Abstract
Healthy human aging can have adverse effects on cortical function and on the brain’s ability to integrate visual information to form complex representations. Facial identification is crucial to successful social discourse, and yet, it remains unclear whether the neuronal mechanisms underlying face perception per se, and the speed with which they process information, change with age. We present face images whose discrimination relies strictly on the shape and geometry of a face at various stimulus durations. Interestingly, we demonstrate that facial identity matching is maintained with age when faces are shown in the same view (e.g. front-front or side-side), regardless of exposure duration, but degrades when faces are shown in different views (e.g. front and turned 20° to the side) and does not improve at longer durations. Our results indicate that perceptual processing speed for complex representations and the mechanisms underlying same-view facial identity discrimination are maintained with age. In contrast, information is degraded in the neural transformations that represent facial identity across views. We suggest that the accumulation of useful information over time to refine a representation within a population of neurons saturates earlier in the aging visual system than it does in the younger system and contributes to the age-related deterioration of face discrimination across views.
Keywords: Aging, Face Perception, View Representation, Processing Speed, Object Perception
INTRODUCTION
Aging humans exhibit a widespread decline of visual function, which includes changes in contrast sensitivity, visual acuity, and motion sensitivity (for recent reviews: Spear, 1993; Sekuler & Sekuler, 2000). These deficits arise in part from deterioration of the aging optics (Weale, 1982), but mainly from changes in neural processing (Weale, 1975; Spear, 1993). Age-related neural change is exemplified by cellular activity in older monkeys, which displays an increase in spontaneous firing, a decrease in signals stronger than background activity (decreased signal to noise ratio) and a decrease in selectivity for both orientation and motion direction in V1 (Schmolesky, Wang, Pu & Leventhal, 2000; Leventhal, Wang, Pu, Zhou & Ma, 2003) and V2 (Yu, Wang, Li, Zhou & Leventhal, 2006). Furthermore, age-related deficits become more apparent at higher stages of cortical processing (Habak & Faubert, 2000). Face perception, which is highly relevant to human social discourse and involves widespread networks of neuronal populations, seems vulnerable to the effects of age. For example, low contrast faces (Owsley, Sekuler & Boldt, 1981) and faces shown at increasing distance (Lott, Haegerstrom-Portnoy, Schneck & Brabyn, 2005) are less visible with age, as expected from age-related changes in contrast sensitivity and visual acuity. Furthermore, older observers are impaired in tasks that require learning, memory, and recognition of faces (Smith & Winograd, 1978; Bartlett & Leslie, 1986; Bartlett, Leslie, Tubbs, & Fulton, 1989; Crook & Larrabee, 1992). However, impairments in learning and memory have been linked to aging (e.g. Grady & Craik, 2000) and might explain the differential effect of age in these studies. The question remains whether aging affects our ability to discriminate among faces based strictly on the shape and geometric change of the human face, and what the implications are for the underlying neural circuitry.
The representation of faces involves distributed cortical networks, which comprise various regions of the temporal lobe and the extensive interactions among them (McIntosh, Grady, Ungerleider, Haxby, Rapoport & Horwitz, 1994; Haxby, Hoffman & Gobbini, 2001). Of particular interest is our ability to identify the face of an individual shown in different views (e.g. front or profile). Human fMRI findings indicate that the fusiform face area (FFA; Kanwisher, McDermott & Chun, 1997) is implicated in the representation of facial identity in a particular view (GrillSpector etal., 1999; Grill-Spector & Malach, 2001; Vuilleumier, Henson, Driver, & Dolan, 2002; Andrews & Ewbank, 2004), whereas the superior temporal sulcus (STS) is involved in changes of face view but not identity (Andrews & Ewbank, 2004). Facial identity and facial view are processed in parallel (Lee, Matsumiya & Wilson, 2006) across different neuronal populations and the complete representation of a face thus requires information to be combined across these populations.
Age-related changes in neural function analogous to those at early stages of visual processing (V1, V2) may exist at higher stages (e.g. FFA, STS) and be especially apparent in networks involving a greater number of neuronal operations. In this scenario, it is expected that healthy aging might have an adverse effect on facial identity discrimination, which would be particularly evident in conditions involving view changes. Furthermore, it has been advanced that changes in performance between older and younger observers might be explained by an age-related general slow-down of information processing (Salthouse, 1996). This idea is physiologically plausible for at least two reasons. First, changes in myelination throughout monkey cortex, including visual cortex (Peters, 2002), could lead to slower signal transmission. Second, because aging cells exhibit a lower signal to noise ratio, the accumulation of information in neuronal populations might occur over longer periods of time. In the case of an age-related slowing, differences in performance between older and younger observers should be exacerbated at shorter stimulus durations and reduced or absent at longer durations.
