Abstract
IMPORTANCE
Unintentional weight loss has been associated with risk of dementia. Since mild cognitive impairment (MCI) is a prodromal stage for dementia, we sought to evaluate whether changes in weight and body mass index (BMI) may predict incident MCI.
OBJECTIVE
To investigate the association of change in weight and BMI with risk of MCI.
DESIGN, SETTING, AND PARTICIPANTS
A population-based, prospective study of participants aged 70 years and older from the Mayo Clinic Study of Aging. Maximum weight and height in midlife (aged 40 to 65 years old) were retrospectively ascertained from the medical records of participants using a medical records linkage system.
MAIN OUTCOMES MEASURES
Participants were evaluated for cognitive outcomes of normal cognition, MCI, or dementia at baseline and prospectively assessed for incident events at each 15-month evaluation. The association of rate of change in weight and body mass index with risk of MCI was investigated using proportional hazards models.
RESULTS
Over a mean follow-up of 4.4 years, 524 of 1895 cognitively normal participants developed incident MCI. The mean (standard deviation) rate of weight change per decade from midlife to study entry was greater for individuals who developed incident MCI vs. those who remained cognitively normal (−2.0 (5.1) vs. −1.2 (4.9) kg; p = 0.006). A greater decline in weight per decade was associated with an increased risk of incident MCI (hazard ratio [HR] 95% confidence interval [CI], 1.04 [1.02, 1.06], p < 0.001) after adjusting for sex, education and apolipoprotein E (APOE) ε4 allele. A weight loss of 5 kg/decade corresponds to a 24% increase in risk of MCI (HR=1.24). Higher decline in BMI per decade was also associated with incident MCI (HR, 1.08, 95% CI = [1.03, 1.13], p = 0.003).
CONCLUSIONS AND RELEVANCE
These findings suggest that declining weight from midlife to late-life is a marker for MCI and may help identify persons at increased risk for MCI.
Keywords: Mild cognitive impairment, weight, body mass index, prospective, cohort study, population based
Introduction
Mild cognitive impairment (MCI) is a prodromal stage of dementia that provides opportunity to study risk factors for and to identify persons at increased risk for dementia. Approximately 5 to 15% of persons with MCI will progress to dementia per year.1,2 Therefore, the delay or prevention of MCI could also reduce the public health impact of dementia. Several modifiable risk factors are associated with an increased risk of MCI, including education, vascular risk factors and related outcomes.1
Changes in body mass index (BMI) and weight are also associated with increased risk of dementia, but overall the findings of different studies have been inconclusive. Some studies have reported associations of lower late life BMI, declining weight or faster decline in BMI in late life with increased risk of dementia;3–7 others suggest that overweight at older ages,8 central obesity in midlife9 or higher midlife BMI10,11 increase the risk of dementia and Alzheimer Disease (AD). In contrast, one study found that being underweight in midlife may increase the risk of dementia in late life. Another study also observed an association between low BMI in midlife and increased dementia deaths in late life. Furthermore, other investigators have reported that obesity in midlife is associated with decreased risk of cognitive decline and dementias12 or have not observed a predictive role of high late life BMI for cognitive decline.13 The association of BMI with MCI is even less certain. Few investigators have observed a decline in BMI prior to a diagnosis of MCI.14,15 The association of declining weight and BMI with MCI may have implications for preventive strategies for MCI. The objective of this study was to examine associations of longitudinal changes in weight and BMI with incident MCI among Mayo Clinic Study of Aging (MCSA).
Methods
Study Participants
The study design and methodology are published and are only briefly described here.16–18 Participants were enrolled in the population-based MCSA established in Olmsted County, Minnesota. At initiation of the study, a sampling frame of Olmsted County residents who were 70 to 89 years old on October 1, 2004 (total population = 9,953) was constructed using the medical records-linkage system of the Rochester Epidemiology Project.16 Participants were randomly selected from this sampling frame and eligible subjects (without dementia or in hospice care) were recruited to the study. Recruitment is ongoing to maintain the sample size, and participants are seen every 15 months. The current study includes participants who were cognitively normal at the baseline evaluation, had at least one follow-up evaluation, and had data on maximum weight and height in midlife (aged 40 to 65 years old; mean, 58.6).
Approval of Study Protocols, and Participant Consent
The study was approved by the Institutional Review Boards of the Mayo Clinic and the Olmsted Medical Center. Written informed consent was obtained from each participant prior to the participation.
Participant Evaluation
Participants were evaluated by a nurse or a study coordinator who assessed their memory, and administered the Clinical Dementia Rating scale,19 and Functional Activities Questionnaire20 to the participant’s informant. Each participant underwent neuropsychological testing using 9 tests to assess performance in four cognitive domains: memory, executive function, language, and visuospatial skills.17,18,21,22 Participants also had a neurological evaluation by a physician.
Identification of MCI/Dementia
The nurse or the study coordinator, the physician who evaluated the participant, and a neuropsychologist reviewed all data collected for each participant. A diagnosis of MCI, dementia or normal cognition was made by consensus.17 Participants were classified as cognitively normal if they performed in the normative range and did not meet criteria for MCI23 or for dementia.24 Incident MCI cases were classified as amnestic (aMCI) if the memory domain was impaired or nonamnestic (naMCI) if the memory domain was not impaired.25
Assessment of Weight, Height, and BMI at Baseline and Follow-Up Evaluations
Weight and height were measured at each evaluation, and body mass index (BMI) was computed (as weight in kilograms/height in meters; kg/m2). The maximum weight and height in midlife (mean age, 58.6 years old) were ascertained from the medical record of each participant by trained nurse abstractors using the medical records linkage system of the Rochester Epidemiology Project.
