Abstract
Neurons in the vestibular nuclei have a vital function in balance maintenance, gaze stabilization, and posture. Although muscarinic acetylcholine receptors (mAChRs) are expressed and involved in regulating vestibular function, it is unclear how individual mAChR subtypes regulate vestibular neuronal activity. In this study, we determined which specific subtypes of mAChRs control synaptic input and excitability of medial vestibular nucleus (MVN) neurons that project to the cerebellum. Cerebellum-projecting MVN neurons were labeled by a fluorescent retrograde tracer and then identified in rat brainstem slices. Quantitative PCR analysis suggested that M2 and M3 were the possible major mAChR subtypes expressed in the MVN. The mAChR agonist oxotremorine-M significantly reduced the amplitude of glutamatergic excitatory postsynaptic currents evoked by stimulation of vestibular primary afferents, and this effect was abolished by the M2-preferring antagonist AF-DX 116. However, oxotremorine-M had no effect on GABA-mediated spontaneous inhibitory postsynaptic currents of labeled MVN neurons. Furthermore, oxotremorine-M significantly increased the firing activity of labeled MVN neurons, and this effect was blocked by the M3-preferring antagonist J104129 in most neurons tested. In addition, AF-DX 116 reduced the onset latency and prolonged the excitatory effect of oxotremorine-M on the firing activity of labeled MVN neurons. Our findings suggest that M3 is the predominant postsynaptic mAChR involved in muscarinic excitation of cerebellum-projecting MVN neurons. Presynaptic M2 mAChR regulates excitatory glutamatergic input from vestibular primary afferents, which in turn influences the excitability of cerebellum-projecting MVN neurons. This new information has important therapeutic implications for treating vestibular disorders with mAChR subtype-selective agents.
Keywords: acetylcholine, brainstem, glutamate, muscarinic receptors, synaptic transmission, vestibular system
Introduction
The vestibular system is essential for everyday life, contributing to the stabilization of gaze and control of balance and posture by detecting the motion of the head in space (Goldberg & Cullen 2011, McCrea & Luan 2003, Roy & Cullen 2004). The peripheral vestibular apparatus, including the semicircular canals and the otolithic organs (i.e., utricle and saccule), provides abundant sensory input to the vestibular nuclei located in the dorsal medullary brainstem. In turn, the neurons in the vestibular nuclei project to neural structures, such as the cerebellum, oculomotor nuclei, and spinal cord, to control eye movements, posture, and balance (Dieterich et al. 2005, Modianos & Pfaff 1976, Barmack et al. 1993, Kotchabhakdi & Walberg 1978). Among the four classical neuronal groups that compose the vestibular complex, the medial vestibular nucleus (MVN) contains a full range of cell types and is, in total volume and neuronal density, the largest of the vestibular nuclei (Alvarez et al. 1998). The MVN is highly heterogeneous and contains inhibitory and excitatory interneurons as well as projection neurons (Kolkman et al. 2011, Gottesman-Davis et al. 2011). The cerebellum receives vestibular input both from the semicircular canals and from the vestibular nuclei, and sends fibers back to the medial and lateral vestibular nuclei (Langer et al. 1985, Kotchabhakdi & Walberg 1978). The MVN neurons projecting to the cerebellum are known to be involved in coordinating the postural adjustments and maintaining balance (Dieterich et al. 2005, Pyykko et al. 1991, Shaikh 2014), but little is known about the specific synaptic input and regulatory mechanisms that control MVN neuronal excitability.
Muscarinic acetylcholine receptors (mAChRs) play a critical role in the processing of sensory information in the vestibular nuclei. Although mAChR antagonists are commonly used to treat motion sickness (Soto et al. 2013, Golding & Gresty 2015), the sites of their action along the vestibular pathways and the mAChR subtypes involved are not clear. Abundant cholinergic neurons and nerve terminals are distributed in the vestibular nuclei (Fukushima et al. 2001). Autoradiographic analysis shows that there are dense mAChR binding sites in the vestibular nuclei (Zanni et al. 1995), and activation of mAChRs increases the excitability of MVN neurons (Sun et al. 2002, Ujihara et al. 1989). Molecular cloning studies have revealed five molecularly distinct mAChR subtypes, referred to as M1-M5 (Caulfield 1993, Caulfield & Birdsall 1998). The five mAChR subtypes are all linked to different types of G proteins. M1, M3, and M5 are selectively linked to the excitatory Gq/11 proteins, while M2 and M4 are preferentially coupled to the inhibitory Gi/o proteins (Felder 1995, Wess 2003). However, the specific mAChR subtypes that control synaptic input and excitability of MVN projection neurons have yet to be identified.
