Abstract
Randomized placebo controlled trials are a mainstay of modern clinical epilepsy research; the success or failure of innovative therapies depends on proving superiority to a placebo. Consequently, understanding what drives response to placebo (including the “placebo effect”) may facilitate evaluation of new therapies. In this review, part one will explore observations about placebos specific to epilepsy, including the relatively higher placebo response in children, apparent increase in placebo response over the past several decades, geographic variation in placebo effect, relationship to baseline epilepsy characteristics, influence of nocebo on clinical trials, the possible increase in (SUDEP) in placebo arms of trials, and patterns that placebo responses appear to follow in individual patients. Part two will discuss the principal causes of placebo responses, including regression to the mean, anticipation, classical conditioning, the Hawthorne effect, expectations from symbols, and the natural history of disease. Included in part two will be a brief overview of recent advances using simulations from large datasets that have afforded new insights into causes of epilepsy related placebo responses. In part three, new developments in study design will be explored, including sequential parallel comparison, two-way enriched design, time to pre-randomization, delayed start, and cohort reduction techniques.
Keywords: Placebo effect, epilepsy, clinical trial
INTRODUCTION
Epilepsy affects at least 2.2 million Americans, costs $9.5–$12.5 billion annually, and has a 10-fold increased risk of sudden death compared to the general public (Institute of Medicine (U.S.) et al., 2012). Approximately 30% of patients remain uncontrolled by anti-seizure drugs (Kwan and Brodie, 2000). Surgery for uncontrolled patients results in 13.5–92.5% seizure-freedom (West et al., 2015), but only 44–45% achieve long-term seizure-freedom (Englot et al., 2012; Schmidt and Stavem, 2009) (Englot et al., 2012; Schmidt and Stavem, 2009). In the face of these challenges, modern clinical epilepsy trials frequently employed a comparison of therapy to placebo as a control. Strangely, the effects of the placebo arm often are quite positive, making an efficacious therapy difficult to validate.
The term placebo had been in use since 1785 or earlier; however, the modern redefinition came with Beecher. (Kaptchuk, 1998) He implied it to mean a non-therapeutic intervention of any kind (Beecher, 1961). The term “placebo effect”, in turn, was popularized in part by Beecher’s influential 1955 paper (Beecher, 1955). At the time, his review of 15 studies concluded that placebos possessed, on average, 35.2% effectiveness. Interestingly, decades later, a reinterpretation of Beecher’s original work suggested that the effectiveness he believed was related directly to the placebo was not a placebo effect at all (Kienle and Kiene, 1997). In fact, other effects accounted for all of the placebo improvements, such as: added treatments, natural improvements, misquotations, and scaling bias. A notable omission from Beecher’s original study—and many randomized controlled trials since then—is the percentage of patients who worsen with placebo treatment (Kaptchuk, 1998). A few years before Beecher’s paper, a study was published on motion sickness remedies that showed no evidence of a placebo effect at all (Tyler, 1946). The study randomly assigned subjects on a boat to drug treatment, placebo, or no treatment. Interestingly, the no treatment arm (35%) and placebo arm (34%) had essentially equivalent levels of severe motion sickness. Such “no treatment” arms are rarely used, but they can highlight effects of study design, such as regression to the mean and natural history of disease.
Within the last 60 years, varying definitions of placebo effects have been suggested (Beecher, 1961). In this review, we will adopt the approach of Kienle and Keine: “(1) A placebo had to be given. (2) The event had to be an effect of the placebo treatment, i.e., the event would not have happened without placebo administration. (3) The event had to be relevant for the disease or symptom, i.e., it had to be a therapeutic event (Kienle and Kiene, 1997).” Additionally, we define here the “placebo response” as any response observed during the trial in the placebo arm, regardless of cause. It is noteworthy that with these definitions, one must be careful not to mis-attribute “placebo effect” when one observes a “placebo response”.
This review will provide the reader with an overview of important observations about placebos in epilepsy trials; the causes believed to influence placebo response; and new trial designs crafted to better control the placebo response.
PART 1: OBSERVATIONS ABOUT PLACEBOS IN EPILEPSY
1.1. Magnitude of Effect
Several meta-analyses of randomized controlled trials (RCTs) report on the responses to placebo in epilepsy. Estimates of the placebo response magnitude (for 50%-responders) in drug trials range from 4–19% (Burneo et al., 2002; Cramer et al., 1999; Guekht et al., 2010; Rheims et al., 2008; Zaccara et al., 2015).
In device studies, a similar range exists for 50%-responders. In a review of trials in transcranial magnetic stimulation (TMS), placebo responder rates were 16–20% (Bae et al., 2011). In trigeminal nerve stimulation (TNS), a phase II RCT found the 25 placebo-arm patients to respond at a rate of 21.1% (DeGiorgio et al., 2013). A trial of responsive neuro-stimulation (RNS) found 27% response rate in the 93 patients assigned to sham stimulation (Morrell, 2011). The large vagal nerve stimulation (VNS) trial found that the 60 patients assigned to placebo (in this case “low stimulation”) had a 13% responder rate (Vagus et al., 1995). In a deep brain stimulation (DBS) RCT of 109 patients (54 stimulated, 55 control) the authors found no “statistically significant treatment group difference” for 50% responders during the blinded phase, but did not actually report the number (Fisher et al., 2010).
Seizure freedom rates in RCTs are much lower than the 50%-responder rates: 8.2% for drug and 2.1% for placebo in one meta-analysis (Beyenburg et al., 2010). In another study, drug treated seizure-free rates were 4.5% and 2.8% in children and adults respectively; placebo-treated children and adults achieved rates of 0.6% and 0.4% respectively (Rheims et al., 2008). Several studies raised the point that use of last-observation-carried-forward (LOCF) method (rather than using full completers) artificially increase seizure freedom-rates and responder rates in both placebo and active drug treated patients (Gazzola et al., 2007; Rheims et al., 2011).
Device trials reported 0% “placebo” seizure-free responders for TMS (Bae et al., 2011), TNS (DeGiorgio et al., 2013), RNS (Morrell, 2011), and VNS (Vagus et al., 1995). A randomized trial of DBS reported 1.8% (1 out of 55) “placebo” patients and 0% “active treatment” patients became seizure-free during the blinded phase (Fisher et al., 2010).
Taken altogether across drugs and devices, seizure-freedom rates on placebo are quite low (0–2.8%), while 50%-responder rates on placebo are quite a bit larger (4–27%).
In addition, a statistically significant publication bias in epilepsy clinical trials was reported in a meta-analysis of 55 trials (Beyenburg et al., 2010). If this bias exists, then the magnitude of the placebo response may be even higher than the levels reported here.
Interestingly, a meta analysis found a difference in placebo response sizes between RCTs of 5 different anti-seizure medications (p=0.01, chi squared) suggesting heterogeneity of placebo responses between trials (Cramer et al., 1999). Also, the rates of response between placebo arm and drug arm were correlated in a large meta-analysis (r = 0.498, P <0.001) (Zaccara et al., 2015). The implication of these findings may be that differing RCT designs and differing populations may influence the magnitude of placebo response.