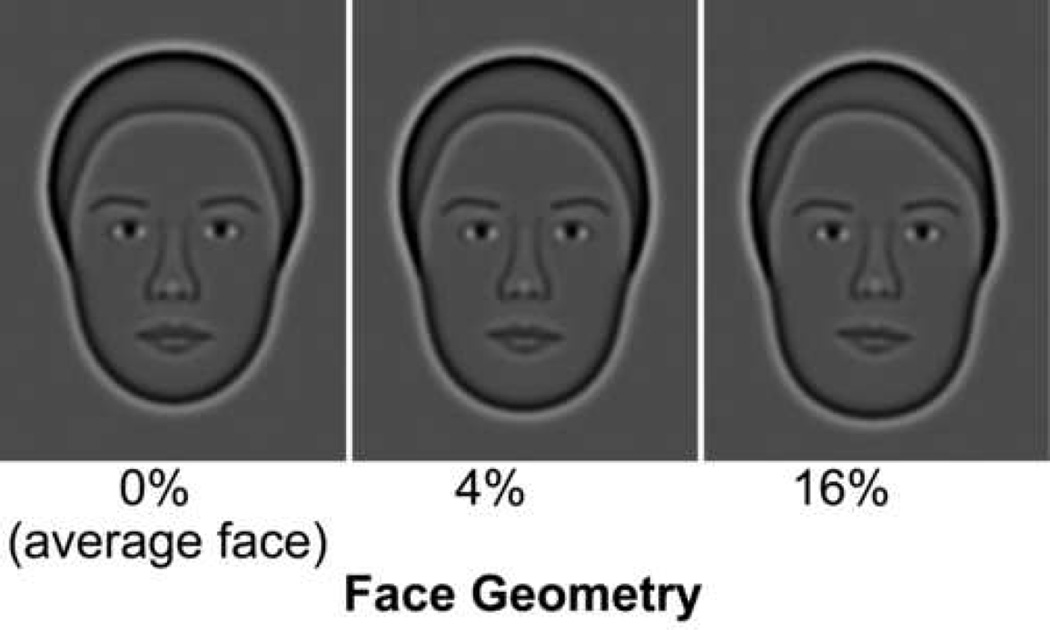
To establish the effect of healthy aging on face perception, we use synthetic faces (Wilson, Loffler & Wilkinson, 2002), which allow for precise control of facial geometry, do not contain any fine detail such as wrinkles, and have been shown to activate human cortical regions preferentially sensitive to human faces (Loffler, Yourganov, Wilkinson & Wilson, 2005). We employed a discrimination task (delayed match-to-sample with brief delays) that consisted of a target face followed by two choice faces, one of which had to be matched to the target face. This task did not require observers to learn or memorize faces. Facial identity discrimination was measured as a function of facial geometry (Fig. 1) at various target stimulus durations, for faces shown in same or different views.
Figure 1.
Synthetic faces shown with increasing geometrical change (in percentage) from the average face or “identity strength”.
METHODS
Participants
Nineteen younger observers (mean age 23.4, SD 2.9 years, range 20–30) and twenty-one community-dwelling older observers (mean age 64.4, SD 3.8 years, range 58–72) participated in this study. Older observers were screened for general health and for medications that could affect visual or brain function; they underwent complete eye exams by an optometrist and only those with healthy eyes participated in these experiments. All observers had a visual acuity of 20/25 or better, except for one older observer whose visual acuity was 20/30. All older observers were corrected for the viewing distance of 131 cm. Older observers were highly educated with 13 holding graduate degrees, 7 had undergraduate degrees, and 1 a technical school degree. Younger observers consisted of undergraduate and graduate students attending York University.
Stimuli
Stimuli were generated and presented on an Apple iMac G3 using routines from the Psychophysics and Video Toolbox (Brainard, 1997; Pelli, 1997). Contrast linearization was carried out using 150 equally spaced grey levels. The monitor was viewed binocularly from a distance of 131 cm (one pixel subtended 41.5 arc sec at the resolution of 1028 × 764), and mean luminance was 65 cd/m2. Stimulus luminance contrast was 99%.
The construction of synthetic faces has been described previously (Wilson et al., 2002). Briefly, photographs of individual faces taken in frontal view and turned 20° to the side, were digitized by defining facial geometry with 37 points, which represented a 37-dimensional vector for each face. A subset of the 37 points each defined head shape, hairline, internal features and placement, so that features and the distance between them were varied. An average male face and average female face were constructed by averaging the 37 parameters from 40 male and 40 female faces, respectively. This was carried out for each view (front and 20° side) using the same set of individual photographs. The Euclidian distance between two vectors represents the geometric difference between two faces. The vector representing a face was normalized relative to the average face, so that geometric difference or “identity strength” from the average face could be manipulated (% face geometry). Face images were generated containing this geometric information and filtered at 10 cycles/face-width, with a bandwidth of 2 octaves, which is optimal for face perception (Gold, Bennett, & Sekuler, 1999; Näsänen, 1999).