In this study, by using a combination of retrograde neuronal labeling in vivo and whole-cell patch-clamp recordings in brainstem slices, we sought to identify mAChR subtypes that regulate synaptic input and excitability in MVN neurons projecting to the cerebellum. Our findings suggest that presynaptic M2 subtype controls glutamatergic input from vestibular primary afferents, whereas M3 is the postsynaptic mAChR predominantly responsible for stimulating the firing of cerebellum-projecting MVN neurons. This new information greatly improves our understanding of the cholinergic mechanism regulating the vestibular system and could help the development of new strategies for treating vestibular disorders.
Materials and methods
Retrograde labeling of cerebellum-projecting MVN neurons
All experiments were performed using male Sprague-Dawley rats (4–6 weeks of age; Harlan Laboratories, Inc., Indianapolis, IN). The surgical procedures and experimental protocols were approved by the Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals. The rats were anesthetized with 2–3% isoflurane in O2, and the head was immobilized by a head holder. The muscles covering the occipital region of the skull were removed, and the occipital bone was drilled to expose the cerebellum. A rhodamine-based fluorescent microsphere suspension (FluoSpheres, 0.04 μm; Molecular Probes, Eugene, OR) was injected bilaterally into lobules IX/X of the cerebellum. FluoSpheres (50 nL) were microinjected using a glass pipette connected to a microinjector (Nanoject II; Drummond Scientific Company, Broomall, PA) and were monitored under a surgical microscope. The pipette tip position in lobules IX/X was at 13 mm posterior to the bregma, 5.5–6.5 mm from the brain surface, and 0.5 mm lateral from the midline. After injection, the rats were allowed to recover for 5–7 days to allow FluoSpheres to be transported to the MVN. Rats were treated prophylactically with enrofloxacin (5 mg/kg, subcutaneously daily for 3 days) and buprenorphine (0.2–0.5 mg/kg, subcutaneously every 12 h for 2 days) after surgery. The electrophysiological properties of the labeled neurons were not influenced by the rhodamine-based microspheres (Tseng et al. 1991, Li et al. 2004). The cerebellum was taken out and sectioned to 30 μm thickness at the injection site immediately after the rats were euthanized. The injection site of FluoSpheres was confirmed under a fluorescence microscope, and data were excluded from analysis if the injection site was not located within lobules IX/X.
Immunocytochemical labeling of MVN neurons and vestibular afferent fibers in the MVN
Using specimens from five rats, we performed immunocytochemical labeling to determine the distribution of primary afferent fibers in the MVN region. We used isolectin-B4 (IB4), a primary afferent nerve marker (Kobayashi & Matsumura 1996, Kitchener et al. 1993) and NeuN, a specific neuronal marker (Chen & Pan 2006, Zhou et al. 2009). Under deep anesthesia induced by sodium pentobarbital (60 mg/kg, intraperitoneal injection), rats were intracardially perfused with 200 mL of ice-cold normal saline containing 1,000 units of heparin followed by 250 mL of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4) and 200 mL of 10% sucrose in 0.1 M PBS (pH 7.4). The brain tissue containing the MVN was quickly removed and post-fixed for 2 h in the same fixative solution and then cryoprotected in 30% sucrose in PBS for 48 h at 4 °C. The tissue was sectioned into 30-μm-thick slices in the coronal plane using a freezing microtome. Free-floating sections were incubated with a mouse anti-NeuN antibody (1:100; EMD Millipore, Billerica, MA) diluted in Tris-buffered saline (TBS) containing 1% normal goat serum for 2 h at 25 °C and overnight at 4 °C. Subsequently, sections were rinsed in TBS and incubated with the secondary antibody (goat anti-mouse IgG conjugated to Alexa Fluor 594, dilution 1: 400; Molecular Probes) diluted in TBS containing 2% normal goat serum for 1.5 h at 25 °C. The sections were rinsed in TBS for 40 min and incubated with IB4 conjugated to Alexa Fluor 488 (dilution 1:500; Molecular Probes) for 2 h. Sections were then mounted on slides, dried, and covered with coverslips. When the primary antibody was omitted, no immunolabeling was observed. The sections were examined under a confocal microscope (Carl Zeiss, Jena, Germany), and the areas of interest were photo-documented.