1.2. Placebo in surgery
Before modern imaging, Penfield and Steelman wrote about craniotomies without resections compared with full resections (Penfield and Steelman, 1947). They found that 5 out of 16 (31%) of patients achieved a 50% reduction in seizures after craniotomy without resection. Also known as “trepanation,” the technique had been in use throughout the world, dating at least as far back as Hippocrates and has been used as a treatment of epilepsy (Wilder Penfield and Jasper, H., 1954). Interestingly, animal data supports the idea that seizures are reduced in the short term after trepanation (Forcelli et al., 2013), and there is evidence that temporary placement of intracranial electrodes alone results in seizure-freedom in some patients (Schulze-Bonhage et al., 2010). The placebo effect is difficult to study in surgery in modern times because of ethical considerations (Wiebe, 2003).
1.3. Increasing Size of the Placebo Response
The placebo response size has been “drifting” upwards over the course of about 25 years in epilepsy trials as well as in other illnesses (Figure 1) (Rheims et al., 2011; Zaccara et al., 2015). In the figure, the year of publication appears to correlate positively with responder rate (r=0.65, p=0.021) in placebo, whereas the arm with anti-epileptic drug (AED) had a non-significant association. A similar trend has been observed in drugs trials for several other conditions, including mania (Sysko and Walsh, 2007), depression (Walsh et al., 2002), and psychosis (Agid et al., 2013).
FIGURE 1.
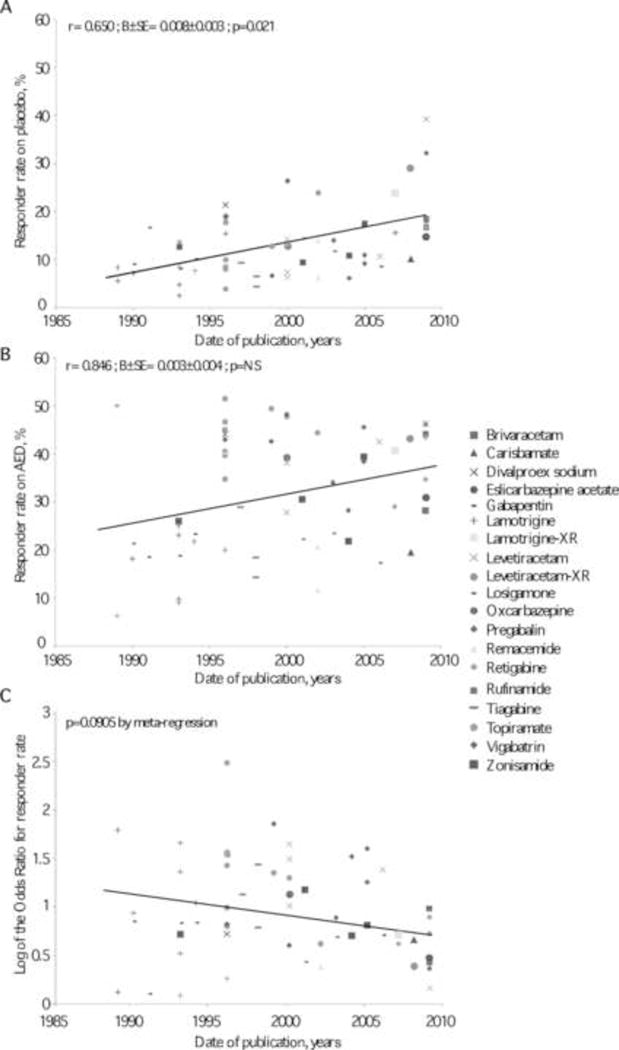
Relationships between responder rates (A, placebo-treated groups; B, active medication-treated groups) and year of trial publication. Both correlations were tested by linear regression adjusted for AEDs. The relationship between the size of the treatment effect and year of publication is shown in panel C and was analyzed by meta-regression. r, correlation coefficient; B, regression coefficient; SE, standard error. (Reproduced with permission (Rheims et al., 2011)).
1.4. Modified by Geography
The effect also may vary with geographic region. For example, in a study of the drug perampanel, placebo responses were found to be higher in Central and South America (33% placebo responders) when compared to North America (21.9% placebo responders) (French et al., 2012). A review of lacosamide drug trials found a similar, though not statistically significant trend (Schmidt et al., 2013). Conversely, a recent meta-analysis did not find a systematic regional difference in placebo response rates (Zaccara et al., 2015).
1.5. Modified by Age
When ages were separated in a meta-analysis of 32 trials, placebo responders were seen in 19% +/− 2.3% of children (age <18 years) and 9.9% +/− 4.6% of adults (Rheims et al., 2008). This difference affected statistical comparison of drug effectiveness - adults had seemingly higher effectiveness for the same drugs as children. The difference was driven by equal rates of drug responders but higher placebo responder rates in children (Figure 2).
FIGURE 2.
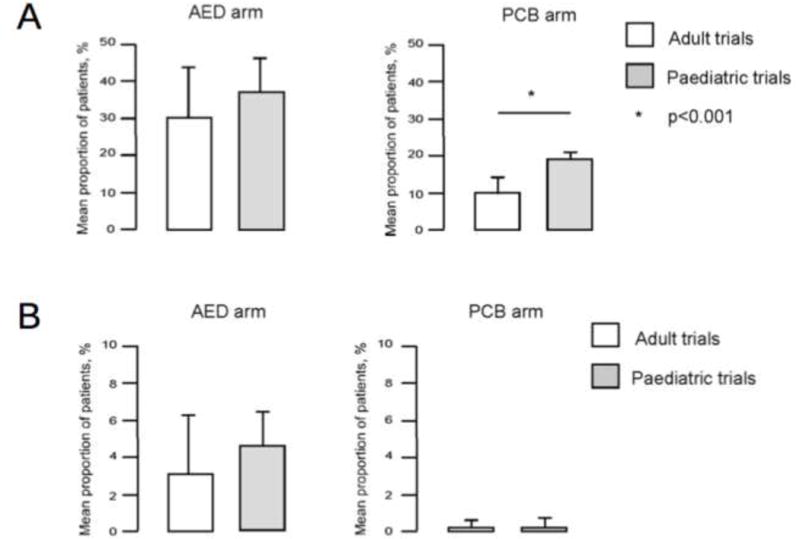
Mean 50% responder rate with placebo and with AED in adult and pediatric trials. * p= 0.001 (logistic regression). (Adapted from (Rheims et al., 2008) with permission).
1.6. Modified by Duration of Test Period
Duration of test period influences the proportion of placebo responders. A meta-analysis took the titration phase and included or excluded it from the placebo test period, finding 14.5% responders when included, and 18.3% responders when excluded (p=0.005) (Rheims et al., 2011). On the other hand, a different analysis found that longer study duration correlated with larger placebo response rates (r=0.264, p=0.030) (Zaccara et al., 2015).
1.7. Modified by Patients’ Baseline Characteristics
There appear to be some baseline characteristics of patients that correlate with placebo response. For example, median seizure frequency seems to be inversely proportional to placebo response (r=−0.312, p=0.018) (Zaccara et al., 2015). A review of 3 studies in lacosamide found that >6 anti-seizure drug exposures (p<0.001) and >10 seizures/28 days (p<0.001), and history of resective surgery (p=0.003) all predicted low placebo response rates (Schmidt et al., 2013).