Assortment of Other Covariates
Demographical variables including age, sex, and education were obtained at the baseline visit for each participant. A history of type 2 diabetes mellitus, hypertension, coronary heart disease, and stroke were abstracted from the medical records at baseline and during follow-up. Depressive symptoms were assessed with the Beck Depression Inventory (BDI). History of cigarette smoking (never, former, current) and diagnosed alcohol problems were assessed from self-report. Current medications were assessed from the medication bottles at each evaluation. Apolipoprotein E (APOE) ε4 allele genotyping was performed at baseline evaluation.
Statistical Analysis
All cognitively normal individuals at baseline were considered at risk for incident MCI. MCI onset was assigned at the midpoint between the last assessment as cognitively normal and the first-ever assessment as MCI. Persons who developed dementia without an intervening MCI diagnosis were presumed to have had an undetected MCI phase. We computed the follow-up duration from baseline to MCI onset for incident cases and through the date of the last follow-up for participants who remained cognitively normal. Subjects who refused participation, could not be contacted, or died during follow-up were censored at their last evaluation.
We computed the rate of weight change (kg/decade) from the maximum weight in midlife through follow-up, including late life weights as a time-dependent variable. We investigated the association of weight change with incident MCI using Cox proportional hazards model and reported hazard ratios (HR) and 95% confidence intervals (CI); in separate models we included maximum weight in midlife or late life weights. We examined confounding by APOE ε4 allele, diabetes mellitus, hypertension, stroke, and cigarette smoking with each variable added separately, and effect modification by age, sex, and APOE ε4 allele by including interaction terms of these covariates with rate of weight change.
Multivariable models included: Model 1: sex (where applicable), education and APOE ε4 genotype (ε4 carrier vs non-carrier); Model 2: included model 1 variables and potential confounders: alcohol problem (yes vs. no), depressive symptoms (BDI score ≥16), use of statins, diabetes mellitus, hypertension, coronary heart disease, cigarette smoking (never vs. former or current) and stroke.
We also examined the effect of including height in the models. In a separate model, we included both maximum weight in midlife and late life weights, but not weight change, in the same model and examined the associations with MCI. We repeated the analyses using rate of change in BMI (unit/decade). All hypothesis testing was conducted assuming an alpha = 0.05 significance level and a two-sided alternative hypothesis. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, North Carolina).
Results
The characteristics of the 1,895 cognitively normal participants at baseline (50.3% men, mean age 78.5 years) are summarized by sex in Table 1. Men had a higher frequency of former or current smoking (61.2% vs. 36.0%, p < 0.001), diabetes mellitus (20.4% vs. 14.3%, p = 0.001), and coronary artery disease (49.2% vs. 29.1%, p <0.001) than women. Women had a non-significantly greater mean (standard deviation [SD]) rate of weight change per decade from midlife to study entry compared to men, but a lower maximum weight and lower BMI in midlife compared to men.
Table 1.
Summary of Participants’ Characteristics by Sex
| Characteristics | All (N = 1895) |
Men (N = 954) |
Women (N = 941) |
p |
|---|---|---|---|---|
| Age, mean (SDa) | 78.5 (5.1) | 78.2 (4.9) | 78.9 (5.4) | 0.016 |
| Men, n (%) | 954 (50.3) | --- | --- | --- |
| Education (years), mean (SD) | 14.0 (2.9) | 14.3 (3.2) | 13.7 (2.4) | <0.001 |
| Ever Smoking, n (%) | 923 (48.7) | 584 (61.2) | 339 (36.0) | <0.001 |
| APOE ε4 allele carrierb n (%) | 489 (25.9) | 246 (25.8) | 243 (26.0) | 0.94 |
| Follow-Up (years), mean (SD) | 4.4 (2.4) | 4.3 (2.5) | 4.5 (2.4) | 0.05 |
| Midlife Weight to Study Entry (years), mean (SD) | 19.9 (8.6) | 20.1 (8.6) | 19.7 (8.6) | 0.16 |
| Body Mass Index (kg/m2), mean (SD) | ||||
| Maximum Midlifec (ages 40–65) | 28.3 (4.9) | 28.7 (4.2) | 27.9 (5.6) | <0.001 |
| Late lifed (study entry) | 27.9 (5.0) | 28.2 (4.4) | 27.6 (5.5) | <0.001 |
| Weight (Kg), mean (SD) | ||||
| Maximum Midlifee (ages 40–65) | 82.0 (16.7) | 89.9 (14.1) | 74.1 (15.3) | <0.001 |
| Late lifef | 79.3 (17.0) | 87.4 (14.9) | 71.0 (15.0) | <0.001 |
| Rate of Weight Changeg, mean (SD)h | −1.4 (5.0) | −1.3 (4.8) | −1.6 (5.1) | 0.87 |
| Clinical conditions | ||||
| Diabetes Mellitus (type 2), n (%) | 330 (17.4) | 195 (20.4) | 135 (14.3) | <0.001 |
| Hypertension, n (%) | 1460 (77.0) | 742 (77.8) | 718 (76.3) | 0.45 |
| Coronary Artery Disease, n (%) | 743 (39.2) | 469 (49.2) | 274 (29.1) | <0.001 |
| Stroke, n (%) | 80 (4.2) | 43 (4.5) | 37 (3.9) | 0.53 |
| Incident Mild Cognitive Impairment, n (%) | 524 (27.7) | 255 (26.7) | 269 (28.6) | 0.37 |
Standard Deviation.
