SUMMARY
Background
Acquired and inherited bleeding disorders may present in the neonatal period with devastating lifelong effects. Diagnosing bleeding disorders in the neonatal population could aid in preventing and treating the associated complications. However, currently available platelet function testing is limited in neonates owing to difficulties obtaining adequate blood volume, lack of normal reference ranges, and an incomplete understanding of the neonatal platelet functional phenotype.
Objective
Develop small-volume, whole blood platelet function assays to quantify and compare neonatal and adult platelet function.
Methods and Results
Peripheral blood was obtained from healthy, full-term neonates at 24-hours of life. Platelet activation, secretion, and aggregation were measured via flow cytometry. Platelet adhesion and aggregation were assessed under static and flow conditions. As compared to adult platelets, peripheral neonatal platelet P-selectin expression and integrin glycoprotein (GP) IIbIIIa activation was significantly reduced in response to the G protein-coupled receptor (GPCR)-agonists thrombin receptor activator peptide-6 (TRAP-6), adenosine 5′-diphosphate (ADP), and U46619 and the immunoreceptor tyrosine-based activation motif (ITAM)-signaling pathway agonists collagen-related peptide (CRP) and rhodocytin. Neonatal platelet aggregation was markedly reduced in response to TRAP-6, ADP, U46619, CRP, and rhodocytin compared to adult platelets. The extent of neonatal and adult platelet adhesion and aggregate formation under static and shear conditions on collagen and von Willebrand factor (VWF) were similar.
Conclusions
As compared to adult platelets, we found neonatal platelet activation and secretion were blunted in response to GPCR- or ITAM-agonists, while the extent of neonatal platelet adhesion and aggregate formation was similar to adult platelets.
Keywords: neonate, platelet activation, platelet adhesiveness, platelet aggregation, platelet function tests
INTRODUCTION
Maintenance of the highly regulated human hemostatic system is dependent on the delicate balance of the pro- and anti-coagulant systems of primary and secondary hemostasis and fibrinolysis. At sites of vascular injury, primary hemostasis is initiated when platelets are recruited to extracellular matrix proteins in the basement membrane through binding of the platelet glycoprotein (GP)Ib complex to collagen-bound von Willebrand factor (VWF), allowing for adhesion via platelet α2β1 and GPIIbIIIa integrins [1]. Following platelet adhesion, intracellular signaling downstream of the immunoreceptor tyrosine-based activation motif (ITAM)-containing receptor, GPVI, results in rapid calcium mobilization, granule release, cytoskeletal reorganization, platelet spreading to increase surface area, and release of secondary mediators, adenosine 5′-diphosphate (ADP) and thromboxane-A2 (TxA2), which activate platelets downstream of the G-protein coupled receptors (GPCRs) P2Y12 and P2Y1 and TxA2 receptor, respectively [2]. Upon platelet activation, the GPIIbIIIa complex undergoes a conformational change into its high affinity state, allowing for binding of fibrinogen, VWF, and fibronectin [3]. Platelets aggregate together via GPIIbIIIa-fibrinogen interactions to form a platelet plug. Furthermore, phosphatidylserine is exposed on the platelet surface following activation, facilitating the assembly and activation of coagulation factors. Concomitant with primary hemostasis is the activation of the coagulation cascade, resulting in the generation of thrombin, which in turn activates platelets via the GPCRs protease-activated receptor (PAR) 1 and 4 and cleaves fibrinogen into fibrin around the platelet plug to firmly establish a hemostatic plug.
As the neonatal hemostatic system is developmentally regulated, significant differences exist between it and the adult hemostatic system [4,5]. Disorders of the secondary hemostatic system have been fairly well-defined, and age-specific reference values for the pro- and anti-coagulant proteins are available [6,7]. Delineating whether differences exist between the neonatal and adult primary hemostatic system has been problematic [8]. Neonatal platelet number and structure does not differ from adult platelet number and structure [4,9]. Yet, neonatal platelet function is thought to be distinct from adult platelet function [10]. The neonatal platelet functional phenotype has thus far been largely inferred from studies with samples derived from cord blood. While some studies have indicated that cord blood platelets may be functionally similar to peripheral blood platelets in certain assays [11], other studies have suggested that cord platelets and peripheral platelets are functionally distinct [12]. Several studies have indicated that neonatal platelets have decreased response to platelet agonists, decreased granule secretion, decreased fibrinogen binding, and decreased platelet aggregation [2,13,14]. This hypo-reactivity is believed to persist for up to several weeks after birth [15]. Despite these differences, bleeding times and platelet function analyzer-100 (PFA-100) closure times in healthy full-term neonates have been reported as shorter than in adults, suggesting more effective primary hemostasis [16].
Despite the characterization of their hemostatic system as immature, healthy full-term newborns are functionally hemostatic. However, premature and sick newborns often present with comorbid disruptions in their hemostatic system, with a relatively high incidence of thrombocytopenia and coagulopathy leading to potentially life-threatening bleeding [13]. Improved understanding of the functional phenotype of neonatal platelets is needed in order to determine the role of potential platelet hypo-reactivity in pathological bleeding.
MATERIALS AND METHODS
Blood collection
Adult venous blood was collected in accordance with an OHSU Review Board approved protocol via venipuncture with a 21-gauge needle from healthy volunteers into trisodium citrate (0.38% w/v; Sigma-Aldrich, St Louis, MO). Adult capillary blood was collected by finger-prick with a lancet into trisodium citrate (0.38% w/v). Blood (500–1000 μL) from full-term (>38 weeks gestation) neonates who were in the newborn nursery and receiving no medications, was collected at 24-hours of life via heel stick into trisodium citrate (0.38% w/v) in accordance with an OHSU Review Board approved protocol. Neonatal blood was obtained following a 3-minute (minimum) application of an infant heel warmer. Samples were collected after obtaining blood for requisite clinical testing and were obtained by scooping blood from the puncture site. Neonates were excluded if any of the following were present: major congenital anomaly, intraventricular hemorrhage, intravenous antibiotics, maternal history of diabetes mellitus, maternal history of immune thrombocytopenia, pre-eclampsia, chorioamnionitis, or known illicit drug or alcohol exposure.
Platelet and white blood cell (WBC) quantification assay
Adult or neonatal citrated whole blood (2.5μL) was diluted 1:10 with HEPES/Tyrode’s solution (129mM NaCl, 12mM NaHCO3, 2.9mM KCl, 20mM HEPES, 1mM MgCl2, 0.34mM Na2HPO4•12H2O, 5.6mM glucose; pH 7.3), added to a fluorescence-activated cell sorting (FACS) tube containing antibodies (Abs) CD41-FITC (1:100; BioLegend, San Diego, CA) and CD45-APC (1:100; BD Biosciences, San Jose, CA), and incubated at 25°C for 20-min. BD Cytofix Fixation Buffer (1:3; BD Biosciences) was added to the FACS tube and incubated for 5-min at 25°C. The sample was then diluted 1:100 with PBS, measured by FACS and analyzed using Flowing 2.5.1 software. The percentage of all recorded events positive for CD41-FITC or CD45-APC was quantified by FACS to enumerate platelets and white blood cells (WBCs).