1.8. Sudden Unexplained Death in Epilepsy (SUDEP)
A meta-analysis of placebo-arm patients found a statistically significant increase in SUDEP (Ryvlin et al., 2011). The rate per 1000 person-years of definite or probable SUDEP was 6.9 in the placebo arm and 0.9 in the efficacious drug arm; calculations were based on 21,224 patients over 5,589 patient-years. Fourteen of 112 trials that reported a total of 20 SUDEP cases (11 definite, 7 probable, 2 possible), comprising of 14 placebo deaths (7 definite SUDEP, 7 probable SUDEP), 3 efficacious drug deaths (3 definite SUDEP) and 3 non-efficacious drug deaths (1 definite SUDEP, 2 possible SUDEP). Perhaps in light of these findings, inclusion of a placebo arm in a randomized controlled study should be reconsidered. On the other hand, the authors found that death under-reporting bias was unclear or present in 15 studies (13%). Therefore, a second approach is to consider the small absolute number of events, the under-reporting bias, the uncertainty in diagnosis, and the extremely large number of patients who were safe. If drug trials no longer included a placebo arm (and therefore were unable to distinguish effective therapies as clearly), millions of patients would be affected. With all these mitigating factors it may be prudent to interpret the results cautiously.
1.9. Placebo Patterns
Placebos are generally noted to be poor tools for curing disease (Kaptchuk and Miller, 2015). Indeed, placebos have rarely resulted in seizure-freedom in medication (Beyenburg et al., 2010; Rheims et al., 2011; Zaccara et al., 2015) and device trials (Bae et al., 2011; DeGiorgio et al., 2013; Fisher et al., 2010; Morrell, 2011; Vagus et al., 1995). Average seizure-free rates in the placebo arm of medication trials were 0.4–2.1% (Beyenburg et al., 2010; Rheims et al., 2008).
In trials of drugs for other medical conditions, there may be unique improvement patterns that can be used to segregate types of response. For instance, a study of placebo-treated depressed patients noted that 23% demonstrated a pattern of abrupt early improvement that did not persist, while 27% showed more gradual, persistent improvement pattern (Quitkin et al., 1991). The authors concluded that perhaps the former pattern represents placebo response, and the latter represents spontaneous remission. A review of 130 trials that included placebo and no intervention found that trials with a binary outcome differed from those with a continuous outcome variable. The binary outcome trials failed to show a placebo effect, while the continuous outcome variables did show a statistically significant effect (Hróbjartsson and Gøtzsche, 2001). The effect seen in that study decreased as the number of patients increased, suggesting that underpowered studies might falsely indicate placebo effects when measuring continuous variables.
1.10. Worsening with Placebo
Placebos can also result in adverse consequences, a concept termed “nocebo”. Interestingly, this phenomenon is not uncommon: a recent study found that nearly two-thirds (60%) of placebo treated patients reported an adverse event. The most common adverse events experienced were headache (12.4%), somnolence (8.6%), dizziness (8.2%), and fatigue (7.9%) (Zaccara et al., 2015). The nocebo effect is not limited to adult patients. In a meta-analysis of 32 RCTs, 0.7% of adults and 5.6% children had worsening of seizures while in the placebo arm of studies (Rheims et al., 2008). The same study found that 7% of adults and 13.3% of children withdrew from trials while taking placebo, making it clear that nocebo effects can result in early dropout from a study. This early dropout can, in turn, result in inflated effect size when using the LOCF method as mentioned above (Section 1.1) (Gazzola et al., 2007). Therefore, a deeper understanding of how to minimize nocebo effects could have sizeable effects on clinical trial power and effect size.
1.11. Placebo Response in Animals
There is little in the medical literature on placebo effects in animals with epilepsy. However, one study, summarizing three different trials with dogs, found evidence of placebo-treated 50%-responder rates. The rates ranged between 0 and 60%, depending on the trial. Seizure-free rates were much lower, ranging 0–7% (Muñana et al., 2010). The same group followed up with a randomized drug trial that failed to show a significant difference between levetiracetam and placebo in dogs (Muñana et al., 2012).
PART 2: Causes of Placebo Response
There are many possible causes of the improvements seen with placebo. They can be divided into psychological, methodological, and inherent.
2.1. Psychological Causes
The psychological causes of placebo effects include classical conditioning, the Hawthorne effect, symbols and expectations, and social learning. These various effects may play a role in epilepsy, though they have never been evaluated in that context.
Classical conditioning, originally described by Ivan Pavlov, pairs a specific stimulus to a response; the traditional example is pairing the sound of a bell (the stimulus) with salivation (the response) (Jarius and Wildemann, 2015). This same effect may be seen in research studies. For some patients, the “stimulus” of taking a pill may result in the “response” of improvement: they have been “conditioned” to respond well when getting a pill. Conversely, in other patients, the opposite effect may be seen: since patients enrolled in typical trials of drugs are often drug-resistant, they may pair the “stimulus” of receiving a pill with the “response” of expecting no improvement. Also, such patients may pair the receiving of anti-seizure pills with adverse side effects, resulting in withdrawal from a clinical trial.
The Hawthorne effect refers to the improvement seen in groups of people when they are being observed, regardless of whether there is actually any intervention. Originally, the Western Electric telephone company found that close supervision, even without any specific intervention, led to increased employee productivity (McCambridge et al., 2014). In epilepsy, seizures are discrete events that can occur with or without observation. Therefore, although the Hawthorne effect is often cited, it is currently unknown if it plays a significant role in epilepsy clinical trials.
Symbols and expectation certainly appear to play an important role in the placebo effect for medical studies broadly (Benedetti, 2014). A recent Parkinson’s disease study found that higher perceived cost of a drug might increase objective measures of its efficacy (Espay et al., 2015). Similarly, brand-name pain pills were found to be more effective than generic pain pills (Branthwaite and Cooper, 1981). A series of studies in Parkinson’s disease patients receiving treatment for pain, anxiety, and stimulation found that hidden administration of true therapy decreased efficacy while open administration increased it. Moreover, they found that open withdrawal of true therapy worsened symptoms, but hidden withdrawal actually attenuated the worsening of symptoms (Benedetti et al., 2003). Even a simple symbol switch can have significant impact: in a study of migraine medicines, the authors found that falsely labeling rizatriptan as “placebo” versus as “rizatriptan” could decrease the efficacy of the drug—while falsely labeling placebo as “rizatriptan” (versus “placebo”) could increase the efficacy of the placebo effect (Kam-Hansen et al., 2014). There is even evidence to suggest that a person can benefit from an “social placebo effect”: just watching others benefit from a placebo can “infect” subjects with placebo potency (Frisaldi et al., 2014).
Thus, it seems evident that knowledge, expectation, or belief about the treatment modified the treatment result (Benedetti, 2014). In light of that, it is important for researchers to be aware that some patients might be able to distinguish which treatment arm they were assigned to in a double-blind study; such awareness by definition means that the study is no longer “double blinded” and results should be interpreted with caution (Margraf et al., 1991).