Number of participants with missing data from: “All” is 6; “Men” is 1; “Women” is 5.
Number of participants with missing data from: “All” is 1; “Men” is 1; “Women” is 0.
Number of participants with missing data from: “All” is 28; “Men” is 11; “Women” is 17.
Number of participants with missing data from: “All” is 1; “Men” is 1; “Women” is 0.
Number of participants with missing data from: “All” is 25; “Men” is 9; “Women” is 16.
From maximum midlife weight to study entry (kg/decade).
Number of participants with missing data from: “All” is 26; “Men” is 10; “Women” is 16.
Over a mean 4.4 years (SD, 2.4) of follow-up, 524 participants developed incident MCI (Table 2). Participants who developed incident MCI were older, more likely to be carriers of APOE ε4 allele, and to have diabetes mellitus, hypertension, stroke, and coronary artery disease compared to participants who remained cognitively normal. The mean (SD) weight change was greater for participants who developed incident MCI vs. those who remained cognitively normal (−2.0 [5.1] vs. −1.2 [4.9] kg; p = 0.006). The decline in weight was greater in men who developed incident MCI vs. men who did not (−2.1 [5.3] vs. −1.0 [4.6] kg; p = 0.019), but was similar for women who developed incident MCI vs. women who did not (−1.9 [4.8] vs. −1.5 [5.3]; p = 0.115).
Table 2.
Summary of Participants’ Characteristics by Incident MCI
| Characteristics | With Incident MCI (N = 524) |
Without MCI (N = 1371) |
p |
|---|---|---|---|
| Age, mean (SDa) | 80.7 (5.2) | 77.7 (4.9) | <0.001 |
| Men, n (%) | 255 (48.7) | 699 (51.0) | 0.37 |
| Education (years), mean (SD) | 13.4 (3.0) | 14.2 (2.8) | <0.001 |
| Ever Smoking, n (%) | 243 (46.4) | 680 (49.6) | 0.21 |
| APOE ε4 allele carrierb, n (%) | 164 (31.4) | 325 (23.8) | <0.001 |
| Duration of Follow-Up (years), mean (SD) | 2.8 (1.9) | 5.1 (2.3) | <0.001 |
| Time from Midlife Weight to Study Entry (years), mean (SD) | 22.1 (8.5) | 19.1 (8.5) | <0.001 |
| Body Mass Index (kg/m2), mean (SD) | |||
| Maximum Midlifec (ages 40–65) | 28.3 (5.0) | 28.3 (4.9) | 0.93 |
| Late lifed (study entry) | 27.7 (5.2) | 27.9 (5.0) | 0.30 |
| Weight (Kg), mean (SD) | |||
| Maximum midlifee | 81.4 (17.1) | 82.3 (16.6) | 0.24 |
| Late lifef | 77.7 (17.0) | 79.9 (17.0) | 0.009 |
| Rate of Weight Changeg, mean (SD)h | −2.0 (5.1) | −1.2 (4.9) | 0.006 |
| Clinical conditions | |||
| Diabetes Mellitus (type 2), n (%) | 115 (21.9) | 215 (15.7) | 0.001 |
| Hypertension, n (%) | 430 (82.1) | 1030 (75.1) | 0.001 |
| Coronary Artery Disease, n (%) | 229 (43.7) | 514 (37.5) | 0.013 |
| Stroke, n (%) | 28 (5.3) | 52 (3.8) | 0.13 |
Standard Deviation.
Number of participants with missing data from: “All” is 6; “With Incident MCI” is 2; “Without MCI” is 4.
Number of participants with missing data from: “All” is 1; “With Incident MCI” is 0; “Without MCI” is 1.
Number of participants with missing data from: “All” is 28; “With Incident MCI” is 10; “Without MCI” is 18.
Number of participants with missing data from: “All” is 1; “With Incident MCI” is 0; “Without MCI” is 1.
Number of participants with missing data from: “All” is 25; “With Incident MCI” is 10; “Without MCI” is 15.
From maximum midlife weight to study entry (kg/decade)..
Number of participants with missing data from: “All” is 26; “With Incident MCI” is 10; “Without MCI” is 16.
A decline in weight from midlife was associated with an increased risk of incident MCI after adjustment for sex, education and APOE ε4 allele (Table 3, Model 1). Based on our proportional hazards models, a weight loss of 5 kg/decade corresponds to a 24% increase in risk of MCI (HR=1.24). Table 3 also describes the effects of adjusting for maximum midlife weight, weights in late life, separately or simultaneously in models with or without weight change.
Table 3.