Platelet activation assay
Adult or neonatal citrated whole blood (2.5μL) was diluted 1:10 with HEPES/Tyrode’s solution, added to FACS tubes containing the Abs PAC-1-FITC (1:100; BD Biosciences), CD62P-APC (1:100; Acris Antibodies, Herford, Germany), and platelet factor-4 (PF4)-Alexa Fluor 350 (1:100; Antibodies Online, Atlanta, GA), and either thrombin receptor activator-6 (TRAP-6; 10μM; Tocris Bioscience, Bristol, UK), ADP (10μM; Sigma-Aldrich), TxA2 receptor agonist (U46619; 10μM; Tocris Bioscience), collagen-related peptide (CRP; 10μg/mL), epinephrine (10μM; Chrono-Log, Havertown, PA), rhodocytin (300nM), calcium ionophore A23187 (10μM), or vehicle, and incubated for 20-min at 25°C. BD Cytofix Buffer (1:3) was added to each FACS tube and incubated at 25°C for 5-min. Samples were diluted 1:100 with PBS, measured by FACS, and analyzed using Flowing software. The platelet gate was determined by CD41-FITC positive events measured in the platelet and WBC quantification assay. This assay required a total of 25μL whole blood.
Platelet aggregation assay
Adult or neonatal citrated whole blood (150μL) was treated with D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK; 40μM) and split evenly into two aliquots. Each aliquot was centrifuged (1000g, 5-min), and plasma was removed and stored at 25°C. Each sample was re-suspended with 75μL of HEPES/Tyrode’s solution and incubated with CD45-APC (1:100; BD Biosciences) and either CD31-eFluor 450 or CD31-FITC (1:100, 15-min, 25°C; eBioscience, San Diego, CA), followed by centrifugation (1000g, 5-min). The supernatants were discarded and the pelleted samples were re-suspended in reserved plasma in order to maintain donor plasma fibrinogen levels. The aliquots were combined and treated with a GPIIbIIIa inhibitor (eptifibatide; 20μg/mL), a monoclonal antibody against GPIb (6D1; 20μg/mL), or vehicle for 10-min at 25°C. Samples (10μL) was then added to tubes containing TRAP-6 (10μM), ADP (10μM), U46619 (10μM), CRP (10μg/mL), epinephrine (10μM), ristocetin (1mg/mL), rhodocytin (300nM), calcium ionophore A23187 (10μM), or vehicle in a BioShake iQ shaker plate (1000rpm, 37°C; Quantifoil Instruments, Jena, Germany). At 0, 0.5, 1, 2, and 5-min after agonist treatment, 1μL of the sample was removed and diluted 1:200 with 12.5% BD Cytofix Buffer in PBS. Samples were measured by FACS and analyzed using Flowing software. The rate and degree of platelet aggregation was determined by quantifying double-colored CD31 events at each time point, as adapted from the method used by De Cuyper et al [17]. In order to distinguish platelet-platelet interactions from platelet-WBC interactions, CD45 positive events were excluded from the platelet aggregation analysis. This assay required 150μL of whole blood.
Static platelet adhesion and aggregation assay
Circles with an inner diameter of 5mm were drawn on rectangular glass coverslips (#1.5, 22×50mm; Corning Inc., Corning, NY) using a peroxidase-antiperoxidase pen (Life Technologies, Eugene, OR). Coverslips were coated with fibrillar equine Type-I collagen (100μg/mL; Chrono-Log) or human VWF (100μg/mL; Haematologic Technologies) for 1-hr at 25°C, followed by washing with PBS. Coverslips were then blocked with fatty acid free bovine serum albumin (BSA; 5mg/mL, 1-hr, 25°C) and washed with PBS [18]. Citrated blood samples were treated with eptifibatide (20μg/mL), 6D1 (20μg/mL), or vehicle for 10-min at 25°C, followed by incubation of 10μL of sample with TRAP-6 (10μM), ADP (10μM), U46619 (10μM), CRP (10μg/mL), epinephrine (10μM), ristocetin (1mg/mL), rhodocytin (300nM), calcium ionophore A23187 (10μM), or vehicle for 10-min at 25°C. Agonist-treated blood (5μL) was added to VWF- or collagen-coated coverslip circles and incubated for 30-min at 25°C. Coverslips were washed with HEPES/Tyrode’s solution and treated with a staining solution containing Abs CD41a-PE (1:100; eBioscience) and CD62P-FITC (1:100; BioLegend) for 10-min at 25°C. Coverslips were fixed with 4% paraformaldehyde (PFA; Santa Cruz Biotechnology, Santa Cruz, CA) and Hoechst 33342 (1:1000; Life Technologies) for 10-min at 25°C and mounted onto glass slides using Fluoromount-G. Slides were imaged using a ×63 oil-coupled, 1.4 numerical aperture objective and a Zeiss Axio Imager.M2 microscope (Carl Zeiss MicroImaging, Gottingen, Germany). Through-focus transverse differential interference contrast images of the samples were separated by a 0.1μm axial increment while the microscope was operated under the control of SlideBook 5.5 software (Intelligent Imaging Innovations, Denver, CO). Volume measurements were obtained using the custom Hilbert-transform differential interference contrast program as previously described [19,20]. This assay required 100μL of whole blood.
Flow chamber assay
Small-volume flow chambers (μ-Slide VI0.1; Ibidi, Munich, Germany) were coated with fibrillar equine Type-I collagen (100μg/mL) or VWF (100μg/mL) for 1-hr at 25°C, followed by washing with PBS. Protein-coated flow chambers were blocked with BSA (5mg/mL, 1-hr, 25°C), washed with PBS, and assembled onto the stage of a Zeiss Axiovert 200M microscope. Citrated whole blood was treated with eptifibatide (20μg/mL), 6D1 (20μg/mL), or vehicle for 10-min at 25°C and perfused through collagen- or VWF-coated chambers at shear rates of 200s−1 (9.4μL blood/chamber) or 1500s−1 (70.3μL blood/chamber) for 30-sec using a pulse-free syringe pump. Flow chambers were washed with HEPES/Tyrode’s solution for 5-min to remove unbound blood components and stained with Abs CD41a-PE and CD62P-FITC (1:100, 5-min) at the same shear rate the sample was perfused. Samples were fixed with 4%PFA and stained with Hoechst 33342 (1:1000) for 5-min at the same shear rate, followed by washing with HEPES/Tyrode’s solution. Fluorescent and differential interference contrast images were acquired using SlideBook software. Percent surface area coverage of CD41a positive cells in a 140×105μm field of view was analyzed using ImageJ software. This assay required 200μL of whole blood.
Statistical analysis
Data are represented as mean±standard error of the mean (SEM). Each experimental condition was repeated with at least 3 donor samples. Statistical analysis was performed using paired Student’s t-test. Significance for all statistical tests required P<0.05.
RESULTS
Quantification of neonatal platelets and white blood cells counts
The mean percentages of events positive for CD41-FITC (binds GPIIb) or CD45-APC for neonatal and adult samples are listed in Table 1. Neonatal samples contained a higher percentage of CD45-APC positive events (WBCs) as compared with adult samples. Equivalent percentages of CD41-FITC positive events (platelets) were observed for neonatal and adult samples.
Table 1.