2.2. Regression to the Mean
An often-cited cause of placebo response is a concept known as regression to the mean (Morton and Torgerson, 2005). This refers to the idea that patients that perceive themselves to be “sicker” than usual are more likely to volunteer to participate in research studies, and as they gradually return to their usual state of being “less sick,” that change is sometimes mis-attributed to the placebo effect. For example, epilepsy patients whose treatment and overall health are relatively stable may notice an increase in their number of seizures compared to their “typical” number. In this situation, they might volunteer for a study. Such patients would be expected to “naturally” resume their mean seizure frequency after awhile, and would thus potentially appear to improve in whatever treatment arm they were assigned. Since typical clinical trials are not designed to detect the difference between placebo effects and regression to the mean, the two effects are often grouped together. Of note, although regression to the mean is considered an important effect in epilepsy, it has not been clinically studied directly. In simulations based on patient-centered seizure diaries, regression to the mean effects appeared to taper off within 3–6 months (Goldenholz et al., 2015).
2.3. Inherent Causes
Natural fluctuations in a disease can account for part of the improvements seen with placebo. In epilepsy, this is an especially important issue, because the natural fluctuations of the disease are not yet well understood. What has been studied is natural remission in certain sub-populations. Additionally, more recent data has been published simulating the details of natural fluctuations expected in a heterogeneous epilepsy population.
In one prospective study, a cohort of 225 drug-resistant patients was followed for 4 years without surgical intervention, to determine prognosis. Each year, the percentage of patients who ended the year with at least 6 months of remission (terminal remission) were: 3% at 1 year, 9% at 2 years, 13% at 3 years, and 14% at 4 years (Callaghan et al., 2007). The same patients were followed for another 3 years, and the cumulative probability of 12-months seizure remission was found to be 33%. The risk of a relapse after such a 12-month remission was 71% at 5 years (Callaghan et al., 2011). Similar rates have been seen by others (Choi et al., 2011; Luciano and Shorvon, 2007).
In children, the expected natural fluctuations seen in epilepsy are perhaps even larger. A cohort of 413 patients were followed for an average of 14.8 years (Geerts et al., 2010). In 25%, seizures went into remission in less than 1 year, while in another 25% the patients suffered from seizures for over 12 years. Over two-thirds of the patients (71%) of patients achieved 5-year terminal remission (Geerts et al., 2010). In another study, a group of 144 children aged 15 or under were followed prospectively for an average of 37 years. That study suggested several patterns: early complete remission (16%), early remission with relapse (16%), delayed remission (mean delay of 9 years, 32%), worsening (16%), and relapsing-remitting (19%) (Sillanpää and Schmidt, 2006). A similar set of patterns has been observed in a group of over a thousand newly diagnosed patients (n=1,098) ranging in age from 9 to 93 years old (Brodie et al., 2012). Both of these studies suggest that the subtype of epilepsy may play a role in possible relatively likelihood of various clinical courses, with idiopathic epilepsy tending toward more favorable outcomes (Brodie et al., 2012; Sillanpää and Schmidt, 2006).
The natural fluctuations seen in epilepsy are not limited to non-surgical patients. For example, a cohort of 915 post-surgical patients was followed for a minimum of 1 year (mean 6.6 years) (Jehi et al., 2010). The study authors found a relationship between the number of post-operative seizures the patient suffered and the risk of recurrence. A single postoperative seizure was associated with a 66% risk of recurrence; 2 or 3 post-op seizures were associated with a 75% and 80% risk of recurrence, respectively. However, 20–34% of patients will have 1–3 seizures after surgery and then remain seizure-free thereafter. These results indicate that even post-operatively, the natural fluctuations of the disease remain relevant and significant.
Taking these observations into consideration, in order to tease apart the difference between a true placebo effect and a “natural fluctuation” effect, a simulation study was undertaken. A patient-centric database of 684,825 seizures reported by 8,228 patients was used to simulate clinical trials, without actually performing these trials (Goldenholz et al., 2015). Surprisingly, although the study, by virtue of being a simulation, lacked all the traditional psychological influences (discussed in section 2.1 above), and accounted for regression to the mean by extending the duration of the study out to 2 years (Figure 3), the simulation did find “placebo responders,” at levels comparable to actual clinical trials. Looking at the results in more detail, the study found that patients who could be categorized as “50% responders”, “worseners, ” etc., would, over time, change their categorization multiple times (Figure 4). As a result, studies that identify “responders” in a 3-month follow up may well discover that the same patients become “non-responders” later on, and vice versa. The authors concluded that the natural fluctuations of the disease itself accounted for the improvements seen in their simulations.
FIGURE 3.
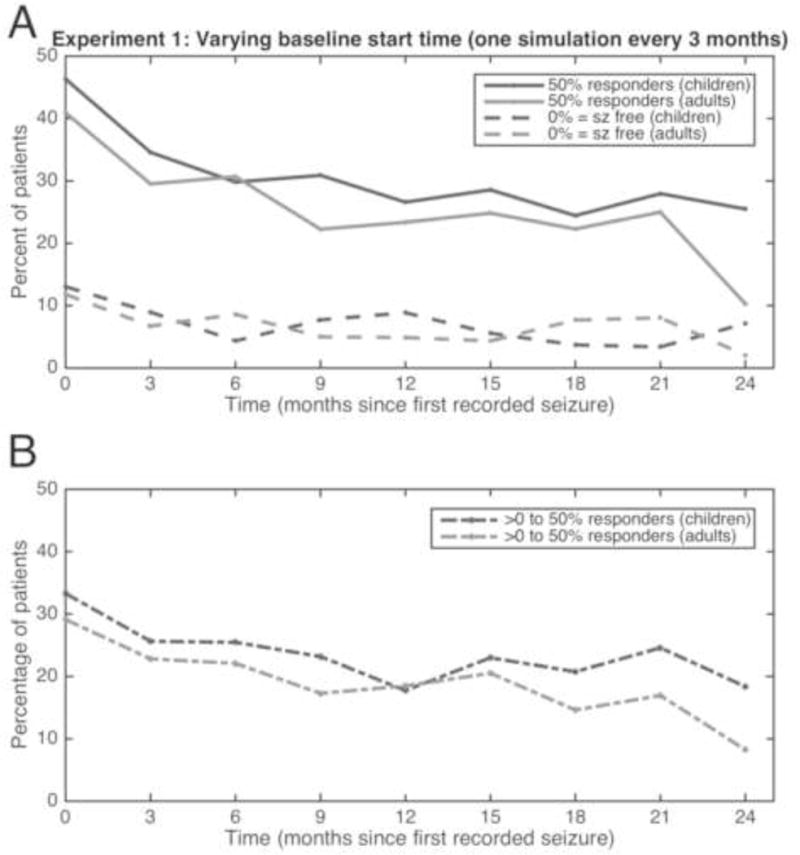
Simulation studies showing “placebo effect.” Each time on the x-axis represents a single experiment, which started a certain number of months after time 0. The experiment consisted of a 6-week baseline compared with a 6-week test period. Each experiment resulted in a certain percentage of patients experiencing 50% reduction in seizures (sz; solid lines, upper graph) and seizure freedom (broken lines, upper graph). The difference between the 50% reduction and seizure-free groups is represented in the lower graph. Children are represented in dark gray, adults in light gray. Adapted with permission from (Goldenholz et al., 2015).
