, mammillary bodies, olfactory tubercle, and dorsomedial thalamus) and other areas (striatum, superior and inferior colliculi, LC, raphé, VTA) as well as the pituitary in primates and rodents (Aguilera et al., 1987; Catt et al., 1987; Millan et al., 1986). In the central nervous system, both CRF1 and CRF2 receptor types are present, with some splice variants located in specific peripheral tissues (Chalmers et al., 1995; Hiroi et al., 2001; Potter et al., 1994). The CRF1 receptor is the primary endocrine transduction pathway for CRF, and it is found at high densities in the anterior pituitary. In the brain CRF1 receptors densely populate the cerebral cortex, hippocampus, amygdala, raphé nuclei and cerebellum. Peripheral CRF1 receptors are located in adrenal gland, ovaries, testes, and skin. The CRF2 receptors are located in brain as the CRF2(a) isoform (Lovenberg et al., 1995), in a variety of subcortical areas including amygdala, BNST, raphé nuclei, hypothalamus, and pituitary. The CRF2(b) isoform is found in retina, cerebellum, cerebral arterioles and choroid plexus as well as peripheral tissues (Lovenberg et al., 1995). Peripheral tissues including adrenal, ovaries, testes, skeletal muscle, GI tract, heart and lungs are populated by CRF2(b) receptors in rodents.
3. The role of stress in depression
Recent work has characterized chronic stress as one of the primary causal factors for the development and persistence of depressive behavioral phenotypes (Iniguez et al., 2014; Kleiman et al., 2014; Mahar et al., 2014; Monroe et al., 2014; Morris et al., 2014; Nederhof et al., 2014; Saravanan and Wilks, 2014; Shapero et al., 2014; Sickmann et al., 2014; Vinkers et al., 2014). In addition, exposure to stressful life events synergistically interacts with residual depressive symptoms (i.e., high depression scores following therapeutic interventions), to increase the risk of relapsing to a depressed state (Harkness et al., 2014). Major neural and endocrine sites of CRF production in the CeA, BNST, E-WN, and PVN tie the systemic and local delivery of CRF and CRF-like peptides, directly or indirectly, to all central and peripheral physiological systems associated with stress response (e.g., HPA axis, sympatho–adreno–medullary axis, central monoamine circuits; Ronan and Summers, 2011). Chronic exposure to stress is associated with dysfunctions in the negative feedback mechanisms that maintain homeostasis within these stress responsive systems, and this in turn, is associated with the increased expression and activation of neural and endocrine CRF, CRF1, and glucocorticoid systems, each of which is a hallmark of depressed patients (Binder and Nemeroff, 2010; Galard et al., 2002). Furthermore, increased central glucocorticoid levels lead to one of the more notable features in depression: reduced dendritic branching in hippocampal pyramidal cells (Sapolsky, 2000). Therefore, stress is linked with depression through its effects on structural and ultra-structural brain integrity as well as the modulation of neurotransmission, and evidence supports a role for CRF in each of these processes (Anacker, 2014; de Kloet et al., 2005; Duman, 2014; Mahar et al., 2014; McEwen, 2007). What is more, stressful events, both early and later in life, appear to be the causative link in gene-by-environment interactions that produce or exacerbate depression, through gene expression related to polymorphisms such as the BDNFVal66/Met, and the serotonin transporter 5-HTTLPR gene polymorphisms (Caspi et al., 2003; Hosang et al., 2014; Kruijt et al., 2014; Pezawas et al., 2008; Ressler et al., 2010). While the exact role of CRF in these gene by environment interactions is not conclusive, both human (Vigod and Stewart, 2009) and animal studies (Yu et al., 2012) demonstrate associations between these polymorphisms, depression (or depression-like behavior), and HPA axis reactivity (initiated by CRF).
Depression is fundamentally and etiologically a stress related disorder (Valdez, 2009) instigated and adjusted in patients by the neuroactive and hormonal modulators of stress responsiveness, including CRF (Goldstein and Klein, 2014; Ising et al., 2007); during clinical depression elevated CRF concentrations are measured in plasma (Galard et al., 2002) and cerebrospinal fluid (Banki et al., 1987; Hartline et al., 1996; Nemeroff et al., 1984). Furthermore, postmortem studies of humans afflicted with depression show evidence of a hyper-activation of CRF neurons in the PVN, cortical areas, pontine nuclei, and LC (Austin et al., 2003; Bissette et al., 2003; Merali et al., 2006; Raadsheer et al., 1994). Concomitant with this increased activation, CRF binding (also measured postmortem) in some regions of the brain, like the frontal cortex, is decreased (Nemeroff, 1998). This may be explained, in part, by the down-regulation of cortical CRF1 (but not CRF2) receptors that is observed in suicide victims (Merali et al., 2004). Noteworthy, a down-regulation of amygdala CRFBP is also observed in patients with bipolar disorder (Herringa et al., 2006). It is clinically significant that elevated plasma CRF levels in depressed individuals are ameliorated with successful electroconvulsive shock therapy (Nemeroff et al., 1991) or antidepressant treatment (De Bellis et al., 1993; Heuser et al., 1998; Holsboer, 2000; Veith et al., 1993). The literature relating central CRF activity in depression to the genetic polymorphisms mentioned earlier is sparse, however their relationship to the role of CRF in the HPA axis is better established; both polymorphisms are associated with hyperactivity in this system (Hosang et al., 2014; Wust et al., 2009). A recent study indicates an interaction between the 5-HTTLPR polymorphism and a polymorphism of the CRF1 gene (rs878886), resulting in an increased response to stress in individuals possessing both genotypes (Heitland et al., 2013). The causative relationship between depression and CRF hyperactivity and receptor expression is not clear. However, improvement in the symptoms of depression, if coupled with persistently elevated CRF in the CSF, is associated with early relapse (Banki et al., 1992).
4. Neurotransmitter systems that influence depression
While this review focuses on the effects and relation of central CRF systems to MDD, numerous other transmitter and modulatory systems clearly influence depression (reviewed in Hamon and Blier, 2013; Hirschfeld, 2012; Krishnan and Nestler, 2008; Mulinari, 2012). The depressive effects of these additional systems are often modulated through, or otherwise involve CRF actions in the brain, demonstrating the prominent and multi-faceted role of CRF in MDD (Hauger et al., 2009). Here, we briefly summarize the evidence of two prevailing neurophysiological theories of MDD, highlighting a role for CRF in these systems.
Extensive evidence suggests that monoamine neurotransmitter systems contribute (Hirschfeld, 2012) to the etiology of MDD. In fact, all approved pharmacological treatments for depression target these neurotransmitter systems (Nestler et al., 2002). Four ligands comprise the major monoamine neurotransmitters, the indoleamine (5-HT), and three catecholamines dopamine (DA), norepinephrine (NE), and epinephrine (Epi) (Hamon and Blier, 2013). The major cell bodies for all of these neurotransmitters (NE in the LC, 5-HT in the dorsal raphé, and DA in the VTA) exhibit high levels of CRF1 and CRF2 receptor expression, and the total expression levels of these receptors, as well as the ratio of their expression, influences an individual’s stress coping ability and risk of developing MDD (Bangasser and Valentino, 2012, 2014; Polter and Kauer, 2014; Wood et al., 2010, 2013b). Thus, the stress induced changes that are observed in these brain systems seem to be involved, or mediated by, CRF activity in either the cell body, or terminal regions, suggesting a pivotal role for this peptide in the established relationship of monoamine neurotransmitters and MDD.
Further studies suggest a close association between volume and synaptic connectivity in the hippocampus and prefrontal cortex with depression (Jha et al., 2011; Miller and Hen, 2014); parameters that are highly dependent on the presence and activity of neurotrophic factors, specifically brain derived neurotrophic factor (BDNF). Through multiple circuits (e.g., extended amygdala –hippocampus, periaqueductal gray, and VTA), CRF influences the production, release, and activity of BDNF, as well as many other neurotrophins (Bennett and Lagopoulos, 2014). A recent study elegantly demonstrates the importance of CRF in BDNF signaling. The authors demonstrate distinct stress mediated changes in BDNF signaling in the dopaminergic reward system (VTA/nucleus accumbens pathway). They go on to show that these changes in BDNF, that result in depression-like behavior, are directly ‘gated’ by CRF activity in the accumbens (Walsh et al., 2014).
Multiple theories describe central neurotransmitter systems that contribute to MDD, highlighting the heterogeneous nature of this disease. As outlined above, and in later sections of this review, the effects of stress on these systems are often mediated through stress related changes in CRF signaling. These observations support the idea that CRF may provide a common pathway for stress related diseases, such as MDD, and could serve as a more comprehensive therapeutic target for this disease.
5. CRF physiology and depression
Animal models have been successfully used to demonstrate the strong link between CRF and behavior associated with stress-mediated pathology, such as MDD. For example, i.c.v. injection of CRF induces these characteristic behaviors, including anxiety, anhedonia, decreased appetite, reduced libido, reduced slow wave sleep, and psychomotor alterations (Binder and Nemeroff, 2010; Keck, 2006). Conversely, CRF1 receptor knockout mice exhibit diminished responses to stressful stimuli (Contarino et al., 1999; Smith et al., 1998; Timpl et al., 1998). Together, these observations suggest that the CRF system plays an important role in depression, and other stress-related psychiatric disorders. In animal models of depression, such as social defeat, learned helplessness, tail suspension, and forced swim, CRF1 receptor antagonists, such as antalarmin, CP-154, CRA1000, 526, DMP696, DMP904, LWH234, R121919/NBI-30775, R278995/CRA0450, and SSR125543A reverse or inhibit the development of depressive-like behavioral traits (Valdez, 2009). Significant antidepressant effects, such as the reversal of shock escape deficit (by CP-154,526, CRA1000, and R278995/CRA0450), tail suspension immobility (by R121919 and DMP696), and forced swim immobility (by LWH234, antalarmin, and SSR125543A) have been demonstrated in these models, however not all of the drugs are equally or universally effective (for the shock escape deficit: DMP696 and DMP904; tail suspension: antalarmin, DMP904, CP154,526 and R278995/CRA0450; forced swim immobility: antalarmin, CP-154,526, R121919, R278995/CRA0450, DMP696, and DMP904 were all ineffective) (Chaki et al., 2004; Griebel et al., 2002; Jutkiewicz et al., 2005; Nielsen et al., 2004; Webster et al., 1996; Yamano et al., 2000). In rodents predisposed to higher baseline levels of forced swim immobility, this behavior is effectively ameliorated with SSR125543A and CP-154,526 (Overstreet et al., 2005).