Percentage of events positive for CD41-FITC or CD45-APC of adult and neonatal whole blood samples measured by fluorescence-activated cell sorting (FACS).
| Antibody | Adult (%) | Neonate (%) |
|---|---|---|
| CD41-FITC | 2.3 ± 1.07 | 2.9 ± 1.09 |
| CD45-APC | 0.6 ± 0.14 | 2.0 ± 0.83* |
| Unlabeled (RBCs) | 97.1 ± 1.16 | 95.2 ± 1.90 |
Data are represented as mean ± SEM; Nadult = 3 and Nneonate = 4;
P < 0.05 with respect to adult samples
Measurement of neonatal platelet activation and secretion
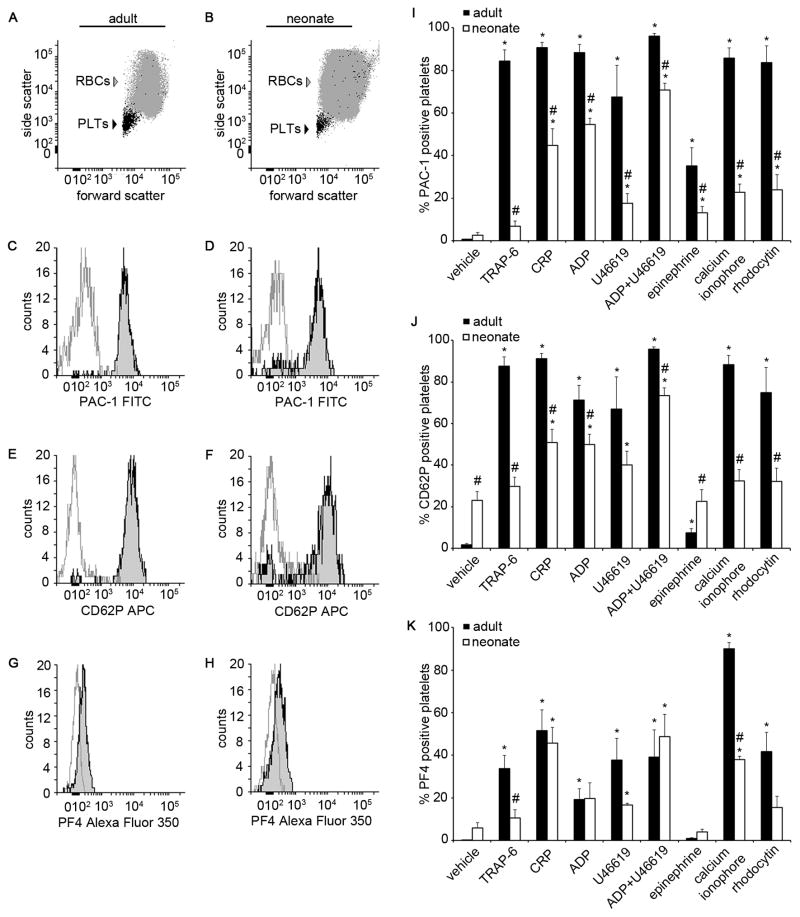
In order to evaluate platelet activation, a small-volume, whole blood, platelet activation assay was utilized to assess the binding of PAC-1-FITC (binds to activated GPIIbIIIa), CD62P-APC (P-selectin antibody), or PF4-Alexa Fluor 350 (binds to platelet factor 4) to adult or neonatal platelets in response to platelet agonists. We first used adult platelets in order to standardize our assays. Consistent with previous reports, adult platelets expressed significantly increased levels of P-selectin, PF4, and activated GPIIbIIIa in response to the GPCR-agonists TRAP-6, ADP (binds receptors P2Y1 and P2Y12), U46619 (TxA2 receptor agonist), or a combination of ADP and U46619, the ITAM-mediated signaling pathway agonists CRP (GPVI ligand) or rhodocytin (CLEC-2 ligand), or a calcium ionophore, whereas only an increase in P-selectin expression and PAC-1 binding was observed following stimulation with the GPCR-agonist epinephrine (Fig. 1G&H).
Figure 1. Adult and neonatal whole blood platelet activation.
Representative flow cytometry forward and side scatter dot plots of a whole blood sample from an adult (A) and a neonate (B). Representative histograms of PAC-1-FITC (C and D), CD62P-APC (E and F), and platelet factor 4 (PF4)-Alexa Fluor 350 (G and H) fluorescence intensity of adult and neonatal whole blood treated with adenosine 5′-diphosphate (ADP) + U46619 (10 μM; black line) or vehicle (gray line). Percent of platelets positive for PAC-1-FITC (I), CD62P-APC (J), and PF4-Alexa Fluor 350 (K) in response to thrombin receptor activator peptide-6 (TRAP-6; 10 μM), collagen related peptide (CRP; 10 μg/mL), ADP (10 μM), U46619 (10 μM), ADP+U46619 (10 μM), epinephrine (10 μM), calcium ionophore A23187 (10 μM), rhodocytin (300 nM), or vehicle treatment. Data are represented as mean ± SEM; Nadult = 6 and Nneonate = 8; *P < 0.05 with respect to vehicle treated samples; #P < 0.05 with respect to adult samples.
We next investigated the responsiveness of neonatal platelets to platelet agonists. Our results showed that neonatal platelets increased their expression of the active form of GPIIbIIIa in response to ADP, U46619, the combination of ADP and U46619, epinephrine, CRP, rhodocytin, or a calcium ionophore (Fig. 1G). However, the degree of GPIIbIIIa activation was markedly blunted for neonatal platelets as compared to adult platelets at equimolar agonist concentrations, with neonatal platelets being unresponsive to stimulation with TRAP-6.
We next examined the ability of agonists to induce neonatal platelet α-granule secretion, as measured by P-selectin expression and PF4 release. Basal expression of P-selectin, but not PF4, was significantly higher for neonatal platelets as compared to adult platelets. The expression levels of neonatal platelet P-selectin significantly increased in response to ADP, U46619, the combination of ADP and U46619, and CRP, whereas neonatal PF4 release was observed in response to U46619, the combination of ADP and U46619, CRP and a calcium ionophore (Fig. 1H). Similar to the trend observed for GPIIbIIIa activation, the increase in P-selectin expression was markedly blunted for neonatal platelets as compared to adult platelets at equimolar agonist concentrations. In contrast, equivalent levels of PF4 release were observed for neonatal and adult platelets in response to CRP or a combination of ADP and U46619. Moreover, stimulation of neonatal platelets with TRAP-6, epinephrine, or rhodocytin failed to increase either the P-selectin expression or PF4 levels above baseline, whereas only PF4 levels were observed to increase in response to a calcium ionophore.
In order to examine the potential effect of blood withdrawal through a capillary bed on the pre-activation of platelets, we next evaluated the response of adult platelets from venous blood and finger prick (capillary) blood collected from the same donor. We did not observe any differences in either baseline or agonist-induced P-selectin expression or GPIIb/IIIa activation for platelets from finger prick blood samples as compared to venous blood samples (Fig. S1).