Association of Weight Measures with Incident MCI overall and by Sex
| Weight-related variablesa | Model 1b | Model 2b | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| All | ||||
| Wt Change/Decadec | 1.04 (1.02, 1.06) | <0.001 | 1.04 (1.02, 1.06) | <0.001 |
| Wt Change/Decadec | 1.05 (1.03, 1.07) | <0.001 | 1.05 (1.02, 1.06) | <0.001 |
| Max midlife wt, kg | 1.00 (0.99, 1.00) | 0.46 | 1.00 (0.99, 1.00) | 0.22 |
| Wt Change/Decadec | 1.04 (1.02, 1.06) | <0.001 | 1.04 (1.02, 1.06) | <0.001 |
| Late life wt, kg | 1.00 (0.99, 1.01) | 0.75 | 1.00 (0.99, 1.00) | 0.33 |
| BMI Change/Decaded | 1.08 (1.03, 1.13) | 0.003 | 1.06 (1.01, 1.12) | 0.014 |
| Max midlife wt, kg | 1.01 (1.01, 1.02) | <0.001 | 1.01 (1.00, 1.02) | 0.008 |
| Late life wt, kg | 0.98 (0.98, 0.99) | <0.001 | 0.98 (0.98, 0.99) | <0.001 |
| Men | ||||
| Wt Change/Decadec | 1.05 (1.02, 1.08) | <0.001 | 1.04 (1.02, 1.07) | 0.002 |
| Wt Change/Decadec | 1.05 (1.02, 1.08) | 0.001 | 1.04 (1.01, 1.07) | 0.004 |
| Max midlife wt, kg | 1.00 (0.99, 1.01) | 0.85 | 100 (0.99, 1.01) | 0.93 |
| Wt Change/Decadec | 1.05 (1.02, 1.08) | <0.001 | 1.04 (1.02, 1.07) | 0.002 |
| Late life wt, kg | 1.00 (0.99, 1.01) | 0.53 | 1.00 (0.99, 1.01) | 0.65 |
| BMI Change/Decaded | 1.08 (1.01, 1.16) | 0.025 | 1.07 (1.00, 1.15) | 0.06 |
| Max midlife wt, kg | 1.02 (1.00, 1.03) | 0.010 | 1.02 (1.00, 1.03) | 0.036 |
| Late life wt, kg | 0.98 (0.97, 1.00) | 0.026 | 0.99 (0.97, 1.00) | 0.05 |
| Women | ||||
| Wt Change/Decadec | 1.04 (1.01, 1.07) | 0.002 | 1.03 (1.01, 1.06) | 0.008 |
| Wt Change/Decadec | 1.05 (1.02, 1.08) | 0.002 | 1.05 (1.02, 1.08) | 0.002 |
| Max midlife wt, kg | 1.00 (0.99, 1.00) | 0.27 | 0.99 (0.98, 1.00) | 0.09 |
| Wt Change/Decadec | 1.04 (1.01, 1.06) | 0.007 | 1.03 (1.00, 1.06) | 0.035 |
| Late life wt, kg | 1.00 (0.99, 1.00) | 0.30 | 0.99 (0.98, 1.00) | 0.07 |
| BMI Change/Decaded | 1.07 (1.00, 1.14) | 0.044 | 1.06 (0.99, 1.13) | 0.09 |
| Max midlife wt, kg | 1.01 (1.00, 1.02) | 0.023 | 1.01 (1.00, 1.02) | 0.07 |
| Late life wt, kg | 0.98 (0.97, 0.99) | 0.005 | 0.98 (0.97, 0.99) | 0.002 |
HR, hazard ratio; CI, confidence interval; wt, weight; max, maximum; late life wt, weights during study as a time dependent variable.
Weight-related variables included simultaneously within the models.
Model 1: Adjusted for sex (where applicable), education and APOE ε4 genotype (ε4 carrier vs non-carrier); Model 2: included model 1 variables and potential confounders: history of diagnosed alcohol problem (yes vs. no), depressive symptoms (BDI score ≥16), use of statins, diabetes mellitus, hypertension, coronary heart disease, cigarette smoking (never vs. former or current) and stroke. 6 participants in model 1 and 20 participants in model 2 with missing data were not included for rate of weight change from max midlife weight per decade through late life; max midlife wt; and late life wt. 34 participants in model 1 and 48 participants in model 2 with missing data were not included for rate of BMI change from max midlife BMI per decade through late life.
Rate of weight change from maximum midlife weight through late life, kg per decade.
Rate of BMI change from maximum midlife BMI per decade through late life, kg/m2 per decade.
When maximum midlife weight and late life weights were included in the same model as weight change, neither was significantly associated with MCI, but weight change remained significantly associated with MCI. However, when both maximum midlife weight and late life weights, but not weight change, were included in the same model, higher maximum midlife weight and low late life weight were associated with an increased risk of MCI. Consistent with results for weight change, a greater decline in BMI per decade (i.e. unit decrease) was associated with an increased risk of MCI (HR = 1.08 [1.03, 113], p = 0.003), but the model fit was better using weight change.
When we simultaneously adjusted for potential confounders, the associations of weight change with MCI persisted (Table 3, Model 2). However, simultaneously adjusting for these variables in a multivariable model with weight change could result in over-controlling since these conditions are in the causal pathway from obesity to cognitive impairment.