Brainstem slice preparation
Brain slices containing the MVN were prepared from the FluoSphere-injected rats. Briefly, the rats were anesthetized with 2% isoflurane and decapitated, and the brain was quickly removed and placed in ice-cold artificial cerebrospinal fluid saturated by a mixture of 95% O2 and 5% CO2. The artificial cerebrospinal fluid solution contained (in mM) 124.0 NaCl, 3.0 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.4 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3. A tissue block containing the MVN was glued onto the stage of a vibrating microtome (Technical Products International, St. Louis, MO). Coronal slices (300 μm thick) were cut and then transferred to an incubation chamber containing the artificial cerebrospinal fluid solution continuously gassed with a mixture of 95% O2 and 5% CO2 at 34 °C for at least 1 h before electrophysiological recordings.
Electrophysiological recordings
Whole-cell recordings were performed in labeled MVN neurons in the brainstem slices. A slice was placed in a recording chamber (Warner Instruments, Hamden, CT) and was held to the bottom of the chamber by a nylon mesh attached to a U-shaped stainless steel weight. The recording chamber was continuously perfused (3 mL/min) with artificial cerebrospinal fluid bubbled with 95% O2 and 5% CO2 at 34 °C, maintained by an in-line solution heater. The volume of the solution needed to fill the recording chamber was approximately 1.0 mL. It took approximately 1.5 min to completely exchange the solution inside the recording chamber. The labeled MVN neurons were first identified by using an upright microscope (BX51WI; Olympus, Tokyo, Japan) with a combination of epifluorescence illumination and differential interference contrast optics.
The recording electrode was pulled from borosilicate capillaries (1.2-mm outer diameter, 0.68-mm inner diameter; World Precision Instruments, Sarasota, FL) by using a micropipette puller (P-97; Sutter Instruments, Novato, CA). The resistance of the pipette was 3–7 MΩ when it was filled with internal solution containing (in mM) 140.0 K+ gluconate, 2.0 MgCl2, 0.1 CaCl2, 10.0 HEPES, 1.1 EGTA, 0.3 Na2-GTP, and 2.0 Na2-ATP, adjusted to pH 7.25 with 1 M KOH (300–310 mOsm). The firing activity of labeled MVN neurons was recorded using the current-clamp mode. Excitatory postsynaptic currents (EPSCs) were recorded at a holding potential of −60 mV in the presence of the GABAA receptor antagonist bicuculline (20 μM) and the specific glycine receptor antagonist strychnine (2 μM). EPSCs were evoked by electrical stimulation (0.2–0.4 ms, 0.6 mA, and 0.1 Hz) through a bipolar stimulating electrode placed on the vestibular afferent fiber path in the lateral margin of the brainstem slice. Monosynaptic EPSCs were identified on the basis of the constant latency and the absence of conduction failure of evoked EPSCs in response to 20-Hz electrical stimulation as we described previously (Li et al. 2002, Zhou et al. 2010). Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded at a holding potential of +10 mV in the presence of the glutamate non-NMDA receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 μM). In some experiments, a general G-protein inhibitor, GDP-β-S (a non-hydrolyzable analog of GDP; 1 mM), was added into the pipette solution to block postsynaptic G-protein signaling (Zhou et al. 2010). Signals were processed using a MultiClamp 700B amplifier (Molecular Devices, Foster City, CA), filtered at 1–2 kHz, and digitized at 20 kHz using Digidata 1440 (Molecular Devices). We used at least three rats for each recording protocol.