Quantification of neonatal platelet aggregation and agglutination
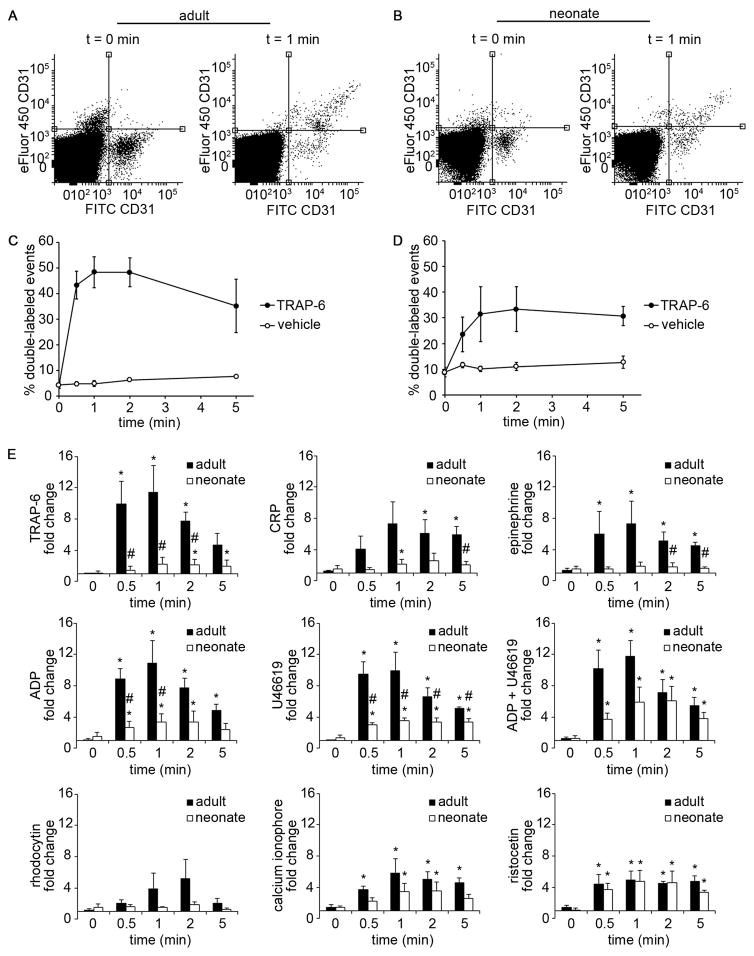
We next employed a small-volume, whole blood, platelet aggregation and agglutination assay to measure and compare adult and neonatal platelet aggregation in response to GPCR- and ITAM-agonists. Two populations of platelets labeled with a red or green fluorescent anti-CD31 Ab in whole blood were mixed together, and the degree of double-labeled events was quantified as a function of time following stimulation. Our results showed that both adult and neonatal platelets aggregated in response to the GPCR-agonists TRAP-6, ADP, U46619, the combination of ADP and U46619, and the ITAM-signaling pathway agonist CRP (Fig. 2). It is noteworthy that the degree of platelet aggregation, as measured by the percent of double-labeled events and reported as fold-change relative to baseline, was dramatically reduced for neonatal samples as compared to adult samples for these agonists. Moreover, only adult platelets were observed to aggregate in response to epinephrine or rhodocytin. In contrast, equivalent levels of adult and neonatal platelet agglutination were observed in response to ristocetin (Fig. 2E). For both adult and neonatal samples, platelet aggregation in response to TRAP-6, CRP, and the combination of ADP and U46619 was eliminated in the presence of a GPIIbIIIa-function blocking antibody, while an anti-GPIb blocking antibody blocked ristocetin-induced agglutination (Table 2).
Figure 2. Adult and neonatal whole blood platelet aggregation.
Representative CD31-FITC and CD31-eFluor 450 dot plots of adult (A) and neonatal (B) blood at t = 0 min (left) and t = 1 min (right) after treatment with thrombin receptor activator peptide-6 (TRAP-6; 10 μM). Double-labeled events (platelet aggregates) are shown in the upper right quadrant. Representative graph of percent double-labeled events of adult (C) and neonatal (D) platelets stimulated with TRAP-6 (10 μM) or vehicle at 0, 0.5, 1, 2, and 5 min. (E) Fold change of percent double-labeled events normalized to vehicle treatment of adult and neonatal blood stimulated with TRAP-6 (10 μM), collagen related peptide (CRP; 10 μg/mL), epinephrine (10 μM), adenosine 5′-diphosphate (ADP; 10 μM), U46619 (10 μM), ADP+U46619 (10 μM), rhodocytin (300 nM), or calcium ionophore A23187 (10 μM). Data are represented as mean ± SEM; Nadult = 3 and Nneonate = 4; *P < 0.05 with respect to vehicle treated samples; #P < 0.05 with respect to adult samples.
Table 2.
Effect of glycoprotein (GP) IIbIIIa and GPIb inhibition on adult and neonatal platelet aggregation.
| Agonist | Antagonist | Adult | Neonate |
|---|---|---|---|
| TRAP-6 | vehicle | 4.6 ± 1.55 | 1.9 ± 0.83** |
| eptifibatide | 0.8 ± 0.12* | 0.6 ± 0.37* | |
| 6D1 | 6.4 ± 3.71 | 2.3 ± 0.75** | |
|
| |||
| CRP | vehicle | 5.9 ± 1.08 | 2.0 ± 0.48** |
| eptifibatide | 2.2 ± 1.33* | 0.7 ± 0.26*,** | |
| 6D1 | 7.3 ± 3.86 | 3.4 ± 1.71** | |
|
| |||
| ADP + U46619 | vehicle | 5.5 ± 0.99 | 3.8 ± 0.84** |
| eptifibatide | 3.1 ± 1.82* | 0.8 ± 0.46*,** | |
| 6D1 | 6.0 ± 1.76 | 5.4 ± 1.90 | |
|
| |||
| Ristocetin | vehicle | 4.7 ± 0.74 | 3.3 ± 0.32 |
| eptifibatide | 4.1 ± 1.36 | 4.5 ± 1.75 | |
| 6D1 | 1.3 ± 0.19* | 2.3 ± 0.87*,** | |
Blood samples were treated with agonists for 5 min at 37°C. Data are represented as mean fold change of percent double-labeled events normalized to vehicle ± SEM; Nadult = 3 and Nneonate = 3;
P < 0.05 with respect to vehicle;
P < 0.05 with respect to adult samples
Measurement of neonatal platelet adhesion and aggregate formation under static conditions