There was no significant interaction of sex with weight change, however, given the higher risk of MCI in men vs. women in our cohort, we have reported results by sex (Table 3). The effect sizes were greater in men than in women. There was a significant interaction between age and maximum midlife weight in regard to MCI risk (p for interaction = 0.019). The associations of a 5-kg difference in maximum midlife weight with MCI were stronger for persons older at baseline: 70–74 years: (HR = 1.05 [1.01, 1.10], p = 0.028); 75–79 years: (HR = 1.07 [1.03, 1.12], p = 0.001); 80–84 years: (HR = 1.08 [1.04, 1.12], p <0.001); 85 years and older: (HR = 1.09 [1.04, 1.14], p <0.001). There was no interaction of vascular risk factors with weight change (data are not presented).
Figure 1 illustrates inter-relationships of midlife weight, weight change and MCI risk. There is no association of midlife weight with MCI after weight change is taken into account. The figure demonstrates that the slopes for the four quartiles of midlife weight do not significantly differ. For persons in the upper midlife weight quartile (Q4), the risk of MCI (hazard ratio [HR], 95% confidence interval) increased by 39% (HR, 1.39 [1.02, 1.82) for a weight change of −10kg/decade. For persons in the lowest quartile (Q1), the risk of MCI increased by 78% (HR, 1.78 [0.99, 3,21]) for a decline of −10kg/decade. The corresponding increases in risk of MCI per −10 kg/decade were 69% (1.69 [1.04, 2.74]) for the second quartile and 61% (1.61 [1.08, 2.38]) for the third quartile. Thus, there is no significant interaction between midlife weight and weight change in determining in the risk of MCI (p = 0.80). Similarly, there was no significant difference in the intercepts of the 4 lines based on the general test comparing the four groups (p = 0.47); i.e. after taking into account the rate of weight change, there was no significant contribution of midlife weight to incident MCI. The specific test directly comparing the slopes in the upper and lower quartiles was not significant (p = 0.43). When we studied the association of weight change with MCI subtypes separately, weight change was significantly associated with aMCI not with naMCI (Table 4), suggesting that decline in weight may involve AD-related mechanisms.
Figure 1.
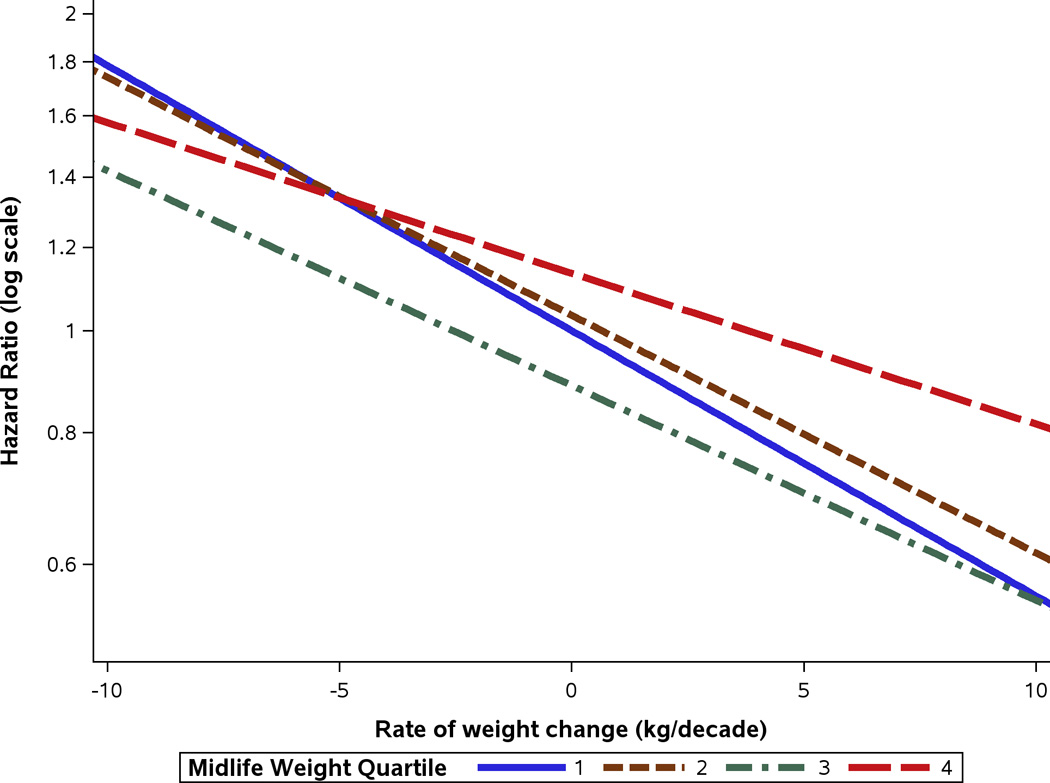
Hazard ratios for midlife weight quartiles (Q1, Q2, Q3, Q4) by the rate of weight change per decade. The estimates are based on a model adjusted for sex, years of education, APOE ε4 allele and weight at study entry, with age as the timescale. The mean (SD) maximum weights in midlife were: 62.03 (5.08) kg for Q1; 75.94 (3.58) kg for Q2; 86.79 (2.86) kg for Q3; and 103.68 (11.83) kg for Q4. The observed median (5th and 95th percentile) rates of weight change (kg/decade) from midlife to study entry for the four weight quartiles were: 1st quartile, −0.36 (−6.50, 5.20); 2nd quartile, −1.02 (−8.24, 5.17); 3rd quartile, −1.68 (−9.37, 6.04); and 4th quartile, −2.26 (−14.44, 7.74) kg/decade. We provided the 5th and 95th percentiles to give a broader sense of the distribution of weight change for each midlife weight quartile.
