Abstract
Background
Allosteric inhibition is a promising approach to developing a new group of anticoagulants with potentially reduced bleeding consequences. Recently, we designed sulfated β-O4 lignin (SbO4L) as an allosteric inhibitor that targets exosite 2 of thrombin to reduce fibrinogen cleavage through allostery and compete with GPIbα to reduce platelet activation.
Objective
To assess 1) the antithrombotic potential of a novel approach of simultaneous exosite 2-dependent allosteric inhibition of thrombin and competitive inhibition of platelet activation and 2) the promise of SbO4L as the first-in-class antithrombotic agent.
Methods
A combination of whole blood thromboelastography, hemostasis analysis, mouse arterial thrombosis models and mouse tail bleeding studies were used to assess antithrombotic potential.
Results and Conclusions
SbO4L extended clot initiation time, reduced maximal clot formation, platelet contractile force and clot elastic modulus suggesting dual anticoagulant and antiplatelet effects. These effects were comparable to those measured for enoxaparin. A dose of 1 mg SbO4L per mouse prevented occlusion in 100% of arteries, while lower doses exhibited proportionally reduced response. Likewise, the time to occlusion increased ~70% with 0.5 mg dose in mouse Rose Bengal thrombosis model. Finally, tail bleeding studies demonstrated that SbO4L does not increase bleeding propensity. In comparison, a 0.3 mg dose of enoxaparin increased bleeding time and blood volume loss. Overall, this work highlights the promise of allosteric inhibition approach and presents SbO4L as a novel anticoagulant with potentially reduced bleeding side effects.
Keywords: Blood Coagulation, Anticoagulant agents, Thrombin, Allosteric Regulation, Glycoprotein Ib, Antiplatelet agents
Introduction
Thrombotic disorders such as ischemic heart disease and venous thrombosis are major causes of mortality in humans [1]. Anticoagulants heparin and warfarin are used to treat such conditions [2–4], although both suffer from serious bleeding risks and adverse reactions. Low molecular weight heparins (LMWHs) have better safety profiles but are not completely devoid of bleeding risks [2–5]. Newer agents introduced in the clinic including direct thrombin and factor Xa inhibitors appear to have considerably improved outcomes but retain significant bleeding risks [6–8]. Likewise, antiplatelet agents, which include P2Y12 receptor antagonists (ticlopidine, clopidogrel and prasugrel), fibrinogen mimetics of glycoprotein IIbIIIa receptor (tirofiban and eptifibatide) and aspirin, the inhibitor of cyclooxygenase-1 [9], are also used to treat prothrombotic conditions but are not devoid of adverse effects. Bleeding remains a common side-effect [10,11]. Additionally, many drug-specific side effects are known such as resistance to P2Y12 receptor antagonists [12], thrombocytopenia [13] and dosing issues in patients with renal insufficiency [14].
Although to date no agent has been developed to simultaneously function as an anticoagulant (prevent fibrin formation) and antiplatelet (prevent platelet aggregation), medical conditions that require the combined use of the two types of drugs do exist [15]. For example, combination therapy has proven vital in management of thrombosis in patients with prosthetic heart valves [16]. Another condition is acute arterial thrombosis, which is a common cause of myocardial infarction [15]. Yet, a common problem with combination therapy is the significantly increased risk of bleeding [17,18], which may arise from complete knockdown of the two key pathways of hemostasis.
Clot formation is a highly inter-connected sequence of events that revolves around chemical bond cleavage and synthesis to generate polymeric fibrin and physical association of cells/molecules to form a platelet plug. One of these inter-connections is the interaction of thrombin with glycoprotein Ibα (GPIbα) present on the platelet surface [19,20]. Detailed studies performed to date show that GPIbα binds to anion-binding exosite 2 of thrombin [21]. A specific sulfated tyrosine-rich anionic peptide sequence 274DLYsDYsYs279 present on GPIbα [21,22] recognizes Arg93, Arg101, Arg126, Arg233, Lys235, Lys236, and Lys240, which are also known to be involved in binding to highly sulfated chains of heparin [23]. This cross-talk between thrombin and platelet GPIbα is important because of its key role in platelet activation and aggregation [19,20,24]. Thrombin bound to GPIbα is capable of activating protease-activated receptor–1 and glycoprotein V on the platelet surface, which contribute to platelet aggregation [25,26]. Interestingly, GPIbα binding to thrombin induces a reduction in the catalytic efficiency for fibrinogen cleavage by thrombin [27–29].
The molecular cross-talk between fibrin formation and platelet activation/aggregation presents a novel avenue for development of new antithrombotics [24]. Our earlier work showed that sulfated molecules that target exosite 2 allosterically inhibit thrombin and exhibit an anticoagulant effect [30–32]. If these sulfated molecules also compete with GPIbα for binding to exosite 2, then they will exhibit an antiplatelet effect. In support of this hypothesis, we had earlier designed a molecule called SbO4L (sulfated β-O4 lignin, see Figure 1) that competed with GPIbα for binding to thrombin and reduced aggregation in platelet-rich plasma [33]. More importantly, SbO4L allosterically inhibited thrombin’s catalytic activity with nanomolar potency (IC50 ~14 nM) and caused prolongation of human plasma clotting times in APTT and PT assays. Interestingly, mutagenesis-based experiments had shown that SbO4L binds to Arg233, Lys235 and Lys236 on thrombin, which defines the site of binding for both GPIbα and heparin. Yet, unlike heparin, SbO4L does not require antithrombin to inhibit thrombin and targets thrombin directly. Thus, SbO4L represents a new class of interesting molecules that exhibits dual anticoagulant and antiplatelet activities, which rely on allostery and could potentially be useful in specialized thrombotic events.
Figure 1.
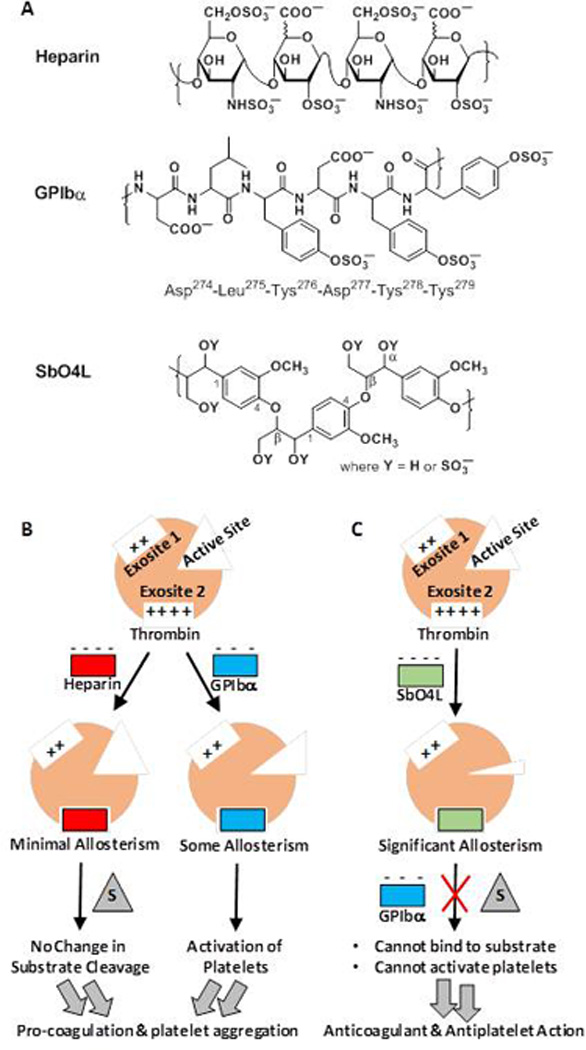
A) Structures of the common sequence of heparin, the sulfated tyrosine containing peptide sequence in GPIbα, and the common sequence of fully synthetic sulfated beta-O4-linked lignin. B) A cartoon representation of the effect of heparin and GPIbα following binding to exosite 2 of thrombin. Heparin induces minimal allosteric changes in the catalytic site, which do not affect hydrolysis of substrates. In contrast, GPIbα induces some allosteric inhibition but contributes more to the activation of platelets. C) A cartoon representation of the effect of SbO4L binding to exosite 2 of thrombin. Significant allosteric changes in the catalytic site of thrombin reduce cleavage of substrate, while simultaneously competing with GPIbα and reducing activation of platelets. These effects generate anticoagulant and antiplatelet function in SbO4L.