CRF2 receptor knockout mice increased immobility in the forced swim test, suggesting that the CRF2 receptor may also be an influential factor in the relationship between CRF systems and depression, and may exert effects that oppose those of the CRF1 receptor (Bale and Vale, 2003). This is supported by the observation that CRF2-selective ligands (Urocortin 2 and Urocortin 3) reduce forced swim immobility (Tanaka and Telegdy, 2008). Surprisingly, female Urocortin 2 knockout mice exhibit less forced swim and tail suspension immobility, although males did not (Chen et al., 2006) – suggesting sex differences in the relationship between the CRF system and depression. In the learned helplessness model, Urocortin 2 injected directly into the dorsal raphé potentiates escape deficits (Hammack et al., 2003). Thus, it seems likely that CRF1 and CRF2 receptor effects on depression are site specific, and it will be important to consider the dynamics of these receptors in opposing brain regions as well as the impact of their relative densities.
Not surprisingly, the activation or inhibition of CRF gene expression can be induced by social stress in adult animals, and thereby invoke symptoms and behavior associated with depression (Elliott et al., 2010). Subjecting rodents to chronic social stress produces defeat, social avoidance, and susceptibility to depression-like behaviors in some subjects, but not others (Arendt et al., 2014; de Kloet et al., 2005; Krishnan et al., 2007; Krishnan and Nestler, 2010, 2011). The mesolimbic DA reward pathway is implicated in depressive behaviors (Krishnan et al., 2007), and pro-depressive effects generated through BDNF in this pathway are modulated by CRF (Walsh et al., 2014). In stress susceptible mice, chronic social stress induces long-term demethylation of the CRF gene, increasing its expression, and resulting in depression-like behavior (Elliott et al., 2010). In the same study, stress resilient mice did not exhibit this effect of social stress on CRF gene induction, nor did they exhibit depressive-like behavior. The causative role for CRF gene expression in depression-like behavior was demonstrated through a site-specific knockdown of the CRF gene in the PVN, which attenuated this depression-like response. Increased CRF gene expression in the PVN also leads to increased HPA axis activation, suggesting that susceptible individuals are also potentially more stress reactive (Shapero et al., 2014; Wood et al., 2010), or perhaps differently stress reactive (Morris et al., 2014), than stress resilient individuals. In effect, stress-induced epigenetic modification and expression of genes for CRF and its CRF1 receptor may be the critical factors for engendering susceptibility or resilience to the potential for depression and depression-like behavior (Elliott et al., 2010; Ressler et al., 2010).
The gene loci for CRF-family ligands and receptors are found in disparate sites in humans, with the CRF gene on chromosome 8q13.1, (containing 2 exons, spanning 2 kb of genomic DNA), and Urocortin 1 (chromosome 2p23.3, 2 exons, 1 kb), Urocortin 2 (chromosome 3p21.3, 2 exons, 2 kb), Urocortin 3 (chromosome 10p15.1, 2 exons, 9 kb), CRF1 (chromosome 17q21.31, 13–14 exons, 20–51 kb) and CRF2 receptors (chromosome 7p15.1, 12–15 exons, 29 kb; 3 different promoter sites/3 different first exons to encode the CRF2(a) CRF2(b) and CRF2(c) isoforms) and CRF binding protein (CRFBP, chromosome 5q13.3, 7exons, 17 kb) distinctively separated (Binder and Nemeroff, 2010). Linkage studies have suggested an association between the 8q region in or distal to the CRF gene and the 3p region containing Urocortin 2 gene with bipolar disorder as well as combined anxiety with the early onset of major depressive symptoms (Camp et al., 2005; Kelsoe et al., 2001; Liu et al., 2003; Marcheco-Teruel et al., 2006; McQueen et al., 2005; Segurado et al., 2003). A rare SNP (rs503875) for the CRF gene appears to interact significantly with a functional SNP (ThtIII1) in the glucocorticoid receptor (GR) gene (Rosmond et al., 2001). Behavioral inhibition in children of parents with anxiety disorders is associated with a repeat polymorphism and three SNPs near the CRF gene (Gu et al., 1993; Smoller et al., 2003, 2005). In addition, SNPs for CRF1 and vasopressinergic V1B receptors were associated with panic disorder (Keck et al., 2008). Three CRF1 SNPs (rs1876828, rs242939 and rs242941) are over-represented in patients with major depression (Liu et al., 2006) or have been associated (rs4792887) with stress, depression, and attempted suicide (Wasserman et al., 2008). Associations between response to antidepressant treatment (fluoxetine, desiprimine, or citalopram) and three SNPs, a promoter SNP, or an intronic SNP (rs110402) within the CRF1 gene have been reported (Licinio et al., 2004; Liu et al., 2006; Papiol et al., 2007). Additionally, CRF1 SNPs interacting with the serotonin transporter (5-HTT) SNPs and child abuse, also predict adult depression (Ressler et al., 2010). Finally, treatment of affective disorders with citalopram was positively associated with CRF2 intronic SNP (Papiol et al., 2007).
Taken together, there is substantial evidence to suggest that neural and endocrine CRF-related gene expression and signaling influence the potential risks and outcomes of major depression and comorbid psychological disorders.
6. The role of CRF in mediating an individual’s risk to social stress-induced psychopathology (chapter contributed by S.K. Wood, PhD)
As noted earlier, stressors of a social nature are the most common type of stress in people that precipitate psychopathologies such as depression and anxiety (Bjorkqvist, 2001). An animal model of social stress useful for studying stress-induced psychopathology is the resident-intruder paradigm. This model is characterized by threats and attacks by a large aggressive male rat (resident) toward a smaller male rat (intruder) (Miczek, 1979). Exposure to this stressor induces a robust neuroendocrine and cardiovascular activation as well as hyperthermia (Bhatnagar et al., 2006; Buwalda et al., 1999; Sgoifo et al., 1999; Tornatzky and Miczek, 1993; Wood et al., 2010). Subsequently, intruder rats exhibit increased behavioral despair, anhedonia, decreased social interactions, and long lasting changes in HPA axis function (Becker et al., 2008; Bhatnagar et al., 2006; Buwalda et al., 1999; Rygula et al., 2005; Sgoifo et al., 2002). As such, social stress exposure produces a depressive-like phenotype in rats that corresponds well with clinical manifestations of affective disorders (American Psychiatric Association, 2013).
Rodents exposed to social stress also vary greatly in their behavioral response, exhibiting either passive behaviors (supine postures) or proactive behaviors characterized by greater territorial control, upright postures and counter attacks (Meerlo et al., 1999; Walker et al., 2009; Wood et al., 2010). These diverse coping strategies are related to divergent physiological responses to stress, as passive behaviors are characterized by higher HPA axis reactivity and active behaviors are associated with enhanced sympathetic activity (Koolhaas et al., 1999). Importantly, rats demonstrating passive behavioral strategies also exhibit heightened vulnerability to stress-related consequences, similar to that observed in humans (Billings and Moos, 1984; Folkman and Lazarus, 1980). For example, passive responses during social stress are related to greater impairment of diurnal rhythms, HPA dysfunction, and behavioral despair while active coping confers resistance to developing many stress-related consequences (Meerlo et al., 1999; Wood et al., 2010). In fact, a study evaluating the effect of repeated intermittent exposures to social stress revealed evidence of sustained HPA activation in passive coping rats, but not in those demonstrating active responses. Furthermore, behavioral despair and anhedonia were evident only in the passive coping rats following social stress (Wood et al., 2010, 2015). These studies mimic findings in melancholic depressed patients indicating that HPA dysregulation co-occurs with depressive behaviors (Swaab et al., 2005).
A growing body of evidence suggests that the stress-sensitive neuropeptide CRF is a critical factor mediating one’s risk of developing stress-induced psychopathology. Initially recognized as the neurohormone that initiates the HPA axis (Vale et al., 1981), CRF in extrahypothalamic regions contributes to anxiety and depressive-like behaviors in animal models (Dunn and Swiergiel, 2008). Overproduction of CRF as evidenced by increased CRF expression in PVN neurons, increased CRF-immunoreactivity in the noradrenergic nucleus locus ceruleus and the 5-HT containing dorsal raphé nucleus (DR) as well as increased CRF levels in cerebrospinal fluid has also linked CRF with depressive disorders in humans (Austin et al., 2003; Bissette et al., 2003; Merali et al., 2004, 2006). The intersection between CRF and monoamines provides a mechanism by which CRF can have a major impact on one’s risk of depression. One monoaminergic target of CRF implicated in depressive disorders is the DR-5-HT system (Valentino and Commons, 2005). Within the DR, CRF has opposing effects on 5-HT neuronal activity dependent upon its actions at CRF1 or CRF2. CRF1 activation in the DR enhances gamma-aminobutyric acid (GABA)ergic inhibition of DR-5-HT neurons and CRF2 activation excites DR-5-HT neurons thereby increasing extracellular 5-HT in DR targets (Kirby et al., 2000, 2008; Price et al., 1998; Roche et al., 2003). In naïve rats, CRF1 is localized predominantly on the plasma membrane, while CRF2 is located within the cytoplasm (Waselus et al., 2009). Repeated intermittent exposure to social stress in the more stress resistant, active coping rats results in a redistribution of these receptors such that CRF2 is recruited to the plasma membrane and CRF1 is internalized. This effect is absent in rats displaying a passive response to social stress (Wood et al., 2013b). Although differences in protein expression of B-arrestin2 or splice variants of the CRF2 receptor could account for differential CRF receptor trafficking, significant differences in protein expression of these molecules within the DR were not evident 24 h after social defeat (Wood et al., 2013b). Consistent with the findings of internalized CRF1 receptors in the active coping phenotype, daily administration of a CRF1 antagonist shifts the behavioral response to a proactive strategy and prevents the development of behavioral despair in the forced swim test and adrenal hypertrophy (Wood et al., 2012). In addition to the convincing role that CRF plays in depressive symptomatology, CRF is also poised to contribute to the pathogenesis of stress-related medical disorders that are comorbid with affective disorders, including urinary disorders and cardiovascular disease. Social stress-induced overexpression of CRF within Barrington’s nucleus, the pontine micturition center of the brain, has been linked to urodynamic dysfunction (Valentino et al., 2011). These physiological manifestations of stress were blocked by either CRF1 antagonism or shRNA knockdown of CRF in Barrington’s nucleus (Wood et al., 2013a). In addition, social stress has been linked to cardiovascular dysfunction such as cardiac hypertrophy and reduced heart rate variability. Importantly, CRF1 antagonist treatment during social stress mitigates both the effects of social stress on depressive-like behaviors and the cardiovascular system (Wood et al., 2012). Taken together results of these studies underscore a critical CRF-related mechanism that is capable of mediating the individual differences in vulnerability to social stress.