Table 4.
Association of Weight Measures with Incident MCI Subtypesa
| Weight-related variablesb | Model 1c | Model 2d | ||||||
|---|---|---|---|---|---|---|---|---|
| aMCI | p | naMCI | p | aMCI | p | naMCI | p | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| Wt Change/Decadee | 1.05 (1.02, 1.07) | <0.001 | 1.00 (0.96, 1.04) | 1.00 | 1.04 (1.02, 1.06) | 0.001 | 0.99 (0.96, 1.04) | 0.79 |
| BMI Change/Decadef | 1.09 (1.03, 1.16) | 0.002 | 1.02 (0.92, 1.13) | 0.747 | 1.07 (1.01, 1.13) | 0.021 | 1.01 (0.91, 1.12) | 0.88 |
| Max midlife wt, kg | 1.01 (1.00, 1.02) | 0.006 | 1.00 (0.98, 1.02) | 0.78 | 1.01 (1.00, 1.02) | 0.075 | 0.99 (0.97, 1.01) | 0.58 |
| Late life wt, kg | 0.98 (0.97, 0.99) | 0.002 | 1.00 (0.98, 1.02) | 0.95 | 0.98 (0.97, 0.99) | 0.004 | 1.00 (0.98, 1.02) | 0.95 |
HR, hazard ratio; CI, confidence interval; Wt, weight; max, maximum; late life wt, weights during study as a time dependent variable.
The number of incident aMCI cases was 366 and the number of incident naMCI cases was 120 in our study. The remaining 38 incident MCI cases could not be characterized in regard to MCI subtype, i.e. they progressed from cognitively normal directly to dementia.
Weight variables included simultaneously within the models.
Model 1: sex (where applicable), education and APOE ε4 genotype (ε4 carrier vs non-carrier). 2 participants with missing data were not included in model 1 for aMCI for rate of weight change from max midlife weight per decade through late life; rate of BMI change from max midlife BMI per decade through late life; max midlife wt; and late life wt. 2 participants with missing data were not included in model 1 for naMCI for rate of weight change from max midlife weight per decade through late life; max midlife wt; and late life wt. 4 participants with missing data were not included in model 1 for naMCI for rate of BMI change from max midlife BMI per decade through late life.
Model 2: included model 1 variables and potential confounders: alcohol problem (yes vs. no), depressive symptoms (BDI score ≥16), use of statins, diabetes mellitus, hypertension, coronary heart disease, cigarette smoking (never vs. former or current) and stroke. 12 participants with missing data were not included in model 2 for aMCI for rate of weight change from max midlife weight per decade through late life; rate of BMI change from max midlife BMI per decade through late life; max midlife wt; and late life wt. 5 participants with missing data were not included in model 2 for naMCI for rate of weight change from max midlife weight per decade through late life; max midlife wt; and late life wt. 7 participants with missing data were not included in model 2 for naMCI for rate of BMI change from max midlife BMI per decade through late life.
Rate of weight change from maximum midlife weight through late life, kg per decade.
Rate of BMI change from maximum midlife BMI through late life, kg/m2 per decade.
Discussion
The results of this population-based elderly cohort demonstrate that a higher weight loss from midlife to late life and decline in weights in late life, are markers of risk for MCI. Greater rate of decline in weight or in BMI from midlife to late life increased risk of MCI. After taking into account rate of weight change, midlife and late life weights do not contribute to risk of MCI. However, without accounting for rate of change in weight, a higher maximum weight in midlife is associated with an increased risk of MCI, whereas a decline in weight in late life is associated with an increased risk of MCI.
The association of greater weight loss with MCI suggests that weight loss may be a marker for risk of MCI. While weight loss may not be causally related to MCI, we hypothesize that weight loss may represent a prodromal stage or an early manifestation of MCI. Consistent with this, there was no interaction of weight loss with midlife weight; even among persons who were of normal weight in midlife, a greater weight loss was associated with an increased risk of MCI in late life.
An important strength of our study is the ability to assess maximum midlife weight from the medical records of participants and from direct measurements in late life. From these measures, we demonstrated that age at which weight is assessed is important when investigating the association of weight with risk of MCI. Specifically, when we simultaneously considered maximum midlife and late life weight in the same model, higher maximum midlife weight and weight loss in late life were both associated with incident MCI. However, our findings suggest that, weight loss is the key weight-related marker of incident MCI in the elderly. The association of weight loss with incident MCI was stronger than estimates for maximum midlife weight or for low weights in late life when these latter variables were simultaneously considered.