In this work, we report on the efficacy of SbO4L as a prototypic, first-in-class, dual action antithrombotic in preventing in vitro and in vivo clot formation. Our work shows that SbO4L effectively reduces the rate and extent of fibrin formation, while also reducing platelet contractile force and clot elastic modulus. In vivo, SbO4L prevents arterial occlusion, while not enhancing tail bleeding propensity in mice. Thus, SbO4L exhibits considerable promise as an allosteric anticoagulant/antiplatelet agent that targets the cross-talk between thrombin and GPIbα highlights the value of studying novel mechanisms as an avenue for realizing new antithrombotic agents with reduced bleeding side effects.
Materials and Methods
Proteins and Chemicals
Human and murine thrombins were obtained from Haematologic Technologies (Essex Junction, VT). Protamine was obtained from Sigma-Aldrich. Spectrozyme TH was obtained from Sekisui Diagnostics (Stamford, CT). Thromboelastograph® Coagulation Analyzer 5000 and its supplies were obtained from Haemoscope Corporation (Niles, IL).
Inhibition of Murine Thrombin
SbO4L inhibition of murine thrombin was studied using substrate hydrolysis assay in the manner described earlier for human thrombin [30–33]. Briefly, 5 µL of either water or SbO4L at 2.3 ng/ml to 2.3 mg/ml was diluted with 185 µL of 20 mM Tris-HCl buffer, pH 7.4, containing 100 mM NaCl, 2.5 mM CaCl2 and 0.1% PEG8000 in a 96-well polystyrene microplate at 37°C. 5 µL murine thrombin was then added (6 nM final concentration) and the solution incubated for 10 min, followed by 5 µL of 5 mM Spectrozyme TH. The residual activity of thrombin was determined by monitoring the A405. The SbO4L concentration that results in 50% of inhibition of thrombin (IC50), Hill slope (HS) and maximal percent residual thrombin activity (YM) were calculated using logistic equation 1, in which Y is the percent residual thrombin activity at each concentration of SbO4L and Y0 is the percent residual thrombin activity in the absence of SbO4L.
| (Eq. 1) |
Reversal of SbO4L Activity by Protamine
Protamine (0 to 14.8 mg/ml) was added to a solution of 6 nM thrombin and 0.58 µg/ml of SbO4L in a 20 mM Tris-HCl buffer, pH 7.4, containing 100 mM NaCl, 2.5 mM CaCl2 and 0.1% PEG8000 at 37°C. Immediately thereafter the residual thrombin activity was measured using Spectrozyme TH hydrolysis, as described above. Control experiments with protamine alone showed that the activity of thrombin was unaffected. The increase in thrombin activity at varying protamine concentrations could be fitted by a modified form of logistic equation 1 to calculate the protamine concentration that recovers thrombin activity by 50% (RC50).
Human Whole Blood Thromboelastograph (TEG®) Analysis of Clot Formation
TEG assays were performed using human whole blood in the presence of SbO4L, as described earlier [34]. Briefly, aliquots of whole blood were incubated with aqueous solution of SbO4L (10 µl) for 5min to yield 0 to 152 µg/ml final concentrations. TEG assays were carried by transferring 20 µl of 200 mM CaCl2 into the TEG cup, incubating at 37 °C, followed by transferring 340 µl of whole blood into the cup to start the assay. The TEG® cup oscillated through 4° 45’ angle at 0.1 Hz until all data was collected, which includes the time to initiation of clot (R), the kinetics of clot formation (α), the maximal stiffness (MA), and elasticity (G) of the clot formed, as discussed earlier [34–36]. All assays were run in duplicate.
Human Whole Blood and Platelet-Rich Plasma (PRP) Hemostatic Analysis
Analysis of platelet function and clot structure was performed using Hemostasis Analysis System (HAS)™ from Hemodyne, Inc., Richmond, VA, as reported earlier [34]. A mixture of 700 µl of citrated whole blood or PRP (platelet count adjusted to 200,000) and 10 µl SbO4L (or ddH2O as control) to give 0 to 74 µg/ml was co-incubated at room temperature for 5 minutes and then placed in a disposable cup. To initiate clotting, 50 µl of 150 mM CaCl2 was added and the HAS measures the contractile forces of platelets (PCF), the platelet force onset time (FOT), and the clot elastic modulus (CEM). All assays were performed in duplicate.
In Vivo FeCl3 Carotid Artery Thrombosis Studies
Procedures involving mice were approved by the Institutional Animal Care and Use Committee of Vanderbilt University and have been previously reported [37,38]. For the FeCl3 carotid artery thrombosis model, wild type C57Bl/6 mice (n=4–8, weights 22–27 gms) were anesthetized with 50 mg/kg intra-peritoneal pentobarbital. SbO4L (0, 100, 300, 500 or 1000 µg in 100 µl PBS) was infused into the right internal jugular vein. Five minutes after infusion, the right common carotid artery was exposed and fitted with a Doppler flow probe (Model 0.5 VB, Transonic System, Ithaca, NY). Thrombus formation was induced by applying two 1×1.5 mm filter papers (GB003, Schleicher & Schuell, Keene, NH) saturated with FeCl3 (3.5% w/v solution) to opposite sides of the artery for three min. After washing the site of injury with PBS, blood flow was monitored for 30 min. Mice were sacrificed using a pentobarbital overdose after conclusion of the experiment and under anesthesia.
In Vivo Rose Bengal Laser Injury Arterial Thrombosis Studies
In vivo testing using Rose Bengal thrombosis model studies were performed following literature reports [38]. Briefly, wild type C57Bl/6 mice (n=3 per group, weights 22–27 gms) were anesthetized, as described above, and 100 µl PBS with or without 500 µg of SbO4L was infused into the right internal jugular vein. Five minutes after infusion, Rose Bengal (75 mg/kg) was infused through the internal jugular vein and the carotid artery was illuminated with a 1.5 mW 540 nm laser (Melles Griot, Carlsbad, CA) positioned 6 cm from the artery. Flow was monitored for 120 min. Mice were sacrificed by a pentobarbital overdose after conclusion of the experiment and under anesthesia.
Tail Bleeding Time Studies
Wild type ICR mice (n ≥ 5, weights 30–45 gms) were anesthetized by an intraperitoneal injection of pentobarbital (50 mg/kg) and tail bleeding was performed as described in the literature [39]. Briefly, following anesthesia the animals were placed on a 37°C heating pad. Either LMWH (0.1 – 0.5 mg), SbO4L (1 mg) or control (PBS), prepared in 50 µl PBS, was injected into the lateral tail vein using a syringe. After 2 min, the tail tip (~4–5 mm) was transected with a scalpel and immediately immersed in a 1.7 mL microtube filled with 1.2 mL of PBS placed in a 37°C heating block. The animals were observed for 15 min. The total bleed time (defined as the sum of all bleeding events occurring within 15 min) and the weight of blood loss was recorded for each mouse.
Results
SbO4L is an Inhibitor of Thrombin Hydrolysis of its Small and Large Substrates
Among the animal models of thrombosis, mouse models have been developed the most [39]. There are small structural differences between mouse and human thrombins, especially within the sodium binding site (PDB ID:2OCV), which is known to allosterically regulate the catalytic triad [40,41]. Literature reports that the sodium binding site is also energetically coupled to exosites 1 and 2 [42], exosite 2 being the site of heparin and GPIbα binding. This raises a question whether SbO4L inhibits murine thrombin. We measured SbO4L inhibition of murine thrombin using Spectrozyme TH hydrolysis assay (Figure 2A), as performed earlier [33], and measured an IC50 of 0.17 µg/ml, which is essentially identical to the potency measured against the human version.