FIGURE 4.
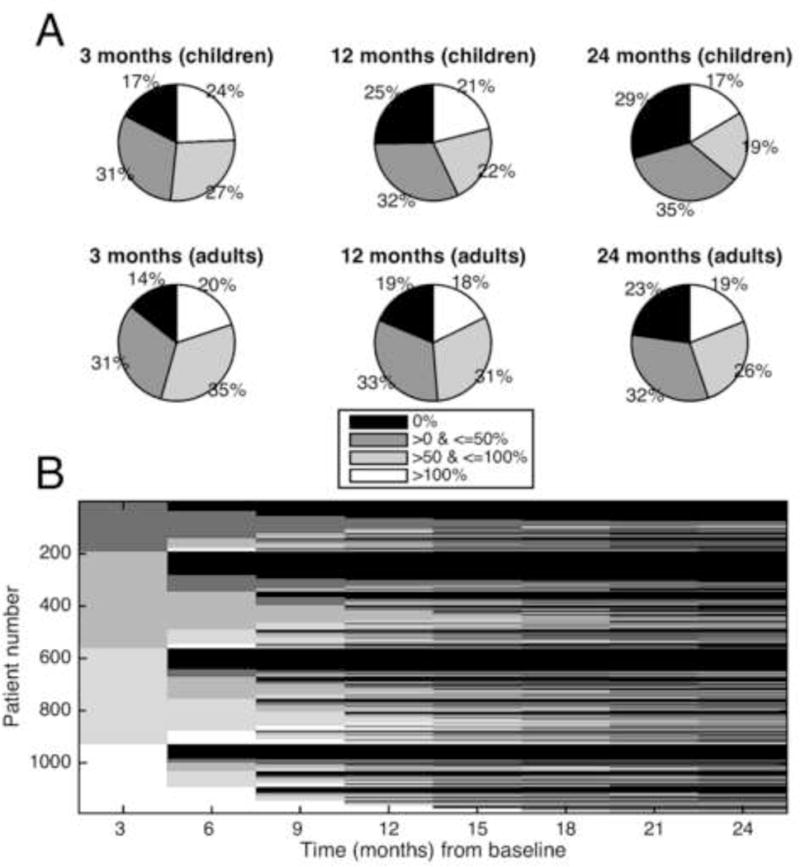
Simulation showing longitudinal category switching. In this simulation, a single 6 week baseline was compared to serial 6 week blocks at 3 month intervals. (A) Response breakdown. Shown are the percentage of patients who had specific levels of change from their baseline seizure rates. The graphs show 3 times after the baseline (3, 12, and 24 months) for children and adults. Roughly a quarter of each graph represents each of the 4 percent- age change categories throughout time. (B) Tracking individuals. The x-axis shows time in months from the baseline period. The y-axis represents individual patients, arbitrarily given a number assignment. The shading represents which improvement category is represented by an individual patient at a given time. Therefore, each patient is represented across time, and the entire stack of patients comprises the figure. Initially the patients separate out neatly into 4 categories of baseline change (0%, 0–50%, 50–100%, and >100% change from baseline). Seen from left to right, the category of an individual horizontal line does not stay constant, rather it changes several times. The figure depicts patients changing categories multiple times, suggesting a dynamic process that evolves for individual patients, even if on aggregate patients segregate into these 4 categories roughly equally. Adapted from with permission (Goldenholz et al., 2015)
Although beyond the scope of this review, a series of interesting findings have been accumulating in other areas of medicine, suggesting that perhaps patients that respond to placebo have unique genetic and anatomic markers (Benedetti, 2014; Kaptchuk and Miller, 2015). Indeed, one group found evidence of the placebo effect influencing single neurons (Frisaldi et al., 2014). At this time, such tantalizing possibilities have yet to be evaluated in epilepsy.
PART 3: NEWER STUDY DESIGNS
3.1. Trial Designs
Clinical trials have clearly evolved over the past century (Perucca, 2012). Many years ago, trials were not blinded, and randomized controls were certainly not the norm. For example, in 1912, Alfred Hauptmann monitored the effects of phenobarbital on patients for many months, noting that the drug was likely effective in bromide-resistant patients. (Perucca, 2012) Thanks in part to regulatory changes from the Kefauver-Harris Drug Amendment of 1962, trials began to improve over time. Between 1970 and 1990, crossover designs that sometimes included placebo were commonly used, but these fell out of favor due to concerns over carry over effects. From 1990 onward, clinical trials in epilepsy began to favor a parallel design randomized controlled trials, which immediately meant larger trials. Because of the larger sample size requirements, multi-site studies have become increasingly common, raising problems of comparing groups from different settings. Fundamental barriers to developing new efficacious treatments for epilepsy - including large sample sizes, high costs, differences in regulatory requirements in the United States versus Europe (and elsewhere), and the increasing magnitude of the placebo response - all of these challenges appear to conspire against drug and device companies. Indeed, the efficacy rates of the whole shelf full of the twenty approved anti-seizure medications are, sadly, not dramatically different than the phenobarbital tested by Dr. Hauptmann over 100 years ago.
3.2. Placebo Run In and Randomized Withdrawal
A common practice outside epilepsy research is a “placebo run-in” design, in which all patients initially enrolled are given a placebo. Those who do not respond are then randomized for either placebo or drug, and those who respond to the initial placebo are excluded from further study. Such a design adds both cost and time to clinical trials.
A second technique is called randomized withdrawal. In this scenario, patients are given the active drug, and drug non-responders are excluded. The remaining subjects are randomized to switch to placebo or continue within the active drug arm. Here too, the design adds cost and duration to the study.
Neither placebo run-in nor randomized withdrawal are currently used in epilepsy trials, and some have raised concerns that taken alone either of these methods would not result in benefit for clinical trials (Fava et al., 2003; Ivanova and Tamura, 2011). It has also been suggested that such methods may artificially increase apparent drug effects.
3.3. Sequential Parallel Comparison Design
In 2003, an improved method of dealing with placebo effect in psychiatric clinical trials was proposed (Fava et al., 2003). Called sequential parallel comparison design (SPCD), the technique offers a balance between larger sample size and increased statistical power. In this paradigm, there are two phases of equal duration, each of which should be shorter than a standard clinical trial duration. The subjects are randomized into 3 unequal groups: drug then placebo (DP), placebo then drug (PD), and placebo then placebo (PP). An example ratio is 2:3:3 for DP:PD:PP. In phase 1, PP and PD subjects get placebo while DP subjects get drug. In phase 2, the drug non-responders from DP are given placebo. Similarly, in phase 2, the placebo non-responders are given drug or placebo for groups PD and PP, respectively. The results of both phases are then pooled together. As with crossover trials, carry-over effects may play a role in these designs, depending on the pharmacodynamics of the drug being tested, requiring additional time for washout periods. Although SPCD has not yet been tested in epilepsy, this design has the potential to decrease costs and increase statistical power. The underlying assumption in this design is that placebo responder patients are unique in some special way, and that removing them from the study would decrease their contribution. That assumption remains to be tested.