We tested the effects of atropine, oxotremorine-M, (2R)-N-[1-(4-methyl-3-pentenyl)piperidin-4-yl]-2-cyclopentyl-2-hydroxy-2-phenylacetamide (J104129), 11-[[2-[(Diethylamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one (AF-DX 116), and pirenzepine on the synaptic or firing activity of labeled MVN neurons. All the drugs were freshly prepared in artificial cerebrospinal fluid before the experiments and delivered by syringe pumps at final concentrations. DNQX was purchased from Abcam (Cambridge, MA), and other agents were purchased from Sigma-Aldrich (St. Louis, MO). The specificity of drugs at concentrations used was validated previously in studies using mAChR subtype knockout mice (Chen et al. 2010, Chen et al. 2014, Zhang et al. 2006).
Quantitative PCR
The brainstem tissue block containing the MVN was glued onto the stage of a vibrating microtome. Coronal slices of 500-μm thickness were cut, and tissues containing the MVN were punched out. Total RNA was extracted from the MVN tissue using TRIzol-chloroform and treated with DNase I (Invitrogen, Carlsbad, CA). cDNA was prepared by using the SuperScript III First-Strand Synthesis kit and treated with RNase H (Invitrogen). Real-time PCR was performed using the iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA) and SYBR Green. The thermal cycle conditions were as follows: one cycle at 95 °C for 1 min, 40 cycles at 95 °C for 15 s, and one cycle at 60 °C for 15 s. The primers used for quantitative PCR were as follows: M1 forward, CCTGGCTCAGAAGTGGTGAT; M1 reverse, TGTATTTGGGGAGCTTTTGG; M2 forward, GCAATGCCTCCGTTATGAAT; M2 reverse, GTGGTCCGCTTAACTGGGTA; M3 forward, TCATCCAGGAGGAAGTACGG; M3 reverse, GCTGTGGTCTTGGTCCATCT; M4 forward, CAGAGCTGTATCCCCGAGAG; M4 reverse, TTGAAAGTGGCATTGCAGAG; M5 forward, CCACCACTGACCCTGTCTTT; M5 reverse, CTGTTTTCAGTCCGGGTGTT. Relative mRNA levels were calculated using the 2−ΔΔCT method.
Data analysis
Data are presented as means ± SEM. The discharge and membrane potentials were analyzed over a period of 3 min before, during and after drug application. The junction potential was corrected offline based on the composition of the internal and external solutions used for the recordings. The EPSCs, sIPSCs and firing frequency were analyzed off-line using a peak detection program (MiniAnalysis; Synaptosoft, Fort Lee, NJ). Events were detected by setting a threshold above the noise level. The effects of drugs on the firing rate, EPSCs, and sIPSCs were analyzed by using one-way or two-way analysis of variance followed by the Dunnett or Tukey post hoc test. P < 0.05 was considered statistically significant.
