Abstract
Recently, the topic of traumatic brain injury has gained attention in both the scientific community and lay press. Similarly, there have been exciting developments on multiple fronts in the area of neurochemistry specifically related to purine biology that are relevant to both neuroprotection and neurodegeneration. At the 2105 meeting of the National Neurotrauma Society, a session sponsored by the International Society for Neurochemistry featured three experts in the field of purine biology who discussed new developments that are germane to both the pathomechanisms of secondary injury and development of therapies for traumatic brain injury. This included presentations by Drs. Edwin Jackson on the novel 2′,3′ cAMP pathway in neuroprotection, Detlev Boison on adenosine in posttraumatic seizures and epilepsy, and Michael Schwarzschild on the potential of urate to treat central nervous system injury. This mini review summarizes the important findings in these three areas and outlines future directions for the development of new purine-related therapies for traumatic brain injury and other forms of central nervous system injury.
Keywords: adenosine, cyclic-AMP, seizure, neuroprotection, urate, uric acid
In the last 5–10 years, the topic of traumatic brain injury (TBI) has garnered incredible attention in both the scientific community and lay press. This has resulted from the emerging recognition of the importance and consequences of both repetitive mild traumatic brain injury in the civilian population (repeated sports concussion) and blast-induced TBI in combat casualty care and terrorist attacks. The potential linkage of TBI to a variety of neurodegenerative diseases such as chronic traumatic encephalopathy, among others has further fueled this interest. There have been exciting developments in the field of purine biology that may have relevance to TBI. At the 2015 meeting of the National Neurotrauma Society, a session sponsored by the International Society for Neurochemistry featured three experts in the field of purine biology who discussed new developments germane to the pathomechanisms and development of therapies in TBI. This included presentations by Drs. Edwin Jackson on the novel 2′,3′ cAMP pathway in neuroprotection, Detlev Boison on adenosine in posttraumatic seizures and epilepsy, and Michael Schwarzschild on the potential of urate to treat CNS injury.
The 2′,3′ cAMP pathway in neuroprotection
Discovery of Nucleoside 2′,3′-Cyclic Monophosphates
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is a powerful tool that couples the resolving capability of ultra-performance liquid chromatography with the sensitivity and specificity of tandem mass spectrometry. Jackson and coworkers measured, using LC-MS/MS, purines in the renal venous outflow from isolated, perfused rat kidneys (Ren et al., 2009). They observed a large chromatographic peak that was due to a 330 m/z precursor ion and 136 m/z product ion consistent with what would be expected for 3′,5′–cAMP. Surprisingly, however, the retention time of the compound was shorter than that for authentic 3′,5′-cAMP, thus eliminating the possibility that the signal was due to 3′5′-cAMP. They identified the unknown compound as adenosine 2′,3′–cyclic monophosphate (2′,3′–cAMP), and these studies were arguably the first unequivocal identification of 2′,3′–cAMP in any biological system. The discovery of 2′,3′-cAMP in the rat kidney was rapidly followed by other publications providing evidence for the existence in biological systems of not only 2′,3′-cAMP, but other nucleoside 2′,3′-cyclic monophosphates (2′,3′-cNMPs) (Pabst et al., 2010; Van Damme et al., 2012; Burhenne et al., 2013; Bähre and Kaever, 2014; Bordeleau et al., 2014; Jia et al., 2014; Van Damme et al., 2014). It is now clear that there exist a family of non-canonical cNMPs with 2′,3′-cyclic, rather than 3′,5′-cyclic, phosphodiester bonds (Figure 1).
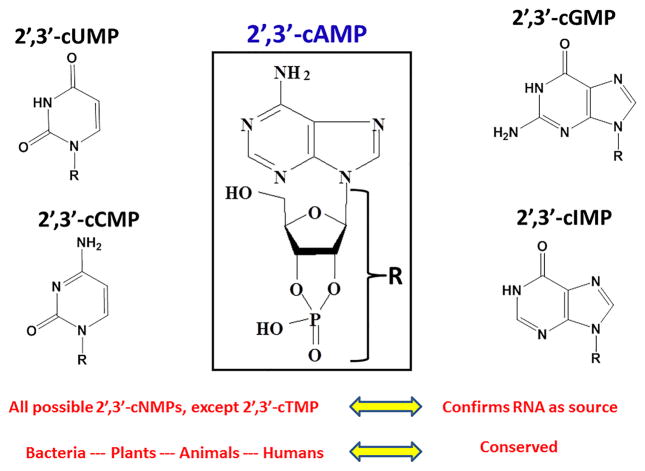
Figure 1. Chemical Structures of Nucleoside 2′, 3′-Cyclic Monophosphates (2′,3′-cNMPs).
2′, 3′-cAMP (center) was discovered to exist in biological systems in 2009. Subsequently, a number of other 2′, 3′-cNMPs were discovered including 2′, 3′-cUPM, 2′, 3′-cCMP, 2′, 3′-cGMP, 2′, 3′-cIMP. The absence of 2′, 3′-cTMP is consistent with the concept that all 2′, 3′-cNMPs derive from RNA, rather than DNA, degradation. That 2′, 3′-cNMPs exist in bacteria, plants, animals, and humans indicate that these molecules are ancient and conserved.
The 3′,5′-cAMP-Adenosine Pathway
Extracellular adenosine biosynthesis occurs via several pathways activated by diverse stimuli to produce adenosine for different purposes. The classical pathway is catalyzed by the ecto-enzyme CD39 working in tandem with the ecto-enzyme CD73; a pathway that produces adenosine in the interstitium as follows: ATP → ADP → 5′-AMP → adenosine. Hypoxia and inflammation activate the “CD39/CD73 pathway” which produces large amounts of extracellular adenosine that restore tissue perfusion and down-regulate inflammation (Eltzschig, 2009; Eltzschig and Carmeliet, 2011; Eltzschig et al., 2012; Eltzschig, 2013). The “extracellular 3′,5′-cAMP-adenosine pathway” is another mechanism for producing adenosine in the interstitium. It involves: 1) the intracellular conversion of ATP to 3′,5′-cAMP by adenylyl cyclases; 2) rapid export of 3′,5′-cAMP from cells by cyclic nucleotide transporters such as MRP4 (Cheng et al., 2010); 3) conversion of extracellular 3′,5′-cAMP to 5′-AMP by ecto-3′,5′-cyclic nucleotide 3′-phosphodiesterases; and 4) metabolism of extracellular 5′-AMP to adenosine by CD73 and tissue non-specific alkaline phosphatase (TNAP; an ecto-enzyme structurally similar to CD73). The extracellular adenosine produced by the extracellular 3′,5′-cAMP-adenosine pathway engages adenosine receptors to expand/modulate the initial effects of adenylyl cyclase activation. The first explicit formulation of the extracellular 3′,5′-cAMP-adenosine pathway was postulated in 1991 (Jackson, 1991); evidence for this mechanism and the role of 3′,5′-cAMP as a “3rd messenger” is extensive (Mi et al., 1994; Mi and Jackson, 1995; Dubey et al., 1996; Jackson et al., 1997; Dubey et al., 1998; Mi and Jackson, 1998; Hong et al., 1999; Dubey et al., 2000a; Dubey et al., 2000b; Jackson and Mi, 2000; Dubey et al., 2001; Jackson et al., 2003; Jackson et al., 2006; Do et al., 2007; Jackson et al., 2007; Chiavegatti et al., 2008; Giron et al., 2008; Jackson and Mi, 2008; Müller et al., 2008; Dubey et al., 2010; Kuzhikandathil et al., 2011; Duarte et al., 2012; Sciaraffia et al., 2014).
The 2′,3′-cAMP-Adenosine Pathway
Since biological systems can express an extracellular 3′,5′-cAMP-adenosine pathway, Jackson and coworkers (Jackson et al., 2009) considered whether an analogous “extracellular 2′,3′-cAMP-adenosine pathway” may also exist: intracellular synthesis of 2′,3′-cAMP → egress of 2′,3′-cAMP → extracellular conversion of 2′,3′-cAMP to 2′-AMP plus 3′-AMP → extracellular catabolism of 2′-AMP and 3′-AMP to adenosine. The rationale for this hypothesis was based on four observations. First, intracellular ribonucleases (RNases) degrade RNA by facilitating the hydrolysis of the P-O5′ bond of RNA via transphosphorylation of RNA to yield 2′,3′-cNMPs (Wilusz et al., 2001). NMR spectroscopy (Thompson et al., 1994) demonstrated that 2′,3′-cNMPs formed by transphosphorylation of RNA are released form RNases as intact 2′,3′-cNMPs. Consequently, most 2′,3′-cNMPs, such as 2′,3′-cAMP, likely are formed from nucleotide bases in RNA via RNase catalyzed transphosphorylation (Thompson et al., 1994). Recent studies (Gu et al., 2013; Sokurenko et al., 2015) using a variety of approaches reveal a detailed molecular mechanism for 2′,3′-cNMP biosynthesis from RNA. Inasmuch as mRNA has a large number of adenosine monophosphates in the poly-A tail (Alberts et al., 1989), mRNA degradation can generate large amounts of 2′,3′-cAMP.
Second, nucleotide transporters, for example MRP4 and MRP5, both rapidly and actively export a diverse number of nucleotides (linear and cyclic), into the extracellular space (Kruh et al., 2001; van Aubel et al., 2002; Deeley et al., 2006; Borst et al., 2007). Likely then nucleotide transporters would also export 2′,3′-cAMP; release of endogenous 2′,3′-cAMP into the renal circulation (Jackson et al., 2009; Ren et al., 2009; Jackson et al., 2011a) unquestionably indicates that 2′,3′-cAMP reaches the extracellular compartment.