Our findings for MCI are consistent with the findings of other prospective studies that correlated weight loss with the increased risk of dementia. In a study of controls for a dementia cohort, greater weight loss preceded the diagnosis of AD dementia.26 In a community-dwelling elderly cohort, men and women who developed probable or possible AD dementia had a significant weight loss preceding the diagnosis compared to persons who remained cognitively normal.27 In a small study of MCI participants, a low initial BMI and weight loss during follow-up were associated with a significantly greater risk of developing dementia. In a population-based cohort, higher midlife BMI was related to higher risk of dementia and AD dementia, independent of obesity-related risk factors and co-morbidities.28 In an elderly African-American cohort, participants who developed MCI had a greater rate of weight loss showed by repeated BMI measures compared to elderly who remained cognitively normal.15 Similarly, in a case-control study in Olmsted County, weight loss was associated with a greater risk of dementia in women, but not in men.29 In the prospective Honolulu Aging Study, weight loss preceded onset of dementia in a cohort of men followed over a 26-year follow-up.7,26,29
Contrary to our findings, higher BMI in both midlife and late life was associated with decreased risk of dementia in a large population-based retrospective study.12 Potential issues that may account for their findings12 include lack of clarity on age at assessment of weight and BMI and onset of dementia. These may have implications on their findings if there is a mix of timings of age of weight assessment and BMI, with the majority being assessed at older ages. In another large population-based prospective study, being overweight or obese in midlife was not associated with increasing risk of dementia.30
Weight loss prior to MCI or dementia may be a component of the predementia syndrome rather than a causal relationship. If weight loss is a prodromal dementia stage, we would expect the association between weight loss and MCI to be similar across midlife weight classes. Indeed, the associations between weight loss and MCI did not differ across midlife weight quartiles, suggesting that the hypothesis of weight loss as a prodromal predementia stage is likely.
The association of weight loss with cognitive impairment may involve direct causal mechanisms, may be due to reverse causality, or to a shared etiology. In regard to causal mechanisms, weight loss prior to cognitive impairment may be related to what has been termed ‘anorexia of aging’. While the direct cause of this anorexia is not clear, we speculate that dysfunctional production of certain hormones (cholecystokinin, leptin, cytokines, dynorphin, neuropeptide Y, serotonin) on dietary intakes and energy metabolism may lead to reduced dietary intakes that impact MCI risk. In regard to reverse causality, neuropsychiatric symptoms such as depression and apathy that are prodromal and predictors of MCI and dementia, may contribute to decreased appetite and weight loss prior to the diagnosis of these conditions.31–33 Finally, in regard to a shared etiology, protein deposits including Lewy bodies, tau or amyloid have been identified in the olfactory bulb and central olfactory pathways prior to the onset of dementia, and olfactory dysfunction is a marker for cognitive impairment and dementia.34–38 Thus, impairment in smell with related changes in taste, may contribute to decreased appetite, reduced dietary intake, and the weight loss observed with MCI, AD dementia and other neurodegenerative conditions.
The association of high midlife weight with MCI may involve effects of obesity on the brain through cerebrovascular disease and metabolic abnormalities (e.g. glucose metabolism, insulin signaling).39 Obesity-related brain pathology likely includes hypoperfusion,40 neuronal injury and death,40 brain atrophy,41 cerebrovascular dysfunction,40 increased levels of amyloid-beta precursor protein,42 increased tau expression,43 blood-brain barrier dysfunction,44 systemic45,46 and central47 inflammation-related pathologies, and dysfunction of microglia and astrocytes.48,49
A potential limitation of this study is that it is not possible to determine whether weight loss was intentional or unintentional. Given the consistency of the association of weight loss with incident MCI across all midlife weight quartiles, it is most likely unintentional weight loss. Despite the limited ethnic diversity of the study cohort, the findings are consistent with findings from an African-American cohort.15
Additional strengths of our study include the large cohort and population-based design. Participants were clinically assessed for risk of MCI or dementia. Furthermore, information on clinical conditions was abstracted from the medical record than from self-report.
Acknowledgments
Dr. Roberts receives funding from the NIH.
Dr. Knopman serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRX Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH.
Dr. Mielke receives research grants from the NIH/NIA, Alzheimer Drug Discovery Foundation, Lewy Body Association.
Ronald C. Petersen serves on data monitoring committees for Pfizer, Inc., Janssen Alzheimer Immunotherapy, and is a consultant for Roche, Inc., Merck, Inc. and Genentech, Inc., Biogen, Inc. and Eli Lilly and Co; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), and receives research support from the National Institute of Health
Role of the Funder/Sponsor: The funders had no role in the design and conduct of study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The study was supported by National Institutes of Health grants U01 AG006786, P50 AG016574; Mayo Foundation for Medical Education and Research, and was made possible by the Rochester Epidemiology Project (R01AG034676). The authors thank Ms. Sondra Buehler for her editorial support, Ms. Mary Dugdale, RN and Ms. Connie Fortner, RN for abstraction of medical record data, and the Mayo Clinic Study of Aging participants and staff.
Footnotes
Authors’ Contributions:
Study concept and design: Roberts, Petersen
Acquisition, analysis, or interpretation of data: Roberts, Vassilaki, Alhurani, Kremers, Aakre Mielke, Machulda, Knopman, Petersen, Geda.
Drafting of the manuscript: Alhurani, Vassilaki, Roberts,
Critical revision of the manuscript for important intellectual content: Roberts, Vassilaki, Kremers, Mielke, Knopman, Petersen, Machulda, Geda
Statistical analysis: Kremers, Aakre
Obtaining funding: Petersen, Roberts, Mielke, Knopman
Administrative, technical, or material support: Petersen, Roberts
Study supervision: Roberts, Kremers
Conflict of Interest Disclosures:
Dr. Machulda reports no disclosures.
Dr. Alhurani reports no disclosures.
Dr. Vassilaki reports no disclosures.