Results
Spatial relationship between vestibular afferent nerve fibers and cerebellum-projecting neurons in the MVN
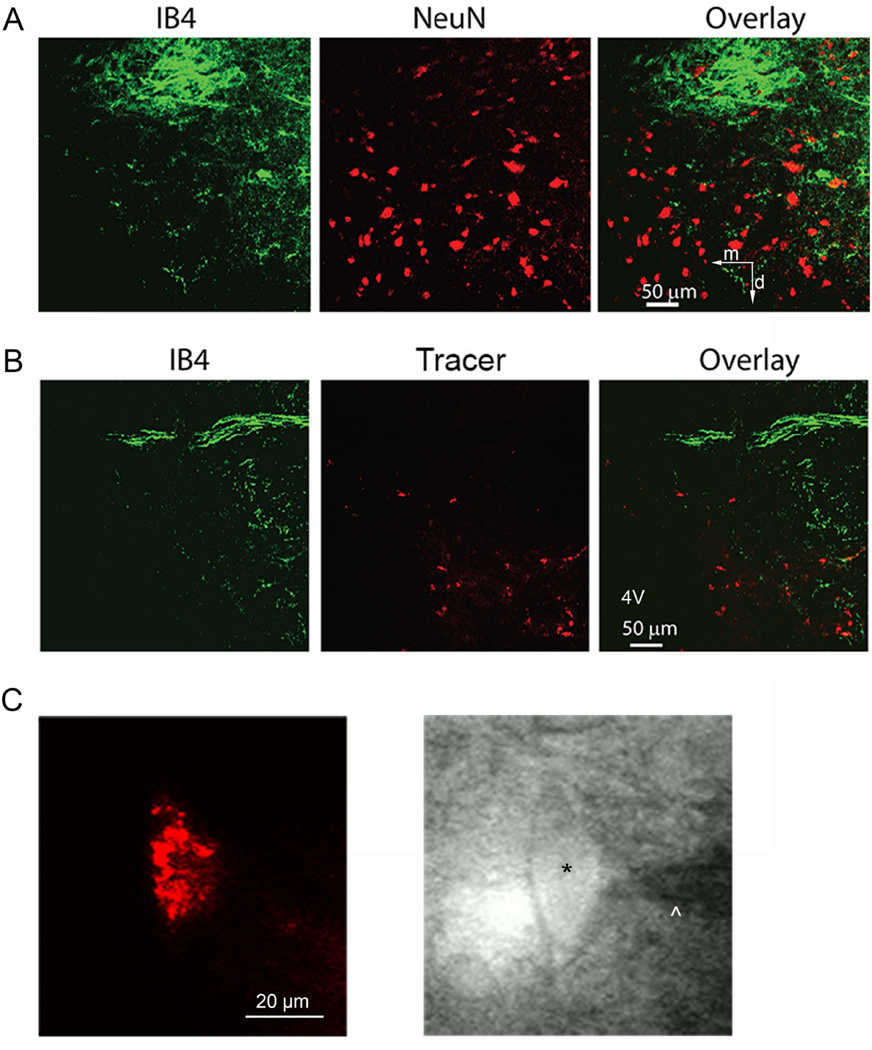
We first used double immunocytochemical labeling and confocal microscopy to validate the efficacy of FluoSphere labeling and determine the distribution of vestibular primary afferent nerve terminals and their spatial relationship to cerebellum-projecting neurons in the MVN. The primary vestibular afferent terminals, labeled with IB4, were densely distributed in the lateral region of the MVN. A large bundle of IB4-positive afferent fibers was present dorsal to the labeled MVN neurons. The MVN neurons, identified by NeuN-immunoreactivity, were in close proximity to IB4-positive afferent fibers in the MVN (Fig. 1A). Also, MVN neurons labeled with rhodamine-based FluoSpheres were adjacent to IB4-positive afferent fibers (Fig. 1B).
Fig. 1. Distribution of vestibular afferent nerve fibers and cerebellum-projecting neurons in the MVN and identification of retrogradely labeled cerebellum-projecting MVN neurons in brainstem slices.
A: Confocal images show the spatial relationship between primary afferent nerve fibers labeled with IB4 (green) and MVN neurons labeled with NeuN (red). B: Confocal images show the distribution of primary afferent nerve fibers identified by IB4 labeling (green) in the MVN region and retrograde tracer-labeled cerebellum-projecting MVN neurons (red). 4V, fourth ventricle. m, medial; d, dorsal. All images are single confocal optical sections. C: FluoSphere-labeled MVN neuron in the brain slice viewed with fluorescence illumination, and photomicrograph of the same labeled neuron (*) with an attached recording electrode (^) in the slice.