Third, there exist enzymes that could catalyze the pathway. For example, 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) can metabolize 2′,3′-cAMP to 2′-AMP in vitro (Vogel and Thompson, 1988; Sprinkle, 1989; Thompson, 1992). Some secreted RNases can hydrolyze 2′,3′-cAMP to 3′-AMP (Sorrentino and Libonati, 1997; Sorrentino, 1998). Rao et al. (Rao et al., 2010) report that six different phosphodiesterases containing three different families of hydrolytic domains can generate 3′-AMP from 2′,3′-cAMP. Consistent with the existence of ecto-2′,3′-cyclic nucleotide 2′-phosphodiesterases and ecto-2′,3′-cyclic nucleotide 3′-phosphodiesterases are the findings that: 1) 3′-AMP is present in rat spleen, kidney, liver, heart, and brain (Bushfield et al., 1990; Fujimori and Pan-Hou, 1998; Fujimori et al., 1998; Miyamoto et al., 2008); and 2) 2′-AMP and 3′-AMP are present in human cerebrospinal fluid (CSF) and their concentrations correlate with the concentrations of 2′,3′-cAMP (Verrier et al., 2012).
Fourth, there are ecto-nucleotidases that process extracellular 2′-AMP and 3′-AMP to adenosine. Ohkubo et al. (Ohkubo et al., 2000) showed that in NG108-15 cells TNAP can release orthophosphate from 2′-AMP or 3′-AMP. Moreover, in kidneys TNAP metabolizes 2′-AMP and 3′-AMP to adenosine (Jackson, E.K.; unpublished observations). Also, adenosine levels in human CSF correlate with those of 2′,3′-cAMP, 2′-AMP, and 3′-AMP (Verrier et al., 2012)—consistent with the metabolism of these compounds to adenosine in the extracellular compartment.
Jackson and coworkers confirmed the existence of the extracellular 2′,3′-cAMP-adenosine pathway. In kidneys, arterial infusions of 2′,3′-cAMP dramatically increased renal venous 3′-AMP, 2′-AMP, and adenosine (Jackson et al., 2009; Jackson et al., 2011a); and infusions of 2′-AMP and 3′-AMP augmented secretion of adenosine similar to that achieved by 5′-AMP (prototypical adenosine precursor). Energy depletion can activate the degradation of RNA (Akahane et al., 2001a; Akahane et al., 2001b; Almeida et al., 2004) and should engage the extracellular 2′,3′-cAMP-adenosine pathways. As predicted, treatment of kidneys with metabolic inhibitors increased renal venous 2′,3′-cAMP, 2′-AMP, 3′-AMP, and adenosine (Jackson et al., 2009; Jackson et al., 2011a). The extracellular 2′,3′-cAMP-adenosine pathway may exist in many cells, tissues, and organs. For example, preglomerular vascular smooth muscle cells (Jackson et al., 2010), preglomeular vascular endothelial cells (Jackson and Gillespie, 2012), glomerular mesangial cells (Jackson et al., 2010), renal epithelial cells (Jackson and Gillespie, 2012; Jackson and Gillespie, 2013), aortic vascular smooth muscle cells (Jackson et al., 2011b), coronary artery vascular smooth muscle cells (Jackson et al., 2011b), microglia (Verrier et al., 2011), astrocytes (Verrier et al., 2011), oligodendrocytes (Verrier et al., 2013), neurons (Verrier et al., 2013), Schwann cells (Verrier et al., 2015), and intact brain in vivo (Verrier et al., 2012) metabolize exogenous 2′,3′-cAMP, 2′-AMP, and 3′-AMP to downstream purines.
CNPase and the Role of the 2′,3′-cAMP-Adenosine Pathway in Neurotrauma
CNPase is the most abundant protein in non-compact CNS myelin, and is the 3rd most abundant protein overall in CNS myelin (Raasakka and Kursula, 2014). Yet, the role for CNPase remained an enigma from the time of its discovery (Whitfeld et al., 1955) until the discovery of 2′,3′-cAMP in rat kidneys in 2009 (Ren et al., 2009). Before 2009, 2′,3′-cAMP was not known to exist in biological systems; therefore the ability of CNPase to convert 2′,3′-cAMP to 2′-AMP in vitro was viewed as an epiphenomenon (Vogel and Thompson, 1988; Thompson, 1992; Schmidt, 1999). With the discovery of 2′,3′-cAMP pathway, the role of the enzymatic activity of CNPase is being reconsidered (Raasakka and Kursula, 2014).
Jackson and coworkers (Verrier et al., 2012) were the first to reveal an important role for the enzymatic activity of CNPase. Employing microdialysis to infuse 2′,3′-cAMP and 2′-AMP into the extracellular compartment of the mouse brain they demonstrated that the brain converts exogenous 2′,3′-cAMP to 2′-AMP and adenosine and metabolizes exogenous 2′-AMP to adenosine. Notably the increase in interstitial levels of 2′-AMP and adenosine following delivery of 2′,3′-cAMP to the brain interstitial compartment caused significant increases in 2′-AMP and adenosine within 30 minutes. Similarly the increase in interstitial levels of adenosine following delivery of 2′-AMP also occurred within 30 minutes. However, the conversion of exogenous 2′,3′-cAMP to 2′-AMP was attenuated in brains from CNPase knockout (KO) mice. In wild-type mice, TBI increased brain interstitial levels of 2′,3′-cAMP, 2′-AMP, 3′-AMP, adenosine, and inosine (adenosine metabolite) within a time frame of 30 minutes. In CNPase KO mice, TBI induced higher levels of interstitial 2′,3′-cAMP, yet lower levels of 2′-AMP, adenosine, and inosine. Thus deficiency of CNPase impairs the 2′,3′-cAMP-adenosine pathway (Verrier et al., 2012) and activity of CNPase importantly participates in this system. Furthermore, histology suggested greater hippocampal neuronal injury in CNPase KO vs. wild-type and functional outcomes were worse in CNPase KO. This is consistent with observations by others that CNPase KO mice have enhanced astrogliosis, microgliosis, axon degeneration, and defects in working memory following brain injury (Wieser et al., 2013); and an aging associated psychiatric disease (catatonia-depression syndrome) (Hagemeyer et al., 2012).
Neuroprotective Mechanism of CNPase and the 2′,3′-cAMP-Adenosine Pathway
Adenosine is neuroprotective (Kochanek et al., 2013) and 2′,3′-cAMP rapidly (within minutes) opens mitochondrial permeability transition pores (mPTPs) (Azarashvili et al., 2009; Azarashvili et al., 2010) possibly triggering apoptosis, necrosis, and autophagy (mitophagy). Therefore, metabolism of endogenous 2′,3′-cAMP to 2′-AMP by CNPase would rid the brain cells of an intracellular neurotoxin (2′,3′-cAMP) while metabolism of 2′-AMP and 3′-AMP to adenosine would provide a neuroprotectant/anti-inflammatory agent (adenosine). With regard to inflammation, 2′,3′-cAMP, 3′-AMP, and 2′-AMP inhibit the release of proinflammatory TNF-α and CXCL10 in murine microglia via production of adenosine leading to activation of A2A receptors (Newell et al., 2015). It is important to note that the effects of brain injury on components of the 2′,3′-cAMP-adenosine pathway occurs within 30 minutes. Thus, the pathway is activated in a time frame rapid enough to affect early events following brain injury. Indeed, in TBI patients, CSF levels of 2′,3′-cAMP are increased for 12 hours after injury and correlate with CSF 2′-AMP, 3′-AMP, adenosine, and inosine (Verrier et al., 2012). These clinical findings suggest that the 2′,3′-cAMP-adenosine pathway is engaged post TBI in humans in a time frame consistent with affecting early events after injury. Although activation of the 2′,3′-cAMP-adenosine pathway requires RNA metabolism, this does not mean that the pathway is activated only in dying cells. In addition, it would be expected that even cells destined to die could produce extracellular adenosine via the 2′3′-cAMP-adenosine pathway, and this adenosine could increase the survival rate of surrounding cells via paracrine effects.
Studies by Lappe-Siefke et al. (Lappe-Siefke et al., 2003) suggested that myelin and axonal morphology are normal in CNPase −/− mice up to about 3.5 months of age. Then axonal pathology begins to emerge and gradually increases with age. In aged CNPase −/− mice, the main axonal pathology is axonal swellings, whereas the myelin sheath remains relatively normal (only minor changes at the paranodal regions). However, subsequent studies by Edgar et al. (Edgar et al., 2009) indicated early changes (i.e. swelling) in the inner tongue of the myelin sheath in paranodal regions of small (but not large) axons associated with some degeneration of small axons. However, these changes were not associated with a significant reduction in axon number until about 6 months of age. In preliminary studies, we examined with transmission electron microscopy white matter tracts of CNPase KO mice and CNPase wild-type mice that were about 3 months old. We did not detect any changes in axonal morphology, autophagosome number and area or mitochondrial number and area. Because our TBI experiments were performed in CNPase KO mice that were about 3 months of age, it is unlikely that background white matter pathology accounted for the differential response to TBI in CNPase KO versus CNPase wild-type mice. Although, we cannot completely rule out this possibility, it is important to consider that subtle changes in background axonal health may indeed be mediated by chronic deficiency of the 2′,3′-cAMP-adenosine pathway. That is to say, not only may acute changes in the pathway determine the response to an acute injury, it is conceivable that chronic deficiency causes underlying pathology that determines the response to acute TBI as well as the risk of neurodegenerative processes such as chronic traumatic encephalopathy.