3.4. Two-Way Enriched Design
Similar to SPCD, Two-Way Enriched Design (TED) utilizes two phases of equal duration, shorter than a typical clinical trial. Again, similar to SPCD, patients are randomly assigned to DP, PD, DD, PP. In the first stage, DP and DD patients are given drug, while PD and PP patients are given placebo. In the second stage, drug non-responders and placebo responders are excluded (Ivanova and Tamura, 2011). As with SPCD, carryover effects may be a concern in some cases. And as with SPCD, this method has yet to be applied to epilepsy research.
3.5. Time to Prerandomization
A different approach that may be somewhat more tailored to the unique issues in epilepsy is the “time to prerandomization baseline” method (French et al., 2015). The measure of efficacy using this method is the time it takes for a patient to reach his/her baseline number of seizures. For example, if a patient had 8 seizures per month during the baseline phase, and took 2 months to have 8 seizures during the treatment phase, the technique would interpret that patient’s outcome as “2 months.” If the median time to prerandomization for all patients was 2 months, it would imply that the treatment could decrease the seizure frequency by 50%. This technique was validated over 3 clinical trials with perampanel, and may be used in future trials as well. The advantages of this method include shorter study duration, decreased exposure to risk–both from placebo and from drug– the possibility of including patients with lower seizure frequency, and endpoints that are clinically meaningful. The disadvantages include less observation time for adverse events, and unequal exposure times between patients with frequent seizures compared to those with infrequent seizures. Further study of the potential value of this trial method is clearly warranted.
3.6. Delayed Start
In the large simulation trial based on patient-centered seizure diaries, the possibility of a new trial design was explored (Goldenholz et al., 2015). By enrolling a patient into a clinical trial but not measuring anything for 6 months—e.g., delaying measurement by 6 months—the effects of regression to the mean should attenuate. This is because the “sicker than usual” patients who rushed to join a clinical trial will gradually return to their mean state, and when this occurs, the measurements begin. In simulation, delaying a trial by 6 months resulted in a significant decrease in placebo responders: from 43% down to 20%. This trial design in theory has the advantage of no additional cost and the possibility of decreasing the impact of regression to the mean. As a consequence, the placebo responder rate would decrease and could therefore increase the statistical power of the study. On the other hand, if patients drop out from the initial enrollment due to the prolonged delay, this would increase the cost and duration of the study, and potentially decrease the statistical power of the study. Strangely, it may be possible to treat the patients who are “sicker than usual” with standard clinical care while patients wait for their delayed entry into the clinical trial. This too could result in greater dropout (as patients feel less sick), or simply reducing the number of eligible patients (as their seizure frequency improves). In spite of these problems, the relative improvement in trial power by this technique is unknown, and may be dramatic. Therefore, this technique awaits clinical validation to confirm or refute its potential.
3.7. Cohort Reduction
In the same simulation trial, a second method was evaluated – cohort reduction (Goldenholz et al., 2015). In this model, the assumption is that certain subsets of patients have more fluctuations in their seizure rates than others. By identifying these and removing them after the baseline assessment period (even retroactively), the trial could potentially lower the rates of placebo responders. In simulation, this technique reduced placebo responders rate from 43% to 33%. The advantage of cohort reduction, as in the case of the delayed start method, is that it could reduce placebo response and therefore increase statistical power. It can also be applied retroactively—a unique characteristic among trial design improvements. The disadvantage is severe however – by definition, the size of the study sample will be reduced. The larger the reduction in sample size, the greater the loss in statistical power. Future studies will need to address these issues to determine if such a technique is appropriate in large clinical trials.
3.8 Methods that avoid placebo
Because of cost and concerns about SUDEP, there are some who wish to avoid placebos in clinical trials altogether. This move would not eliminate the placebo effect – it is included in the effect of each treatment. Example approaches include the use of active comparator arms, or historical controls, both of which have been used in epilepsy trials (Friedman and French, 2012).
Although attractive in certain aspects, lack of placebo prevents direct quantification of non-therapy related contributions to effect size, including natural fluctuation, psychological influence, regression to the mean, geographic variation, and so forth. Maintaining placebo as a control therefore permits the investigator to also quantify the magnitude of the true therapeutic effect. True effects sizes that are clinically large can save many patients from SUDEP over many years (Thurman et al., 2014). Therefore, this may outweigh the theoretical short-term risks of including placebo controls.
Conclusions
The placebo effect has been well known for over 60 years, but it been blamed for effects that are quite unrelated, such as regression to the mean and natural fluctuations of disease. Surprising findings emerged when placebo response was studied directly in epilepsy, including the effects of age, year of publication, and geography. Some of these findings may be better explained within the context of the natural fluctuations of disease rather than placebo effects, though this remains speculative. However, by recognizing the significance of the natural fluctuations of disease, understanding the parameters of regression to the mean, and implementing more advanced clinical trial paradigms, future studies may help to uncover more effective therapies for a disease that remains, for the most part, incurable.
Hilghtlights.
This review provides a set of observations about placebo responses in epilepsy trials over the years
A point by point review of the components of the placebo response is given
Old and newer methods for clinical trial design are discussed in the context of the placebo response
Acknowledgments
The authors would like to acknowledge the guidance from Dr. William Theodore and Dr. Sara Inati. This work was supported by the Intramural Research Program of the NIH.
ABBREVIATIONS
- RCT
randomized clinical trial
- TNS
trigeminal nerve stimulation
- TMS
transcranial magnetic stimulation
- VNS
vagal nerve stimulation
- RNS
responsive neuro-stimulation
- DBS
deep brain stimulation
- LOCF
last observation carried forward
- AED
anti-epileptic drug
- SUDEP
sudden unexplained death in epilepsy
- SPCD
sequential parallel comparison design
- TED
two-way enriched design
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest.