Oxotremorine-M inhibits glutamatergic input from vestibular primary afferents to cerebellum-projecting MVN neurons
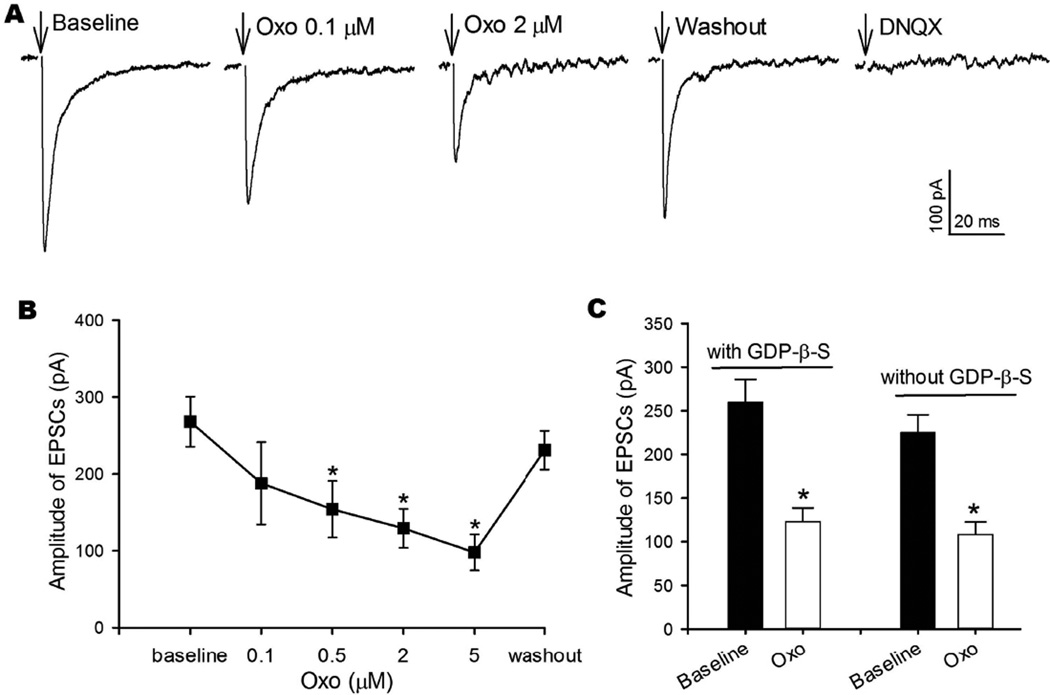
mAChRs are present in the vestibular ganglia and the vestibular end-organs in both humans and rats (Wackym et al. 1996). To determine how mAChRs regulate the excitatory synaptic input from vestibular primary afferents to cerebellum-projecting MVN neurons, we recorded monosynaptically evoked EPSCs by placing a bipolar stimulating electrode on the vestibular afferent fiber path on the lateral margin of the brainstem slice. We first obtained baseline EPSCs and then bath perfused oxotremorine-M while stimulation continued to be delivered until a stable effect of oxotremorine-M was observed (typically within 2–3 min after the start of oxotremorine-M application). Bath application of 0.1−5 μM oxotremorine-M, a specific mAChR agonist that is not subtype-selective (Bevan 1984, Chen et al. 2014), significantly reduced the amplitude of monosynaptic EPSCs in a concentration-dependent manner (n = 7, Fig. 2, A and B). The inhibitory effect of oxotremorine-M was near maximum at 2 μM and was reversible after washout of oxotremorine-M. Monosynaptic EPSCs were completely blocked by 20 μM DNQX (Fig. 2A), a glutamate non-NMDA receptor antagonist, indicating that the evoked EPSCs were mediated by glutamate release from vestibular afferent terminals.
Fig. 2. Oxotremorine-M inhibits glutamatergic input from vestibular afferent terminals to cerebellum-projecting MVN neurons.
A: Representative recordings show evoked monosynaptic EPSCs during control, application of 0.1 and 2 μM oxotremorine-M (Oxo), and 20 μM DNQX. Note that the stimulating artifact was removed for clarity, and arrows indicate the original location of the stimulating artifact. B: Concentration-dependent effects of oxotremorine-M on the amplitude of evoked EPSCs of 7 labeled MVN neurons. C: Summary data showing the effect of 2 μM oxotremorine-M on the amplitude of EPSCs in 6 labeled MVN neurons recorded with GDP-β-S in internal solution and 5 labeled MVN neurons recorded without GDP-β-S in internal solution. Data are presented as means ± SEM. *P < 0.05, compared with baseline.