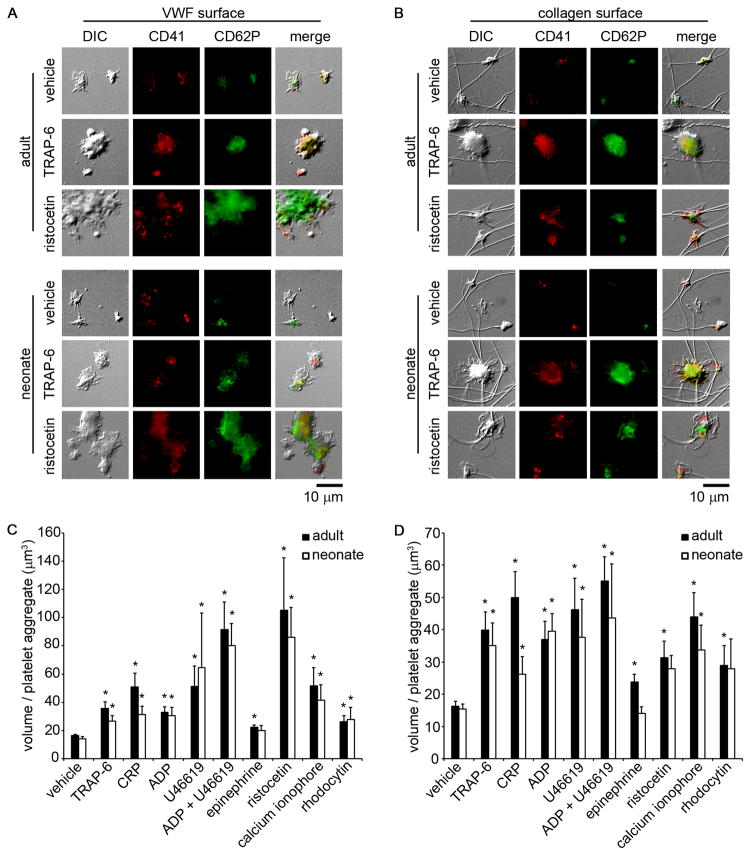
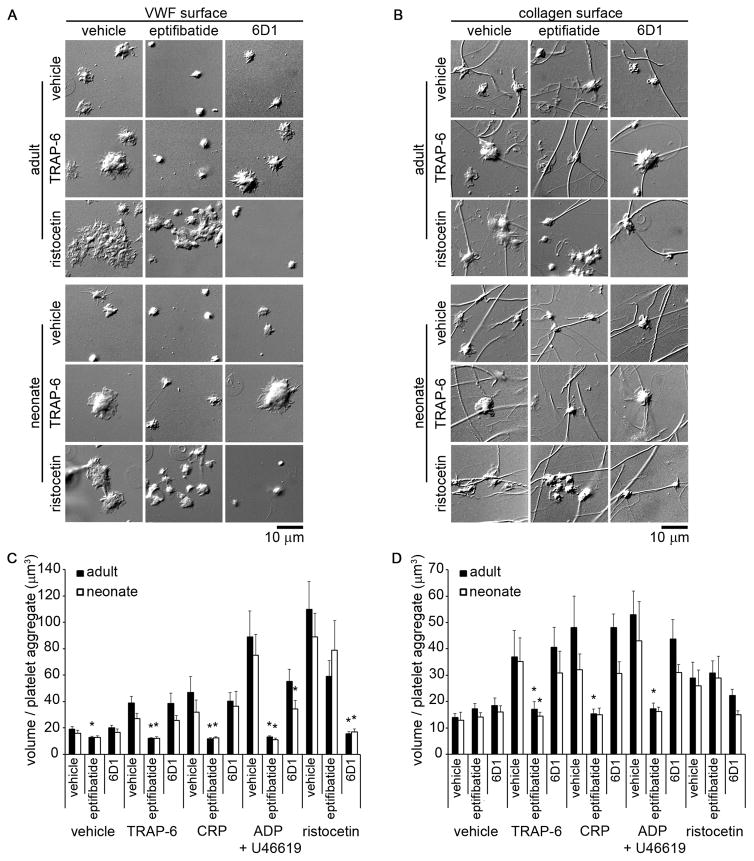
A small-volume, whole blood, static platelet adhesion and aggregation assay was developed to quantify neonatal platelet adhesion. Both adult and neonatal platelets bound to immobilized VWF (Fig. 3A) or collagen (Fig. 3B). When whole blood was treated with the agonists TRAP-6, ADP, U46619, the combination of ADP and U46619, CRP, or a calcium ionophore, both adult and neonatal platelets were observed to form aggregates on immobilized VWF and collagen, as observed by both differential interference contrast imaging (Fig. 3A&B) and quantified as the volume per platelet aggregate (Fig. 3C&D). Moreover, both adult and neonatal platelet aggregates were observed to increase their P-selectin expression levels. Equivalent levels of platelet adhesion, P-selectin expression, and aggregate formation in response to either GPCR- or ITAM-signaling pathway agonists were observed for both adult and neonatal samples, with the exception of a reduction in neonatal platelet aggregation formation on collagen following stimulation with either epinephrine or CRP as compared to adult platelets. Both adult and neonatal platelet aggregate formation on VWF and collagen in response to TRAP-6, CRP, or the combination of ADP and U46619 was eliminated in the presence of a function-blocking anti-GPIIbIIIa antibody, whereas the robust ristocetin-induced platelet agglutinate formation observed on VWF was eliminated by a GPIb-blocking antibody (Fig. 4).
Figure 3. Adult and neonatal platelet adhesion and aggregation under static conditions.
Representative differential interference contrast images and corresponding fluorescent CD41a-PE and CD62P-FITC images of adult and neonatal platelet aggregates formed on coverslips coated with 100 μg/mL von Willebrand factor (VWF) (A) or 100 μg/mL fibrillar collagen (B). Adult and neonatal citrated whole blood were pretreated with vehicle, thrombin receptor activator peptide-6 (TRAP-6; 10 μM), collagen related peptide (CRP; 10 μg/mL), adenosine 5′-diphosphate (ADP; 10 μM), U46619 (10 μM), ADP+U46619 (10 μM), epinephrine (10 μM), ristocetin (1 mg/mL), calcium ionophore A23187 (10 μM), or rhodocytin (300 nM) for 10 min prior to coverslip incubation. Mean volume of adult and neonatal platelet aggregates positive for CD62P-FITC formed on coverslips coated with VWF (C) or fibrillar collagen (D). Data are represented as mean ± SEM; Nadult = 3 and Nneonate = 3; *P < 0.05 with respect to vehicle treated samples.
Figure 4. Effect of glycoprotein (GP) IIbIIIa and GPIb inhibition on adult and neonatal platelet adhesion and aggregation under static conditions.
Representative differential interference contrast images of adult and neonatal platelet aggregates formed on coverslips coated with 100 μg/mL von Willebrand factor (VWF) (A) or 100 μg/mL fibrillar collagen (B). Adult and neonatal citrated whole blood were incubated with a glycoprotein (GP) IIbIIIa inhibitor (eptifibatide; 20 μg/mL), a GPIb function blocking antibody (6D1; 20 μg/mL), or vehicle for 10 min at 25°C and then treated with thrombin receptor activator peptide-6 (TRAP-6; 10 μM), collagen related peptide (CRP; 10 μg/mL), adenosine 5′-diphosphate (ADP) + U46619 (10 μM), ristocetin (1 mg/mL), or vehicle for 10 min. Mean volume of adult and neonatal platelet aggregates positive for CD62P-FITC formed on coverslips coated with VWF (C) or fibrillar collagen (D). Data are represented as mean ± SEM; Nadult = 3 and Nneonate = 3; *P < 0.05 with respect to vehicle treated samples.