7. Sex differences in corticotropin releasing factor receptors (chapter contributed by D.A. Bangasser, PhD)
Women are twice as likely to suffer from depression as men (Kessler, 2003; Kessler et al., 1993). Given the link between CRF and depression, it is possible that sex differences in CRF sensitivity could contribute to this sex bias in depression rates. Specifically, there is mounting preclinical evidence for sex differences in CRF receptors. For example, sex differences in CRF receptor binding have been identified in nearly every brain region examined (Weathington et al., 2014). CRF1 receptor binding is greater in adult female than male rats in regions implicated in depression, including the cingulate cortex, accumbens, and amygdala (Weathington et al., 2014). In contrast, males have greater CRF2 receptor binding than females in many regions, such as the BNST (Lim et al., 2005; Weathington et al., 2014). The functional relevance of these sex differences remains unknown. However, as noted, stress sensitivity is thought to be increased via CRF1 receptor activation, but decreased via CRF2 receptor activation (Bale and Vale, 2004). While this statement is likely an oversimplification of CRF receptor effects on behavior universally, if it holds true for the aforementioned brain regions (cingulate cortex, accumbens, amygdala), then higher CRF1 but lower CRF2 receptor binding observed in these regions in females could render them more sensitive to stress.
Sex differences in CRF1 receptor coupling and signaling also have been identified. As stated earlier in this review, the CRF1 receptor is a G protein-coupled receptor that preferentially binds the Gs protein to activate the cAMP-PKA signaling cascade (Grammatopoulos et al., 2001; Hillhouse and Grammatopoulos, 2006). Female rats have greater cortical CRF1 receptor coupling to the Gs protein than males (Bangasser et al., 2010). This suggests that when activated, female CRF1 receptors would initiate greater cAMP-PKA signaling than male CRF1 receptors. In fact LC neurons of female rats are more sensitive to CRF than those of males, and this increased sensitivity is linked to greater activation of the cAMP-PKA signaling cascade in females (Bangasser et al., 2010; Curtis et al., 2006). Thus, sex differences at the level of receptor signaling can translate into sex differences in stress physiology. In turn, these physiological sex differences could result in sex differences in stress-induced arousal, which is mediated by the effects of CRF in the LC. Typically, activation of the LC during a stressful event is thought to be adaptive, as it promotes appropriate cognitive and behavioral coping responses (Snyder et al., 2012; Valentino and Van Bockstaele, 2005). However, inappropriate activation of this system by benign stimuli could be disruptive. Because sex differences in the CRF1 receptor render female LC neurons more sensitive to CRF, females are more likely to have these inappropriate arousal responses than males.
CRF1 receptors are also differentially affected by stressor exposure or excessive CRF release in males compared to females. In vitro, CRF1 receptors become desensitized and internalized (i.e., trafficked from the membrane to the cytosol) in response to saturating concentrations of CRF (Hauger et al., 2000; Teli et al., 2005). This internalization is considered a cellular adaptation to high levels of CRF release because the internalized receptors cannot be activated. CRF1 receptor internalization also occurs in vivo in males. Both local CRF administration and forced swim stress result in the internalization of CRF1 receptors in LC dendrites in male rats (Bangasser et al., 2010; Reyes et al., 2006, 2008). Additionally, CRF1 receptor internalization in LC dendrites is observed in male CRF overexpressing mice (Bangasser et al., 2013). In contrast, CRF1 receptor internalization fails to occur in stressed female rats and female mice that overexpress CRF (Bangasser et al., 2010, 2013). Rather, these females have more CRF1 receptors in the plasma membrane than their unstressed or wild type same sex counterparts. This suggests that females lack the compensatory response of internalization, which could make their LC neurons more sensitive to conditions of CRF hypersecretion. In support of this, LC neurons of female CRF overexpressing mice fire roughly 3× faster than neurons of wild type mice (Bangasser et al., 2013). However, LC neurons of male CRF overexpressing mice maintain their firing rate at wild-type levels, despite excessive CRF release in their LC (Bangasser et al., 2013). The CRF1 receptor internalization observed in male, but not female CRF overexpressing mice can explain these physiological sex differences.
Sex differences in CRF1 receptor internalization can be attributed to sex differences in binding of β-arrestin, the protein that initiates CRF1 receptor internalization (Hauger et al., 2009; Holmes et al., 2006; Oakley et al., 2007). In cortical tissue, stressor exposure induced β-arrestin binding to the CRF1 receptor in male but not female rats, an effect that may explain why CRF1 receptors of females do not internalize following excessive CRF release (Bangasser et al., 2010). In addition to its role in initiating internalization, β-arrestin also activates its own suite of signaling cascades that are often distinct from signaling pathways activated by G proteins (DeWire et al., 2007; Lefkowitz and Shenoy, 2005). This has led to the proposal that the signaling of the CRF1 receptor is sex biased, such that it signals more through β-arrestin mediated pathways in males and Gs-mediated pathways in females (Bangasser and Valentino, 2012; Valentino et al., 2013a,b). Sex biased CRF1 receptor signaling may be an important, yet underexplored, mechanism by which sex differences in CRF responses are established.
Overactivation of the LC-NE system can lead to symptoms of hyperarousal, including agitation, restlessness, and sleep disturbances (Gold and Chrousos, 1999, 2002). Sex differences in CRF1 receptors render LC neurons of females more sensitive to CRF and less adaptable to conditions of CRF hypersecretion, effects that could translate into increased hyperarousal in females during stressful conditions. If these sex differences occur in humans, they may explain the sex bias in the rates of depression, as well as the prevalence of certain hyperarousal symptoms in depressed women (Nolen-Hoeksema et al., 1999; Plante et al., 2012). Additionally, sex differences in CRF receptor function may affect the treatment of depression. CRF1 receptor antagonists that are being designed to treat depression may work differently in males and females due to sex differences in CRF1 receptors. Indeed, administration to the dorsal raphé of a small molecule CRF1 receptor antagonist reduced stress-related behavior in male but not female mice (Howerton et al., 2014). Importantly though, by comparing CRF receptors in males and females, novel therapeutic targets can be identified (Bangasser, 2013). For example, a drug that biases the CRF1 receptor toward β-arrestin binding, thereby promoting internalization, may be an effective treatment for depression, especially in women. Together these studies underscore the need to consider sex differences when trying to understand the etiology of depression and develop effective treatments for the disorder.
8. Dissecting CRH-controlled neurocircuitries of stress and anxiety – (chapter contributed by J.M. Deussing, PhD)
While the role of CRF as an indispensable initiator of the neuroendocrine cascade of the HPA axis is well defined, we are just starting to comprehend the role of extrahypophysiotropic CRF with regards to emotionality and behavioral responses to stress. This is somewhat surprising considering that already soon after its discovery (Vale et al., 1981), numerous groups demonstrated that intracerebroventricular (i.c.v.) application of CRF in rodents can elicit a broad spectrum of physiological and behavioral reactions sharing typical characteristics with stress (reviewed in: Dunn and Berridge, 1990a). Independent of its effect on the HPA axis, exogenous CRF acts within the central nervous system to increase sympathoadrenal outflow, which in turn activates cardiovascular function, increases plasma glucose levels and oxygen consumption – typical signs of the fight-or-flight response (Brown et al., 1982; Dunn and Berridge, 1987; Fisher et al., 1982). In agreement with the established positive relationships of CRF with multiple modes of stress related behavior (Berridge and Dunn, 1986; Swerdlow et al., 1986; Britton et al., 1982, 1986a; Koob et al., 1993; Sutton et al., 1982), CRF antagonists are capable of attenuating or reversing the effects of various stressors, strongly suggesting that CRF itself is a mediator of these responses (Berridge and Dunn, 1987; Britton et al., 1986b; Dunn and Berridge, 1990a). Taken together, these findings indicate that the extrahypophysiotropic CRF system on its own may be both necessary and sufficient to execute stress responses (Dunn and Berridge, 1990b; Heinrichs et al., 1995).
In the previous two decades, the CRF system has extensively been studied using gain- and loss-of-function mouse models. Numerous models of CRF overexpression have been developed to further refine previous experiments of i.c.v. CRF application and to mimic CRF hyperactivity observed in depressed patients (Nemeroff et al., 1984). These mouse lines have proven extremely valuable given their ability to reflect different degrees of the activating, arousing and anxiogenic-like properties of CRF (Dedic et al., 2012; Dirks et al., 2002; Kolber et al., 2010; Lu et al., 2008; Refojo et al., 2011; Stenzel-Poore et al., 1994; Vicentini et al., 2009). However, a direct comparison of different CRF overexpressing mouse models reveals substantial discrepancies and inconsistencies, which are largely related to differences in the design of these animal models, including the expression level and the spatio-temporal properties of the utilized promoters driving CRF overexpression (reviewed in: Laryea et al., 2012). Another confounding factor appearing in several CRF overexpressing mouse lines is the hypercorticosteronism accompanied by a Cushing-like phenotype. In recent years, approaches applying viral expression systems (lenti- and adeno-associated viruses) have been used that provide means to overexpress CRF in a spatially more restricted manner allowing for more refined analyses of CRF actions in different brain nuclei (Flandreau et al., 2012; McFadden et al., 2012; Qi et al., 2014; Regev et al., 2011, 2012). Nevertheless, all approaches of CRF excess published until now are confounded by a substantial amount of ectopic expression. Although it can be assumed that even ectopic CRF mediates its biological effects only via its cognate receptors, it remains unclear which receptor is involved, and where the overexpressed CRF is released, i.e. locally or in a far distance to the site of overexpression.
In contrast, targeted inactivation of the CRF gene in the mouse enabled the direct assessment of in vivo functions of CRF with respect to HPA axis activity, emotionality and behavioral stress responses. As expected, constitutive CRF knockout mice display a blunted HPA axis (Muglia et al., 1995). Surprisingly, these animals do not show any alterations in anxiety-related behavior under basal conditions or in response to stress. Moreover, different selective CRF1 antagonists are still capable of attenuating stress-induced behaviors in constitutive CRF knockout mice, suggesting that stress-induced behaviors require CRF1 but not CRF (Weninger et al., 1999).
The effects observed in CRF knockout mice are even more surprising in the light of CRF1 mutant mice, which show distinct behavioral phenotypes. Constitutive CRF1 knockout mice fully phenocopy the HPA axis phenotype of CRF knockout mice, including a chronic corticosterone deficit and a blunted stress response. In addition, the two independently generated CRF1-deficient mouse lines show a clear decrease in anxiety-related behaviors in various test paradigms compared to wild-type littermates (Smith et al., 1998; Timpl et al., 1998). CRF1 is a prime example of the power of mouse genetic tools, which enable the dissection of gene function in a very refined manner. In the first instance, conditional mutagenesis was used to specifically delete CRF1 in the forebrain, including limbic structures. Thereby, it could be demonstrated that CRF1 modulates anxiety-related behavior in a completely HPA axis independent manner (Muller et al., 2003). These mutants further demonstrate that the absence of CRF1 can protect from aversive effects of chronic stress during early life or adulthood (Wang et al., 2011a,b, 2012). The fact that CRF1 is expressed throughout the brain in different types of neurons suggested that the receptor might have specific functions depending on the cellular context (Refojo et al., 2011). Consequently, the function of CRF1 was probed in different neurotransmitter circuits using respective Cre driver lines that provided selectivity for glutamatergic, GABAergic, dopaminergic and serotonergic neurons. This approach revealed a previously unknown bidirectional control of anxiety-related behavior via CRF1, suggesting that an imbalance of CRF1-controlled anxiogenic glutamatergic and anxiolytic dopaminergic circuits might be involved in the manifestation of stress-related disorders (Refojo et al., 2011). Along these lines, Lemos and colleagues could demonstrate that stress can switch the action of CRF from appetitive to aversive, involving dopamine release within the nucleus accumbens (Lemos et al., 2012).