Figure 2.
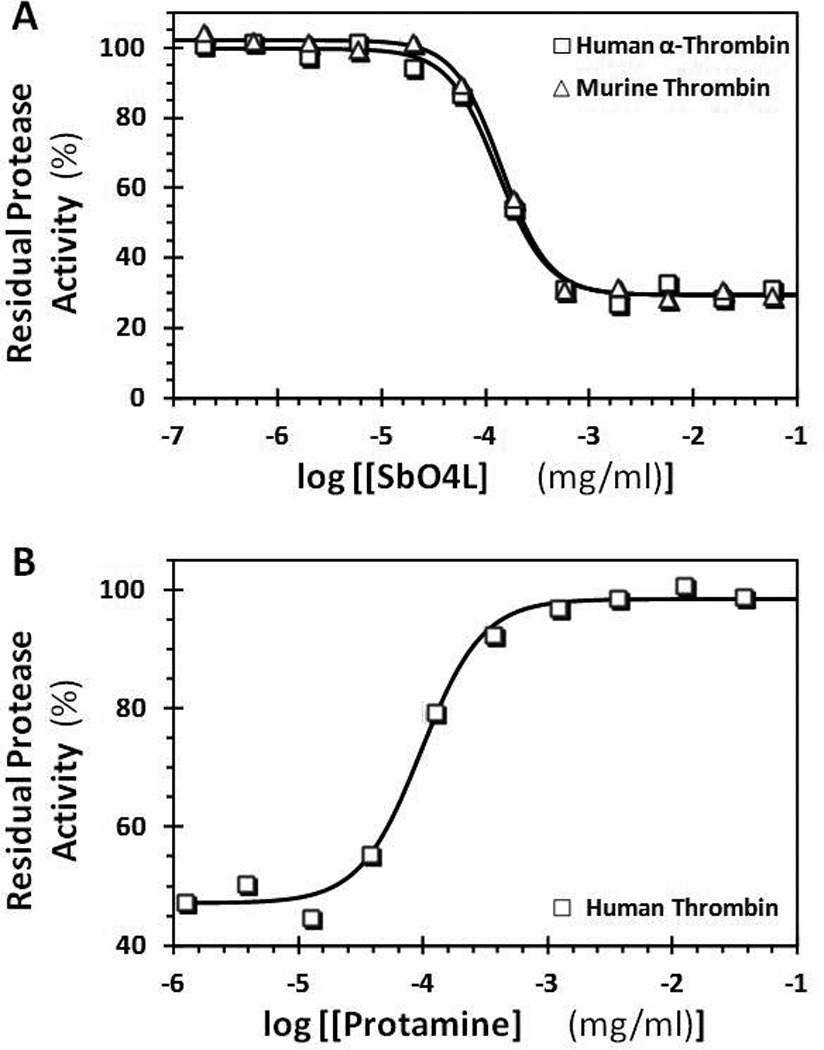
A comparison of direct inhibition of human and murine thrombins by SbO4L (A) and recovery of human thrombin activity in the presence of protamine (B). The concentration of residual thrombin activity at pH 7.4 and 37 °C was measured by chromogenic substrate hydrolysis assay, as described earlier [33]. Solid lines represent sigmoidal dose-response fits (Equation 1) to the data to obtain IC50 (A) or RC50 (B), Hill slope (HS) and maximal inhibition or recovery efficacy (ΔY). The definitions of these terms are provided in the methods section.
To also assess whether SbO4L inhibits thrombin cleavage of fibrinogen, we utilized a transmittance assay that quantify the formation of fibrin in buffer. Using this assay, we found that SbO4L inhibits thrombin cleavage of fibrinogen with an IC50 of 0.19 µg/ml, which is comparable to that measured using small molecule chromogenic substrate (see Supplementary Figure S1) and further confirming its possible value as an anticoagulant.
SbO4L Inhibition of Thrombin can be Reversed by Protamine
Protamine, an arginine-rich highly cationic protein mixture, is used to neutralize effects of highly sulfated heparin in vitro as well as in vivo. It is an FDA approved antidote for treating heparin overdose, which offers a major advantage to heparin-based anticoagulation therapy. Because SbO4L is also a sulfated agent, we reasoned that protamine may be able to neutralize its thrombin inhibition potential. To test this, we studied reversibility of SbO4L inhibition of thrombin by protamine using Spectrozyme TH hydrolysis assay. Figure 2B shows the gain in thrombin catalytic activity in the presence of increasing levels of protamine. The profile essentially mirrors the inhibition profile and the recovery was instantaneous. The concentration of protamine necessary to recover 50% of thrombin activity, i.e., RC50, was found to be ~0.1 µg/ml, which is comparable to the IC50 for SbO4L inhibition of thrombin. Also, protamine reversed SbO4L inhibition of thrombin by 100%, which can be expected to have significant advantages.
SbO4L Exhibits Good Anticoagulant Potential in Human Whole Blood Thromboelastography
To evaluate SbO4L as an anticoagulant in whole blood, we employed TEG, which attempts to simulate clotting under low shear conditions. TEG has been approved by the FDA for the diagnosis and management of coagulation disorders [43]. TEG is also quite often used to monitor LMWH therapy and is especially useful to assess the anticoagulant state during surgeries and coagulopathies [35,36]. TEG can easily monitor the time to initiation and rate of clot formation; however, it is also useful to assess the nature of physical forces within a clot. A good anticoagulant should slow clot formation as well as reduce the inter-molecular forces within the clot. Both these effects decrease the extent and integrity of clot, which are quantified by TEG parameters including maximum amplitude (MA), the shear elastic modulus (G), the reaction time (R) and the angle (α) [34–36].
Figure 3 shows the change in R, α, MA and G parameters as a function of the level of SbO4L or enoxaparin, a representative LMWH. We chose enoxaparin as a control because it is similar in homogeneity, size and charge to SbO4L. Since SbO4L and enoxaparin are active at different concentrations levels, the effect of the TEG parameters is displayed as a percentage of the maximal concentration of each agent used in the experiment. As the level of SbO4L increases from 0% to 100%, R increases from 7.7 to 25.9 min, while α decreases from 56° for normal blood to 22° indicating that the kinetics of fibrin polymerization and network formation is significantly depressed in the presence of SbO4L (Figures 3A and 3B). Essentially identical profiles for R and α were measured for enoxaparin at the respective % level used in the experiment. Likewise, the extent of fibrin polymerization and integrity of clot, i.e., MA and G, respectively, decreased with increasing levels of SbO4L (Figures 3C and 3D). As expected, enoxaparin results were found to be similar to SbO4L. Thus overall both agents introduced very similar changes in TEG parameters; however their dosage levels were different. SbO4L was active in the range of 5 to 150 µg/ml, whereas enoxaparin was active at levels of 1 to 5 µg/ml.
Figure 3.