Measurement of neonatal platelet aggregate formation under shear
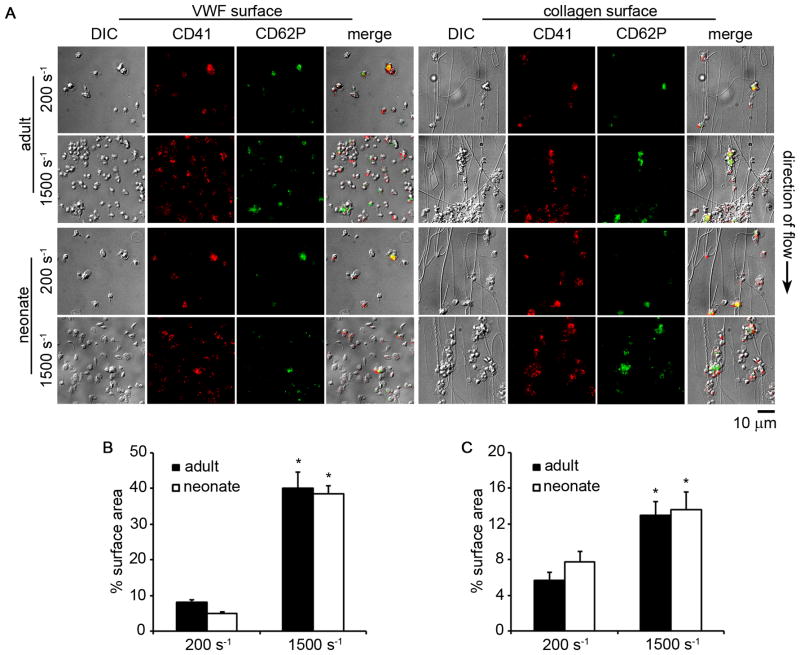
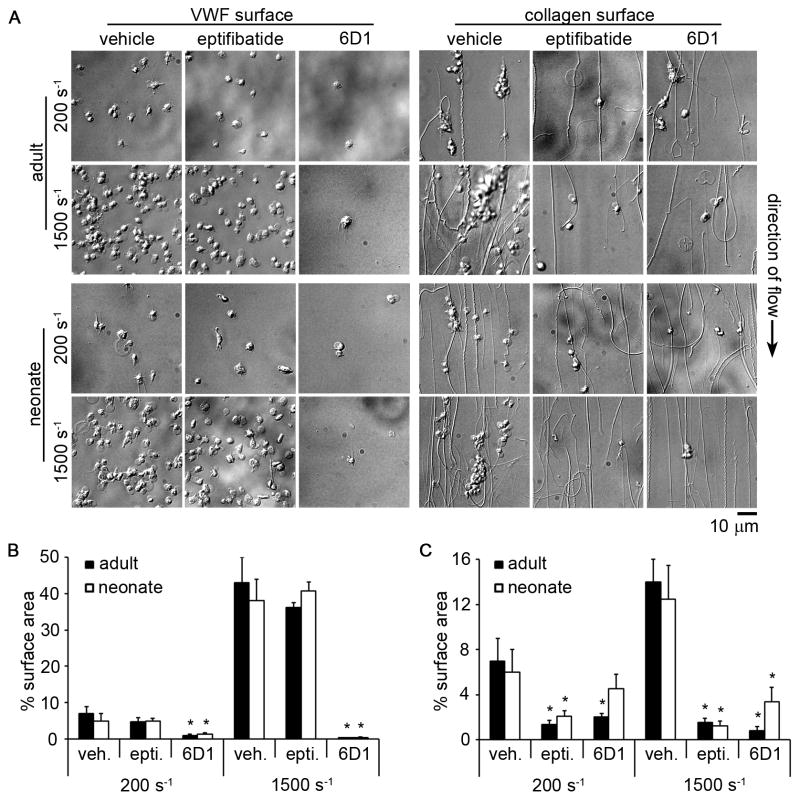
We next developed a small-volume assay to study and compare adult and neonatal platelet aggregate formation on collagen and VWF under both venous and arterial shear rates. Both adult and neonatal platelets bound to and aggregated on VWF or collagen surfaces in a shear rate-dependent manner, with an increased degree of aggregate formation observed at the higher shear rate (Fig. 5). Both the rate and extent of aggregate formation were equivalent for adult and neonatal samples. Adult and neonatal platelet aggregate formation on collagen was eliminated in the presence of a function-blocking anti-GPIIbIIIa antibody, whereas a GPIb-blocking antibody eliminated platelet adhesion to VWF under shear (Fig. 6).
Figure 5. Adult and neonatal platelet adhesion and aggregation under fluid shear conditions.
(A) Representative differential interference contrast images and corresponding fluorescent CD41a-PE and CD62P-FITC images of adult and neonatal platelet aggregates formed in a small-volume flow chamber coated with 100 μg/mL von Willebrand factor (VWF) or 100 μg/mL fibrillar collagen at 200 or 1500 s−1 shear rate for 30 s. The black arrow denotes direction of blood flow. Mean percent surface area coverage of a 140 × 105 μm field of view of adult and neonatal platelet aggregates positive for CD41a-PE formed in flow chambers coated with VWF (B) or collagen (C). Data are represented as mean ± SEM; Nadult = 3 and Nneonate = 3; *P < 0.05 with respect to 200 s−1 shear rate.
Figure 6. Effect of glycoprotein (GP) IIbIIIa and GPIb inhibition on adult and neonatal platelet adhesion and aggregation under fluid shear conditions.
(A) Representative differential interference contrast images of adult and neonatal platelet aggregates formed in a small-volume flow chamber coated with 100 μg/mL von Willebrand factor (VWF) or 100 μg/mL fibrillar collagen at 200 or 1500 s−1 shear rate for 30 s. Adult and neonatal citrated whole blood were incubated with a glycoprotein (GP) IIbIIIa inhibitor (eptifibatide; 20 μg/mL), a GPIb function blocking antibody (6D1; 20 μg/mL), or vehicle for 10 min at 25°C prior to perfusion through flow chambers. Mean percent surface area coverage of a 140 × 105 μm field of view of adult and neonatal platelet aggregates positive for CD41a-PE formed in flow chambers coated with VWF (B) or collagen (C). Data are represented as mean ± SEM; Nadult = 3 and Nneonate = 3; *P < 0.05 with respect to vehicle treated samples.
DISCUSSION
The hemostatic system is developmentally regulated, characterized by age-dependent qualitative and quantitative differences in both primary and secondary hemostasis. Determining the presence of an acquired or inherited hemostatic defect in the neonatal population is dependent upon the availability of assays that can be carried out in patients with limited venous access and limited blood volume. This is particularly challenging when evaluating the primary hemostatic system. Available platelet function data have demonstrated that neonatal platelets obtained from cord blood are hypo-reactive compared to adult platelets when evaluating platelet response to agonists in terms of granule secretion, receptor conformational changes, intracellular calcium changes, and aggregation [4,9]. However, primary hemostasis as measured by bleeding time and PFA-100 closure times is shortened in neonatal blood compared to adult blood [21]. Further, healthy newborns do not have a primary hemostatic defect clinically. Our understanding of the functional phenotype of neonatal platelets and the implications of the differences from the functional phenotypes of adult platelets is limited by the lack of assays that can be utilized in the neonatal population.
Here we present an explorative pilot study utilizing four small-volume, whole blood, platelet function assays to assess the functional phenotype of peripherally obtained neonatal platelets. In total, the four assays require only 500μL of whole blood, which can be safely obtained from low birth weight infants. Importantly, we ensured that our assays used whole blood in order to more closely mimic the interactions between each of the blood components that occur in vivo and minimize the handling and manipulation of neonatal blood samples. With FACS technology becoming available in many clinical laboratories [22] and the inherent small sample volume required for FACS, the development of FACS-based platelet function assays may be ideal for the study of primary hemostasis in the neonatal population. Point-of-care microfluidic platforms that offer easy to use and simple biological readouts, such as time to occlusion or aggregation times, would need to be developed to allow for the translation of our studies on platelet adhesion and aggregation towards clinical utility in the neonatal population.