Brain Cells that Mediate the 2′,3′-cAMP-Adenosine Pathway
The aforementioned findings suggest that: 1) the 2′,3′-cAMP-adenosine pathway exists in vivo in the CNS of mice and humans; 2) brain CNPase converts endogenously generated 2′,3′-cAMP to 2′-AMP; and 3) the 2′,3′-cAMP-adenosine pathway and CNPase are neuroprotective. What CNS cell types mediate the 2′,3′-cAMP-adenosine pathway? Although astrocytes, microglia, oligodendrocytes, and neurons all can metabolize 2′,3′-cAMP to 2′-AMP, oligodendrocytes are preeminent in this regard (Verrier et al., 2013); likely because oligodendrocytes are enriched in CNPase. In oligodendrocytes from CNPase KO mice, the metabolism of 2′,3′-cAMP to 2′-AMP is impaired (Verrier et al., 2013). In contrast, microglia are the most efficient at converting 2′-AMP to adenosine (Verrier et al., 2011). Although brain injury increases extracellular 2′,3′-cAMP levels, the major sources of 2′,3′-cAMP have yet to be identified. Likely a collaboration among CNS cell types is required to constitute a complete brain 2′,3′-cAMP-adenosine pathway.
Summary
Evidence is mounting that 2′,3′-cAMP is an important molecule that is metabolized to adenosine. TBI activates the 2′,3′-cAMP-adenosine pathway, and this mechanism is a determinant of outcome. The challenge going forward is to discover ways to manipulate this pathway to benefit patients after TBI. In this regard, there are a number of feasible strategies for using the knowledge generated by studying the 2′,3′-cAMP-adenosine pathway in the brain to treat TBI. For example, using the structure of 2′,3′-cAMP as a starting point, it is feasible to develop antagonists that block the effects of 2′,3′-cAMP on mPTPs thus preventing 2′,3′-cAMP-induced apoptosis, necrosis, and autophagy (mitophagy). Also, inhibitors of RNases that manufacture 2′,3′-cAMP could be developed to temporarily reduced 2′,3′-cAMP production. Other approaches would be to induce the expression (with pharmacological agents) of transporters that mediate cellular egress of 2′,3′-cAMP, CNPase, or TNAP so as to increase the rate at which 2′,3′-cAMP is exported and converted to adenosine. In addition to treating TBI, polymorphisms in CNPase, the relevant transport proteins, and TNAP may serve to identify individuals susceptible to TBI so that they can be advised not to participate in contact sports or other activities that increase the risk of TBI or chronic traumatic encephalopathy.
Role of adenosine in posttraumatic seizures and epilepsy
Posttraumatic epilepsy accounts for ~10–20% of all symptomatic epilepsies in the general population (Englander et al. 2003). Predicting persons who might develop epilepsy and preventing its development are consequently of utmost importance. Adenosine is a well-known endogenous anticonvulsant and seizure terminator. Since adenosine deficiency, caused by enhanced metabolic clearance through reactive astrocytes and overexpression of the adenosine removing enzyme adenosine kinase (ADK), is a hallmark of epilepsy, therapeutic adenosine augmentation is a rational approach to suppress seizures in the epileptic brain (Boison 2012). Seizure suppression by adenosine is mediated by increased activation of adenosine A1 receptors, whereas a lack of A1 receptors is associated with lethal seizures after exposure of the brain to trauma or an excitotoxin (Kochanek et al. 2006, Fedele et al. 2006). Whereas the receptor-dependent effects of adenosine are well characterized and have been the subject of drug development efforts (Chen et al. 2013) new findings demonstrate that adenosine has additional, adenosine receptor independent, unprecedented properties to prevent the development of epilepsy through an epigenetic mechanism.
Epileptogenic brain areas in chronic epilepsy, in the clinic and in rodent models, are characterized by overexpression of ADK in reactive astrocytes (Aronica et al. 2011, Boison 2012) and a hypermethylated state of DNA (Miller-Delaney et al. 2015, Williams-Karnesky et al. 2013, Kobow et al. 2013). As stated in the “methylation hypothesis of epileptogenesis” originally proposed by Kobow and Blumcke in 2011 (Kobow & Blumcke 2011) seizures by themselves may induce epigenetic chromatin modifications, thereby aggravating the epileptogenic condition. Consequently, hypermethylation of DNA was considered a driving force for the progression of epilepsy (Kobow & Blumcke 2012). DNA methylation is an epigenetic modification whereby S-adenosylmethionine (SAM) contributes a methyl group to the formation of 5-methylcytosine bases in the DNA. This leads to the formation of S-adenosylhomocysteine (SAH), which is cleaved by S-adenosylhomocysteine hydrolase (SAHH) into adenosine and homocysteine. Since the thermodynamic equilibrium of the SAHH reaction is on the side of SAH formation and since SAH is a product inhibitor of DNA methyltransferases (DNMTs), DNA methylation can only occur if adenosine is effectively removed by ADK. Consequently, increased expression of ADK, as occurs in epilepsy, drives increased DNA methylation, whereas therapeutic adenosine augmentation blocks DNA methylation and induces a hypomethylated status of DNA (Williams-Karnesky et al. 2013) (Figure 2).
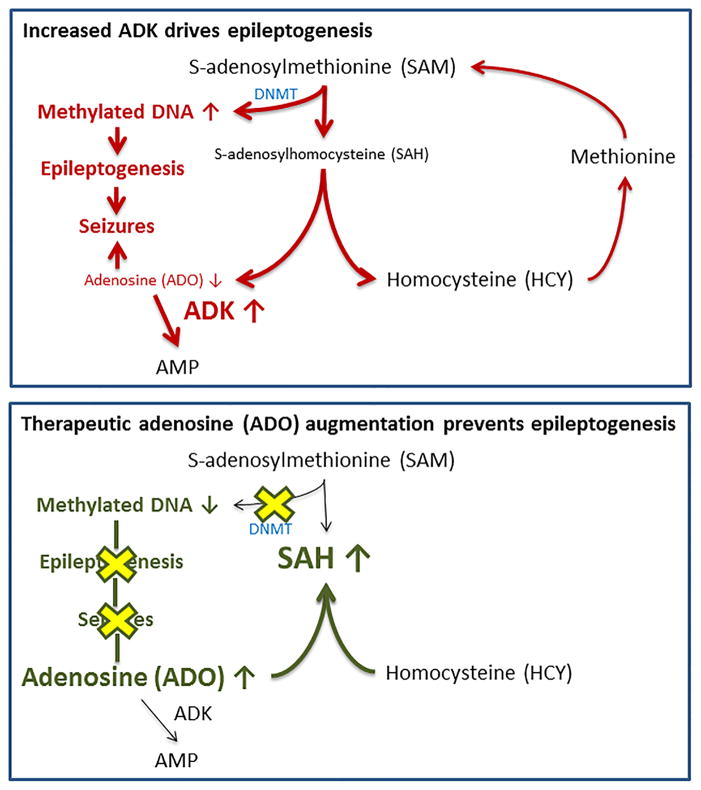
Figure 2. The epigenetics of epileptogenesis.
Increased ADK expression (top) drives increased DNA methylation as a prerequisite for progressive epileptogenesis and seizure generation. Conversely, adenosine therapy (bottom) restores normal DNA methylation preventing epileptogenesis. ADO: adenosine; ADK: adenosine kinase; SAM: S-adenosylmethionine; SAH: S-adenosylhomocysteine; DNMT: DNA-methyltransferase.
If increased DNA methylation is functionally implicated in epilepsy progression, then therapeutic adenosine augmentation, by reducing the methylation status of DNAshould block epileptogenesis. To test this hypothesis we used a rat model of status epilepticus-induced progressive temporal lobe epilepsy and silk-based brain implants engineered to release a defined dose of adenosine (250 ng adenosine/day/per ventricle) only transiently for 10 days (Williams-Karnesky et al. 2013). Transient drug use followed by a drug-free ‘washout’ period is a standard strategy to distinguish between acute antiictogenic effects of a drug and longer-lasting antiepileptogenic effects (Silver et al. 1991). Adenosine-releasing polymers, or corresponding silk-only control rods, were implanted into the lateral brain ventricles of rats after the onset of epilepsy. Compared to naïve controls, hippocampal DNMT activity was elevated in the epileptic controls prior to the adenosine delivery, whereas local silk-based adenosine delivery almost completely abrogated any DNMT activity. Consistent with those findings, DNA in the epileptic controls was hypermethylated vs. healthy controls, however the transient delivery of adenosine for only 10 days reverted the DNA methylation status in the epileptic animals back to normal; Importantly, normal methylation was maintained even weeks after cessation of adenosine release from the polymer. To assess epilepsy progression following silk-polymer implantation, the animals were monitored for an additional 3 months after expiration of active adenosine delivery. Sham treated controls and those that received control silk implants progressed in frequency and severity of seizures. Conversely, animals receiving a transient dose of adenosine for 10 days did not progress further in epilepsy development. Three month after treatment the seizure rate stabilized at ~2 per week, whereas controls progressed to at least 8 seizures per week and some controls died from excessive seizure activity. Consistent with those findings a transient dose of adenosine halted mossy fiber sprouting, a characteristic marker for epileptogenesis. Methylated DNA immunoprecipitation arrays and bisulfite sequencing revealed distinct sets of genes whose methylation status increased during epileptogenesis and was corrected by adenosine therapy. Among the targets with reduced DNA methylation during adenosine therapy several interact with DNA, or play a role in gene transcription or translation (PolD1, Polr1e, Rps6kl1, Snrpn, Znf524, Znf541, Znf710), making them candidates for mediating adenosine-dependent changes in major homeostatic functions (Williams-Karnesky et al. 2013). Further research into epigenetically regulated antiepileptogenic mechanisms may reveal transcriptional activators as epigenetic meta-regulators such as those linked to the mTOR pathway (Cho 2011). In further support of an antiepileptogenic role of hypermethylated DNA, we kindled rats in the presence of the DNMT inhibitor 5-Aza2dC (5AZA). Under those conditions, kindling epileptogenesis was suppressed, and when re-stimulated after an 11 day drug-free washout period, rats kindled in the presence of 5AZA showed a robust reduction of the seizure phenotype (stage 3 instead of stage 5 seizures) compared to control animals kindled in the absence of 5AZA. Thus changes in DNA methylation patterns are a key determinant of epilepsy progression and adenosine augmentation may reverse DNA hypermethylation and break the cycle of increasing seizure severity (Williams-Karnesky et al. 2013).