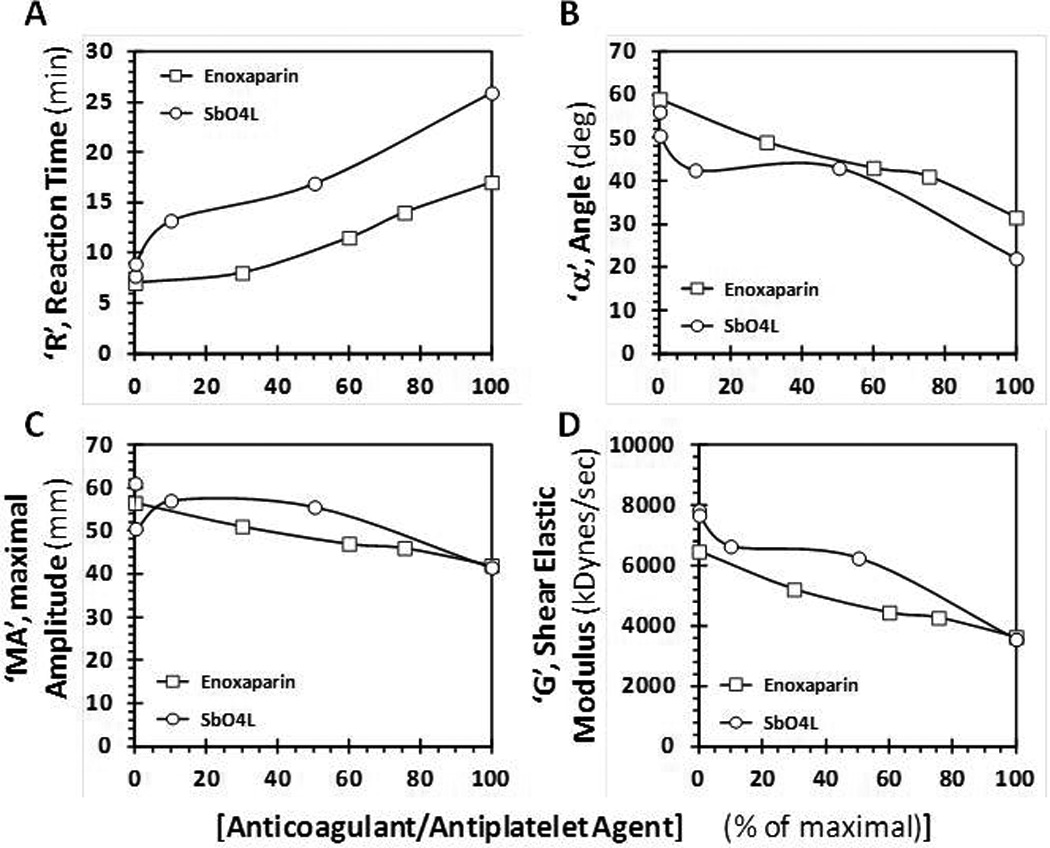
A comparison of the effects of SbO4L and enoxaparin on clot formation in human whole blood using thromboelastography (TEG). (A) and (B) show the changes in reaction time, ‘R’, and angle ‘α’, respectively, while (C) and (D) show the changes maximal amplitude, ‘MA’, and clot elastic modulus, ‘G’, respectively. These parameters are automatically calculated by the instrument manufacturer’s algorithm following an experiment, as described earlier [34–36]. SbO4L and enoxaparin concentrations are plotted as percent of the maximum used.
SbO4L Exhibit Good Anti-Platelet Activity in Human Whole Blood Hemostatic Analysis
To further assess the antithrombotic potential of SbO4L, we utilized HAS™, which evaluates platelet contribution to clot formation [44–46]. This technique provides information on contractile forces between platelets adhering to surfaces, which restrict the relative movement of two cups. The force measured, called the platelet contractile force (PCF) depends on several factors including the number and status of platelets and the presence of inhibitors that affect platelet activation [44–47]. HAS also assesses the internal micro-structure of a growing thrombus in the form of clot elastic modulus (CEM), which is the ratio of stress induced by platelets to strain arising from the change in clot thickness. CEM also depends on several factors including fibrinogen levels, activity and level of factor XIII, and thrombin formation rate [44–48]. It has been suggested that changes in PCF and CEM correlate with susceptibility to bleeding and/or thrombotic tendency [44,49,50].
The effects of SbO4L on PCF and CEM are illustrated in Figure 4 and Table 1. Both PCF and CEM are affected by SbO4L in a dose-dependent manner. As the concentration of SbO4L increases from 0 to 74 µg/ml, PCF and CEM decrease from 8.0 to 0.7 kDynes and 18.3 to 0.6 kDynes/cm2, respectively. These results parallel those measured through TEG. When comparisons are made with enoxaparin, essentially identical results are observed except for the difference in the concentration of enoxaparin necessary to prevent clotting. Whereas a reduction in PCF value of <1.0 kDynes was achieved at 74 µg/ml (or 6.2 µM) for SbO4L, it was achieved at 2.0 µg/ml (0.4 µM) for enoxaparin. Comparable results were also obtained at these doses when using PRP (platelet count ~200,000) insteadof human whole blood (see Supplementary Figure S2). These results suggest that SbO4L-mediated targeting of the GPIbα site of thrombin exosite 2 inhibits platelet activation resulting in significant reduction in PCF and CEM.
Figure 4.
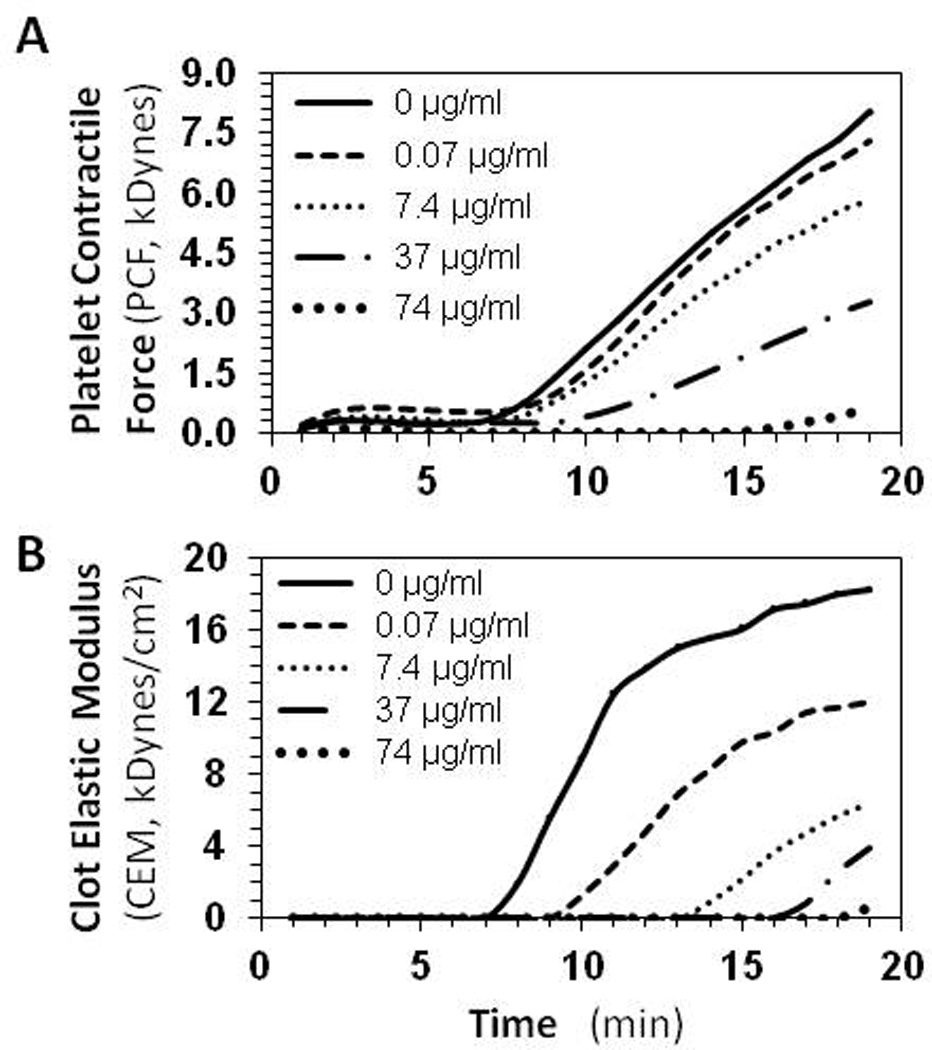
The effect of SbO4L on platelet function in human whole blood using hemostasis analysis system (HAS™). (A) and (B) show the change in platelet contractile force (PCF) and clot elastic modulus (CEM), respectively, with time at varying concentrations of SbO4L (0 to 74 µg/ml). See also Table 1 for quantitative variation of PCF and CEM at different SbO4L levels.