These recent findings indicate a previously unexpected complexity of the CRF system, which is further complicated by the existence of a second receptor, a binding protein and soluble variants of CRF2 that are regulating the bioavailability of CRF. The discrepancy between CRF and CRF1 mouse mutants with respect to behavioral outcomes might originate from different causes: (1) The early deletion of CRF might trigger compensatory processes; (2) the corticosterone deficiency might mask potential phenotypes; (3) Urocortin 1 as the single other ligand activating CRF1 might compensate for the loss of CRF; (4) CRF might exert its action primarily under conditions of chronic or severe stress; (5) the CRF1 receptor might comprise ligand-independent activity, e.g. due to constitutive activity or heteromerization with other receptors.
To better understand the role of CRF in emotionality and stress responses it will be of major importance to gain insights at the neurocircuit level. It will be crucial to understand under which circumstances CRF is released and where it will be active. Earliest hints came from i.c.v. application studies (Bittencourt and Sawchenko, 2000) but again, these have not shed light on the endogenous CRF system, which might involve local as well as distant release. CRF is a neuropeptide and thus stored within dense core vesicles but it is fully unclear which physiological stimuli trigger the release within the extrahypophysiotropic CRF system in vivo and whether CRF is synaptically released or rather in a mode of volume transmission (van den Pol, 2012). Moreover, it will be of major importance to unravel the identity of CRF expressing neurons in stress-involved brain structures in greater detail. In this regard, it will be mandatory to develop better tools, such as conditional CRF mice as well as Cre drivers that provide genetic access to subpopulations of CRF and Urocortin 1 neurons (Taniguchi et al., 2011).
9. Clinical biomarkers for central CRF overexpression –identifying the right patient for CRF1 antagonistic treatment (chapter contributed by M. Ising, PhD and F. Holsboer, MD, PhD)
Depression is a typical example of a stress-related disorder of the brain. Severe early life stress increases the risk of adult depression, certain stressors trigger the incidence of a depressive episode, and features of acute depression resemble symptoms of chronic stress (Hammen, 2005; Paykel, 2003). A large number of clinical findings including post-mortem studies in suicide victims and results from cerebrospinal fluid studies in acutely depressed patients point to a pathological overactivity of CRF in major depression. Neuroendocrine challenge tests like the combined dexamethasone (dex)/CRF test conducted in acute depression indicate a profound dysregulation of the stress response in the majority of patients (Holsboer and Ising, 2010). These disturbances typically disappear under successful antidepressant medication (Ising et al., 2005). Animal studies of anxiety and chronic stress also point to a prominent role of CRF, which mainly exerts its stress effects mainly via CRF1 receptors. For instance, CRF overexpressing mice show features of hyperarousal and anxiety, while specific deletion of forebrain CRF1 led to a marked reduction of anxiety-like behavior (Muller et al., 2003).
A number of small-molecule non-peptide antagonists with high affinity for CRF1 have been developed for the treatment of depression and anxiety disorders. While these compounds displayed substantial anxiolytic effects in diverse animal models of CRF overactivity and chronic stress, controlled clinical trials in patients with major depression or anxiety disorders were mostly negative. As a result, no CRF1 antagonist has yet been approved for clinical use in depression or anxiety disorders (Griebel and Holsboer, 2012).
However, it is important to note that not all acutely depressed patients show symptoms of pathological CRF overactivity in terms of an impaired stress response regulation. Accordingly, specific CRF1 antagonistic treatments are likely to be effective in only those patients characterized by such a CRF-related pathology. Nevertheless, all clinical trials with CRF1 antagonists have so far been conducted in unselected patient samples, which might explain the disappointing results (Griebel and Holsboer, 2012). Validated biomarkers for identifying patients with central CRF-related depression pathology are currently not available; however, there are promising approaches for the development of such biomarkers.
Overexpression of hypothalamic neuropeptides including CRF seems to be a major factor contributing to an impaired stress response regulation in many patients suffering from depression, and sensitive laboratory tests to identify these impairments like the combined dex/CRF test are readily available. However, in addition to CRF, other neuropeptides like arginine-vasopressin and an altered sensitivity of corticosteroid receptors contribute to a disturbed stress response regulation (Holsboer and Ising, 2010), which limits the CRF specificity of such laboratory tests.
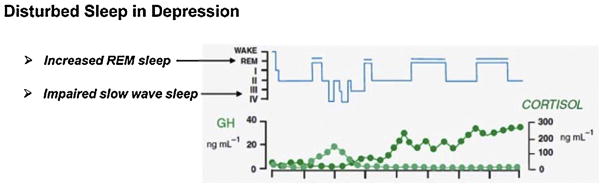
Another core feature of depression physiology is disinhibited rapid eye movement (REM) sleep (Fig. 2), which specifically associates with central CRF activity (Steiger, 2003). Accordingly, elevated REM sleep should indicate increased central CRF activity (concomitant with decreased levels of the slow sleep wave-promoting hormone, growth hormone releasing hormone [GHRH]), and thus characterize patients with CRF-related depression pathology.
Fig. 2.
Disturbed sleep in depression is characterized by impaired slow-wave sleep and increased REM sleep resulting from an imbalance between GHRH and CRF (after Steiger, 2003).
In fact, conditional mice mutants with forebrain CRF overexpression display increased REM sleep, which reverts to normal sleep under CRF1 antagonistic (DMP696) treatment (Kimura et al., 2010). These REM suppressive effects of CRF1 antagonists could be replicated in further animal models (Ahnaou et al., 2012), and with diverse CRF1 antagonists (Kimura and Romanowski, personal communication).
One single clinical trial evaluating safety and tolerability of the CRF1 antagonist R121919 included recordings of sleep EEG before and after 28 days of treatment (Held et al., 2004). A recent re-analysis of the EEG data revealed that baseline REM density indicating impaired REM sleep predicted treatment outcome with the CRF1 antagonist (Holsboer and Ising, 2010). The majority of patients with substantial REM sleep abnormality (Fig. 3, shaded area) showed between 50 and 90% improvement of depression symptoms, while improvement in patients without REM sleep abnormality was consistently below 50%.
Fig. 3.
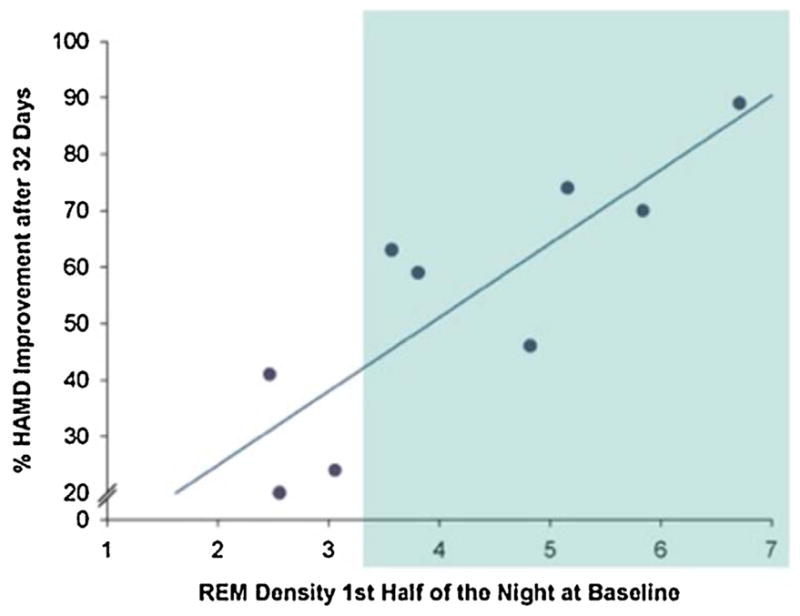
Elevated REM density (shaded area) indicating central CRF over-activity in depression potentially characterizes the appropriate target population for successful CRF1 antagonistic treatment in depression and anxiety disorders. [HAMD: Hamilton rating scale for Depression] (after Holsboer and Ising, 2010).
Neuropeptide receptor ligands like CRF1 antagonists are highly selective treatments that require the availability of appropriate biomarkers to identify the right patients who would optimally benefit from such specific intervention. This benefit of targeting subpopulations of individuals that suffer from MDD has been previously demonstrated (Mehta et al., 2013). The summarized findings suggest that besides laboratory tests of stress response regulation, REM sleep disinhibition is a promising candidate for such a biomarker to reflect central CRF overexpression, and thus, to identify the appropriate target population for successful CRF1 antagonistic treatment in depression and anxiety disorders.
10. An integrated view of CRF and depression
Depression is a stress-induced psychopathology and disorder of the brain. It is especially well realized by social stressors, the most common type of stress in humans, and the most intensely stressful (Koolhaas et al., 1997). Women are twice as likely to suffer from depression as men, and animal models of depression reveal similar sex-related physiological and behavioral differences. Social stress in animal models corresponds well with clinical manifestations of affective disorders. As in humans, animal models describe significant variability of stress responsiveness in populations, which include more passive and proactive individual proclivities. Passive coping is associated with increased susceptibility to the consequences of stress and depression in animals and in humans. Clinically, major depression is characterized by a pathological over activity of CRF systems. In addition to CRF, altered arginine vasopressin and glucocorticoid receptor sensitivity contribute to disturbed stress responses in depressed individuals. Neuroendocrine challenges (dexamethasone/CRF) indicate a profound dysregulation of stress responsiveness in the majority of depressed patients (Holsboer and Ising, 2010; Ising et al., 2005). Typically, this lack of negative feedback sensitivity to challenge or stress-induced elevated hormonal concentrations disappears under successful antidepressant treatment.
Therefore, dysregulation of HPA function co-occurs with depression, and with this extrahypothalamic CRF appears to drive anxiety and depressive behavior. What is more, CRF1 receptors modulate anxious behaviors independently of the HPA axis. Specific deletion of CRF1 receptors results in a marked reduction of anxious behavior. Absence of CRF1 receptors protects individuals form aversive effects of chronic stress during early life or adulthood. It is important to note that CRF1 receptors have specific functions depending on cellular context. Recent work has suggested bidirectional control of anxious behavior via CRF1 receptor activities.