Procedure
Experiments were conducted using the method of constant stimuli and a 2AFC match-to-sample paradigm, in which a target face appeared briefly, followed by a random noise mask for 200 ms, and two choice faces shown side-by-side. Observers were required to indicate which of the two choice faces matched the target face. Such brief sequential comparisons of visual stimuli are shown to be unaffected by age for delays as long as 4 s (McIntosh et al., 1999; Bennett et al., 2001; Sekuler, Kahana, McLaughlin, Golomb, & Wingfield, 2005). Target faces were shown for one of three exposure durations (200, 500, and 1000 ms), which were run in separate blocks. Choice faces remained on the screen until observers made a selection. The noise field that separated the target and choice faces was bandpass filtered using the same filter and characteristics as that used for the synthetic faces (see previous section: stimuli). Each trial was initiated by a mouse click and the selected face was indicated by moving the cursor over the image and clicking the mouse. To avoid key-press errors, the experimenter entered the older observers’ responses. Within a block, four levels of face geometry were each shown 20 times, for a total of 80 trials per condition. A single target duration and view condition (front-front, front-side, or view-change) was run in a block of trials, and observers were instructed as to which condition they were running. A different set of faces was used in each block of trials and testing order was randomized across observers. Testing was conducted over 3 sessions that each lasted 1 hour. Observers were given at least one practice run of 25 trials. For the view-change condition, half the observers in each group were shown a front-view target face and side-view choice faces, and the remaining half was shown a side-view target face and front-view choice faces. Earlier work from our laboratory has shown that face geometry thresholds are similar for these two conditions (Lee et al., 2006).
Analysis
Measures from each block were fit with a Quick (Quick, 1974) or Weibull (Weibull, 1951) function using maximum likelihood estimation, and thresholds were defined at 75% correct responses. A three-way repeated measures analysis of variance (ANOVA; age group (2) × view condition (3) × exposure duration (3)) was carried out on face geometry thresholds. Additional repeated measures ANOVAs were conducted on subsets of the data to explore significant interactions (see results for details), and comparisons were assessed using Scheffé’s method.
RESULTS
Facial Identity Discrimination Between Same and Different Views
Facial identity discrimination thresholds were measured by varying geometrical difference from the mean face. Data were collected as a function of target face duration for three view conditions: target and choice faces are all facing forward (front view), faces are all turned 20° to the side (side view), target face is facing forward and choice faces are turned 20° and vice-versa (view change). We confirm earlier findings that thresholds for matching facial identity across a 20° change in view are approximately 1.5× higher than thresholds for matching faces in the same view (Lee et al., 2006). This suggests that cortical processing between views requires more visual information than it does for same views.
Results are shown in figure 2, where solid symbols indicate data for older observers and hollow symbols data for younger observers. The performance of two of the 21 older observers on the View Change condition was extremely poor despite numerous runs: threshold criterion could not be reached at certain exposure durations (200 ms for 1 observer, and 500 and 1000 ms for the other) and their data were left out of all analyses. A three-way repeated measures analysis of variance (ANOVA; age group (2) × view condition (3) × exposure duration (3)) revealed a significant effect of view condition (F[2,34] = 140.1, p < .0001) and of exposure duration (F[2,34] = 36.8, p < .0001), with a significant interaction between them (F[4,68] = 140.1, p < .0001). The effect of age alone is very weak (F[1,17] = 3.8, p = .066), but the interaction between age and view condition is significant (F[2,34] = 6.83, p < .005), suggesting that age has a significant effect on certain view conditions. As such, we investigated this interaction through a series of two-way repeated measures ANOVAs (age group (2) × exposure duration (3)) for each of the view conditions.