Based on our new findings we propose an amended version of our original “ADK hypothesis of epileptogenesis” (Boison 2008) by including a biphasic response of the DNA methylome in response to an epileptogenesis triggering insult: Acute DNA hypomethylation, also seen within 24 h after TBI and associated with microglial activation (Zhang et al. 2007), may contribute to the initiation of epileptogenesis, whereas chronic DNA hypermethylation associated with astroglial activation and adenosine deficiency (Williams-Karnesky et al. 2013) might be required to maintain the epileptic state and to promote disease progression; this biphasic response may be directly related to biphasic expression changes of ADK during the course of epileptogenesis (Boison 2008, Li et al. 2008, Williams-Karnesky et al. 2013). We acknowledge that at this time our hypothesis is largely based on correlative evidence. Although key data, such as long-term epigenetic and antiepileptogenic effects of a short-term adenosine dose and antiepileptogenic activity of a conventional DNMT inhibitor, strongly support a causal relationship between increased DNA methylation and increased epileptogenesis, more research is needed to identify relevant epigenetic targets and mechanisms.
Intriguingly, genetic variants of ADK were associated with the development of posttraumatic epilepsy in humans (Diamond et al. 2015). Therefore, changes in adenosine metabolism, such as those triggered by pathological overexpression of ADK or by genetic mutations, emerge as an attractive biomarker for the prediction of epileptogenesis and a therapeutic target to prevent posttraumatic epilepsy.
Rehabilitating Urate — the Maligned and Forgotten Purine
Urate’s Generally Bad Reputation
Urate (a.k.a. uric acid) is often referred to as a waste product of purine metabolism (Johnson et al., 2009; Rock et al., 2013). It circulates at high levels in humans and other hominoids due to mutations in the gene encoding the urate-catabolizing enzyme urate oxidase (UOx) during primate evolution (Wu et al. 1992, Oda et al. 2002). In our species its circulating concentrations are so high they approach the limits of solubility. Urate is best known clinically for the pain and damage that results when these limits are exceeded and urate crystallizes. When this occurs in joints it results in gout, a form of inflammatory arthritis triggered by urate crystals. Similarly, when urate (or more typically its acid form, uric acid) crystallizes in the urine then it can cause kidney stones.
In addition to its direct, causal contributions to these crystallopathic disorders, higher blood levels of urate have been found to carry an increased risk of heart disease, hypertension, kidney disease and diabetes (Feig et al., 2008; Edwards, 2009; Johnson et al., 2013). Although these adverse associations are partially explained by other co-morbidities of elevated urate such as obesity (Palmer et al., 2013), they have fostered concerns that higher urate may mediate as well as mark major classes of human disease. The advent of multiple new urate-lowering therapies may be adding to the unfavorable image of urate even in the absence of gout or stones (Gaffo & Saag, 2012; Bach et al., 2014; Borghi et al., 2014).
Urate’s Protective Potential for Parkinson’s and other Neurodegenerative Diseases
Despite these known and theoretical adverse effects of higher urate levels, the evolutionary biology and biochemistry of urate have suggested that its salubrious actions may offset and possibly outweigh is detrimental effects (Álvarez-Lario & Macarrón-Vicente, 2010). Because the urate-elevating inactivation of the urate oxidase enzyme in chimpanzees, gorillas and humans can be attributed to multiple independent mutations in UOx during the speciation of primates (Wu et al. 1992, Oda et al. 2002), it is reasonably presumed that urate elevation conveyed a critical survival advantage to our ancestors. The discovery that urate possesses strong antioxidant properties, with a comparable or greater activity than ascorbate at their physiological concentrations in humans (Ames et al., 1981), suggested potential benefits of protection against oxidative stress.
The findings for urate’s antioxidant actions converged with evidence that neurodegenerative diseases like Parkinson’s disease (PD) result from excessive oxidative damage to neurons (Jenner, 2003). They hypothesized that higher levels of urate may help protect the brain from PD and prompted epidemiologists to investigate the relationship between blood urate levels and the risk of PD. Studies of prospectively followed healthy cohorts have repeatedly demonstrated that higher but ‘normal’ blood urate among healthy individuals conveys a reduced risk for developing PD later in life in men (Davis et al., 1996; de Lau et al., 2005; Weisskopf et al., 2007; Chen et al., 2009), with findings less consistent in women (O’Reilly et al., 2010; Jain et al., 2011) who have lower serum urate levels on average. For example, men in the top quartile of plasma urate levels had a significantly (55%) lower risk of later developing PD than men in the bottom quartile in a rigorously followed cohort of health professionals (Weisskopf et al., 2007). The decrease in risk was even greater (with an 80% PD risk reduction in highest compared to the lowest quartile; p<0.01 for trend) in those with blood collected at least four years before diagnosis, suggesting that the lower urate in those who develop PD precedes symptom onset and is thus unlikely to be a consequence of changes in diet, activity or medical treatment early in the clinical course of PD. Complementary epidemiological findings that a urate-elevating diet (Gao et al., 2008) and gout (Alonso et al., 2007; De Vera et al., 2008) are also associated with a lower risk of PD strengthen the link. Similarly, many cross-sectional studies have reported lower urate levels (Bakshi et al., 2015b) or urate-lowering genotypes (Gonzalez-Aramburu et al., 2013) are more likely in PD than controls. The link appears robust as it has been demonstrated across nationalities and races (Jesus et al., 2012; Sun et al., 2012), and in community (Winquist et al., 2010) as well as academic center-based (Cipriani et al., 2010) cohorts.
This epidemiological association between urate and PD risk in healthy populations prompted investigatation of whether urate might also be linked to PD progression among those already diagnosed with PD. In multiple prospectively followed PD cohorts, higher blood (or CSF) urate was strongly associated with a slower clinical progression (Schwarzschild et al., 2008; Ascherio et al., 2009; Moccia et al., 2015). A similarly significant, inverse association between serum urate and subsequent rates radiographic progression was measured by serial measurement of dopamine transporter (DAT) binding sites from the striatum using DAT brain scan imaging (Schwarzschild et al., 2008). Similarly, lower urate levels have been linked to the development or more rapid progression of other neurodegenerative diseases including amyotrophic lateral sclerosis (ALS) and Huntington’s disease (Bakshi et al., 2015b).
Preclinical studies of pharmacological (Gong et al., 2012) or genetic (Chen et al., 2013) strategies to elevated brain urate levels in animal models of PD provided biological evidence of protection by urate against the dopaminergic neuron degeneration characteristic of PD. Interestingly, an indirect antioxidant effect of urate, via its activation of the Nrf2 antioxidant response pathway in astrocytes (Zhang et al., 2014; Bakshi et al., 2015a), may account for much of urate’s protection potential in PD.
Together these epidemiological, clinical and neurobiological data identified urate as a promising molecular biomarker and possible mediator of favorable clinical progression of PD. They prompted an initial clinical trial of the urate precursor inosine as a potential urate-elevating strategy for disease modification in PD. The Safety of Urate Elevation in PD (SURE-PD) study (Parkinson Study Group, 2014) assessed three primary outcomes for safety, tolerability and urate-elevating ability of oral inosine in early PD. Despite known risks of gout and uric acid kidney stones, inosine demonstrated overall good safety and tolerability and significant elevation of both CSF and serum urate, supporting further clinical development of inosine for PD.
Prospect for protection against acute neuronal injury: from stroke to TBI
Urate elevation has emerged as a neuroprotective strategy in acute neuronal injury, as well as in chronic neuronal degeneration. As with targeting urate in PD, that in stroke has undergone rapid translation to phase 2/3 clinical trials based on a combination of laboratory and clinical data. Building on its well-established antioxidant properties (Ames et al., 1981), stroke biologists administered urate just before or during transient unilateral cerebral ischemia and found reduced striatal or cortical damage as well as preserved neurological function (Yu et al., 1998; Romanos et al., 2007). Clinical epidemiology studies found that people with higher serum urate levels when presenting with an ischemic stroke had better clinical outcomes upon hospital discharge ~two weeks later (Chamorro et al., 2002).