Repeated stress stimulates CRF activity, and modifies the functionality of the CRF system distinctively for males and females through unique CRF1 and CRF2 receptor cell physiology. Repeated stress results in CRF1 receptor internalization, while CRF2 receptors are recruited to the membrane. Internalization of CRF1 receptors is absent in individuals coping with social stress by means of passive responses. This is particularly important for the demographics of depression, as CRF1 receptor responses are sex biased; CRF1 binding and coupling to Gs proteins is greater in females than males, in regions implicated in depression. In CRF-overexpressing mice, CRF1 receptors in females fail to bind β-arrestin and become internalized. In contrast there is greater CRF2 receptor binding in males in related brain regions such as the BNST. Daily CRF1 antagonist treatment shifts behavioral responses to proactive coping and also prevents despair in animals. What is more, daily CRF1 antagonism mitigates urinary and cardiovascular dysfunctions associated with depression.
The evidence suggests that CRF1 controlled glutamatergic (anxiogenic), noradrenergic (anxiogenic) and dopaminergic (anxiolytic) circuits are involved in the manifestation of stress-related disorders such as depression. In this type of bi-directionally controlled system, stress can switch the action of CRF from appetitive to aversive involving DA in the nucleus accumbens. Similarly, individuals lacking the compensatory response of CRF1-β-arrestin cellular internalization (as observed in females) exhibit more rapid firing of the LC. In this light it is important to remember that CRF1 receptors have specific functions depending on cellular context, and that not all acutely depressed patients show symptoms of CRF over activity. Individual and sex related differences in overexpression of CRF, receptor binding, and cellular response may be reasons that in controlled clinical trials for small-molecule non-peptide CRF1 antagonists were ineffective for some patients. It is reasonable to think that specific CRF1 antagonists will only be effective in patients with a CRF over expression pathology. An essential feature of depression physiology in these patients is disinhibited REM sleep, which specifically interacts with CRF activity. The majority of patients with REM irregularities exhibit reduced symptoms of depression after CRF1 antagonist treatment (Holsboer and Ising, 2010). As differences in CRF1 function in women may lead to increased depressive symptoms, treatments for both women and men may also require customization to address sex responsiveness criteria.
Footnotes
Conflict of interest
Parts of the presented findings in Section 9 have been supported by Neurocrine Biosciences Inc., San Diego, CA, USA, the Janssen Research Foundation, Beerse, Belgium, and by NeuroNova gGmbH, Munich, Germany. Otherwise, no conflicts exist.
Contributor Information
R. Parrish Waters, Email: rwaters@umw.edu.
Marion Rivalan, Email: marion.rivalan@charite.de.
Cliff H. Summers, Email: cliff@usd.edu.
References
- Aguilera G, Millan MA, Hauger RL, Catt KJ. Corticotropin-releasing factor receptors: distribution and regulation in brain, pituitary, and peripheral tissues. Ann N Y Acad Sci. 1987;512:48–66. doi: 10.1111/j.1749-6632.1987.tb24950.x. [DOI] [PubMed] [Google Scholar]
- Ahnaou A, Steckler T, Heylen A, Kennis L, Nakazato A, Chaki S, Drinkenburg WH. R278995/cra0450, a corticotropin-releasing factor (CRF(1)) receptor antagonist modulates REM sleep measures in rats: implication for therapeutic indication. Eur J Pharmacol. 2012;680:63–68. doi: 10.1016/j.ejphar.2012.01.023. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. (5) 2013 http://dx.doi.org/10.1176/appi.books.9780890425596.744053.
- Anacker C. Adult hippocampal neurogenesis in depression: behavioral implications and regulation by the stress system. Curr Top Behav Neurosci. 2014 doi: 10.1007/7854_2014_275. [DOI] [PubMed] [Google Scholar]
- Arendt DH, Hassell J, Li H, Achua JK, Guarnieri DJ, Dileone RJ, Ronan PJ, Summers CH. Anxiolytic function of the orexin 2/hypocretin a receptor in the basolateral amygdala. Psychoneuroendocrinology. 2014;40:17–26. doi: 10.1016/j.psyneuen.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8:324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- Bale T, Vale W. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci. 2003;23:5295–5301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA. Sex differences in stress-related receptors: “micro” differences with “macro” implications for mood and anxiety disorders. Biol Sex Differ. 2013;4:2. doi: 10.1186/2042-6410-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(877):896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2013;18:166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banki CM, Bissette G, Arato M, O’Connor L, Nemeroff CB. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987;144:873–877. doi: 10.1176/ajp.144.7.873. [DOI] [PubMed] [Google Scholar]
- Banki CM, Karmacsi L, Bissette G, Nemeroff CB. CSF corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. Eur Neuropsychopharmacol. 1992;2:107–113. doi: 10.1016/0924-977x(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry. 2008;13:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of crf and related peptides. Front Neuroendocrinol. 1995a;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Behan DP, Maciejewski D, Chalmers D, De Souza EB. Corticotropin releasing factor binding protein (CRF-BP) is expressed in neuronal and astrocytic cells. Brain Res. 1995b;698:259–264. doi: 10.1016/0006-8993(95)01014-m. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Dunn AJ. Corticotropin-releasing factor elicits naloxone sensitive stress-like alterations in exploratory behavior in mice. Regul Pept. 1986;16:83–93. doi: 10.1016/0167-0115(86)90196-5. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Dunn AJ. A corticotropin-releasing factor antagonist reverses the stress-induced changes of exploratory behavior in mice. Horm Behav. 1987;21:393–401. doi: 10.1016/0018-506x(87)90023-7. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic–pituitary–adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Billings AG, Moos RH. Coping, stress, and social resources among adults with unipolar depression. J Pers Soc Psychol. 1984;46:877–891. doi: 10.1037//0022-3514.46.4.877. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28:1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors? An analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Boorse GC, Denver RJ. Widespread tissue distribution and diverse functions of corticotropin-releasing factor and related peptides. Gen Comp Endocrinol. 2006;146:9–18. doi: 10.1016/j.ygcen.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Britton KT, Lee G, Dana R, Risch SC, Koob GF. Activating and ‘anxiogenic’ effects of corticotropin releasing factor are not inhibited by blockade of the pituitary–adrenal system with dexamethasone. Life Sci. 1986a;39:1281–1286. doi: 10.1016/0024-3205(86)90189-x. [DOI] [PubMed] [Google Scholar]
- Britton KT, Lee G, Vale W, Rivier J, Koob GF. Corticotropin releasing factor (CRF) receptor antagonist blocks activating and ‘anxiogenic’ actions of CRF in the rat. Brain Res. 1986b;369:303–306. doi: 10.1016/0006-8993(86)90539-1. [DOI] [PubMed] [Google Scholar]
- Brown MR, Fisher LA, Spiess J, Rivier C, Rivier J, Vale W. Corticotropin-releasing factor: actions on the sympathetic nervous system and metabolism. Endocrinology. 1982;111:928–931. doi: 10.1210/endo-111-3-928. [DOI] [PubMed] [Google Scholar]
- Buwalda B, de Boer SF, Schmidt ED, Felszeghy K, Nyakas C, Sgoifo A, Van der Vegt BJ, Tilders FJ, Bohus B, Koolhaas JM. Long-lasting deficient dexamethasone suppression of hypothalamic–pituitary–adrenocortical activation following peripheral CRF challenge in socially defeated rats. J Neuroendocrinol. 1999;11:513–520. doi: 10.1046/j.1365-2826.1999.00350.x. [DOI] [PubMed] [Google Scholar]
- Camp NJ, Lowry MR, Richards RL, Plenk AM, Carter C, Hensel CH, Abkevich V, Skolnick MH, Shattuck D, Rowe KG, Hughes DC, Cannon-Albright LA. Genome-wide linkage analyses of extended utah pedigrees identifies loci that influence recurrent, early-onset major depression and anxiety disorders. Am J Med Genet B: Neuropsychiatr Genet. 2005;135B:85–93. doi: 10.1002/ajmg.b.30177. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-htt gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Catt KJ, Millan MA, Wynn PC, Mendelsohn FA, Aguilera G. Brain receptors for hypothalamic hormones. Adv Biochem Psychopharmacol. 1987;43:51–67. [PubMed] [Google Scholar]
- Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, Vinken P, Ashton D, Langlois X, Steckler T. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, r278995/cra0450. Eur J Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Zorrilla E, Smith S, Rousso D, Levy C, Vaughan J, Donaldson C, Roberts A, Lee KF, Vale W. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus–pituitary–adrenal axis and depressive-like behavior. J Neurosci. 2006;26:5500–5510. doi: 10.1523/JNEUROSCI.3955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Furay A, Evanson N, Ostrander M, Ulrich-Lai Y, Herman J. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic–pituitary–adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic–pituitary–adrenocortical axis responses to acute and chronic stress. Endocrinology. 2008;149:818–826. doi: 10.1210/en.2007-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Anderson W, Dhansay MA, Phillips A, Shurin S, Walport M, Ewart W, Savill SJ, Bordin IA, Costello EJ, Durkin M, Fairburn C, Glass RI, Hall W, Huang Y, Hyman SE, Jamison K, Kaaya S, Kapur S, Kleinman A, Ogunniyi A, Otero-Ojeda A, Poo MM, Ravindranath V, Sahakian BJ, Saxena S, Singer PA, Stein DJ Scientific Advisory B., the Executive Committee of the Grand Challenges on Global Mental H. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale W, Gold LH. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3:1355–1368. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Wille S, Braun S, Hauger RL. Grk3 regulation during CRF- and urocortin-induced CRF1 receptor desensitization. Biochem Biophys Res Commun. 2002;298:303–308. doi: 10.1016/s0006-291x(02)02463-4. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Gold PW, Geracioti TD, Jr, Listwak SJ, Kling MA. Association of fluoxetine treatment with reductions in csf concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry. 1993;150:656–657. doi: 10.1176/ajp.150.4.656. [DOI] [PubMed] [Google Scholar]
- Dedic N, Touma C, Romanowski CP, Schieven M, Kuhne C, Ableitner M, Lu A, Holsboer F, Wurst W, Kimura M, Deussing JM. Assessing behavioural effects of chronic hpa axis activation using conditional crh-overexpressing mice. Cell Mol Neurobiol. 2012;32:815–828. doi: 10.1007/s10571-011-9784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing JM, Breu J, Kühne C, Kallnik M, Bunck M, Glasl L, Yen YC, Schmidt MV, Zurmühlen R, Vogl AM, Gailus-Durner V, Fuchs H, Hölter SM, Wotjak CT, Landgraf R, de Angelis MH, Holsboer F, Wurst W. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J Neurosci. 2010;27:9103–9116. doi: 10.1523/JNEUROSCI.1049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Dirks A, Groenink L, Bouwknecht JA, Hijzen TH, Van Der Gugten J, Ronken E, Verbeek JS, Veening JG, Dederen PJ, Korosi A, Schoolderman LF, Roubos EW, Olivier B. Overexpression of corticotropin-releasing hormone in transgenic mice and chronic stress-like autonomic and physiological alterations. Eur J Neurosci. 2002;16:1751–1760. doi: 10.1046/j.1460-9568.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, Rivier JE, Vaughan JM, Vale WW. Cloning and characterization of human urocortin. Endocrinology. 1996;137:2167–2170. doi: 10.1210/endo.137.5.8612563. [DOI] [PubMed] [Google Scholar]