Studies using FACS analysis have reported that neonatal platelets from peripheral blood in the first days of life are hypo-responsive to ADP, TRAP-6, the GPVI-ligand CRP, and the TxA2 receptor agonist U46619 [3,13,15,23,24], have reduced P-selectin expression following agonist stimulation [11,25,26], and have fewer α-granules [27] relative to adult platelets. At 24-hours of life, we found that neonatal platelets expressed significantly less P-selectin in response to both GPCR- and ITAM-signaling pathway agonists as compared to adult platelets. The lack of response to epinephrine may be explained by the reported decrease in α2-adrenergic receptors on neonatal platelets as compared to adults [28]. The fact that we did not observe platelet α-granule secretion in response to TRAP-6 is in line with previous studies demonstrating reduced calcium mobilization upon PAR-1 activation in neonatal platelets as compared to adults [29]. Along these lines, the reduced neonatal platelet α-granule secretion in response to calcium ionophore in our study further suggests reduced levels or mobilization of intracellular calcium in neonatal platelets as compared to adults.
Our study demonstrates that neonatal platelets expressed an increased baseline level of P-selectin as compared to adult platelets, whereas neither adult nor neonatal platelets expressed significant levels of the α-granule cytokine PF4 or the activated form of GPIIbIIIa at rest. In addition, platelets from adult finger prick blood had equivalent basal P-selectin expression and GPIIbIIIa activation as platelets from adult venous blood, suggesting that the withdrawal of blood through a capillary bed had a negligible effect on platelet activation. Along these lines, Schmugge et al. reported that platelets from 2–3 day old neonates collected peripherally by dripping blood from the end of a needle into a tube were found to have increased P-selectin, annexin-V, microparticles, and CD41 (inactive GPIIbIIIa) levels, but not activated GPIIbIIIa when compared with adult samples collected via venipuncture [30]. They tested five adult samples collected with the same method used with neonates and found no difference in platelet baseline P-selectin expression levels compared with venipuncture collection, suggesting that the different P-selectin expression levels observed in the neonatal samples were likely not due to a collection artifact. Future studies are required to understand the mechanisms by which P-selectin expression levels are developmentally regulated, and to understand whether this plays a physiological role in neonatal hemostasis. As P-selectin is constitutively expressed by megakaryocytes, perhaps these young platelets express an increased constitutive surface level of P-selectin during development. This might explain why the baseline P-selectin levels for neonatal platelets were increased as compared to adult platelets, whereas the levels of the α-granule cytokine PF4 were equivalent at baseline.
It has been shown that neonatal and adult platelet baseline GPIIbIIIa expression levels are equivalent [11]; however, neonatal platelets have been shown to have reduced PAC-1 binding (which only binds the active form of GPIIbIIIa) following agonist stimulation relative to adult platelets [11,25,26]. Our data support this paradigm in that we found that neonatal platelets expressed less active GPIIbIIIa in response to both GPCR- and ITAM-mediated agonists as compared to adults. These results indicate that signaling pathways regulating GPIIbIIIa activation may be differently regulated in neonatal platelets compared with adults. Additionally, it has been previously reported that neonatal platelets from cord blood or peripheral blood have a reduced aggregation response compared to adult platelets [31,32]. Interestingly, while we observed a decrease in platelet aggregation for neonatal platelets relative to adult platelets in response to both GPCR- and ITAM-signaling pathway agonists, we did find that the extent of platelet agglutination in response to ristocetin was equivalent between neonatal and adult platelets. As expected, we found that the inhibition of GPIb reduced adult and neonatal platelet agglutination or aggregate formation on VWF in response to ristocetin. Although neonatal platelet activation and aggregation are reduced relative to adults in vitro, full-term neonates do not clinically display a bleeding tendency [13]. Interestingly, the whole blood flow chamber assay revealed no significant difference in the degree of platelet adhesion between adult and neonatal platelets. This may be due in part to enhanced platelet/vessel wall interactions due to the presence of higher molecular weight VWF, higher concentrations of VWF, higher hematocrits, and higher red blood cell mean corpuscular volumes in neonates compared to adults [7,12,21].
This study was aimed to assess the feasibility of a collection of small-volume assays to study neonatal platelet function from heel-stick blood samples. Thus, the sample sizes used in our current study were limited to repeats of 3–4 samples per test, which in turn limits the conclusions that can be drawn from this study regarding the population distribution of neonatal platelet function. Further work is required to expand sample sizes to directly compare cord and peripherally obtained platelets, and to evaluate neonatal platelet responses in disease states. Our current study, utilizing small sample sizes, demonstrates the utility and feasibility of a collection of small-volume assays to assess neonatal platelet activation, adhesion and aggregation.
New platelet function assays are needed to aid in the diagnosis of platelet dysfunction in the neonatal population, to guide treatment decisions in the event of clinically significant bleeding, as well as to understand the physiological differences between neonatal and adult platelets. Defining the functional phenotype of neonatal platelets may eventually provide guidance in diagnosing disease states and directing therapy in the neonatal population. Future work will assess differences in platelet function at varying gestational ages and in pre-term neonates.
Supplementary Material
ESSENTIALS.
Assays are needed to aid in the diagnosis of platelet dysfunction in the neonatal population.
Development of small-volume assays to assess neonatal platelet activation and aggregation.
Compared to adult platelets, neonatal platelet activation and secretion was reduced.
The extent of neonatal and adult platelet adhesion and aggregate formation were similar.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (RO1 HL101972). S. Baker-Groberg is a Whitaker International Fellow. O. McCarty is an American Heart Association Established Investigator (13EIA12630000). The authors would like to thank A. Cox and J. Wright and her team at the Oregon Health & Science University for their aid in consenting donors and neonatal sample collection.
The authors also acknowledge N. Dovlatova at the University of Nottingham for her insightful comments and technical assistance.
Footnotes
ADDENDUM
S. M. Baker-Groberg, S. Lattimore, K. M. Haley, M. Recht, and O. J. McCarty conceived and designed the experiments. S. M. Baker-Groberg performed the experiments. S. M. Baker-Groberg, K. M. Haley, M. Recht, and O. J. McCarty analyzed the data. S. M. Baker-Groberg, K. M. Haley, M. Recht, S. Lattimore, and O. J. McCarty wrote the paper.
DISCLOSURE OF CONFLICT OF INTERESTS
The authors state that they have no conflict of interest.