Table 1.
Hemostasis Analysis System Parameters for SbO4L Anticoagulation in Comparison to Enoxaparin.
| Hemostasis Analysis* | |||||
|---|---|---|---|---|---|
| [Conc] (µg/ml) |
[Conc]δ (µM) |
FOT* (min) |
PCF* (kDynes) |
CEM* (kDynes/cm2) |
|
| SbO4L | 0 | 0 | 6.3 | 8.0 | 18.3 |
| 0.07 | 0.006 | 5.1 | 7.3 | 12.0 | |
| 7.4 | 0.617 | 6.6 | 5.8 | 6.3 | |
| 37 | 3.080 | 11.3 | 3.3 | 3.9 | |
| 74 | 6.170 | 13.6 | 0.7 | 0.6 | |
| Enoxaparin | 0 | 0 | 6.2 | 7.6 | 21.6 |
| 0.7 | 0.156 | 5.2 | 5.3 | 15.1 | |
| 1.0 | 0.222 | 9.1 | 3.6 | 12.7 | |
| 1.6 | 0.356 | 11.0 | 2.8 | 8.5 | |
| 2.0 | 0.444 | 12.5 | 0.9 | 2.9 | |
Calculated using average molecular weights of 12,000 for SbO4L and 4,500 for enoxaparin.
Analysis was performed using Hemostasis Analysis System (HAS) on human whole blood as described. Parameters deduced from this analysis included FOT (force onset time), PCF (platelet contractile force) and CEM (clot elastic modulus).
SbO4L shows Good Antithrombotic Efficacy in Mouse Arterial Thrombosis Models
To assess whether the anticoagulant and antiplatelet activities of SbO4L translate into in vivo antithrombotic activity, we utilized two well-characterized mouse arterial thrombosis models including the FeCl3-induced [39] and the Rose Bengal-induced [39,51] artery injury models. Both models have been extensively utilized in the literature for studying anticoagulants [39,52], although neither model exactly simulates the pro-thrombotic state that humans typically experience. In fact, FeCl3 is considered to produce a severe injury, in comparison to a pro-thrombotic state in humans, and is typically accompanied by desquamation of vascular endothelium [53]. This implies that an agent that suppresses arterial occlusion following FeCl3 injury is likely to exhibit good antithrombotic potential in higher animals. Finally, detailed studies of dose-dependent reduction in thrombosis using the FeCl3-induced arterial injury model have been published in the literature for enoxaparin [54,55] and full-length heparin [37].
Exposure of the carotid artery of wild type mice to a 3.5% solution of FeCl3 resulted in the formation of an occlusive platelet-rich thrombus within 15 min. When SbO4L was injected approximately 10 mins before the application of FeCl3 patch, distinct reduction in extent of thrombus formation was observed (Figure 5). At 100 µg per animal, no significant effect was observed probably because thrombin was not saturated but at 250 or 500 µg dose, SbO4L prevented occlusion in ~35 to 50% of arteries studied. In contrast, administration of 1 mg SbO4L prevented occlusion in 100% of arteries studied (Figure 5). In comparison, literature reports indicate that a comparable ~1 mg dose of enoxaparin results in approximately 50% reduction in thrombosis in identical FeCl3-induced thrombosis models [54,55].
Figure 5.
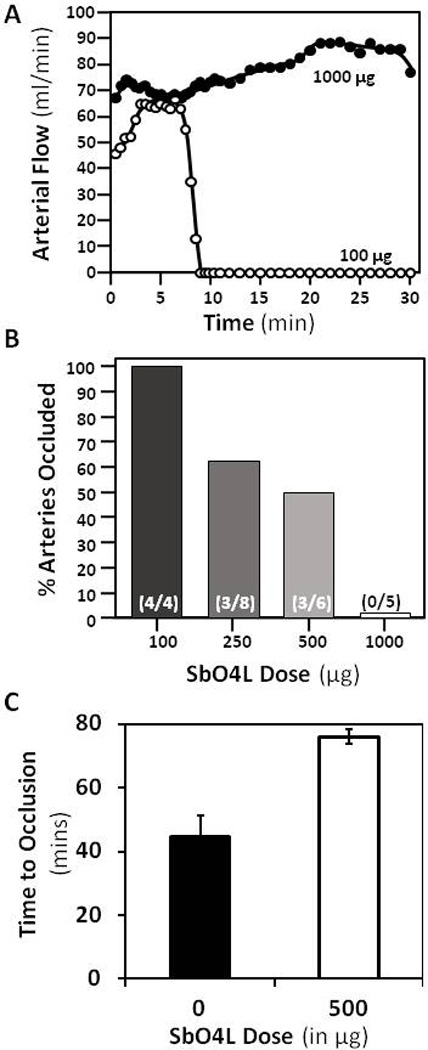
In vivo studies on the effect of SbO4L in two mouse models of arterial thrombosis. (A) Time-dependence of the formation of occlusive platelet-rich thrombus in the carotid artery of mice using a 3.5% FeCl3 solution with two doses of SbO4L, i.e., 100 µg and 1,000 µg. (B) Dose-dependent decrease in occlusion as the levels of SbO4L increase from 100 to 1,000 µg per animal. The number in brackets shows the fraction of mice that showed complete and sustained occlusion for 15 min. (C) Results obtained in the Rose Bengal laser injury induced arterial thrombosis model using a dose of 500 µg SbO4L per animal (or PBS injection).
The effects of SbO4L were also tested in the Rose Bengal-laser injury model of arterial thrombosis, in which the primary mechanism of generating a vessel injury is through local production of free radicals [39,51]. A focused beam of light converts the Rose Bengal dye to free radicals, which initiates the formation of a platelet-rich thrombus. Control experiments indicated that vessel occlusion was completed in approximately 45±7 min for wild type mice. In contrast, vessel occlusion occurred in 76±21 minutes in animals dosed with 500 µg of SbO4L approximately 10 min before laser injury (Figure 5C). This represents a significant prolongation in clotting suggesting potent antithrombotic effect of SbO4L. An important point to note here is that SbO4L appears to be more potent in comparison to enoxaparin in in vivo antithrombotic assays, while the reverse is true for in vitro assays. Although the basis for the better performance of SbO4L in vivo is not clear, it is likely to be synergy brought about the unique dual anticoagulant–antiplatelet function.
SbO4L does not Enhance Tail Bleeding
To assess bleeding propensity of SbO4L, the murine tail bleeding experiment, utilized in a large number of studies, was performed [39]. Figure 6 shows total bleeding time and total blood loss for 1 mg SbO4L, 0.1 mg, 0.3 mg and 0.5 mg enoxaparin and PBS injected approximately 10 min before the transaction of tail. For SbO4L, the tail bleeding time was found to be 7.6±0.4 min, which was essentially same as that noted for PBS (8.3±0.7 min). Likewise, the total blood lost following tail clipping for SbO4L was 0.043±0.008 mg, which was also identical to that noted for PBS (0.044±0.016 mg). In comparison, a 3-fold lower dose of enoxaparin (0.3 mg per animal) showed a bleeding time of 14.3±0.2 mins and a blood loss of 0.085±0.022 mg. These represent a ~1.7-fold higher bleeding time and a ~1.9-fold more blood loss for enoxaparin than SbO4L. Similar results have been reported earlier in the literature [54,55]. To identify which dose of enoxaparin would produce results similar to SbO4L, we studied 0.1, 0.2, 0.4 and 0.5 mg doses. In all cases, enoxaparin induced more bleeding time and equal or more blood weight than SbO4L (Figure 6, data shown only for 0.1 and 0.5 mg doses). Thus, SbO4L appears to display reduced bleeding tendency in comparison to enoxaparin.
Figure 6.