Based on these human and animal data Charmorro and colleagues conducted a phase 2b/3 trial of intravenous urate in acute ischemic stroke (Charmorro et al. 2014). Although it did not demonstrate a statistically significant overall benefit of urate, it was sufficiently suggestive to warrant for fuller phase 3 clinical testing. Interestingly, post hoc analysis stratifying by gender indicated significantly better anatomical and clinical outcomes after urate treatment in women, who at baseline have substantially lower serum urate levels than do men (Llull et al., 2015). Interestingly, this sex difference appeared to be mirrored for PD in the SURE-PD study (Parkinson Study Group SURE-PD Investigators, 2014; Schwarzschild et al. 2014) and warrants further attention in future studies.
TBI like stroke entails a sudden profound metabolic (as well as mechanical) injury of neurons, and thus may trigger a common cascades of excitotoxic, inflammatory and oxidative factors that contribute to functional disability. Thus the rapid clinical translation and potential of urate as a therapeutic target in stroke as well as neurodegenerative disease warrants consideration of its ‘lateral translation’ to TBI. Although urate itself has not been systematically investigated in TBI models, its precursor inosine (which rapidly metabolized to urate, and is currently in clinical development for PD) has been found to improve outcomes in rodent models of TBI (Dachir et al., 2014) and spinal cord injury (SCI) (Kim et al., 2013). Evidence that astrocytic Nrf2 pathway activation confers protection against neuronal cell death in TBI/SCI (Mao et al., 2012; Miller et al., 2014) and neurodegeneration, and that urate confers protection in PD models via this pathway (Zhang et al., 2014; Bakshi et al., 2015a), lends support to the rationale for investigating urate in TBI and SCI.
Alternatively, inosine may have direct beneficial effects independent of its metabolism to urate (Cipriani et al., 2014) as it can also protect via extracellular engagement of adenosine receptors (Gomez & Sitkovsky, 2003; Shen et al., 2005) and intracellular activation of the Mst3b signaling cascade (Kim et al., 2013). However, the metabolism of inosine to urate by way of peripheral xanthine oxidase could in theory generate deleterious oxidative stress via its hydrogen peroxide byproduct (Kelley et al., 2009), potentially offsetting some of its putative benefits. Thus in traumatic CNS injury, as in acute stroke, the intravenous administration of urate itself may be the most effective as well as expedient strategy to take advantage of its benefits. Urate elevation represents a readily testable candidate neuroprotective strategy across disorders of neurodegeneration and acute neuronal injury.
Acknowledgments
EKJ is supported by NIH grants HL069846, DK068575, DK079307, and DK091190 and EKJ and PMK by NS070003 and DOD grants W81XWH-10-1-0623 and W81XWH-14-2-0018; and MAS by NIH grant NS084710 and DoD grant W81XWH1110150.
Abbreviations
- 2′,3′-cNMPs
nucleoside 2′,3′-cyclic monophosphates
- ADK
adenosine kinase
- CNPase
2′,3′-cyclic nucleotide 3′phosphodiesterase
- DNMTs
DNA methyltransferases
- KO
knockout
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- mPTPs
mitochondrial permeability transition pores
- m/z
mass-to-charge ratio
- SAHH
S-adenosylhomocysteine hydrolase
- SAM
S-adenosylmethionine
- SRM
selected reaction monitoring
- TNAP
tissue non-specific alkaline phosphatase
- TBI
traumatic brain injury
References
- Akahane M, Ono H, Ohgushi H, Takakura Y. Viability of ischemia/reperfused bone determined at the gene expression level. J Reconstr Microsurg. 2001a;17:203–209. doi: 10.1055/s-2001-14352. [DOI] [PubMed] [Google Scholar]
- Akahane M, Ono H, Ohgushi H, Tamai S. Viability of ischemia/reperfused muscles in rat: a new evaluation method by RNA degradation. J Orthop Res. 2001b;19:559–564. doi: 10.1016/S0736-0266(00)00062-0. [DOI] [PubMed] [Google Scholar]
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Mol Biol Cell. Garlan Pbulishing, Inc; New York & London: 1989. The Cell Nucleus; pp. 481–550. [Google Scholar]
- Almeida A, Paul Thiery J, Magdelenat H, Radvanyi F. Gene expression analysis by real-time reverse transcription polymerase chain reaction: influence of tissue handling. Anal Biochem. 2004;328:101–108. doi: 10.1016/j.ab.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Alonso A, Rodriguez LA, Logroscino G, Hernan MA. Gout and risk of Parkinson disease: a prospective study. Neurology 2007. 2007;69:1696–700. doi: 10.1212/01.wnl.0000279518.10072.df. [DOI] [PubMed] [Google Scholar]
- Álvarez-Lario B, Macarrón-Vicente J. Uric acid and evolution. Rheumatology (Oxford) 2010;49:2010–5. doi: 10.1093/rheumatology/keq204. [DOI] [PubMed] [Google Scholar]
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Zurolo E, Iyer A, et al. Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia. 2011;52:1645–1655. doi: 10.1111/j.1528-1167.2011.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, LeWitt PA, Xu K, Eberly S, Watts A, Matson WR, Marras C, Kieburtz K, Rudolph A, Bogdanov MB, Schwid SR, Tennis M, Tanner CM, Beal MF, Lang AE, Oakes D, Fahn S, Shoulson I, Schwarzschild MA. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2008;66:1460–8. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarashvili T, Krestinina O, Galvita A, Grachev D, Baburina Y, Stricker R, Evtodienko Y, Reiser G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2′,3′-cyclic nucleotides and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Am J Physiol Cell Physiol. 2009;296:1428–1439. doi: 10.1152/ajpcell.00006.2009. [DOI] [PubMed] [Google Scholar]
- Azarashvili T, Stricker R, Reiser G. The mitochondria permeability transition pore complex in the brain with interacting proteins - promising targets for protection in neurodegenerative diseases. Biol Chem. 2010;391:619–629. doi: 10.1515/BC.2010.070. [DOI] [PubMed] [Google Scholar]
- Bach MH, Simkin PA. Uricosuric drugs: the once and future therapy for hyperuricemia? Curr Opin Rheumatol. 2014;26:169–75. doi: 10.1097/BOR.0000000000000035. [DOI] [PubMed] [Google Scholar]
- Bähre H, Kaever V. Measurement of 2′,3′-cyclic nucleotides by liquid chromatography–tandem mass spectrometry in cells. J Chromatography B. 2014;964:208–211. doi: 10.1016/j.jchromb.2014.02.046. [DOI] [PubMed] [Google Scholar]
- Bakshi R, Zhang H, Logan R, Joshi I, Xu Y, Chen X, Schwarzschild MA. Neuroprotective effects of urate are mediated by augmenting astrocytic glutathione synthesis and release. Neurobiol Dis. 2015a Aug 25; doi: 10.1016/j.nbd.2015.08.022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi R, Logan R, Schwarzschild MA. Purines in Parkinson’s: Adenosine A2A Receptors and Urate as Targets for Neuroprotection. In: Morelli M, et al., editors. The Adenosinergic System, Current Topics in Neurotoxicity. Vol. 10. Springer International Publishing; Switzerland: 2015b. in press. [DOI] [Google Scholar]
- Boison D. The adenosine kinase hypothesis of epileptogenesis. Progress in Neurobiology. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine dysfunction in epilepsy. Glia. 2012;60:1234–1243. doi: 10.1002/glia.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Aronica E. Comorbidities in Neurology: Is adenosine the common link? Neuropharmacology. 2015;97:18–34. doi: 10.1016/j.neuropharm.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Huber A, Padrun V, Deglon N, Aebisher P, Mohler H. Seizure suppression by adenosine-releasing cells is independent of seizure frequency. Epilepsia. 2002;43:788–796. doi: 10.1046/j.1528-1157.2002.33001.x. [DOI] [PubMed] [Google Scholar]
- Bordeleau E, Oberc C, Ameen E, da Silva AM, Yan H. Identification of cytidine 2′,3′-cyclic monophosphate and uridine 2′,3′-cyclic monophosphate in Pseudomonas fluorescens pfo-1 culture. Bioorg Med Chem Lett. 2014;24:4520–4522. doi: 10.1016/j.bmcl.2014.07.080. [DOI] [PubMed] [Google Scholar]
- Borghi C, Verardi FM, Pareo I, Bentivenga C, Cicero AF. Hyperuricemia and cardiovascular disease risk. Expert Rev Cardiovasc Ther. 2014;12:1219–25. doi: 10.1586/14779072.2014.957675. [DOI] [PubMed] [Google Scholar]
- Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- Burhenne H, Tschirner S, Seifert R, Kaever V. Identification and quantitation of 2′,3′-cGMP in murine tissues. BMC Pharmacology and Toxicology. 2013;14:P12. [Google Scholar]
- Bushfield M, Shoshani I, Johnson RA. Tissue levels, source, and regulation of 3′-AMP: an intracellular inhibitor of adenylyl cyclases. Mol Pharmacol. 1990;38:848–853. [PubMed] [Google Scholar]
- Chamorro A, Obach V, Cervera A, Revilla M, Deulofeu R, Aponte JH. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke. 2002;33:1048–52. doi: 10.1161/hs0402.105927. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Amaro S, Castellanos M, Segura T, Arenillas J, Martí-Fábregas J, Gállego J, Krupinski J, Gomis M, Cánovas D, Carné X, Deulofeu R, Román LS, Oleaga L, Torres F, Planas AM URICO-ICTUS Investigators. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13:453–60. doi: 10.1016/S1474-4422(14)70054-7. [DOI] [PubMed] [Google Scholar]