Because mAChRs are coupled to G proteins, we included 1 mM GDP-β-S, a non-hydrolyzable analog of GDP, in the internal recording solution to inhibit possible postsynaptic G protein signaling via mAChRs. The efficacy of postsynaptic dialysis of GDP-β-S has been shown in our previous study (Zhou et al. 2010). In all 6 labeled MVN neurons tested, oxotremorine-M at 2 μM still significantly decreased the amplitude of evoked EPSCs (Fig. 2C). This effect was similar to that observed in labeled neurons recorded without GDP-β-S in the internal pipette solution (n = 5, Fig. 2C). These findings suggest that presynaptic mAChRs regulate glutamatergic input to cerebellum-projecting MVN neurons.
Oxotremorine-M has no effect on inhibitory synaptic input to cerebellum-projecting MVN neurons
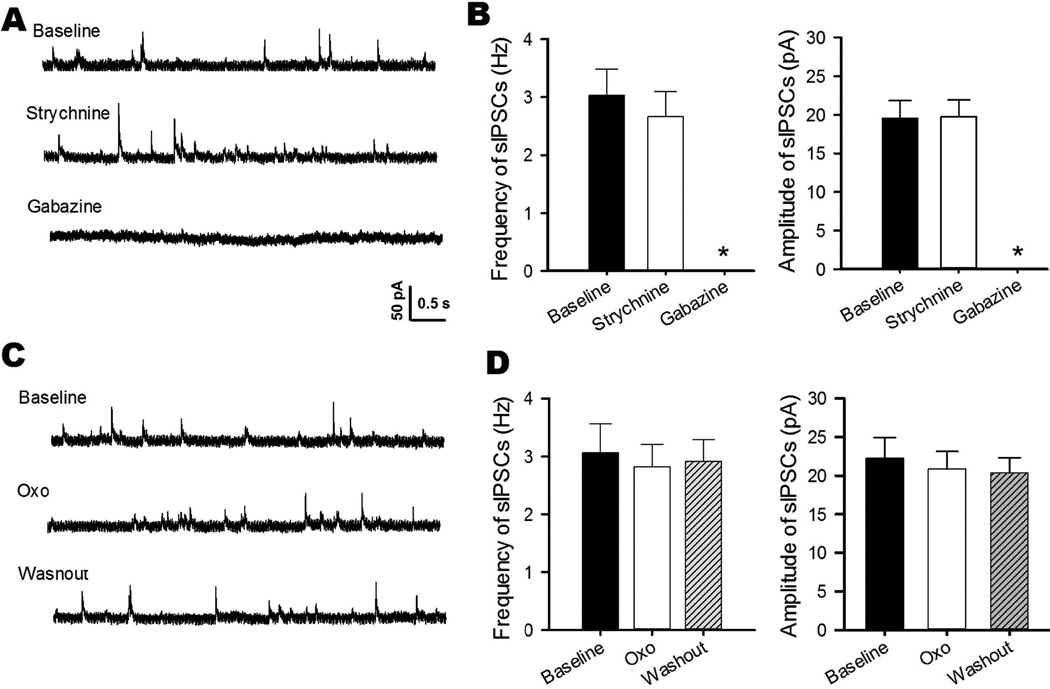
GABA- and glycine-immunoreactive neurons are scattered in the vestibular nuclei (Walberg et al. 1990), and GABAergic input influences the excitability of MVN neurons (Grassi et al. 1995). We first recorded sIPSCs to determine the neurochemical nature of inhibitory inputs to cerebellum-projecting MVN neurons. Bath application of 2 μM strychnine, a specific glycine receptor antagonist, had no significant effect on sIPSCs (n = 7, Fig. 3, A and B). However, the selective GABAA receptor antagonist gabazine at 10 μM completely blocked sIPSCs in all labeled MVN neurons tested (n = 7, Fig. 3, A and B), indicating that cerebellum-projecting MVN neurons receives inhibitory GABAergic, but not glycinergic, input.