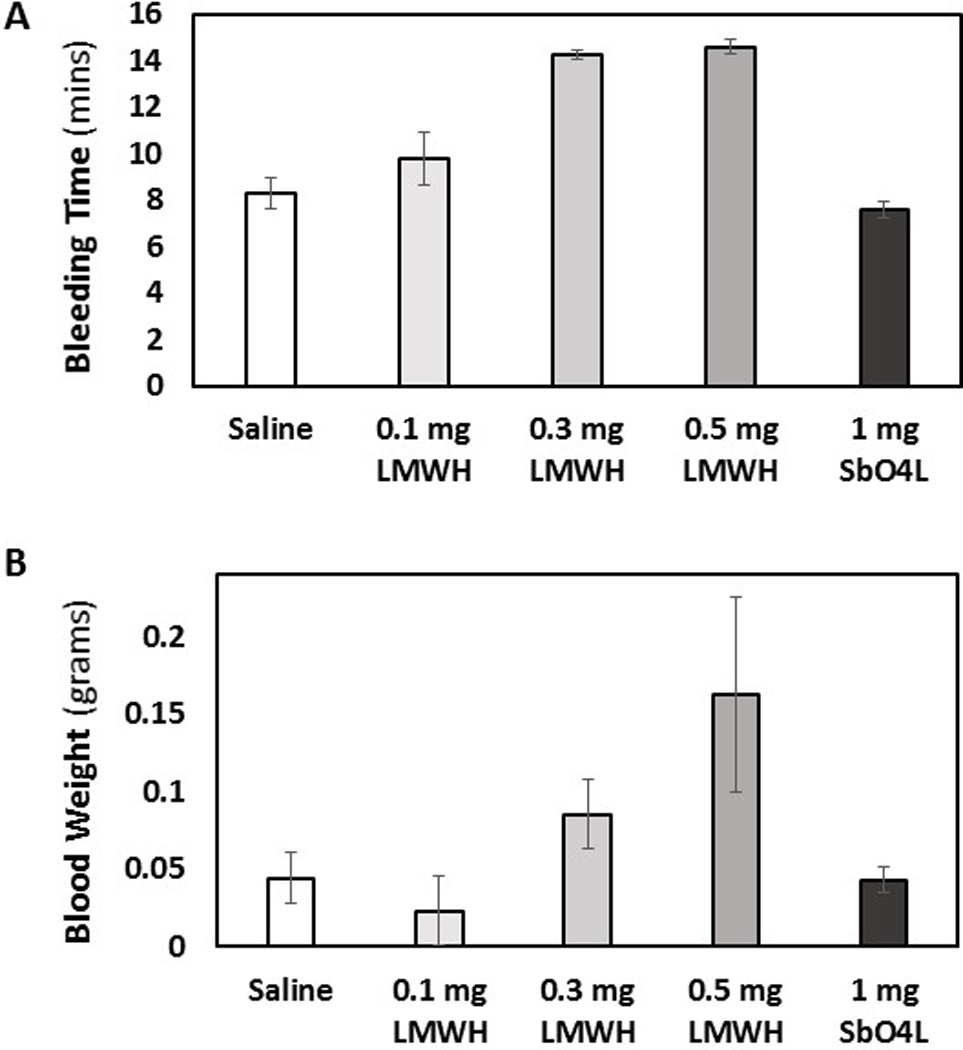
A comparison of mouse tail bleeding following IV injection of either 1 mg SbO4L, with 0.1, 0.3 or 0.5 mg enoxaparin or PBS. Two parameters were followed including bleeding time in min (A) and total blood loss in gms (B). Error bars represent ±1 SEM using N = 5. SbO4L was found to be display less bleeding tendency in comparison to enoxaparin and not significantly different from vehicle.
Discussion
This work brings forth two primary ideas: 1) direct, allosteric inhibition of thrombin can generate sufficient antithrombotic potential and may also provide an advantage in terms of reducing bleeding risk and 2) SbO4L is a novel prototypic, first-in-class molecule that simultaneously displays good anticoagulant and antiplatelet properties by targeting the GPIbα/heparin-binding site on thrombin. Except for hirudin/bivalirudin, all direct inhibitors used in the clinic today are active site inhibitors. Even hirudin/bivalirudin bind in the active site, while also engaging exosite 1 of thrombin. Thus, SbO4L attempts to establish a novel approach of direct, allosteric inhibition in developing new antithrombotics.
Secondary concepts being highlighted include a) targeting the heparin- and GPIbα- binding site of thrombin and b) dual anticoagulant and antiplatelet function in the same molecule. Although unfractionated heparin binds in exosite 2 of thrombin, it alone does not induce inhibition of thrombin and has to rely on antithrombin, wherein it bridges thrombin and antithrombin to form a covalent complex. This implies that structural features of SbO4L, although mimicking the highly sulfated feature of heparin, are important for inducing an allosteric conformational change. We have shown earlier that SbO4L recognizes hydrophobic sub-domains present within exosite 2, which induces allosteric structural changes [33,56]. SbO4L targets overlapping binding sites of heparin and GPIbα, which advantageously elicits the dual anticoagulant and antiplatelet function. No therapeutic has been developed to date that targets this overlapping binding site, which suggests that better analogs may be possible to develop through structure-based drug discovery.
A common concern with dual anticoagulant and antiplatelet agents is the possibility of elevated bleeding. This work shows that this may not necessarily be true and additional studies should be performed to further establish the concept of reduced bleeding by targeting the dual heparin- and GPIbα- binding site of thrombin. The exact reason why SbO4L displays lower bleeding tendency in tail bleeding studies is yet to be deciphered. We have pursued allosterism as a mechanism to discover new anticoagulants because of its potential in enhancing the specificity of action. Allosteric binding sites on proteins are thought to be more structurally diverse, especially for related proteins, which should reduce the chances of cross-reactivity [57]. In fact, SbO4L is highly selective in targeting thrombin and does not affect the closely related factor Xa [33]. Another reason why SbO4L is not saddled with higher bleeding tendency could be its reduced charge density in comparison to heparins. Whereas heparins possess an average of 2.7 negative charges per repeating unit [58], SbO4L possesses only 1.7 sulfate group per monomer [33]. Higher charge density tends to induce binding to many blood proteins and cells, which may enhance bleeding [59].
This work presents SbO4L as the first-in-class allosteric agent that simultaneously induces anticoagulation and antiplatelet actions. SbO4L is not the final therapeutic agent that is ready for clinical studies. Yet, it offers a number of advantages. Its antithrombotic activity was possible to reverse in vitro. Second, although not presented here, SbO4L is a fully synthetic molecule that can be prepared in two simple steps using common raw materials in fairly high yields [33]. SbO4L is also much better structurally defined than the heterogeneous heparins used in the clinic. SbO4L itself has a fairly uniform composition (see Figure 1) that can be monitored with ease using common analytical techniques, which implies that advanced versions of this molecule should be more easily assessed for quality control. Overall, direct, selective, allosteric inhibition of thrombin through SbO4L appears to be a promising approach worth advanced antithrombotic and toxicological studies.
Supplementary Material
Essentials.
Allosteric inhibition is a promising method for reducing bleeding risk associated with anticoagulants.
Sulfated b-O4 lignin (SbO4L) targets exosite 2 of thrombin to induce antithrombotic effects.
SbO4L exhibits dual anticoagulant and antiplatelet effects without increasing tail bleeding time.
This work presents a novel molecule exploiting a novel mechanism of anticoagulation.
Acknowledgments
This work was supported by grants HL090586, HL107152 and HL128639 from the National Institutes of Health to URD.
A. Y. Mehta and U. R. Desai have a patent Sulfated Beta-O4 Low Molecular Weight Lignins pending.
D. Gailani reports grants from National Heart, Lung and Blood Institute during the conduct of the study; non-financial support from Aronora, Bayer, Bristol-Myers Squibb, Dyax, personal fees from Isis, Novartis, Ono Phmaraceutical, Instrument Laboratory, and Merck outside the submitted work; In addition, D. Gailani has a patent Anti-Factor XI Antibodies with royalties paid.