References
- 1.Wei AH, Schoenwaelder SM, Andrews RK, Jackson SP. New insights into the haemostatic function of platelets. Br J Haematol. 2009;147:415–30. doi: 10.1111/j.1365-2141.2009.07819.x. [DOI] [PubMed] [Google Scholar]
- 2.Del Vecchio A, Motta M, Romagnoli C. Neonatal platelet function. Clin Perinatol. 2015;42:625–38. doi: 10.1016/j.clp.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Kühne T, Imbach P. Neonatal platelet physiology and pathophysiology. Eur J Pediatr. 1998;157:87–94. doi: 10.1007/s004310050776. [DOI] [PubMed] [Google Scholar]
- 4.Haley KM, Recht M, McCarty OJT. Neonatal platelets: mediators of primary hemostasis in the developing hemostatic system. Pediatr Res. 2014;76:230–7. doi: 10.1038/pr.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Powers P. Development of the human coagulation system in the full-term infant. Blood. 1987;70:165–72. [PubMed] [Google Scholar]
- 6.Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998–2005. [PubMed] [Google Scholar]
- 7.Andrew M, Paes B, Johnston M. Development of the hemostatic system in the neonate and young infant. Am J Pediatr Hematol Oncol. 1990;12:95–104. doi: 10.1097/00043426-199021000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, De Rosa L, Hamilton S, Ragg P, Robinson S, Auldist A, Crock C, Roy N, Rowlands S. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95:362–72. doi: 10.1160/TH05-01-0047. [DOI] [PubMed] [Google Scholar]
- 9.Israels SJ, Rand ML, Michelson AD. Neonatal platelet function. Semin Thromb Hemost. 2003;29:363–72. doi: 10.1055/s-2003-42587. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer-Marin F, Stanworth S, Josephson C, Sola-Visner M. Distinct differences in platelet production and function between neonates and adults: implications for platelet transfusion practice. Transfusion (Paris) 2013;53:2814–21. doi: 10.1111/trf.12343. [DOI] [PubMed] [Google Scholar]
- 11.Sitaru AG, Holzhauer S, Speer CP, Singer D, Obergfell A, Walter U, Grossmann R. Neonatal platelets from cord blood and peripheral blood. Platelets. 2005;16:203–10. doi: 10.1080/09537100400016862. [DOI] [PubMed] [Google Scholar]
- 12.Saxonhouse MA, Garner R, Mammel L, Li Q, Muller KE, Greywoode J, Miller C, Sola-Visner M. Closure times measured by the platelet function analyzer PFA-100 are longer in neonatal blood compared to cord blood samples. Neonatology. 2010;97:242–9. doi: 10.1159/000253755. [DOI] [PubMed] [Google Scholar]
- 13.Sola-Visner M. Platelets in the neonatal period: developmental differences in platelet production, function, and hemostasis and the potential impact of therapies. ASH Educ Program Book. 2012;2012:506–11. doi: 10.1182/asheducation-2012.1.506. [DOI] [PubMed] [Google Scholar]
- 14.Israels SJ, Cheang T, Roberston C, McMillan-Ward EM, McNicol A. Impaired signal transduction in neonatal platelets. Pediatr Res. 1999;45:687–91. doi: 10.1203/00006450-199905010-00014. [DOI] [PubMed] [Google Scholar]
- 15.Strauss T, Sidlik-Muskatel R, Kenet G. Developmental hemostasis: primary hemostasis and evaluation of platelet function in neonates. Semin Fetal Neonatal Med. 2011;16:301–4. doi: 10.1016/j.siny.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Roschitz B, Sudi K, Köstenberger M, Muntean W. Shorter PFA-100 closure times in neonates than in adults: role of red cells, white cells, platelets and von Willebrand factor. Acta Paediatr Oslo Nor 1992. 2001;90:664–70. [PubMed] [Google Scholar]
- 17.De Cuyper IM, Meinders M, van de Vijver E, de Korte D, Porcelijn L, de Haas M, Eble JA, Seeger K, Rutella S, Pagliara D, Kuijpers TW, Verhoeven AJ, van den Berg TK, Gutiérrez L. A novel flow cytometry-based platelet aggregation assay. Blood. 2013;121:e70–80. doi: 10.1182/blood-2012-06-437723. [DOI] [PubMed] [Google Scholar]
- 18.Baker-Groberg SM, Cianchetti FA, Phillips KG, McCarty OJT. Development of a method to quantify platelet adhesion and aggregation under static conditions. Cell Mol Bioeng. 2014;7:285–90. doi: 10.1007/s12195-014-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker SM, Phillips KG, McCarty OJT. Development of a label-free imaging technique for the quantification of thrombus formation. Cell Mol Bioeng. 2012;5:488–92. doi: 10.1007/s12195-012-0249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker-Groberg SM, Bornstein S, Zilberman-Rudenko J, Schmidt M, Tormoen GW, Kernan C, CRT, Wong MH, Phillips KG, McCarty OJT. Effect of ionizing radiation on the physical biology of head and neck squamous cell carcinoma cells. Cell Mol Bioeng. 2015;8:517–25. doi: 10.1007/s12195-015-0393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Israels SJ, Cheang T, McMillan-Ward EM, Cheang M. Evaluation of primary hemostasis in neonates with a new in vitro platelet function analyzer. J Pediatr. 2001;138:116–9. doi: 10.1067/mpd.2001.109794. [DOI] [PubMed] [Google Scholar]
- 22.Brown M, Wittwer C. Flow cytometry: principles and clinical applications in hematology. Clin Chem. 2000;46:1221–9. [PubMed] [Google Scholar]
- 23.Israels SJ. Diagnostic evaluation of platelet function disorders in neonates and children: an update. Semin Thromb Hemost. 2009;35:181–8. doi: 10.1055/s-0029-1220326. [DOI] [PubMed] [Google Scholar]
- 24.Schlegel N, Bardet V, Kenet G, Muntean W, Zieger B, Nowak-Göttl U. Diagnostic and therapeutic considerations on inherited platelet disorders in neonates and children. Klin Pädiatr. 2010;222:209–14. doi: 10.1055/s-0030-1249065. [DOI] [PubMed] [Google Scholar]
- 25.Rajasekhar D, Kestin AS, Bednarek FJ, Ellis PA, Barnard MR, Michelson AD. Neonatal platelets are less reactive than adult platelets to physiological agonists in whole blood. Thromb Haemost. 1994;72:957–63. [PubMed] [Google Scholar]
- 26.Wasiluk A, Mantur M, Szczepański M, Kemona H, Baran E, Kemona-Chetnik I. The effect of gestational age on platelet surface expression of CD62P in preterm newborns. Platelets. 2008;19:236–8. doi: 10.1080/09537100701882046. [DOI] [PubMed] [Google Scholar]
- 27.Saving KL, Mankin PE, Gorman MJ. Differences in adhesion receptor expression between immature and older platelets and red blood cells of neonates and adults. J Pediatr Hematol Oncol. 2002;24:120–4. doi: 10.1097/00043426-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Corby DG, O’Barr TP. Decreased alpha-adrenergic receptors in newborn platelets: cause of abnormal response to epinephrine. Dev Pharmacol Ther. 1981;2:215–25. [PubMed] [Google Scholar]
- 29.Gelman B, Setty BN, Chen D, Amin-Hanjani S, Stuart MJ. Impaired mobilization of intracellular calcium in neonatal platelets. Pediatr Res. 1996;39:692–6. doi: 10.1203/00006450-199604000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Schmugge M, Rand ML, Bang KWA, Mody M, Dunn MS, Amankwah KS, Blanchette VS, Freedman J. The relationship of von Willebrand factor binding to activated platelets from healthy neonates and adults. Pediatr Res. 2003;54:474–9. doi: 10.1203/01.PDR.0000081294.26060.4B. [DOI] [PubMed] [Google Scholar]
- 31.Uçar T, Gurman C, Arsan S, Kemahli S. Platelet aggregation in term and preterm newborns. Pediatr Hematol Oncol. 2005;22:139–45. doi: 10.1080/08880010590907230. [DOI] [PubMed] [Google Scholar]
- 32.Ferrer-Marin F, Chavda C, Lampa M, Michelson AD, Frelinger AL, Sola-Visner M. Effects of in vitro adult platelet transfusions on neonatal hemostasis. J Thromb Haemost JTH. 2011;9:1020–8. doi: 10.1111/j.1538-7836.2011.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.