References
- Agid O, Siu CO, Potkin SG, Kapur S, Watsky E, Vanderburg D, Zipursky RB, Remington G. Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am J Psychiatry. 2013;170:1335–1344. doi: 10.1176/appi.ajp.2013.12030315. [DOI] [PubMed] [Google Scholar]
- Bae EH, Theodore WH, Fregni F, Cantello R, Pascual-Leone A, Rotenberg A. An estimate of placebo effect of repetitive transcranial magnetic stimulation in epilepsy. Epilepsy Behav. 2011;20:355–359. doi: 10.1016/j.yebeh.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher HK. Surgery as placebo: a quantitative study of bias. J Am Med Assoc. 1961;176:1102–1107. doi: 10.1001/jama.1961.63040260007008. [DOI] [PubMed] [Google Scholar]
- Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159:1602–1606. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- Benedetti F. Placebo Effects: From the Neurobiological Paradigm to Translational Implications. Neuron. 2014;84:623–637. doi: 10.1016/j.neuron.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Maggi G, Lopiano L, Lanotte M, Rainero I, Vighetti S, Pollo A. Open versus hidden medical treatments: The patient’s knowledge about a therapy affects the therapy outcome. Prev Treat. 2003;6 doi: 10.1037/1522-3736.6.1.61a. [DOI] [Google Scholar]
- Beyenburg S, Stavem K, Schmidt D. Placebo-corrected efficacy of modern antiepileptic drugs for refractory epilepsy: Systematic review and meta-analysis. Epilepsia. 2010;51:7–26. doi: 10.1111/j.1528-1167.2009.02299.x. [DOI] [PubMed] [Google Scholar]
- Branthwaite A, Cooper P. Analgesic effects of branding in treatment of headaches. Br Med J (Clin Res Ed) 1981;282:1576–1578. doi: 10.1136/bmj.282.6276.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MJ, Barry SJE, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burneo JG, Montori VM, Faught E. Magnitude of the placebo effect in randomized trials of antiepileptic agents. Epilepsy Behav. 2002;3:532–534. doi: 10.1016/s1525-5050(02)00531-0. [DOI] [PubMed] [Google Scholar]
- Callaghan B, Schlesinger M, Rodemer W, Pollard J, Hesdorffer D, Hauser WA, French J. Remission and relapse in a drug-resistant epilepsy population followed prospectively. Epilepsia. 2011;52:619–626. doi: 10.1111/j.1528-1167.2010.02929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol. 2007;62:382–389. doi: 10.1002/ana.21166. [DOI] [PubMed] [Google Scholar]
- Choi H, Heiman GA, Munger Clary H, Etienne M, Resor SR, Hauser WA. Seizure remission in adults with long-standing intractable epilepsy: An extended follow-up. Epilepsy Res. 2011;93:115–119. doi: 10.1016/j.eplepsyres.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer JA, Fisher R, Ben-Menachem E, French J, Mattson RH. New antiepileptic drugs: comparison of key clinical trials. Epilepsia. 1999;40:590–600. doi: 10.1111/j.1528-1157.1999.tb05561.x. [DOI] [PubMed] [Google Scholar]
- DeGiorgio CM, Soss J, Cook IA, Markovic D, Gornbein J, Murray D, Oviedo S, Gordon S, Corralle-Leyva G, Kealey CP, Heck CN. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology. 2013;80:786–791. doi: 10.1212/WNL.0b013e318285c11a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Wang DD, Rolston JD, Shih TT, Chang EF. Rates and predictors of long-term seizure freedom after frontal lobe epilepsy surgery: a systematic review and meta-analysis. J Neurosurg. 2012;116:1042–1048. doi: 10.3171/2012.1.JNS111620. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Norris MM, Eliassen JC, Dwivedi A, Smith MS, Banks C, Allendorfer JB, Lang AE, Fleck DE, Linke MJ, Szaflarski JP. Placebo effect of medication cost in Parkinson disease: A randomized double-blind study. Neurology. 2015;84:794–802. doi: 10.1212/WNL.0000000000001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: Culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72:115–127. doi: 10.1159/000069738. [DOI] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Krishnamurthy KB, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, Dichter M, Elias W, Francel P, Frysinger R, Graber K, Grant J, Heit G, Herman S, Kandula P, Kanner A, King JA, Kobylarz E, Lapp K, LaRoche S, Lippmann S, Maganti R, Mapstone T, Sabau D, Schrader L, Sharan A, Smith M, Treiman D, Wilkinson S, Wong S, Zangaladze A, Adderley S, Bridges B, Callanan M, Cordero D, Fields C, Johnson M, Kavalir M, Kretschmar P, Macpherson C, Mancl K, Manley M, Marsh S, Montgomery J, Mundt P, Nekkalapu PP, Nikolov B, Palmer B, Perdue L, Randall A, Smith D, Smith L, Strybing K, Stott L, Taylor R, Thompson S, Timenova Z, Vogelsong B, Balbona V, Broshek D, Cahn-Weiner D, Clift L, Davidson M, Drake E, Frutiger S, Featherstone L, Grote C, Han D, Henry D, Horsfall J, Hovick A, Gray J, Kareken D, Kirlin K, Livingood D, Meyer M, Minniti N, Strupinsky JM, Schultz W, Scott J, Tracy J, Waltonen S, Ziefert P, Van Amburg C, Burdelle M, Clements S, Cox R, Dolin R, Fulk M, Kaur HR, Hirsch L, Hoeppner T, Hurt A, Komosa M, Krahl S, Ponticello L, Quigg M, Quinn H, Rossi M, Schaefer P, Skidmore C, Sundstrom D, Trudeau P, Volz M, Wang N, Will L, Young C. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- Forcelli PA, Kalikhman D, Gale K. Delayed effect of craniotomy on experimental seizures in rats. PLoS One. 2013;8:e81401. doi: 10.1371/journal.pone.0081401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, Kumar D, Rogawski MA. Adjunctive perampanel for refractory partial-onset seizures: Randomized phase III study 304. Neurology. 2012;79:589–596. doi: 10.1212/WNL.0b013e3182635735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Gil-Nagel A, Malerba S, Kramer L, Kumar D, Bagiella E. Time to prerandomization monthly seizure count in perampanel trials: A novel epilepsy endpoint. Neurology. 2015;84:2014–20. doi: 10.1212/WNL.0000000000001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, French JA. Clinical trials for therapeutic assessment of antiepileptic drugs in the 21st century: obstacles and solutions. Lancet Neurol. 2012;11:827–34. doi: 10.1016/S1474-4422(12)70177-1. [DOI] [PubMed] [Google Scholar]
- Frisaldi E, Carlino E, Lanotte M, Lopiano L, Benedetti F. Characterization of the thalamic–subthalamic circuit involved in the placebo response through single-neuron recording in Parkinson patients. Cortex. 2014;60:3–9. doi: 10.1016/j.cortex.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Gazzola DM, Balcer LJ, French JA. Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs. Epilepsia. 2007;48:1303–1307. doi: 10.1111/j.1528-1167.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- Geerts A, Arts WF, Stroink H, Peeters E, Brouwer O, Peters B, Laan L, van Donselaar C. Course and outcome of childhood epilepsy: a 15-year follow-up of the Dutch Study of Epilepsy in Childhood. Epilepsia. 2010;51:1189–1197. doi: 10.1111/j.1528-1167.2010.02546.x. [DOI] [PubMed] [Google Scholar]