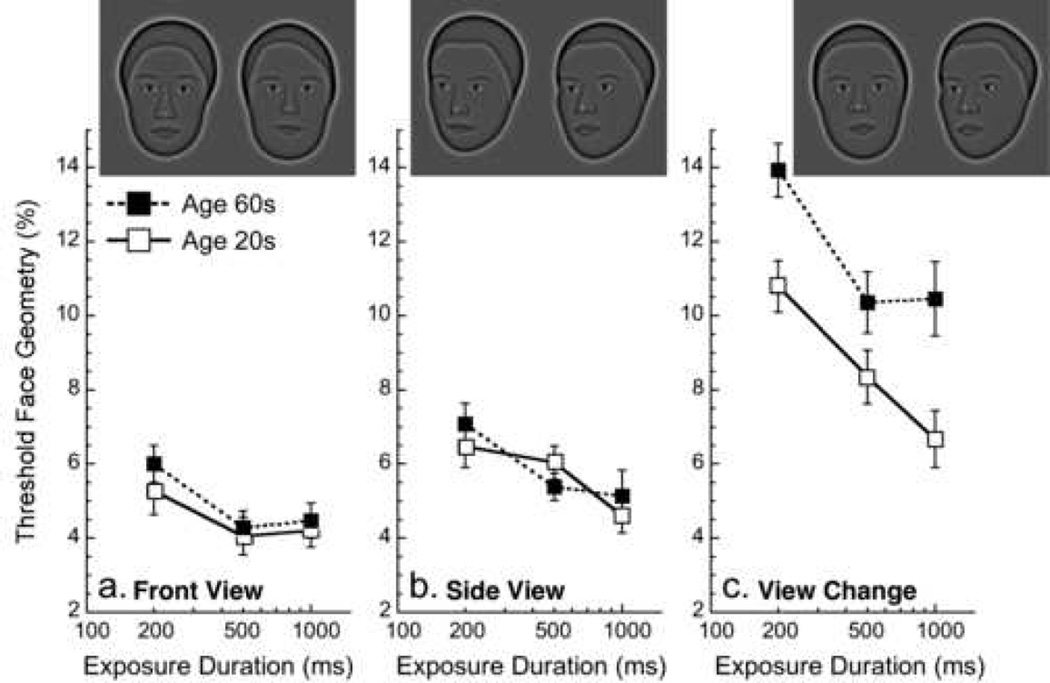
Figure 2.
Effect of aging on face geometry thresholds (smaller values = better performance) as a function of target exposure duration. Hollow symbols indicate younger observers and solid symbols older observers. a) target and choice faces facing forward, b) target and choice faces turned 20° to the side, c) target face facing forward and choice faces turned to side or vice versa. Error bars = 1 SEM. Face discrimination does not change with age for same view faces, but deteriorates for view changes.
For Front View identity matching (Fig. 2a), there is no main effect of age (p > 0.4), but there is a main effect of exposure duration (F[2,36] = 6.6, p < .004). The interaction between the two is not significant. However, we proceeded to explore the within-group effects of exposure duration because older and younger observers appeared to exhibit differences that were driven mainly by a single duration (this same rationale is used for the view conditions below). To address this, a one-way within-group repeated-measures ANOVA was conducted, and comparisons assessed using Scheffé’s method. One-way ANOVAs indicate that performance improves with exposure duration for older observers (F[2,36] = 5.4, p < .01) between 200 and 500 ms (p < 0.03), but not between 500 and 1000 ms (p > 0.5). Younger observers’ performance remained constant with increasing exposure duration, though there was a trend for improvement (F[2,36] = 2.75, p < .08).
The Side View identity matching condition (Fig. 2b) shows a similar pattern, where there is no main effect of age (p > 0.5) but there is a significant effect of exposure duration (F[2,36] = 14.4, p < .0001). One-way ANOVAs reveal that performance improves with exposure duration for older observers (F[2,36] = 9.63, p < .0005) between 200 and 500 ms (p < 0.015) but not between 500 and 1000 ms (p > 0.5). There is also an effect of exposure duration for younger observers (F[2,36] = 4.8, p < .015), whose performance improves between 200 and 1000 ms (p < 0.019).
Results for the View Change condition are shown in figure 2c and indicate that there is a main effect of age, as older observers are worse at discriminating faces across views than younger observers (F[1,18] = 10.6, p < .005) and a main effect of exposure duration (F[2,36] = 23.6, p < .0001). One-way ANOVAs indicate an effect of exposure duration for older observers (F[2,36] = 7.9, p < .0015), where performance improves between target exposure durations of 200 and 500 ms (p < 0.005), but not between 500 and 1000 ms and longer (p > 0.5). A significant effect of exposure duration appears for younger observers (F[2,36] = 14.4, p < .0001) who display a continuous decrease in threshold (improvement) as a function of exposure duration (p < 0.015 between 200 and 500 ms, a trend between 500 to 1000 ms with p < 0.09, and p < .0001 between 200 and 1000 ms).