- Duman RS. Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depress Anxiety. 2014;31:291–296. doi: 10.1002/da.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A, Swiergiel A. Effects of acute and chronic stressors and CRF in rat and mouse tests for depression. Ann N Y Acad Sci. 2008;1148:118–126. doi: 10.1196/annals.1410.022. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Corticotropin-releasing factor administration elicits a stress-like activation of cerebral catecholaminergic systems. Pharmacol Biochem Behav. 1987;27:685–691. doi: 10.1016/0091-3057(87)90195-x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Is corticotropin-releasing factor a mediator of stress responses? Ann N Y Acad Sci. 1990a;579:183–191. doi: 10.1111/j.1749-6632.1990.tb48360.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990b;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the CRF gene in adult mice. Nat Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Fisher LA, Rivier J, Rivier C, Spiess J, Vale W, Brown MR. Corticotropin-releasing factor (CRF): central effects on mean arterial pressure and heart rate in rats. Endocrinology. 1982;110:2222–2224. doi: 10.1210/endo-110-6-2222. [DOI] [PubMed] [Google Scholar]
- Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces hpa axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37:27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S, Lazarus RS. An analysis of coping in a middle-aged community sample. J Health Soc Behav. 1980;21:219–239. [PubMed] [Google Scholar]
- Galard R, Catalan R, Castellanos JM, Gallart JM. Plasma corticotropin-releasing factor in depressed patients before and after the dexamethasone suppression test. Biol Psychiatry. 2002;51:463–468. doi: 10.1016/s0006-3223(01)01273-2. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low crh/ne states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Goldstein BL, Klein DN. A review of selected candidate endophenotypes for depression. Clin Psychol Rev. 2014;34:417–427. doi: 10.1016/j.cpr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos DK, Dai Y, Randeva HS, Levine MA, Karteris E, Easton AJ, Hillhouse EW. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol Endocrinol. 1999;13:2189–2202. doi: 10.1210/mend.13.12.0391. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple g proteins. J Neurochem. 2001;76:509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. 2012;11:462–478. doi: 10.1038/nrd3702. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-n-[(1s)-2-cyclopropyl-1-(3-fluoro-4-methylp henyl)ethyl]5-methyl-n-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (ssr 125543a), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Therap. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Gu J, Sadler L, Daiger S, Wells D, Wagner M. Dinucleotide repeat polymorphism at the crh gene. Hum Mol Genet. 1993;2:85. doi: 10.1093/hmg/2.1.85. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Ann Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. in or out? [DOI] [PubMed] [Google Scholar]
- Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Progr Neuropsychopharmacol Biol Psychiatry. 2013:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Theriault JE, Stewart JG, Bagby RM. Acute and chronic stress exposure predicts 1-year recurrence in adult outpatients with residual depression symptoms following response to treatment. Depress Anxiety. 2014;31:1–8. doi: 10.1002/da.22177. [DOI] [PubMed] [Google Scholar]
- Hartline KM, Owens MJ, Nemeroff CB. Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Ann N Y Acad Sci. 1996;780:96–105. doi: 10.1111/j.1749-6632.1996.tb15114.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Nishiyama M, Tanaka Y, Noguchi T, Asaba K, Hossein PN, Nishioka T, Makino S. Urocortins and corticotropin releasing factor type 2 receptors in the hypothalamus and the cardiovascular system. Peptides. 2004;25:1711–1721. doi: 10.1016/j.peptides.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International union of pharmacology. XXXVI Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Smith RD, Braun S, Dautzenberg FM, Catt KJ. Rapid agonist-induced phosphorylation of the human CRF receptor, type 1: a potential mechanism for homologous desensitization. Biochem Biophys Res Commun. 2000;268:572–576. doi: 10.1006/bbrc.2000.2183. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Merlo Pich E, Britton KT, Koob GF. The role of CRF in behavioral aspects of stress. Ann N Y Acad Sci. 1995:92–104. doi: 10.1111/j.1749-6632.1995.tb44673.x. [DOI] [PubMed] [Google Scholar]
- Heitland I, Groenink L, Bijlsma EY, Oosting RS, Baas JM. Human fear acquisition deficits in relation to genetic variants of the corticotropin releasing hormone receptor 1 and the serotonin transporter. PLOS ONE. 2013;8:e63772. doi: 10.1371/journal.pone.0063772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held K, Kunzel H, Ising M, Schmid DA, Zobel A, Murck H, Holsboer F, Steiger A. Treatment with the crh1-receptor-antagonist r121919 improves sleep-eeg in patients with depression. J Psychiatr Res. 2004;38:129–136. doi: 10.1016/s0022-3956(03)00076-1. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Roseboom PH, Kalin NH. Decreased amygdala CRF-binding protein mRNA in post-mortem tissue from male but not female bipolar and schizophrenic subjects. Neuropsychopharmacology. 2006;31:1822–1831. doi: 10.1038/sj.npp.1301038. [DOI] [PubMed] [Google Scholar]
- Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Nemeroff CB, Holsboer F. Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety. 1998;8:71–79. [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Wong ML, Licinio J, Park C, Young M, Gold PW, Chrousos GP, Bornstein SR. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001;6:540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM. Depression epidemiology and its treatment evolution. J Clin Psychiatry. 2012;73:e29. doi: 10.4088/JCP.11096tx3c. [DOI] [PubMed] [Google Scholar]
- Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and rab gtpases. J Neurochem. 2006;96:934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol. 2010;61:81–109. C101–111. doi: 10.1146/annurev.psych.093008.100321. [DOI] [PubMed] [Google Scholar]
- Hosang GM, Shiles C, Tansey KE, McGuffin P, Uher R. Interaction between stress and the bdnf val66met polymorphism in depression: a systematic review and meta-analysis. BMC Med. 2014;12:7. doi: 10.1186/1741-7015-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, Beck SG, Bale TL. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol Psychiatry. 2014;75:873–883. doi: 10.1016/j.biopsych.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- Huising MO, Flik G. The remarkable conservation of corticotropin-releasing hormone (crh)-binding protein in the honeybee (apis mellifera) dates the crh system to a common ancestor of insects and vertebrates. Endocrinology. 2005;146:2165–2170. doi: 10.1210/en.2004-1514. [DOI] [PubMed] [Google Scholar]
- Iniguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, Cruz B, Warren BL. Social defeat stress induces a depression-like phenotype in adolescent male c57bl/6 mice. Stress. 2014;17:247–255. doi: 10.3109/10253890.2014.910650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Kunzel HE, Binder EB, Nickel T, Modell S, Holsboer F. The combined dexamethasone/crh test as a potential surrogate marker in depression. Progr Neuro-psychopharmacol Biol Psychiatry. 2005;29:1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Ising M, Zimmermann US, Kunzel HE, Uhr M, Foster AC, Learned-Coughlin SM, Holsboer F, Grigoriadis DE. High-affinity CRF1 receptor antagonist nbi-34041: preclinical and clinical data suggest safety and efficacy in attenuating elevated stress response. Neuropsychopharmacology. 2007;32:1941–1949. doi: 10.1038/sj.npp.1301328. [DOI] [PubMed] [Google Scholar]
- Jha S, Dong B, Sakata K. Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Transl Psychiatry. 2011;1:e40. doi: 10.1038/tp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G, Han ZS. Coexistence of corticotropin releasing factor and neurotensin within oval nucleus neurons in the bed nuclei of the stria terminalis in the rat. Neurosci Lett. 1989;99(3):246–250. doi: 10.1016/0304-3940(89)90454-0. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. J Comp Neurol. 1989;280(4):587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Wood SK, Houshyar H, Hsin LW, Rice KC, Woods JH. The effects of CRF antagonists, antalarmin, cp154,526, lwh234, and r121919, in the forced swim test and on swim-induced increases in adrenocorticotropin in rats. Psychopharmacology (Berlin) 2005;180:215–223. doi: 10.1007/s00213-005-2164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama K, Bradbury MJ, Zhao L, Blount AL, Vale WW. Urocortin messenger ribonucleic acid: tissue distribution in the rat and regulation in thymus by lipopolysaccharide and glucocorticoids. Endocrinology. 1999;140:5651–5658. doi: 10.1210/endo.140.12.7223. [DOI] [PubMed] [Google Scholar]
- Keck ME. Corticotropin-releasing factor, vasopressin and receptor systems in depression and anxiety. Amino Acids. 2006;31:241–250. doi: 10.1007/s00726-006-0333-y. [DOI] [PubMed] [Google Scholar]
- Keck ME, Kern N, Erhardt A, Unschuld PG, Ising M, Salyakina D, Muller MB, Knorr CC, Lieb R, Hohoff C, Krakowitzky P, Maier W, Bandelow B, Fritze J, Deckert J, Holsboer F, Muller-Myhsok B, Binder EB. Combined effects of exonic polymorphisms in crhr1 and avpr1b genes in a case/control study for panic disorder. Am J Med Genet B: Neuropsychiatr Genet. 2008;147B:1196–1204. doi: 10.1002/ajmg.b.30750. [DOI] [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of gpcr desensitization. Br J Pharmacol. 2008;153(Suppl 1):S379–S388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsoe JR, Spence MA, Loetscher E, Foguet M, Sadovnick AD, Remick RA, Flodman P, Khristich J, Mroczkowski-Parker Z, Brown JL, Masser D, Ungerleider S, Rapaport MH, Wishart WL, Luebbert H. A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc Natl Acad Sci U S A. 2001;98:585–590. doi: 10.1073/pnas.011358498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the national comorbidity survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kimura M, Muller-Preuss P, Lu A, Wiesner E, Flachskamm C, Wurst W, Holsboer F, Deussing JM. Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol Psychiatry. 2010;15:154–165. doi: 10.1038/mp.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases gaba synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kleiman EM, Liu RT, Riskind JH, Hamilton JL. Depression as a mediator of negative cognitive style and hopelessness in stress generation. Br J Psychol. 2014 doi: 10.1111/bjop.12066. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lefkowitz RJ. Regulation of g protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles SA, Vogt SK, Muglia LJ. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci. 2010;30:2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. discussion 290–5. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Lengvari I, Liposits Z, Vigh S, Schally AV, Flerko B. Corticotropin-releasing factor (CRF)-immunoreactive neurons in the mammillary body of the rat. Cell Tissue Res. 1985;240:455–460. doi: 10.1007/BF00222359. [DOI] [PubMed] [Google Scholar]