- Goldenholz DM, Moss R, Scott J, Auh S, Theodore WH. Confusing placebo effect with natural history in epilepsy: A big data approach. Ann Neurol. 2015;78:329–336. doi: 10.1002/ana.24470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guekht AB, Korczyn AD, Bondareva IB, Gusev EI. Placebo responses in randomized trials of antiepileptic drugs. Epilepsy Behav. 2010;17:64–69. doi: 10.1016/j.yebeh.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (U.S.), Committee on the Public Health Dimensions of the Epilepsies. England MJ. Epilepsy across the spectrum promoting health and understanding. National Academies Press; Washington, D.C: 2012. [PubMed] [Google Scholar]
- Ivanova A, Tamura RN. A two-way enriched clinical trial design: combining advantages of placebo lead-in and randomized withdrawal. Stat Methods Med Res. 2011 doi: 10.1177/0962280211431023. [DOI] [PubMed] [Google Scholar]
- Jarius S, Wildemann B. And Pavlov still rings a bell: summarising the evidence for the use of a bell in Pavlov’s iconic experiments on classical conditioning. J Neurol. 2015 doi: 10.1007/s00415-015-7858-5. [DOI] [PubMed] [Google Scholar]
- Jehi L, Sarkis R, Bingaman W, Kotagal P, Najm I. When is a postoperative seizure equivalent to “epilepsy recurrence” after epilepsy surgery? Epilepsia. 2010;51:994–1003. doi: 10.1111/j.1528-1167.2010.02556.x. [DOI] [PubMed] [Google Scholar]
- Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, Burstein R. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med. 2014;6:218ra5. doi: 10.1126/scitranslmed.3006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ. Powerful placebo: The dark side of the randomised controlled trial. Lancet. 1998;351:1722–1725. doi: 10.1016/S0140-6736(97)10111-8. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ, Miller FG. Placebo Effects in Medicine. N Engl J Med. 2015;373:8–9. doi: 10.1056/NEJMp1504023. [DOI] [PubMed] [Google Scholar]
- Kienle GS, Kiene H. The powerful placebo effect: Fact or fiction? J Clin Epidemiol. 1997;50:1311–1318. doi: 10.1016/S0895-4356(97)00203-5. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Luciano AL, Shorvon SD. Results of treatment changes in patients with apparently drug-resistant chronic epilepsy. Ann Neurol. 2007;62:375–381. doi: 10.1002/ana.21064. [DOI] [PubMed] [Google Scholar]
- Margraf J, Ehlers A, Roth WT, Clark DB, Sheikh J, Agras WS, Taylor CB. How “blind” are double-blind studies? J Consult Clin Psychol. 1991;59:184–187. doi: 10.1037/0022-006X.59.1.184. [DOI] [PubMed] [Google Scholar]
- McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- Morton V, Torgerson DJ. Regression to the mean: Treatment effect without the intervention. J Eval Clin Pract. 2005;11:59–65. doi: 10.1111/j.1365-2753.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- Muñana KR, Thomas WB, Inzana KD, Nettifee-Osborne JA, McLucas KJ, Olby NJ, Mariani CJ, Early PJ. Evaluation of Levetiracetam as Adjunctive Treatment for Refractory Canine Epilepsy: A Randomized, Placebo-Controlled, Crossover Trial. J Vet Intern Med. 2012;26:341–348. doi: 10.1111/j.1939-1676.2011.00866.x. [DOI] [PubMed] [Google Scholar]
- Muñana KR, Zhang D, Patterson EE. Placebo effect in canine epilepsy trials. J Vet Intern Med. 2010;24:166–170. doi: 10.1111/j.1939-1676.2009.0407.x. [DOI] [PubMed] [Google Scholar]
- Penfield W, Steelman H. The Treatment of Focal Epilepsy by Cortical Excision *. Ann Surg. 1947;126:740–761. doi: 10.1097/00000658-194711000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca E. What clinical trial designs have been used to test antiepileptic drugs and do we need to change them? Epileptic Disord. 2012;14:124–131. doi: 10.1684/epd.2012.0511. [DOI] [PubMed] [Google Scholar]
- Quitkin M, Rabkin G, Ph D, Mcgrath J, Tricamo E, Ph D, Markowitz J, Klein F, Stewart W. Heterogeneity of clinical response during placebo treatment. Am J Psychiatry. 1991;148:193–196. doi: 10.1176/ajp.148.2.193. [DOI] [PubMed] [Google Scholar]
- Rheims S, Cucherat M, Arzimanoglou A, Ryvlin P. Greater response to placebo in children than in adults: A systematic review and meta-analysis in drug-resistant partial epilepsy. PLoS Med. 2008;5:1223–1237. doi: 10.1371/journal.pmed.0050166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheims S, Perucca E, Cucherat M, Ryvlin P. Factors determining response to antiepileptic drugs in randomized controlled trials. A systematic review and meta-analysis. Epilepsia. 2011;52:219–233. doi: 10.1111/j.1528-1167.2010.02915.x. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Cucherat M, Rheims S. Risk of sudden unexpected death in epilepsy in patients given adjunctive antiepileptic treatment for refractory seizures: A meta-analysis of placebo-controlled randomised trials. Lancet Neurol. 2011;10:961–968. doi: 10.1016/S1474-4422(11)70193-4. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Beyenburg S, D’Souza J, Stavem K. Clinical features associated with placebo response in refractory focal epilepsy. Epilepsy Behav. 2013;27:393–398. doi: 10.1016/j.yebeh.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Stavem K. Long-term seizure outcome of surgery versus no surgery for drug-resistant partial epilepsy: a review of controlled studies. Epilepsia. 2009;50:1301–9. doi: 10.1111/j.1528-1167.2008.01997.x. [DOI] [PubMed] [Google Scholar]
- Schulze-Bonhage A, Dennig D, Wagner K, Cordeiro J, Carius A, Fauser S, Trippel M. Seizure control resulting from intrahippocampal depth electrode insertion. J Neurol Neurosurg Psychiatry. 2010;81:352–353. doi: 10.1136/jnnp.2009.180075. [DOI] [PubMed] [Google Scholar]
- Sillanpää M, Schmidt D. Natural history of treated childhood-onset epilepsy: Prospective, long-term population-based study. Brain. 2006;129:617–624. doi: 10.1093/brain/awh726. [DOI] [PubMed] [Google Scholar]
- Sysko R, Walsh BT. A systematic review of placebo response in studies of bipolar mania. J Clin Psychiatry. 2007;68:1213–1217. doi: 10.4088/JCP.v68n0807. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: Assessing the public health burden. Epilepsia. 2014:1–7. doi: 10.1111/epi.12666. [DOI] [PubMed] [Google Scholar]
- Tyler DB. The influence of a placebo, body position and medication on motion sickness. Am J Physiol Content. 1946;146:458–466. doi: 10.1152/ajplegacy.1946.146.3.458. [DOI] [PubMed] [Google Scholar]
- Vagus T, Stimulation N, Group S, Vns C. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology. 1995;45:224–230. doi: 10.1212/WNL.45.2.224. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- West S, Nolan SJ, Cotton J, Gandhi S, Weston J, Sudan A, Ramirez R, Newton R. Surgery for epilepsy. Cochrane database Syst Rev. 2015;7:CD010541. doi: 10.1002/14651858.CD010541.pub2. [DOI] [PubMed] [Google Scholar]
- Wiebe S. Randomized controlled trials of epilepsy surgery. Epilepsia. 2003;44(Suppl 7):38–43. doi: 10.1046/j.1528-1157.44.s7.9.x. [DOI] [PubMed] [Google Scholar]
- Penfield Wilder, Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. 1rst. W. Little, Brown and Co; Boston: 1954. [Google Scholar]
- Zaccara G, Giovannelli F, Cincotta M, Loiacono G, Verrotti A. Adverse events of placebo-treated, drug-resistant, focal epileptic patients in randomized controlled trials: a systematic review. J Neurol. 2015;262:501–515. doi: 10.1007/s00415-014-7391-y. [DOI] [PubMed] [Google Scholar]