Exposure Duration Control
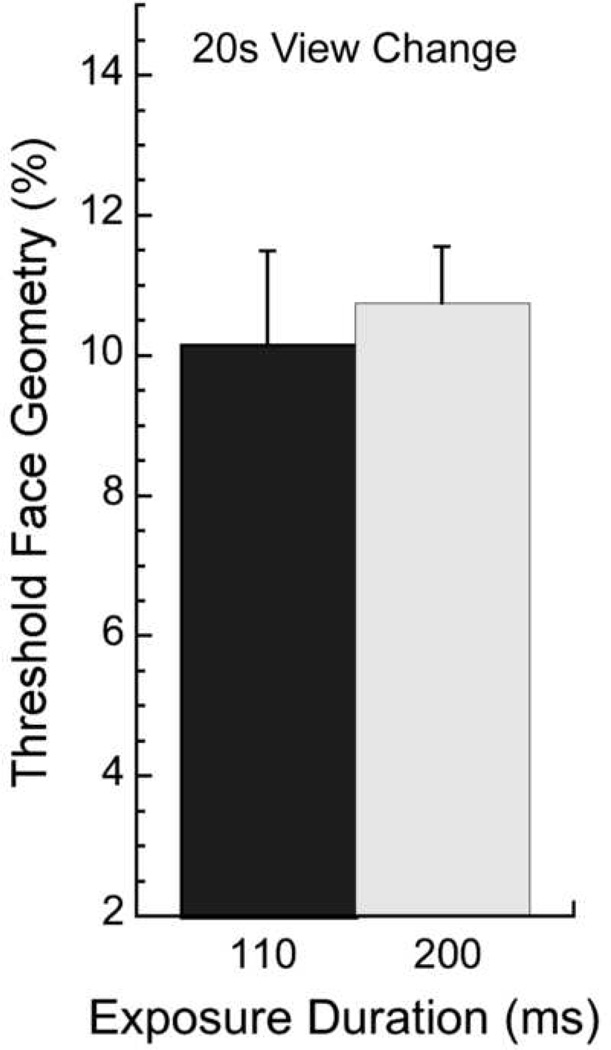
The present difference between older and younger observers in the view-change condition might arise from an age-related slow-down (Cerella, 1985; Salthouse, 1996) that would affect general processing speed or mental rotation speed. Although it has been suggested that face-matching across views is independent of mental rotation (Perrett, Oram & Ashbridge, 1998), we address the issue here. If speed alone were the critical factor, then younger observers’ performance (n = 10, subset from group tested in main experiment) should worsen for a very brief exposure duration of 110 ms and approach that of older observers at longer durations. However, this is not the case, as a paired t-test reveals that younger observers’ performance for facial identity matching across views at 110 ms is similar to performance at 200 ms (p > 0.5; Fig. 3; 110 ms grey bars; 200 ms hatched bars). Furthermore, in the original view-change condition of Fig. 2c, the largest difference between older and younger observers was at the longest duration of 1000 ms. It is therefore unlikely that a general slow-down in the elderly accounts for the difference in performance between older and younger observers in the view-change condition, and changes in the neural representation must be addressed.
Figure 3.
Processing speed control: View-change face discrimination thresholds for decreasing target exposure duration from 200 ms (light gray; replotted from Fig. 2) to 110 ms (dark gray) for 10 younger observers. Performance does not change with a decrease in exposure duration.
Information
The main experiment shows that all observers exhibit higher facial identity thresholds for view changes than for same views, which suggests that the cortical processing of facial identity between face views requires more visual information than it does for same views. Furthermore, aging affects facial identity matching when there is a change of view, but not when the view remains the same. The difference in performance between older and younger observers likely arises from a deterioration of the way in which information is processed in the aging brain. To illustrate this idea, we sought to evaluate younger observers’ performance with a degraded facial image that would mimic older observer’s performance. We use images in which only the upper half of the face is visible. We ran pilot experiments on other stimulus manipulations, such as maintaining only the head contour or only the features, but observers were unable to make matches across views (view change) with these stimuli, and only 2 participants could make the match across views with the bottom half of the face. Furthermore, using low-contrast images would lead to a deterioration in performance for same-view faces (Owsley et al., 1981).
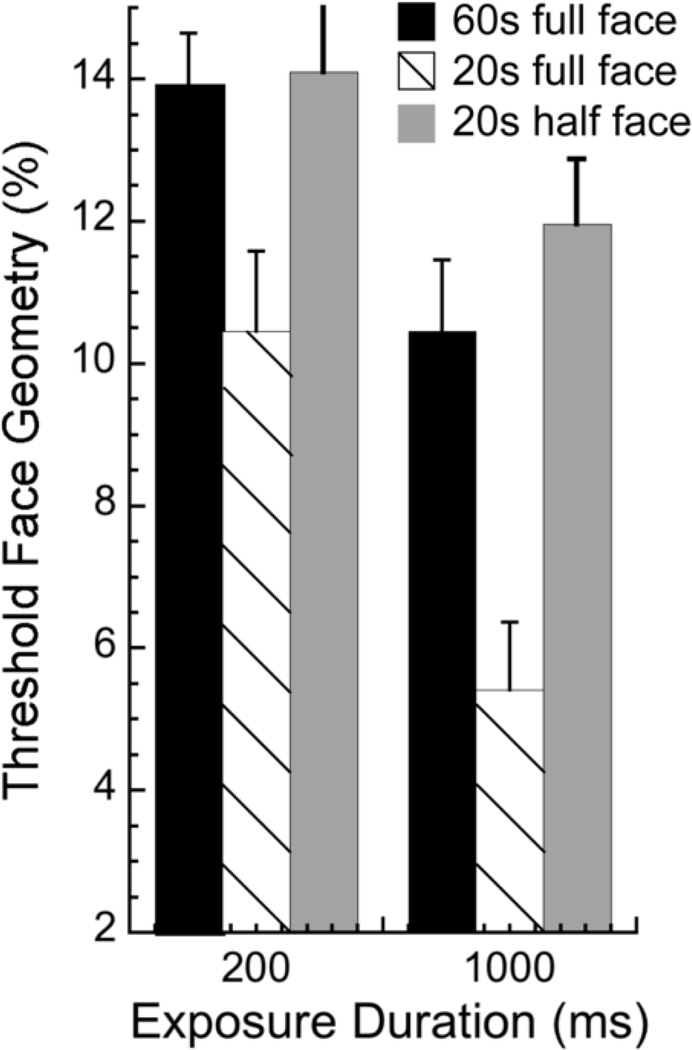
We evaluate a subset of younger observers who participated in the main experiment (n = 8) on facial identity matching across views and for the same view, when only the top half of the face (half of nose and up) is visible for both target and choice faces. Target exposure durations of 200 and 1000 ms were tested. Results for the view-change condition are shown in Figure 4. Thresholds for younger observers tested with half faces (grey bars) are presented alongside thresholds for the full face, which are replotted from Figure 2 for the older group (60s, black bars) and the 8 younger observers (hatched bars) tested here. A two-way repeated-measures ANOVA was conducted on Facial Condition (2; younger full face, younger half face) × Exposure Duration (2; 200 and 1000 ms) and indicates main effects of facial condition (F[1,6] = 30, p < .0015) and exposure duration (F[1,6] = 20.1, p < .0045). Younger observers’ performance with half the face is similar to that of older observers with the whole face (black bars), as 6 younger observers’ thresholds in this condition are within 95% confidence intervals of older observers’ thresholds at a duration of 1000 ms, and 4 at 200 ms. The remaining younger observers’ thresholds with the half face are slightly higher than the upper end of the confidence interval. For same view facial matching there is no significant effect for younger observer’s performance to change with front-view half faces (p > .4), but there is a trend for performance to worsen with side-view half faces (p = .053; data not shown). This illustrates further that cortical processing between views requires more visual information than it does for same views, and that the transformation between different views is degraded with age.
Figure 4.
Information processing: View-change face discrimination thresholds for younger observers (n = 8) with only the top half of the face (grey bars) at 200 and 1000 ms. View change data with the full face for older observers (black bars) and those 8 younger observers (hatched bars) replotted from Fig. 2 for comparison. Younger observers’ performance with the degraded face is similar to that of older observers with the full face.
DISCUSSION
Healthy aging does not affect discrimination of faces shown in the same view regardless of exposure duration, but does adversely affect the discrimination of faces compared across different views. Furthermore, the accumulation of information over time to refine a representation, saturates earlier in older observers than it does in younger observers. This suggests that our findings did not result from age-related low-level visual factors, such as decreased retinal illumination (Weale, 1982) or lower contrast sensitivity (Owsley et al., 1981), nor from factors inherent to the cognitive demands of the task. A possible explanation for the decline of facial identity matching across views is facial symmetry. Front-view faces contain symmetrical information, whereas side-view faces do not. We found no effect of age on either of the same-view matching conditions, suggesting that the age-related deterioration of matching faces across views did not result from the lack of symmetrical face information in side-view faces. Furthermore, an age-dependent difference in processing speed (Cerella, 1985; Salthouse, 1996) does not determine the decline of facial identity discrimination across views for at least two reasons. First, younger observers tested at much briefer stimulus durations performed as they had for slightly longer ones (110 vs. 200 ms). Second, the largest difference in performance between older and younger observers occurred at the longest stimulus duration (1000 ms). Finally, younger observers’ performance on cross-view matching with a degraded face resembled that of older observers with the full face. Taken together, these findings suggest that aging affects the way in which information is accumulated and processed within neural populations that code for view change.
Work on aging and cortical activation is suggestive of age-related changes in neural populations. Cortical activation for same-view face discrimination appears more extensive in older than younger observers, as it involves activation in prefrontal cortex, the thalamus, and the hippocampus in addition to the face-responsive regions activated in younger observers (Grady et al., 2000). This would suggest an age-mediated deterioration of face-responsive populations, and that the recruitment of additional regions is evidence for neural plasticity that may reflect compensatory activity in the aging cortex (Grady et al., 2000; Cabeza, Anderson, Locantore & McIntosh, 2002). Such compensatory activity appears insufficient for the more complex neural transformations involved in face matching across views or it may partially usurp networks that would otherwise participate in view transformations.