- Krasel C, Bunemann M, Lorenz K, Lohse MJ. Beta-arrestin binding to the beta2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J Biol Chem. 2005;280:9528–9535. doi: 10.1074/jbc.M413078200. [DOI] [PubMed] [Google Scholar]
- Krieger DT, Liotta A, Brownstein MJ. Corticotropin releasing factor distribution in normal and brattleboro rat brain, and effect of deafferentation, hypophysectomy and steroid treatment in normal animals. Endocrinology. 1977;100:227–237. doi: 10.1210/endo-100-1-227. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijt AW, Putman P, Van der Does W. The 5-httlpr polymorphism, early and recent life stress, and cognitive endophenotypes of depression. Cognit Emot. 2014;28:1149–1163. doi: 10.1080/02699931.2013.873018. [DOI] [PubMed] [Google Scholar]
- Laryea G, Arnett MG, Muglia LJ. Behavioral studies and genetic alterations in corticotropin-releasing hormone (crh) neurocircuitry: insights into human psychiatric disorders. Behav Sci. 2012;2:135–171. doi: 10.3390/bs2020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, O’Kirwan F, Irizarry K, Merriman B, Thakur S, Jepson R, Lake S, Tantisira KG, Weiss ST, Wong ML. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in mexican-americans. Mol Psychiatry. 2004;9:1075–1082. doi: 10.1038/sj.mp.4001587. [DOI] [PubMed] [Google Scholar]
- Lim MM, Nair HP, Young LJ. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. J Compar Neurol. 2005;487:75–92. doi: 10.1002/cne.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Juo SH, Dewan A, Grunn A, Tong X, Brito M, Park N, Loth JE, Kanyas K, Lerer B, Endicott J, Penchaszadeh G, Knowles JA, Ott J, Gilliam TC, Baron M. Evidence for a putative bipolar disorder locus on 2p13–16 and other potential loci on 4q31, 7q34, 8q13, 9q31, 10q21–24, 13q32, 14q21 and 17q11–12. Mol Psychiatry. 2003;8:333–342. doi: 10.1038/sj.mp.4001254. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, Wang X, Qiu D, Liu W, Cao Z, Li W. Association of corticotropin-releasing hormone receptor1 gene snp and haplotype with major depression. Neurosci Lett. 2006;404:358–362. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M, Refojo D, Ekker M, Rubenstein JL, Stalla GK, Singewald N, Holsboer F, Wotjak CT, Wurst W, Deussing JM. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol Psychiatry. 2008;13:1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- Mahar I, Bambico FR, Mechawar N, Nobrega JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Marcheco-Teruel B, Flint TJ, Wikman FP, Torralbas M, Gonzalez L, Blanco L, Tan Q, Ewald H, Orntoft T, Kruse TA, Borglum AD, Mors O. A genome-wide linkage search for bipolar disorder susceptibility loci in a large and complex pedigree from the eastern part of cuba. Am J Med Genet B: Neuropsychiatr Genet. 2006;141B:833–843. doi: 10.1002/ajmg.b.30314. [DOI] [PubMed] [Google Scholar]
- Marcus M, Yasami T, van Ommeren M, Chisholm D. Depression: A Global Public Health Concern. World Health Organization; 2014. http://www.who.int/mental_health/management/depression/who_paper_depression_wfmh_2012.pdf?ua=1. [Google Scholar]
- Markovic D, Punn A, Lehnert H, Grammatopoulos DK. Intracellular mechanisms regulating corticotropin-releasing hormone receptor-2beta endocytosis and interaction with extracellularly regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling cascades. Mol Endocrinol. 2008;22:689–706. doi: 10.1210/me.2007-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McFadden K, Griffin TA, Levy V, Wolfe JH, Valentino RJ. Overexpression of corticotropin-releasing factor in barrington’s nucleus neurons by adeno-associated viral transduction: effects on bladder function and behavior. Eur J Neurosci. 2012;36:3356–3364. doi: 10.1111/j.1460-9568.2012.08250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW, Abou Jamra R, Albus M, Bacanu SA, Baron M, Barrett TB, Berrettini W, Blacker D, Byerley W, Cichon S, Coryell W, Craddock N, Daly MJ, Depaulo JR, Edenberg HJ, Foroud T, Gill M, Gilliam TC, Hamshere M, Jones I, Jones L, Juo SH, Kelsoe JR, Lambert D, Lange C, Lerer B, Liu J, Maier W, Mackinnon JD, McInnis MG, McMahon FJ, Murphy DL, Nothen MM, Nurnberger JI, Pato CN, Pato MT, Potash JB, Propping P, Pulver AE, Rice JP, Rietschel M, Scheftner W, Schumacher J, Segurado R, Van Steen K, Xie W, Zandi PP, Laird NM. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet. 2005;77:582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, De Boer SF, Koolhaas JM. Long-lasting consequences of a social conflict in rats: behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behav Neurosci. 1999;113:1283–1290. doi: 10.1037//0735-7044.113.6.1283. [DOI] [PubMed] [Google Scholar]
- Mehta D, Raison CL, Woolwine BJ, Haroon E, Binder EB, Miller AH, Felger JC. Transcriptional signatures related to glucose and lipid metabolism predict treatment response to the tumor necrosis factor antagonist infliximab in patients with treatment-resistant depression. Brain Behav Immun. 2013;31:205–215. doi: 10.1016/j.bbi.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and gaba(a) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Bedard T, Anisman H. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin b alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berlin) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Millan MA, Jacobowitz DM, Hauger RL, Catt KJ, Aguilera G. Distribution of corticotropin-releasing factor receptors in primate brain. Proc Natl Acad Sci U S A. 1986;83:1921–1925. doi: 10.1073/pnas.83.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2014;30C:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283(3):315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM, Gotlib IH. Life stress and family history for depression: the moderating role of past depressive episodes. J Psychiatr Res. 2014;49:90–95. doi: 10.1016/j.jpsychires.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by grks and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Morris MC, Rao U, Wang L, Garber J. Cortisol reactivity to experimentally manipulated psychosocial stress in young adults at varied risk for depression. Depress Anxiety. 2014;31:44–52. doi: 10.1002/da.22125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- Mulinari S. Monoamine theories of depression: historical impact on biomedical research. J Hist Neurosci. 2012;21:366–392. doi: 10.1080/0964704X.2011.623917. [DOI] [PubMed] [Google Scholar]
- Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kuhn R, Reul JM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Nederhof E, Ormel J, Oldehinkel AJ. Mismatch or cumulative stress: the pathway to depression is conditional on attention style. Psychol Sci. 2014;25:684–692. doi: 10.1177/0956797613513473. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The neurobiology of depression. Sci Am. 1998;278:42–49. doi: 10.1038/scientificamerican0698-42. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bissette G, Akil H, Fink M. Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy. Corticotrophin-releasing factor, beta-endorphin and somatostatin. Br J Psychiatry. 1991;158:59–63. doi: 10.1192/bjp.158.1.59. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of csf corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nielsen DM, Carey GJ, Gold LH. Antidepressant-like activity of corticotropin-releasing factor type-1 receptor antagonists in mice. Eur J Pharmacol. 2004;499:135–146. doi: 10.1016/j.ejphar.2004.07.091. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. J Pers Soc Psychol. 1999;77:1061–1072. doi: 10.1037//0022-3514.77.5.1061. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Compar Physiol. 2007;293:R209–R222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschowka JA, O’Donohue TL, Mueller GP, Jacobowitz DM. The distribution of corticotropin releasing factor-like immunoreactive neurons in rat brain. Peptides. 1982;3:995–1015. doi: 10.1016/0196-9781(82)90071-7. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathe AA, Yadid G. The flinders sensitive line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Papiol S, Arias B, Gasto C, Gutierrez B, Catalan R, Fananas L. Genetic variability at hpa axis in major depression and clinical response to antidepressant treatment. J Affect Disord. 2007;104:83–90. doi: 10.1016/j.jad.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Paull WK, Phelix CF, Copeland M, Palmiter P, Gibbs FP, Middleton C. Immunohistochemical localization of corticotropin releasing factor (CRF) in the hypothalamus of the squirrel monkey, saimiri sciureus. Peptides. 1984;5(Suppl 1):45–51. doi: 10.1016/0196-9781(84)90264-x. [DOI] [PubMed] [Google Scholar]
- Paull WK, Scholer J, Arimura A, Meyers CA, Chang JK, Chang D, Shimizu M. Immunocytochemical localization of CRF in the ovine hypothalamus. Peptides. 1982;3:183–191. doi: 10.1016/0196-9781(82)90049-3. [DOI] [PubMed] [Google Scholar]
- Paykel ES. Life events and affective disorders. Acta Psychiatr Scand Suppl. 2003:61–66. doi: 10.1034/j.1600-0447.108.s418.13.x. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchinski BA, Chen G, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. Evidence of biologic epistasis between bdnf and slc6a4 and implications for depression. Mol Psychiatry. 2008;13:709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Plante DT, Landsness EC, Peterson MJ, Goldstein MR, Riedner BA, Wanger T, Guokas JJ, Tononi G, Benca RM. Sex-related differences in sleep slow wave activity in major depressive disorder: a high-density eeg investigation. BMC Psychiatry. 2012;12:146. doi: 10.1186/1471-244X-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter AM, Kauer JA. Stress and vta synapses: implications for addiction and depression. Eur J Neurosci. 2014;39:1179–1188. doi: 10.1111/ejn.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cdnas for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349:423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci U S A. 1992;89:4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Qi X, Shan Z, Ji Y, Guerra V, Alexander JC, Ormerod BK, Bruijnzeel AW. Sustained aav-mediated overexpression of CRF in the central amygdala diminishes the depressive-like state associated with nicotine withdrawal. Transl Psychiatry. 2014;4:e385. doi: 10.1038/tp.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raadsheer FC, Tilders FJ, Swaab DF. Similar age related increase of vasopressin colocalization in paraventricular corticotropin-releasing hormone neurons in controls and alzheimer patients. J Neuroendocrinol. 1994;6:131–133. doi: 10.1111/j.1365-2826.1994.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schutz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of crhr1. Science. 2011;333:1903–1907. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, Chen A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2011;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Bradley B, Mercer KB, Deveau TC, Smith AK, Gillespie CF, Nemeroff CB, Cubells JF, Binder EB. Polymorphisms in crhr1 and the serotonin transporter loci: gene x gene x environment interactions on depressive symptoms. Am J Med Genet B: Neuropsychiatr Genet. 2010;153B:812–824. doi: 10.1002/ajmg.b.31052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B, Fox K, Valentino R, Van Bockstaele E. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reyes B, Valentino R, Van Bockstaele E. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan PJ, Summers CH. Molecular signaling and translational significance of the corticotropin releasing factor system. Progr Mol Biol Transl Sci. 2011;98:235–292. doi: 10.1016/B978-0-12-385506-0.00006-5. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Chagnon M, Bouchard C, Bjorntorp P. A polymorphism in the regulatory region of the corticotropin-releasing hormone gene in relation to cortisol secretion, obesity, and gene–gene interaction. Metabolism. 2001;50:1059–1062. doi: 10.1053/meta.2001.25598. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Tsivkovskaia NO, Ryabinin SA. Urocortin 1-containing neurons in the human edinger-westphal nucleus. Neuroscience. 2005;134:1317–1323. doi: 10.1016/j.neuroscience.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Rybnikova EA, Pelto-Huikko M, Rakitskaya VV, Shalyapina VG. Localization of corticoliberin receptors in the rat brain. Neurosci Behav Physiol. 2003;33:399–404. doi: 10.1023/a:1022807926406. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, McGeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berlin) 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Compar Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Saravanan C, Wilks R. Medical students’ experience of and reaction to stress: the role of depression and anxiety. Sci World J. 2014;2014:737382. doi: 10.1155/2014/737382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seasholtz AF, Valverde RA, Denver RJ. Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammals. J Endocrinol. 2002;175:89–97. doi: 10.1677/joe.0.1750089. [DOI] [PubMed] [Google Scholar]
- Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger JI, Jr, Craddock N, DePaulo JR, Baron M, Gershon ES, Ekholm J, Cichon S, Turecki G, Claes S, Kelsoe JR, Schofield PR, Badenhop RF, Morissette J, Coon H, Blackwood D, McInnes LA, Foroud T, Edenberg HJ, Reich T, Rice JP, Goate A, McInnis MG, McMahon FJ, Badner JA, Goldin LR, Bennett P, Willour VL, Zandi PP, Liu J, Gilliam C, Juo SH, Berrettini WH, Yoshikawa T, Peltonen L, Lonnqvist J, Nothen MM, Schumacher J, Windemuth C, Rietschel M, Propping P, Maier W, Alda M, Grof P, Rouleau GA, Del-Favero J, Van Broeckhoven C, Mendlewicz J, Adolfsson R, Spence MA, Luebbert H, Adams LJ, Donald JA, Mitchell PB, Barden N, Shink E, Byerley W, Muir W, Visscher PM, Macgregor S, Gurling H, Kalsi G, McQuillin A, Escamilla MA, Reus VI, Leon P, Freimer NB, Ewald H, Kruse TA, Mors O, Radhakrishna U, Blouin JL, Antonarakis SE, Akarsu N. Genome scan meta-analysis of schizophrenia and bipolar disorder, Part III: Bipolar disorder. Am J Hum Genet. 2003;73:49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgoifo A, Koolhaas JM, Musso E, De Boer SF. Different sympathovagal modulation of heart rate during social and nonsocial stress episodes in wild-type rats. Physiol Behav. 1999;67:733–738. doi: 10.1016/s0031-9384(99)00134-1. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Pozzato C, Meerlo P, Costoli T, Manghi M, Stilli D, Olivetti G, Musso E. Intermittent exposure to social defeat and open-field test in rats: acute and long-term effects on ecg, body temperature and physical activity. Stress. 2002;5:23–35. doi: 10.1080/102538902900012387. [DOI] [PubMed] [Google Scholar]
- Shapero BG, Black SK, Liu RT, Klugman J, Bender RE, Abramson LY, Alloy LB. Stressful life events and depression symptoms: the effect of childhood emotional abuse on stress reactivity. J Clin Psychol. 2014;70:209–223. doi: 10.1002/jclp.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Sun T, Cong B, He P, Zhang Y, Yan J, Lu C, Ni X. Corticotropin-releasing hormone stimulates sgk-1 kinase expression in cultured hippocampal neurons via crh-r1. Am J Physiol Endocrinol Metab. 2008;295:E938–E946. doi: 10.1152/ajpendo.90462.2008. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Li Y, Mork A, Sanchez C, Gulinello M. Does stress elicit depression? Evidence from clinical and preclinical studies. Curr Top Behav Neurosci. 2014 doi: 10.1007/7854_2014_292. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, Laird NM, Kagan J, Snidman N, Hirshfeld-Becker D, Tsuang MT, Sklar PB, Slaugenhaupt SA. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry. 2003;54:1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, Kagan J, Snidman N, Faraone SV, Hirshfeld-Becker D, Tsuang MT, Slaugenhaupt SA, Rosenbaum JF, Sklar PB. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol Psychiatry. 2005;57:1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–530. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger A. Sleep and endocrinology. J Intern Med. 2003;254:13–22. doi: 10.1046/j.1365-2796.2003.01175.x. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology (Berlin) 1986;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Telegdy G. Antidepressant-like effects of the CRF family peptides, urocortin 1, urocortin 2 and urocortin 3 in a modified forced swimming test in mice. Brain Res Bull. 2008;75:509–512. doi: 10.1016/j.brainresbull.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of cre driver lines for genetic targeting of gabaergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for g protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19:474–490. doi: 10.1210/me.2004-0275. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Valdez GR. CRF receptors as a potential target in the development of novel pharmacotherapies for depression. Curr Pharm Des. 2009;15:1587–1594. doi: 10.2174/138161209788168083. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Bangasser D, Van Bockstaele EJ. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol Pharmacol. 2013a;83:737–745. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Commons KG. Peptides that fine tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, Bangasser D. Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends Pharmacol Sci. 2013b;34:437–444. doi: 10.1016/j.tips.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele EJ. Chapter 4.4 – functional interactions between stress neuromediators and the locus coeruleus–norepinephrine system. In: Steckler T, Kalin NH, Reul JMHM, editors. Techniques in the Behavioral and Neural Sciences. Elsevier; 2005. pp. 465–486. [Google Scholar]
- Valentino RJ, Wood SK, Wein AJ, Zderic SA. The bladder-brain connection: putative role of corticotropin-releasing factor. Nat Rev Urol. 2011;8:19–28. doi: 10.1038/nrurol.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veith RC, Lewis N, Langohr JI, Murburg MM, Ashleigh EA, Castillo S, Peskind ER, Pascualy M, Bissette G, Nemeroff CB, et al. Effect of desipramine on cerebrospinal fluid concentrations of corticotropin-releasing factor in human subjects. Psychiatry Res. 1993;46:1–8. doi: 10.1016/0165-1781(93)90002-x. [DOI] [PubMed] [Google Scholar]
- Vicentini E, Arban R, Angelici O, Maraia G, Perico M, Mugnaini M, Ugolini A, Large C, Domenici E, Gerrard P, Bortner D, Mansuy IM, Mangiarini L, Merlo-Pich E. Transient forebrain over-expression of CRF induces plasma corticosterone and mild behavioural changes in adult conditional CRF transgenic mice. Pharmacol Biochem Behav. 2009;93:17–24. doi: 10.1016/j.pbb.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Vigod SN, Stewart DE. Emergent research in the cause of mental illness in women across the lifespan. Curr Opin Psychiatry. 2009;22:396–400. doi: 10.1097/YCO.0b013e3283297127. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Joels M, Milaneschi Y, Kahn RS, Penninx BW, Boks MP. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety. 2014;31:737–745. doi: 10.1002/da.22262. [DOI] [PubMed] [Google Scholar]
- Vitoratos N, Papatheodorou DC, Kalantaridou SN, Mastorakos G. “Reproductive” corticotropin-releasing hormone. Ann N Y Acad Sci. 2006;1092:310–318. doi: 10.1196/annals.1365.029. [DOI] [PubMed] [Google Scholar]
- Walker D, Miles L, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progr Neuro-Psychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, Burnham VL, Mazei-Robison MS, Ferguson D, Golden SA, Koo JW, Chaudhury D, Christoffel DJ, Pomeranz L, Friedman JM, Russo SJ, Nestler EJ, Han MH. Stress and CRF gate neural activation of bdnf in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Chen Y, Wolf M, Wagner KV, Liebl C, Scharf SH, Harbich D, Mayer B, Wurst W, Holsboer F, Deussing JM, Baram TZ, Muller MB, Schmidt MV. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol Dis. 2011a;42:300–310. doi: 10.1016/j.nbd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Labermaier C, Holsboer F, Wurst W, Deussing JM, Muller MB, Schmidt MV. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur J Neurosci. 2012;36:2360–2367. doi: 10.1111/j.1460-9568.2012.08148.x. [DOI] [PubMed] [Google Scholar]
- Wang XD, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, Rice CJ, Wurst W, Holsboer F, Deussing JM, Baram TZ, Stewart MG, Muller MB, Schmidt MV. Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. J Neurosci. 2011b;31:13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. BPS. 2009:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D, Sokolowski M, Rozanov V, Wasserman J. The crhr1 gene: a marker for suicidality in depressed males exposed to low stress. Genes Brain Behav. 2008;7:14–19. doi: 10.1111/j.1601-183X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- Weathington JM, Hamki A, Cooke BM. Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. J Compar Neurol. 2014;522:1284–1298. doi: 10.1002/cne.23475. [DOI] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (crh) receptor antagonist: suppression of pituitary acth release and peripheral inflammation. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Tsivkovskaia NO, Ryabinin AE. Urocortin 1 distribution in mouse brain is strain-dependent. Neuroscience. 2005;132:729–740. doi: 10.1016/j.neuroscience.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, Berridge CW, Majzoub JA. Stress-induced behaviors require the corticotropin-releasing hormone (crh) receptor, but not crh. Proc Natl Acad Sci U S A. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, al-Shekhlee A, Bongiorno PB, Esposito A, Khatri P, Sternberg EM, Gold PW, Licinio J. Localization of urocortin messenger rna in rat brain and pituitary. Mol Psychiatry. 1996;1:307–312. [PubMed] [Google Scholar]
- Wood SK, McFadden K, Griffin T, Wolfe JH, Zderic S, Valentino RJ. A corticotropin-releasing factor receptor antagonist improves urodynamic dysfunction produced by social stress or partial bladder outlet obstruction in male rats. Am J Physiol Regul Integr Compar Physiol. 2013a;304:R940–R950. doi: 10.1152/ajpregu.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berlin) 2012:325–336. doi: 10.1007/s00213-012-2648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Wood CS, Lombard CM, Lee CS, Zhang XY, Finnell JE, Valentino RJ. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol Psychiatry. 2015;78:38–48. doi: 10.1016/j.biopsych.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Zhang XY, Reyes BA, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013b;73:1087–1094. doi: 10.1016/j.biopsych.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust S, Kumsta R, Treutlein J, Frank J, Entringer S, Schulze TG, Rietschel M. Sex-specific association between the 5-htt gene-linked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology. 2009;34:972–982. doi: 10.1016/j.psyneuen.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Yamano M, Yuki H, Yasuda S, Miyata K. Corticotropin-releasing hormone receptors mediate consensus interferon-alpha ym643-induced depression-like behavior in mice. J Pharmacol Exp Therap. 2000;292:181–187. [PubMed] [Google Scholar]
- Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. Variant brain-derived neurotrophic factor val66met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, Slominski AT. Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim Pol. 2010;57:1–13. [PMC free article] [PubMed] [Google Scholar]