- Chen H, Mosley TH, Alonso A, Huang X. Plasma urate and Parkinson’s disease in the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2009;169:1064–9. doi: 10.1093/aje/kwp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Burdett TC, Desjardins CA, Logan R, Cipriani S, Xu Y, Schwarzschild MA. Disrupted and transgenic urate oxidases alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A. 2013;110:300–5. doi: 10.1073/pnas.1217296110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Ren J, Jackson EK. Multidrug resistance protein 4 mediates cAMP efflux from rat preglomerular vascular smooth muscle cells. Clin Exp Pharmacol Physiol. 2010;37:205–207. doi: 10.1111/j.1440-1681.2009.05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavegatti T, Costa VL, Jr, Araujo MS, Godinho RO. Skeletal muscle expresses the extracellular cyclic AMP-adenosine pathway. Br J Pharmacol. 2008;153:1331–1340. doi: 10.1038/sj.bjp.0707648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S, Bakshi R, Schwarzschild MA. Protection by inosine in a cellular model of Parkinson’s disease. Neuroscience. 2014;274:242–249. doi: 10.1016/j.neuroscience.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S, Chen X, Schwarzschild MA. Urate: a novel biomarker of Parkinson’s disease risk, diagnosis and prognosis. Biomark Med. 2010;4:701–12. doi: 10.2217/bmm.10.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CH. Frontier of epilepsy research - mTOR signaling pathway. Exp Mol Med. 2011;43:231–274. doi: 10.3858/emm.2011.43.5.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley YP, Alexander S. Genomic, transcriptomic, and epigenomic approaches to recovery after acquired brain injury. PM R. 2011;3:S52–58. doi: 10.1016/j.pmrj.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Dachir S, Shabashov D, Trembovler V, Alexandrovich AG, Benowitz LI, Shohami E. Inosine improves functional recovery after experimental traumatic brain injury. Brain Res. 2014;1555:78–88. doi: 10.1016/j.brainres.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am J Epidemiol. 1996;144:480–4. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SPC. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- Diamond ML, Ritter AC, Jackson EK, Conley YP, Kochanek PM, Boison D, Wagner AK. Genetic variation in the adenosine regulatory cycle is associated with post-traumatic epilepsy development. Epilepsia. 2015;56:1198–1206. doi: 10.1111/epi.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson Disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- De Vera M, Rahman MM, Rankin J, Kopec J, Gao X, Choi H. Gout and the risk of Parkinson’s disease: a cohort study. Arthritis Rheum. 2008;59:1549–54. doi: 10.1002/art.24193. [DOI] [PubMed] [Google Scholar]
- Do T, Sun Q, Beuve A, Kuzhikandathil EV. Extracellular cAMP inhibits D1 dopamine receptor expression in CAD catecholaminergic cells via A2a adenosine receptors. J Neurochem. 2007;101:619–631. doi: 10.1111/j.1471-4159.2006.04388.x. [DOI] [PubMed] [Google Scholar]
- Duarte T, Menezes-Rodrigues FS, Godinho RO. Contribution of the extracellular cAMP-adenosine pathway to dual coupling of β2-adrenoceptors to Gs and Gi proteins in mouse skeletal muscle. J Pharmacol Exp Ther. 2012;341:820–828. doi: 10.1124/jpet.112.192997. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway induces nitric oxide synthesis in aortic smooth muscle cells. Hypertension. 1998;31:296–302. doi: 10.1161/01.hyp.31.1.296. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Jackson EK. Cardiac fibroblasts express the cAMP-adenosine pathway. Hypertension. 2000a;36:337–342. doi: 10.1161/01.hyp.36.3.337. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Jackson EK. Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension. 2001;37:1095–1100. doi: 10.1161/01.hyp.37.4.1095. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Jackson EK. Extracellular 3′,5′-cyclic AMP-adenosine pathway inhibits glomerular mesangial cell growth. J Pharmacol Exp Ther. 2010;333:808–815. doi: 10.1124/jpet.110.166371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Shue H, Jackson EK. A2B receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension. 2000b;35:267–272. doi: 10.1161/01.hyp.35.1.267. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Mi Z, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway inhibits vascular smooth muscle cell growth. Hypertension. 1996;28:765–771. doi: 10.1161/01.hyp.28.5.765. [DOI] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Werner HB, McCulloch MC, Barrie JA, Brown A, Faichney AB, Snaidero N, Nave KA, Griffiths IR. Early ultrastructural defects of axons and axon–glia junctions in mice lacking expression of Cnp1. GLIA. 2009;57:1815–1824. doi: 10.1002/glia.20893. [DOI] [PubMed] [Google Scholar]
- Edwards NL. The role of hyperuricemia in vascular disorders. Curr Opin Rheumatol. 2009;21:132–7. doi: 10.1097/BOR.0b013e3283257b96. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK. Extracellular adenosine signaling in molecular medicine. J Mol Med. 2013;91:141–146. doi: 10.1007/s00109-013-0999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander J, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, Hughes R, Bergman W. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84:365–373. doi: 10.1053/apmr.2003.50022. [DOI] [PubMed] [Google Scholar]
- Fedele DE, Li T, Lan JQ, Fredholm BB, Boison D. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp Neurol. 2006;200:184–190. doi: 10.1016/j.expneurol.2006.02.133. [DOI] [PubMed] [Google Scholar]
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori H, Pan-Hou H. Formation of adenosine 3′-monophosphate in rat liver mitochondria. Biol Pharm Bull. 1998;21:624–627. doi: 10.1248/bpb.21.624. [DOI] [PubMed] [Google Scholar]
- Fujimori H, Sato R, Yasuda M, Pan-Hou H. A specific and rapid method for determination of adenosine 3′-monophosphate (3′-AMP) content and 3′-AMP forming enzyme activity in rat liver mitochondria, using reversed-phase HPLC with fluorescence detection. Biol Pharm Bull. 1998;21:1348–1351. doi: 10.1248/bpb.21.1348. [DOI] [PubMed] [Google Scholar]
- Gaffo AL, Saag KG. Drug treatment of hyperuricemia to prevent cardiovascular outcomes: are we there yet? Am J Cardiovasc Drugs. 2012;12:1–6. doi: 10.2165/11594580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson’s disease risk in men. Am J Epidemiol. 2008;167:831–8. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron MC, Bin A, Brun P, Etteri S, Bolego C, Florio C, Gaion RM. Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology. 2008;134:1116–1126. doi: 10.1053/j.gastro.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Gomez G, Sitkovsky MV. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood. 2003;102:4472–4478. doi: 10.1182/blood-2002-11-3624. [DOI] [PubMed] [Google Scholar]
- Gong L, Zhang QL, Zhang N, Hua WY, Huang YX, Di PW, Huang T, Xu XS, Liu CF, Hu LF, Luo WF. Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: linking to Akt/GSK3beta signaling pathway. J Neurochem. 2012;123:876–85. doi: 10.1111/jnc.12038. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aramburu I, Sanchez-Juan P, Jesus S, Gorostidi A, Fernandez-Juan E, Carrillo F, Sierra M, Gomez-Garre P, Caceres-Redondo MT, Berciano J, Ruiz-Martinez J, Combarros O, Mir P, Infante J. Genetic variability related to serum uric acid concentration and risk of Parkinson’s disease. Mov Disord. 2013;28:1737–40. doi: 10.1002/mds.25507. [DOI] [PubMed] [Google Scholar]
- Gu H, Zhang S, Wong KY, Radak BK, Dissanayake T, Kellerman DL, Dai Q, Miyagi M, Anderson VE, York DM, Piccirilli JA, Harris ME. Experimental and computational analysis of the transition state for ribonuclease A-catalyzed RNA 2′-O-transphosphorylation. Proc Natl Acad Sci U S A. 2013;110:13002–13007. doi: 10.1073/pnas.1215086110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeyer N, Goebbels S, Papiol S, Kästner A, Hofer S, Begemann M, Gerwig UC, Boretius S, Wieser GL, Ronnenberg A, Gurvich A, Heckers SH, Frahm J, Nave KA, Ehrenreich H. A myelin gene causative of a catatonia-depression syndrome upon aging. EMBO Mol Med. 2012;4:528–539. doi: 10.1002/emmm.201200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KW, Shin HK, Kim HH, Choi JM, Rhim BY, Lee WS. Metabolism of cAMP to adenosine: role in vasodilation of rat pial artery in response to hypotension. Am J Physiol. 1999;276:H376–382. doi: 10.1152/ajpheart.1999.276.2.H376. [DOI] [PubMed] [Google Scholar]
- Jackson EK. Adenosine: a physiological brake on renin release. Ann Rev Pharmacol Toxicol. 1991;31:1–35. doi: 10.1146/annurev.pa.31.040191.000245. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Gillespie DG. Extracellular 2′,3′-cAMP and 3′,5′-cAMP stimulate proliferation of preglomerular vascular endothelial cells and renal epithelial cells. Am J Physiol Renal Physiol. 2012;303:F954–F962. doi: 10.1152/ajprenal.00335.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Gillespie DG. Extracellular 2′,3′-cAMP-adenosine pathway in proximal tubular, thick ascending limb, and collecting duct epithelial cells. Am J Physiol Renal. 2013;304:F49–F55. doi: 10.1152/ajprenal.00571.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Mi Z. Preglomerular microcirculation expresses the cAMP-adenosine pathway. J Pharmacol Exp Ther. 2000;295:23–28. [PubMed] [Google Scholar]