References
- 1.Roberts R, Knopman DS. Classification and Epidemiology of MCI. Clinics in Geriatric Medicine. 2013;29(4):753–772. doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009 Apr;119(4):252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 3.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003 Jan 14;60(1):117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 4.Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc. 2008 Jan;56(1):111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 5.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005 Sep 27;65(6):892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009 Mar;66(3):336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005 Jan;62(1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 8.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003 Jul 14;163(13):1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 9.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008 Sep 30;71(14):1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 10.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005 Oct;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 11.Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011 May 3;76(18):1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qizilbash N, Gregson J, Johnson ME, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015 Jun;3(6):431–436. doi: 10.1016/S2213-8587(15)00033-9. [DOI] [PubMed] [Google Scholar]
- 13.Sturman MT, de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008 Jan 29;70(5):360–367. doi: 10.1212/01.wnl.0000285081.04409.bb. [DOI] [PubMed] [Google Scholar]
- 14.Cronk BB, Johnson DK, Burns JM. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2010 Apr-Jun;24(2):126–130. doi: 10.1097/WAD.0b013e3181a6bf3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao S, Nguyen JT, Hendrie HC, et al. Accelerated weight loss and incident dementia in an elderly African-American cohort. J Am Geriatr Soc. 2011 Jan;59(1):18–25. doi: 10.1111/j.1532-5415.2010.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012 Dec;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010 Sep 7;75(10):889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 Nov;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982 May;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 21.Smith GE, Malec JF, Ivnik RJ. Validity of the construct of nonverbal memory: a factor-analytic study in a normal elderly sample. J Clin Exp Neuropsychol. 1992 Mar;14(2):211–221. doi: 10.1080/01688639208402824. [DOI] [PubMed] [Google Scholar]
- 22.Ivnik RJ, Smith GE, Cerhan JH, Boeve BF, Tangalos EG, Petersen RC. Understanding the diagnostic capabilities of cognitive tests. Clin Neuropsychol. 2001 Feb;15(1):114–124. doi: 10.1076/clin.15.1.114.1904. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004 Sep;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 24.Association AP. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th. Washington (DC): American Psychiatric Association; 1994. [Google Scholar]
- 25.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011 Jun 9;364(23):2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006 Sep;63(9):1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 27.Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. Journal of the American Geriatrics Society. 1996 Oct;44(10):1147–1152. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 28.Tolppanen AM, Ngandu T, Kareholt I, et al. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38(1):201–209. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- 29.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007 Aug 21;69(8):739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 30.Albanese E, Davis B, Jonsson PV, et al. Overweight and Obesity in Midlife and Brain Structure and Dementia 26 Years Later: The AGES-Reykjavik Study. Am J Epidemiol. 2015 May 1;181(9):672–679. doi: 10.1093/aje/kwu331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014 May;171(5):572–581. doi: 10.1176/appi.ajp.2014.13060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brodaty H, Heffernan M, Draper B, et al. Neuropsychiatric symptoms in older people with and without cognitive impairment. J Alzheimers Dis. 2012;31(2):411–420. doi: 10.3233/JAD-2012-120169. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2013 Jul;21(7):685–695. doi: 10.1016/j.jagp.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement. 2015 Jan;11(1):70–98. doi: 10.1016/j.jalz.2014.04.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doty RL. Olfaction in Parkinson's disease and related disorders. Neurobiol Dis. 2012 Jun;46(3):527–552. doi: 10.1016/j.nbd.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovacs T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer's disease: olfactory bulb is involved in early Braak's stages. Neuroreport. 2001 Feb 12;12(2):285–288. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- 37.Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology. 2015 Jan 13;84(2):182–189. doi: 10.1212/WNL.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007 Jul;64(7):802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;8:375. doi: 10.3389/fnins.2014.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011 Sep;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 2008 Jan;16(1):119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 42.Lee YH, Martin JM, Maple RL, Tharp WG, Pratley RE. Plasma amyloid-beta peptide levels correlate with adipocyte amyloid precursor protein gene expression in obese individuals. Neuroendocrinology. 2009;90(4):383–390. doi: 10.1159/000235555. [DOI] [PubMed] [Google Scholar]
- 43.Koga S, Kojima A, Kuwabara S, Yoshiyama Y. Immunohistochemical analysis of tau phosphorylation and astroglial activation with enhanced leptin receptor expression in diet-induced obesity mouse hippocampus. Neurosci Lett. 2014 Jun 13;571:11–16. doi: 10.1016/j.neulet.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 44.Banks WA, Burney BO, Robinson SM. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides. 2008 Nov;29(11):2061–2065. doi: 10.1016/j.peptides.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009 Jan;33(1):151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 46.Gemma C, Bickford PC. Interleukin-1beta and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev Neurosci. 2007;18(2):137–148. doi: 10.1515/revneuro.2007.18.2.137. [DOI] [PubMed] [Google Scholar]
- 47.Andre C, Dinel AL, Ferreira G, Laye S, Castanon N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun. 2014 Oct;41:10–21. doi: 10.1016/j.bbi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Erion JR, Wosiski-Kuhn M, Dey A, et al. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014 Feb 12;34(7):2618–2631. doi: 10.1523/JNEUROSCI.4200-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cano V, Valladolid-Acebes I, Hernandez-Nuno F, et al. Morphological changes in glial fibrillary acidic protein immunopositive astrocytes in the hippocampus of dietary-induced obese mice. Neuroreport. 2014 Jun 6; doi: 10.1097/WNR.0000000000000180. [DOI] [PubMed] [Google Scholar]