Footnotes
Author Contributions
A. Y. Mehta performed SbO4L synthesis, biochemical experiments, helped with TEG and HAS experiments and prepared the initial draft of manuscript; B. M. Mohammed, E. J. Martin, D. F. Brophy helped with TEG, HAS and tail bleeding studies; D. Gailani supervised arterial thrombosis studies; U. R. Desai supervised the entire study and finalized the paper.
Disclosure
Other authors have nothing to disclose.
References
- 1.Day ISCfWT. Thrombosis: A major contributor to global disease burden. Thromb Res. 2014;134:931–938. doi: 10.1016/j.thromres.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Henry BL, Desai UR. Anticoagulants. In: Abraham DJ, Rotella DP, editors. Burger's Medicinal Chemistry, Drug Discovery and Development. 7th. Hoboken, NJ: John Wiley; 2010. pp. 365–408. [Google Scholar]
- 3.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW American College of Chest P. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 4.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ American College of Chest P. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 5.Lim W, Dentali F, Eikelboom JW, Crowther MA. Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med. 2006;144:673–684. doi: 10.7326/0003-4819-144-9-200605020-00011. [DOI] [PubMed] [Google Scholar]
- 6.Werth S, Breslin T, NiAinle F, Beyer-Westendorf J. Bleeding Risk, Management and Outcome in Patients Receiving Non-VKA Oral Anticoagulants (NOACs) Am J Cardiovasc Drugs. 2015;15:235–242. doi: 10.1007/s40256-015-0123-6. [DOI] [PubMed] [Google Scholar]
- 7.Baber U, Mastoris I, Mehran R. Balancing ischaemia and bleeding risks with novel oral anticoagulants. Nat Rev Cardiol. 2014;11:693–703. doi: 10.1038/nrcardio.2014.170. [DOI] [PubMed] [Google Scholar]
- 8.Miesbach W, Seifried E. New direct oral anticoagulants--current therapeutic options and treatment recommendations for bleeding complications. Thromb Haemost. 2012;108:625–632. doi: 10.1160/TH12-05-0319. [DOI] [PubMed] [Google Scholar]
- 9.Capodanno D, Ferreiro JL, Angiolillo DJ. Antiplatelet therapy: new pharmacological agents and changing paradigms. J Thromb Haemost. 2013;111(Suppl):316–329. doi: 10.1111/jth.12219. [DOI] [PubMed] [Google Scholar]
- 10.Chassot PG, Delabays A, Spahn DR. Perioperative antiplatelet therapy: the case for continuing therapy in patients at risk of myocardial infarction. Br J Anaesth. 2007;99:316–328. doi: 10.1093/bja/aem209. [DOI] [PubMed] [Google Scholar]
- 11.Levi M, Eerenberg E, Kamphuisen PW. Bleeding risk and reversal strategies for old and new anticoagulants and antiplatelet agents. J Thromb Haemost. 2011;9:1705–1712. doi: 10.1111/j.1538-7836.2011.04432.x. [DOI] [PubMed] [Google Scholar]
- 12.Tantry US, Gesheff M, Liu F, Bliden KP, Gurbel PA. Resistance to antiplatelet drugs: what progress has been made? Expert Opin Pharmacother. 2014;15:2553–2564. doi: 10.1517/14656566.2014.968126. [DOI] [PubMed] [Google Scholar]
- 13.Hochtl T, Pachinger L, Unger G, Geppert A, Wojta J, Harenberg J, Huber K. Antiplatelet drug induced isolated profound thrombocytopenia in interventional cardiology: a review based on individual case reports. J Thromb Thrombolysis. 2007;24:59–64. doi: 10.1007/s11239-006-9052-1. [DOI] [PubMed] [Google Scholar]
- 14.Capodanno D, Angiolillo DJ. Antithrombotic therapy in patients with chronic kidney disease. Circulation. 2012;125:2649–2661. doi: 10.1161/CIRCULATIONAHA.111.084996. [DOI] [PubMed] [Google Scholar]
- 15.Schneider DJ, Sobel BE. Conundrums in the combined use of anticoagulants and antiplatelet drugs. Circulation. 2007;116:305–315. doi: 10.1161/CIRCULATIONAHA.106.655910. [DOI] [PubMed] [Google Scholar]
- 16.Massel DR, Little SH. Antiplatelet and anticoagulation for patients with prosthetic heart valves. Cochrane Database Syst Rev. 2013;7:CD003464. doi: 10.1002/14651858.CD003464.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oldgren J, Wallentin L, Alexander JH, James S, Jonelid B, Steg G, Sundstrom J. New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: a systematic review and meta-analysis. Eur Heart J. 2013;34:1670–1680. doi: 10.1093/eurheartj/eht049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess CN, James S, Lopes RD, Wojdyla DM, Neely ML, Liaw D, Hagstrom E, Bhatt DL, Husted S, Goodman SG, Lewis BS, Verheugt FW, De Caterina R, Ogawa H, Wallentin L, Alexander JH. Apixaban Plus Mono Versus Dual Antiplatelet Therapy in Acute Coronary Syndromes: Insights From the APPRAISE-2 Trial. J Am Coll Cardiol. 2015;66:777–787. doi: 10.1016/j.jacc.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Adam F, Guillin MC, Jandrot-Perrus M. Glycoprotein Ib-mediated platelet activation. A signalling pathway triggered by thrombin. Eur J Biochem. 2003;270:2959–2970. doi: 10.1046/j.1432-1033.2003.03670.x. [DOI] [PubMed] [Google Scholar]
- 20.Soslau G, Class R, Morgan DA, Foster C, Lord ST, Marchese P, Ruggeri ZM. Unique pathway of thrombin-induced platelet aggregation mediated by glycoprotein Ib. J Biol Chem. 2001;276:21173–21183. doi: 10.1074/jbc.M008249200. [DOI] [PubMed] [Google Scholar]
- 21.Lechtenberg BC, Freund SM, Huntington JA. GpIbα Interacts Exclusively with Exosite II of Thrombin. J Mol Biol. 2014;426:881–893. doi: 10.1016/j.jmb.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabo TM, Maurer MC. Biophysical investigation of GpIbα binding to thrombin anion binding exosite II. Biochemistry. 2009;48:7110–7122. doi: 10.1021/bi900745b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Johnson DJ, Esmon CT, Huntington JA. Structure of the antithrombin-thrombin-heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat Struct Mol Biol. 2004;11:857–862. doi: 10.1038/nsmb811. [DOI] [PubMed] [Google Scholar]
- 24.De Candia E. Mechanisms of platelet activation by thrombin: a short history. Thromb Res. 2012;129:250–256. doi: 10.1016/j.thromres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 25.De Candia E, Hall SW, Rutella S, Landolfi R, Andrews RK, De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J Biol Chem. 2001;276:4692–4698. doi: 10.1074/jbc.M008160200. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan V, DeGuzman F, Bao M, Hall SW, Leung LL, Phillips DR. A thrombin receptor function for platelet glycoprotein Ib-IX unmasked by cleavage of glycoprotein V. Proc Natl Acad Sci U S A. 2001;98:1823–1828. doi: 10.1073/pnas.98.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celikel R, McClintock RA, Roberts JR, Mendolicchio GL, Ware J, Varughese KI, Ruggeri ZM. Modulation of α-thrombin function by distinct interactions with platelet glycoprotein Ibα. Science. 2003;301:218–221. doi: 10.1126/science.1084183. [DOI] [PubMed] [Google Scholar]
- 28.Jandrot-Perrus M, Clemetson KJ, Huisse MG, Guillin MC. Thrombin interaction with platelet glycoprotein Ib: effect of glycocalicin on thrombin specificity. Blood. 1992;80:2781–2786. [PubMed] [Google Scholar]
- 29.Li CQ, Vindigni A, Sadler JE, Wardell MR. Platelet glycoprotein Ibα binds to thrombin anion-binding exosite II inducing allosteric changes in the activity of thrombin. J Biol Chem. 2001;276:6161–6168. doi: 10.1074/jbc.M004164200. [DOI] [PubMed] [Google Scholar]