- Jackson EK, Mi Z. Regulation of renal ectophosphodiesterase by protein kinase C and sodium diet. J Pharmacol Exp Ther. 2008;325:210–216. doi: 10.1124/jpet.107.134445. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Mi Z, Gillespie DG, Dubey RK. Metabolism of cAMP to adenosine in the renal vasculature. J Pharmacol Exp Ther. 1997;283:177–182. [PubMed] [Google Scholar]
- Jackson EK, Mi Z, Zhu C, Dubey RK. Adenosine biosynthesis in the collecting duct. J Pharmacol Exp Ther. 2003;307:888–896. doi: 10.1124/jpet.103.057166. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Cheng D, Mi Z. Extracellular cAMP-adenosine pathways in the mouse kidney. Am J Physiol Renal Physiol. 2011a;301:F565–F573. doi: 10.1152/ajprenal.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Gillespie DG. 2′,3′-cAMP, 3′-AMP and 2′-AMP inhibit human aortic and coronary vascular smooth muscle cell proliferation via A2B receptors. Am J Physiol Heart Circ Physiol. 2011b;301:H391–H401. doi: 10.1152/ajpheart.00336.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Gillespie DG, Dubey RK. Extracellular 2′,3′-cyclic adenosine monophosphate is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth. Hypertension. 2010;56:151–158. doi: 10.1161/HYPERTENSIONAHA.110.152454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem. 2009;284:33097–33106. doi: 10.1074/jbc.M109.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Zacharia LC, Mi Z. Characterization of renal ecto-phosphodiesterase. J Pharmacol Exp Ther. 2007;321:810–815. doi: 10.1124/jpet.106.119057. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Zacharia LC, Zhang M, Gillespie DG, Zhu C, Dubey RK. cAMP-adenosine pathway in the proximal tubule. J Pharmacol Exp Ther. 2006;317:1219–1229. doi: 10.1124/jpet.106.101360. [DOI] [PubMed] [Google Scholar]
- Jain S, Ton TG, Boudreau RM, Yang M, Thacker EL, Studenski S, Longstreth WT, Jr, Strotmeyer ES, Newman AB. The risk of Parkinson disease associated with urate in a community-based cohort of older adults. Neuroepidemiology. 2011;36:223–9. doi: 10.1159/000327748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53:S26–36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Jesus S, Perez I, Caceres-Redondo MT, Carrillo F, Carballo M, Gomez-Garre P, Mir P. Low serum uric acid concentration in Parkinson’s disease in southern Spain. Eur J Neurol. 2012;20:208–10. doi: 10.1111/j.1468-1331.2012.03745.x. [DOI] [PubMed] [Google Scholar]
- Jia X, Fontaine BM, Strobel F, Weinert EE. A facile and sensitive method for quantification of cyclic nucleotide monophosphates in mammalian organs: basal levels of eight cNMPs and identification of 2′,3′-cIMP. Biomolecules. 2014;4:1070–1092. doi: 10.3390/biom4041070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Sautin YY, Oliver WJ, Roncal C, Mu W, Sanchez-Lozada LG, Rodriguez-Iturbe B, Nakagawa T, Benner SA. Lessons from comparative physiology: could uric acid represent a physiologic alarm signal gone awry in western society? J Comp Physiol B. 2009;179:67–76. doi: 10.1007/s00360-008-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY, Lanaspa MA. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62:3307–15. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley EE, Khoo NK, Hundley NJ, Malik UZ, Freeman BA, Tarpey MM. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med. 2010;48:493–498. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Zai L, Liang P, Schaffling C, Ahlborn D, Benowitz LI. Inosine enhances axon sprouting and motor recovery after spinal cord injury. PLoS One. 2013;8:e81948. doi: 10.1371/journal.pone.0081948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobow K, Blumcke I. The methylation hypothesis: do epigenetic chromatin modifications play a role in epileptogenesis? Epilepsia. 2011;52(Suppl 4):14–19. doi: 10.1111/j.1528-1167.2011.03145.x. [DOI] [PubMed] [Google Scholar]
- Kobow K, Blumcke I. The emerging role of DNA methylation in epileptogenesis. Epilepsia. 2012;53(Suppl 9):11–20. doi: 10.1111/epi.12031. [DOI] [PubMed] [Google Scholar]
- Kobow K, Kaspi A, Harikrishnan KN, et al. Deep sequencing reveals increased DNA methylation in chronic rat epilepsy. Acta Neuropathol. 2013;126:741–756. doi: 10.1007/s00401-013-1168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek PM, Vagni VA, Janesko KL, et al. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:565–575. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Verrier JD, Wagner AK, Jackson EK. Adenosine. Springer; New York: 2013. The many roles of adenosine in traumatic brain injury; pp. 307–322. [Google Scholar]
- Kruh GD, Zeng H, Rea PA, Liu G, Chen ZS, Lee K, Belinsky MG. MRP subfamily transporters and resistance to anticancer agents. J Bioenerg Biomembr. 2001;33:493–501. doi: 10.1023/a:1012827221844. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Clark L, Li Y. The extracellular cAMP-adenosine pathway regulates expression of renal D1 dopamine receptors in diabetic rats. J Biol Chem. 2011 doi: 10.1074/jbc.M111.268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Wang HD, Wang XL, Tian L, Xu JY. Disruption of Nrf2 exacerbated the damage after spinal cord injury in mice. J Trauma Acute Care Surg. 2012;72:189–98. doi: 10.1097/TA.0b013e31821bf541. [DOI] [PubMed] [Google Scholar]
- Mi Z, Herzer WA, Zhang Y, Jackson EK. 3-isobutyl-1-methylxanthine decreases renal cortical interstitial levels of adenosine and inosine. Life Sci. 1994;54:277–282. doi: 10.1016/0024-3205(94)00846-9. [DOI] [PubMed] [Google Scholar]
- Mi Z, Jackson EK. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. J Pharmacol Exp Ther. 1995;273:728–733. [PubMed] [Google Scholar]
- Mi Z, Jackson EK. Evidence for an endogenous cAMP-adenosine pathway in the rat kidney. J Pharmacol Exp Ther. 1998;287:926–930. [PubMed] [Google Scholar]
- Miller DM, Wang JA, Buchanan AK, Hall ED. Temporal and spatial dynamics of nrf2-antioxidant response elements mediated gene targets in cortex and hippocampus after controlled cortical impact traumatic brain injury in mice. J Neurotrauma. 2014;31:1194–201. doi: 10.1089/neu.2013.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Delaney SF, Bryan K, Das S, McKiernan RC, Bray IM, Reynolds JP, Gwinn R, Stallings RL, Henshall DC. Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain. 2015;138:616–631. doi: 10.1093/brain/awu373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A, Takeshita M, Pan-Hou H, Fujimori H. Hepatic changes in adenine nucleotide levels and adenosine 3′-monophosphate forming enzyme in streptozotocin-induced diabetic mice. J Toxicol Sci. 2008;33:209–217. doi: 10.2131/jts.33.209. [DOI] [PubMed] [Google Scholar]
- Moccia M, Picillo M, Erro R, Vitale C, Longo K, Amboni M, Santangelo G, Palladino R, Capo G, Orefice G, Barone P, Pellecchia MT. Presence and progression of non-motor symptoms in relation to uric acid in de novo Parkinson’s disease. Eur J Neurol. 2015;22:93–8. doi: 10.1111/ene.12533. [DOI] [PubMed] [Google Scholar]
- Müller G, Wied S, Over S, Frick W. Inhibition of lipolysis by palmitate, H2O2 and the sulfonylurea drug, glimepiride, in rat adipocytes depends on cAMP degradation by lipid droplets. Biochemistry. 2008;47:1259–1273. doi: 10.1021/bi701413t. [DOI] [PubMed] [Google Scholar]
- Newell EA, Exo JL, Verrier JD, Jackson TC, Gillespie DG, Janesko-Feldman K, Kochanek PM, Jackson EK. 2′,3′-cAMP, 3′-AMP, 2′-AMP and adenosine inhibit TNF-α and CXCL10 production from activated primary murine microglia via A2A receptors. Brain Res. 2015;12:27–35. doi: 10.1016/j.brainres.2014.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M, Satta Y, Takenaka O, et al. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640–53. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- Ohkubo S, Kimura J, Matsuoka I. Ecto-alkaline phosphatase in NG108-15 cells : a key enzyme mediating P1 antagonist-sensitive ATP response. Br J Pharmacol. 2000;131:1667–1672. doi: 10.1038/sj.bjp.0703750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly EJ, Gao X, Weisskopf MG, Chen H, Schwarzschild MA, Spiegelman D, Ascherio A. Plasma urate and Parkinson’s disease in women. Am J Epidemiol. 2010;172:666–70. doi: 10.1093/aje/kwq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M, Grass J, Fischl R, Léonard R, Jin C, Hinterkörner G, Borth N, Altmann F. Nucleotide and nucleotide sugar analysis by liquid chromatography-electrospray ionization-mass spectrometry on surface-conditioned porous graphitic carbon. Anal Chem. 2010;82:9782–9788. doi: 10.1021/ac101975k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TM, Nordestgaard BG, Benn M, Tybjærg-Hansen A, Davey Smith G, Lawlor DA, Timpson NJ. Association of plasma uric acid with ischaemic heart disease and blood pressure: mendelian randomisation analysis of two large cohorts. BMJ. 2013;347:f4262. doi: 10.1136/bmj.f4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson Study Group SURE-PD Investigators. Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, Hare JM, Hooper DC, Kieburtz KD, Macklin EA, Oakes D, Rudolph A, Shoulson I, Tennis MK, Espay AJ, Gartner M, Hung A, Bwala G, Lenehan R, Encarnacion E, Ainslie M, Castillo R, Togasaki D, Barles G, Friedman JH, Niles L, Carter JH, Murray M, Goetz CG, Jaglin J, Ahmed A, Russell DS, Cotto C, Goudreau JL, Russell D, Parashos SA, Ede P, Saint-Hilaire MH, Thomas CA, James R, Stacy MA, Johnson J, Gauger L, Antonelle de Marcaida J, Thurlow S, Isaacson SH, Carvajal L, Rao J, Cook M, Hope-Porche C, McClurg L, Grasso DL, Logan R, Orme C, Ross T, Brocht AF, Constantinescu R, Sharma S, Venuto C, Weber J, Eaton K. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014;71:141–50. doi: 10.1001/jamaneurol.2013.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasakka A, Kursula P. The myelin membrane-associated enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase: on a highway to structure and function. Neurosci Bull. 2014;30:956–966. doi: 10.1007/s12264-013-1437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao F, Qi Y, Murugan E, Pasunooti S, Ji Q. 2′,3′-cAMP hydrolysis by metal-dependent phosphodiesterases containing DHH, EAL, and HD domains is non-specific: Implications for PDE screening. Biochem Biophys Res Commun. 2010;398:500–505. doi: 10.1016/j.bbrc.2010.06.107. [DOI] [PubMed] [Google Scholar]
- Ren J, Mi Z, Stewart NA, Jackson EK. Identification and quantification of 2′,3′-cAMP release by the kidney. J Pharmacol Exp Ther. 2009;328:855–865. doi: 10.1124/jpet.108.146712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9:13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanos E, Planas AM, Amaro S, Chamorro A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab. 2007;27:14–20. doi: 10.1038/sj.jcbfm.9600312. [DOI] [PubMed] [Google Scholar]
- Schmidt S. Candidate autoantigens in multiple sclerosis. Mult Scler. 1999;5:147–160. doi: 10.1177/135245859900500303. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Schwid SR, Marek K, Watts A, Lang AE, Oakes D, Shoulson I, Ascherio A, Hyson C, Gorbold E, Rudolph A, Kieburtz K, Fahn S, Gauger L, Goetz C, Seibyl J, Forrest M, Ondrasik J. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2009;65:716–23. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzschild M, Macklin EA, Ascherio A. Urate and Neuroprotection Trials. Lancet Neurology (Correspondence) 2014;13:758. doi: 10.1016/S1474-4422(14)70138-3. [DOI] [PubMed] [Google Scholar]
- Sciaraffia E, Riccomi A, Lindstedt R, Gesa V, Cirelli E, Patrizio M, De Magistris MT, Vendetti S. Human monocytes respond to extracellular cAMP through A2A and A2B adenosine receptors. J Leukoc Biol. 2014;96:113–122. doi: 10.1189/jlb.3A0513-302RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Chen GJ, Harvey BK, Bickford PC, Wang Y. Inosine reduces ischemic brain injury in rats. Stroke. 2005;36:654–659. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- Silver JM, Shin C, McNamara JO. Antiepileptogenic effects of conventional anticonvulsants in the kindling model of epilepsy. Ann Neurol. 1991;29:356–363. doi: 10.1002/ana.410290404. [DOI] [PubMed] [Google Scholar]
- Sokurenko YV, Zelenikhin PV, Ulyanova VV, Kolpakov AI, Muller D, Ilinskaya ON. Identification of 2′,3′-cGMP as an intermediate of RNA catalytic cleavage by binase and evaluation of its biological action. Russ J Bioorg Chem. 2015;41:31–36. doi: 10.1134/s1068162015010136. [DOI] [PubMed] [Google Scholar]
- Sorrentino S. Human extracellular ribonucleases: multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell Mol Life Sci. 1998;54:785–794. doi: 10.1007/s000180050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino S, Libonati M. Structure-function relationships in human ribonucleases: main distinctive features of the major RNase types. FEBS Letters. 1997;404:1–5. doi: 10.1016/s0014-5793(97)00086-0. [DOI] [PubMed] [Google Scholar]
- Sprinkle TJ. 2′,3′-cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol. 1989;4:235–301. [PubMed] [Google Scholar]
- Sun CC, Luo FF, Wei L, Lei M, Li GF, Liu ZL, Le WD, Xu PY. Association of serum uric acid levels with the progression of Parkinson’s disease in Chinese patients. Chin Med J (Engl) 2012;125:583–7. [PubMed] [Google Scholar]
- Thompson JE, Venegas FD, Raines RT. Energetics of catalysis by ribonucleases: fate of the 2′,3′-cyclic phosphodiester intermediate. Biochemistry. 1994;33:7408–7414. doi: 10.1021/bi00189a047. [DOI] [PubMed] [Google Scholar]
- Thompson RJ. 2′,3′-cyclic nucleotide-3′-phosphohydrolase and signal transduction in central nervous system myelin. Biochem Soc Trans. 1992;20:621–626. doi: 10.1042/bst0200621. [DOI] [PubMed] [Google Scholar]
- van Aubel R, Smeets PHE, Peters JGP, Bindels RJM, Russel FGM. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13:595. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- Van Damme T, Blancquaert D, Couturon P, Van Der Straeten D, Sandra P, Lynen F. Wounding stress causes rapid increase in concentration of the naturally occurring 2′,3′-isomers of cyclic guanosine- and cyclic adenosine monophosphate (cGMP and cAMP) in plant tissues. Phytochemistry. 2014;103:59–66. doi: 10.1016/j.phytochem.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Van Damme T, Zhang Y, Lynen F, Sandra P. Determination of cyclic guanosine- and cyclic adenosine monophosphate (cGMP and cAMP) in human plasma and animal tissues by solid phase extraction on silica and liquid chromatography-triple quadrupole mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;909:14–21. doi: 10.1016/j.jchromb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Verrier JD, Exo JL, Jackson TC, Ren J, Gillespie DG, Dubey RK, Kochanek PM, Jackson EK. Expression of the 2′,3′-cAMP-adenosine pathway in astrocytes and microglia. J Neurochem. 2011;118:979–987. doi: 10.1111/j.1471-4159.2011.07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier JD, Jackson TC, Bansal R, Kochanek PM, Puccio AM, Okonkwo DO, Jackson EK. The brain in vivo expresses the 2′,3′-cAMP-adenosine pathway. J Neurochem. 2012;122:115–125. doi: 10.1111/j.1471-4159.2012.07705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier JD, Jackson TC, Gillespie DG, Janesko-Feldman K, Bansal R, Goebbels S, Nave KA, Kochanek PM, Jackson EK. Role of CNPase in the oligodendrocytic extracellular 2′,3′-cAMP-adenosine pathway. GLIA. 2013;61:1595–1606. doi: 10.1002/glia.22523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier JD, Kochanek PM, Jackson EK. Schwann cells metabolize extracellular 2′,3′-cAMP to 2′-AMP. J Pharmacol Exp Ther. 2015 doi: 10.1124/jpet.115.225219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel US, Thompson RJ. Molecular structure, localization, and possible functions of the myelin-associated enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase. J Neurochem. 1988;50:1667–1677. doi: 10.1111/j.1471-4159.1988.tb02461.x. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166:561–7. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfeld PR, Heppel LA, Markham R. The enzymic hydrolysis of ribonucleoside-2′:3′ phosphates. Biochem J. 1955;60:15–19. doi: 10.1042/bj0600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser GL, Gerwig UC, Adamcio B, Barrette B, Nave K-A, Ehrenreich H, Goebbels S. Neuroinflammation in white matter tracts of Cnp1 mutant mice amplied by a minor brain injury. GLIA. 2013 doi: 10.1002/glia.22480. [DOI] [PubMed] [Google Scholar]
- Williams-Karnesky RL, Sandau US, Lusardi TA, Lytle NK, Farrell JM, Pritchard EM, Kaplan DL, Boison D. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Inv. 2013;123:3552–3563. doi: 10.1172/JCI65636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz CJ, Wormington M, Peltz SW. The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol. 2001;2:237–246. doi: 10.1038/35067025. [DOI] [PubMed] [Google Scholar]
- Winquist A, Steenland K, Shankar A. Higher serum uric acid associated with decreased Parkinson’s disease prevalence in a large community-based survey. Mov Disord. 2010;25:932–6. doi: 10.1002/mds.23070. [DOI] [PubMed] [Google Scholar]
- Wu XW, Muzny DM, Lee CC, et al. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78–84. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53:613–25. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Zhang Z, Fauser U, Schluesener HJ. Global hypomethylation defines a sub-population of reactive microglia/macrophages in experimental traumatic brain injury. Neurosci Lett. 2007;429:1–6. doi: 10.1016/j.neulet.2007.09.061. [DOI] [PubMed] [Google Scholar]
- Zhang N, Shu HY, Huang T, Zhang QL, Li D, Zhang GQ, Peng XY, Liu CF, Luo WF, Hu LF. Nrf2 signaling contributes to the neuroprotective effects of urate against 6-OHDA toxicity. PLoS One. 2014;9:e100286. doi: 10.1371/journal.pone.0100286. [DOI] [PMC free article] [PubMed] [Google Scholar]