The precise nature of the neural transformations underlying face representation across views remains unclear, but it is evident that numerous neuronal populations are acting in these operations. Single cells in monkey temporal cortex display relatively broad tuning (60°) to an individual face in a particular view, with peak responses for front, 45° profile, and 90° profile views (Perrett et al., 1991). This suggests that this neuronal population selects for facial identity and that the relative response strength among neuronal subgroups determines the view of the face. Furthermore, other cells in temporal cortex respond to a face in several different views (Hasselmo, Rolls, Baylis & Nalwa, 1989), suggesting a population of neurons that represents the changing aspects of a face. It is therefore the relative activity among these neural populations that links an individual’s identity across views.
How might aging affect these processes? Age-related changes in cellular function analogous to those reported in V1 (Schmolesky et al., 2000; Leventhal et al., 2003) could take effect in the higher cortical areas involved in face processing. If this is the case, then a combination of decreased signal to noise ratio and response selectivity could lead to weaker facial specificity in the aging visual system. If the neural representation of faces is less accurate, then under view-change conditions where specific cues change, the aging visual system would require larger geometric differences to discriminate among faces. This proposal is supported by our finding that discrimination in older observers does not continue to improve between 500 and 1000 ms: performance does not improve with additional viewing time in the older observer, suggesting that the accumulation of information in the aging visual system reaches a maximum. Furthermore, our finding that performance (all observers) is worse across views than it is for same views suggests that more information is required for view transformations than for same views. The adverse effect of aging on performance across views suggests that information is coded poorly in aging neural populations. This is illustrated by younger observers’ performance with half the face resembling that of older observers with the full face. This does not imply that half the facial information is not represented in the aging visual system, but rather that facial representations are simply incomplete or at least poorly linked across populations. Congenital prosopagnosia (CP) serves as an example of how poor or degraded links among neural populations coding for faces can lead to deficits in facial representation. CP constitutes a lifelong impairment in facial recognition (even for same view faces) in the absence of deficits in cognitive function, memory and low-level visual processes (Behrmann & Avidan, 2005). Recent evidence has shown that the fibers linking numerous cortical regions involved in face perception are disrupted in this group (Thomas, Avidan, Jung, & Behrmann, 2006). In addition, unpublished data from our laboratory indicate that individuals with CP are especially poor at matching facial identity across views.
Interestingly, developmental work on facial identity matching across views suggests that this aspect of face perception matures later than do other facial representations, such as identity matching across facial expression, discrimination of gaze direction, or discrimination of facial expression (Mondloch, Geldart, Maurer & Le Grand, 2003). This suggests further that facial identity discrimination across views requires more complex integration of visual information than do other facial representations and lends support to our conclusion that the representation of facial identity across views requires more information than does the representation of same-view facial identity.
Age-related deterioration of neural networks is a subtle change that involves numerous processes, for which we find evidence. Our findings demonstrate both preserved and degraded function with age and provide strong support for changes in the representation and accumulation of useful information in the aging brain.
Acknowledgments
This work was supported by an NIH grant to HRW (EY002158), an NSEC grant to FW (OP0007551), and by a CIHR Postdoctoral Fellowship to CH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bartlett JC, Leslie JE. Aging and memory for faces versus single views of faces. Memory and Cognition. 1986;14:371–381. doi: 10.3758/bf03197012. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Leslie JE, Tubbs A, Fulton A. Aging and memory for pictures of faces. Psychology and Aging. 1989;4:276–283. doi: 10.1037//0882-7974.4.3.276. [DOI] [PubMed] [Google Scholar]
- Bennett PJ, Sekuler AB, McIntosh AR, Della-Maggiore V. The effects of aging on visual memory: Evidence for functional reorganization of cortical networks. Acta Psychologica. 2001;107:249–273. doi: 10.1016/s0001-6918(01)00037-3. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G. Congenital prosopagnosia: face-blind from birth. Trends Cognitive Science. 2005;9:180–187. doi: 10.1016/j.tics.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:443–446. [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cerella J. Information processing rates in the elderly. Psychological Bulletin. 1985;98:67–83. [PubMed] [Google Scholar]
- Crook TH, Larrabee GJ. Changes in facial recognition memory across the adult life span. Journal of Gerontology. 1992;47:138–141. doi: 10.1093/geronj/47.3.p138. [DOI] [PubMed] [Google Scholar]