- 30.Sidhu PS, Abdel Aziz MH, Sarkar A, Mehta AY, Zhou Q, Desai UR. Designing allosteric regulators of thrombin. Exosite 2 features multiple subsites that can be targeted by sulfated small molecules for inducing inhibition. J Med Chem. 2013;56:5059–5070. doi: 10.1021/jm400369q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidhu PS, Liang A, Mehta AY, Abdel Aziz MH, Zhou Q, Desai UR. Rational design of potent, small, synthetic allosteric inhibitors of thrombin. J Med Chem. 2011;54:5522–5531. doi: 10.1021/jm2005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry BL, Monien BH, Bock PE, Desai UR. A novel allosteric pathway of thrombin inhibition: Exosite II mediated potent inhibition of thrombin by chemo-enzymatic, sulfated dehydropolymers of 4-hydroxycinnamic acids. J Biol Chem. 2007;282:31891–31899. doi: 10.1074/jbc.M704257200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta AY, Thakkar JN, Mohammed BM, Martin EJ, Brophy DF, Kishimoto T, Desai UR. Targeting the GPIbα binding site of thrombin to simultaneously induce dual anticoagulant and antiplatelet effects. J Med Chem. 2014;57:3030–3039. doi: 10.1021/jm4020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henry BL, Thakkar JN, Martin EJ, Brophy DF, Desai UR. Characterization of the plasma and blood anticoagulant potential of structurally and mechanistically novel oligomers of 4-hydroxycinnamic acids. Blood Coagul Fibrinolysis. 2009;20:27–34. doi: 10.1097/MBC.0b013e328304e077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowry R, Fraser S, Archeval-Lao JM, Parker SA, Cai C, Rahbar MH, Grotta JC. Thrombelastography detects the anticoagulant effect of rivaroxaban in patients with stroke. Stroke. 2014;45:880–883. doi: 10.1161/STROKEAHA.113.004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lance MD. A general review of major global coagulation assays: thrombelastography, thrombin generation test and clot waveform analysis. Thromb J. 2015;13:1. doi: 10.1186/1477-9560-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renne T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–3989. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gailani D, Cheng Q, Ivanov IS. Murine models in the evaluation of heparan sulfate-based anticoagulants. Methods Mol Biol. 2015;1229:483–496. doi: 10.1007/978-1-4939-1714-3_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marino F, Chen ZW, Ergenekan CE, Bush-Pelc LA, Mathews FS, Di Cera E. Structural basis of Na+ activation mimicry in murine thrombin. J Biol Chem. 2007;282:16355–16361. doi: 10.1074/jbc.M701323200. [DOI] [PubMed] [Google Scholar]
- 41.Bush LA, Nelson RW, Di Cera E. Murine thrombin lacks Na+ activation but retains high catalytic activity. J Biol Chem. 2006;281:7183–7188. doi: 10.1074/jbc.M512082200. [DOI] [PubMed] [Google Scholar]
- 42.Huntington JA. How Na+ activates thrombin--a review of the functional and structural data. Biol Chem. 2008;389:1025–1035. doi: 10.1515/BC.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omert L, Popovsky MA. Laboratory test utilization. Am J Clin Pathol. 2012;137:165–166. doi: 10.1309/AJCPU9X4GZNNHVBR. author reply 6. [DOI] [PubMed] [Google Scholar]
- 44.Carr ME., Jr Development of platelet contractile force as a research and clinical measure of platelet function. Cell Biochem Biophys. 2003;38:55–78. doi: 10.1385/CBB:38:1:55. [DOI] [PubMed] [Google Scholar]
- 45.Carr ME, Jr, Martin EJ, Carr SL. Delayed, reduced or inhibited thrombin production reduces platelet contractile force and results in weaker clot formation. Blood Coagul Fibrinolysis. 2002;13:193–197. doi: 10.1097/00001721-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Carr ME, Jr, Carr SL. Fibrin structure and concentration alter clot elastic modulus but do not alter platelet mediated force development. Blood Coagul Fibrinolysis. 1995;6:79–86. doi: 10.1097/00001721-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Carr ME., Jr Measurement of platelet force: the Hemodyne hemostasis analyzer. Clin Lab Manage Rev. 1995;9:312–314. 6–8, 20. [PubMed] [Google Scholar]
- 48.Kurniawan NA, Grimbergen J, Koopman J, Koenderink GH. Factor XIII stiffens fibrin clots by causing fiber compaction. J Thromb Haemost. 2014;12:1687–1696. doi: 10.1111/jth.12705. [DOI] [PubMed] [Google Scholar]
- 49.Krishnaswami A, Carr ME, Jr, Jesse RL, Kontos MC, Minisi AJ, Ornato JP, Vetrovec GW, Martin EJ. Patients with coronary artery disease who present with chest pain have significantly elevated platelet contractile force and clot elastic modulus. Thromb Haemost. 2002;88:739–744. [PubMed] [Google Scholar]
- 50.Carr ME, Jr, Hackney MH, Hines SJ, Heddinger SP, Carr SL, Martin EJ. Enhanced platelet force development despite drug-induced inhibition of platelet aggregation in patients with thromboangiitis obliterans--two case reports. Vasc Endovascular Surg. 2002;36:473–480. doi: 10.1177/153857440203600610. [DOI] [PubMed] [Google Scholar]
- 51.Perez P, Alarcon M, Fuentes E, Palomo I. Thrombus formation induced by laser in a mouse model. Exp Ther Med. 2014;8:64–68. doi: 10.3892/etm.2014.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whinna HC. Overview of murine thrombosis models. Thromb Res. 2008;122(Suppl 1):S64–S69. doi: 10.1016/S0049-3848(08)70022-7. [DOI] [PubMed] [Google Scholar]
- 53.Eckly A, Hechler B, Freund M, Zerr M, Cazenave JP, Lanza F, Mangin PH, Gachet C. Mechanisms underlying FeCl3-induced arterial thrombosis. J Thromb Haemost. 2011;9:779–789. doi: 10.1111/j.1538-7836.2011.04218.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Lowenberg EC, Crosby JR, MacLeod AR, Zhao C, Gao D, Black C, Revenko AS, Meijers JC, Stroes ES, Levi M, Monia BP. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116:4684–4692. doi: 10.1182/blood-2010-04-277798. [DOI] [PubMed] [Google Scholar]
- 55.Toomey JR, Blackburn MN, Storer BL, Valocik RE, Koster PF, Feuerstein GZ. Comparing the antithrombotic efficacy of a humanized anti-factor IX(a) monoclonal antibody (SB 249417) to the low molecular weight heparin enoxaparin in a rat model of arterial thrombosis. Thromb Res. 2000;100:73–79. doi: 10.1016/s0049-3848(00)00299-1. [DOI] [PubMed] [Google Scholar]
- 56.Mehta AY, Desai UR. Substantial non-electrostatic forces are needed to induce allosteric disruption of thrombin's active site through exosite 2. Biochem Biophys Res Commun. 2014;452:813–816. doi: 10.1016/j.bbrc.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merdanovic M, Monig T, Ehrmann M, Kaiser M. Diversity of allosteric regulation in proteases. ACS Chem Biol. 2013;8:19–26. doi: 10.1021/cb3005935. [DOI] [PubMed] [Google Scholar]
- 58.Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 59.Young E, Cosmi B, Weitz J, Hirsh J. Comparison of the non-specific binding of unfractionated heparin and low molecular weight heparin (Enoxaparin) to plasma proteins. Thromb Haemost. 1993;70:625–630. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