- Gold J, Bennett PJ, Sekuler AB. Identification of band-pass filtered letters and faces by human and ideal observers. Vision Research. 1999;39:3537–3560. doi: 10.1016/s0042-6989(99)00080-2. [DOI] [PubMed] [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age. Current Opinion in Neurobiology. 2000;10:224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Rapoport SI. Age-related changes in the neural correlates of degraded and non-degraded face processing. Cognitive Neuropsychology. 2000;17:165–186. doi: 10.1080/026432900380553. [DOI] [PubMed] [Google Scholar]
- Habak C, Faubert J. Larger effect of aging on the perception of higher-order stimuli. Vision Research. 2000;40:943–950. doi: 10.1016/s0042-6989(99)00235-7. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Rolls ET, Baylis GC, Nalwa V. Object-centered encoding by face-selective neurons in the cortex in the superior temporal sulcus of the monkey. Experimental Brain Research. 1989;75:417–429. doi: 10.1007/BF00247948. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Science. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in extrastriate cortex specialised for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Matsumiya K, Wilson HR. Size-invariant but viewpoint-dependent representation of faces. Vision Research. 2006;46:1901–1910. doi: 10.1016/j.visres.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Loffler G, Yourganov G, Wilkinson F, Wilson HR. fMRI evidence for the neural representation of faces. Nature Neuroscience. 2005;8:1386–1390. doi: 10.1038/nn1538. [DOI] [PubMed] [Google Scholar]
- Lott LA, Haegerstrom-Portnoy G, Schneck ME, Brabyn JA. Face recognition in the elderly. Optometry and Vision Science. 2005;82:874–881. doi: 10.1097/01.opx.0000180764.68737.91. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Grady CL, Ungerleider LG, Haxby JV, Rapoport SI, Horwitz B. Network analysis of cortical visual pathways mapped with PET. Journal of Neuroscience. 1994;14:655–666. doi: 10.1523/JNEUROSCI.14-02-00655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A, Sekuler AB, Penpeci C, Rajah M, Grady C, Sekuler R, Bennett P. Recruitment of unique neural systems to support visual memory in normal aging. Current Biology. 1999;9:1275–1278. doi: 10.1016/s0960-9822(99)80512-0. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Boldt C. Aging and low-contrast vision: face perception. Investigative Ophthalmology and Visual Science. 1981;21:362–365. [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Research. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Geldart S, Maurer D, Le Grand R. Developmental changes in face processing skills. Journal of Experimental Child Psychology. 2003;86:67–84. doi: 10.1016/s0022-0965(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Näsänen R. Spatial frequency bandwidth used in the recognition of facial images. Vision Research. 1999;39:3824–3833. doi: 10.1016/s0042-6989(99)00096-6. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Perrett DI, Oram MW, Ashbridge E. Evidence accumulation in cell populations responsive to faces: an account of generalisation of recognition without mental transformations. Cognition. 1998;67:111–145. doi: 10.1016/s0010-0277(98)00015-8. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Oram MW, Harries MH, Bevan R, Hietanen JK, Benson PJ, Thomas S. Viewer-centred and object-centred coding of heads in the macaque temporal cortex. Experimental Brain Research. 1991;86:159–173. doi: 10.1007/BF00231050. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. Journal of Neurocytology. 2002;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Quick RF. A vector magnitude model of contrast detection. Kybernetik. 1974;16:1299–1302. doi: 10.1007/BF00271628. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nature Neuroscience. 2000;3:384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Kahana MJ, McLaughlin C, Golomb J, Wingfield A. Preservation of Episodic Visual Recognition Memory in Aging. Experimental Aging Research. 2005;31:1–13. doi: 10.1080/03610730590882800. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Sekuler AB. Visual perception and cognition. In: Evans JG, Williams F, Beattie BL, Michel J-P, Wilcock GK, editors. Oxford Textbook of Geriatric Medicine. 2nd. Oxford University Press; 2000. pp. 874–880. [Google Scholar]
- Smith AD, Winograd E. Adult age differences in remembering faces. Developmental Psychology. 1978;14:443–444. [Google Scholar]
- Spear PD. Neural bases of visual deficits during aging. Vision Research. 1993;33:2589–2609. doi: 10.1016/0042-6989(93)90218-l. [DOI] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Jung K-J, Behrmann M. Disruption in structural connectivity in ventral cortex in congenital prosopagnosia. Journal of Vision. 2006;6:88a. doi: 10.1038/nn.2224. http://journalofvision.org/6/6/88/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weale R. Senile changes in visual acuity. Transactions of the Ophthalmologcal Society UK. 1975;95:36–38. [PubMed] [Google Scholar]
- Weale R. Senile ocular changes, cell death, and vision. In: Sekuler R, Kline D, Dismukes K, editors. Aging and visual function. New York: Liss; 1982. pp. 161–171. [Google Scholar]
- Weibull W. A statistical distribution function of wide applicability. Journal of Applied Mechanics. 1951;18:292–297. [Google Scholar]
- Wilson HR, Loffler G, Wilkinson F. Synthetic faces, face cubes, and the geometry of face space. Vision Research. 2002;42:2909–2923. doi: 10.1016/s0042-6989(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Yu S, Wang Y, Li X, Zhou Y, Leventhal AG. Functional degradation of extrastriate visual cortex in senescent rhesus monkeys. Neuroscience. 2006;140:1023–1029. doi: 10.1016/j.neuroscience.2006.01.015. [DOI] [PubMed] [Google Scholar]