Fig. 3. Oxotremorine-M has no effect on GABAergic sIPSCs in cerebellum-projecting MVN neurons.
A: Representative traces of a labeled MVN neuron show sIPSCs during application of 2 μM strychnine or 10 μM gabazine. B: Summary data show the effect of strychnine and gabazine on the frequency and amplitude of sIPSCs in 7 labeled MVN neurons. C: Representative traces of a labeled MVN neuron show the effect of 2 μM oxotremorine-M (Oxo) on sIPSCs. D: Group data show the lack of an oxotremorine-M effect on the frequency or amplitude of sIPSCs in 8 labeled MVN neurons. Data are presented as means ± SEM. *P < 0.05, compared with baseline.
We then determined whether mAChRs regulate GABAergic synaptic input to MVN neurons. In 8 labeled MVN neurons, bath application of oxotremorine-M at 2 and 5 μM had no significant effect on the frequency or amplitude of sIPSCs (Fig. 3, C and D). These data suggest that mAChRs do not control synaptic GABA release to cerebellum-projecting MVN neurons.
Oxotremorine-M increases the firing activity of cerebellum-projecting MVN neurons
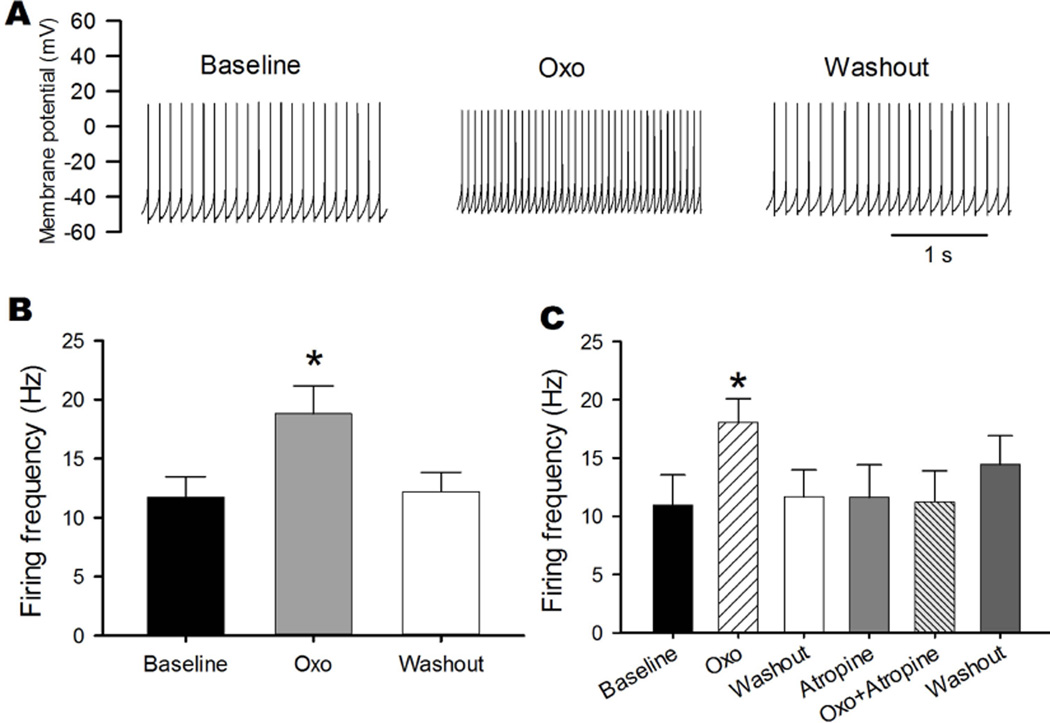
We next recorded action potentials of labeled MVN neurons to determine how activation of mAChRs affects the excitability of cerebellum-projecting MVN neurons. We selected labeled neurons with resting membrane potentials of −50 mV or lower and with action potential overshoot greater than 10 mV. Of the total 53 labeled MVN neurons recorded in our study, 49 displayed spontaneous discharges with an average firing rate of 11.73 ± 1.74 Hz. Bath application of 2 μM oxotremorine-M significantly depolarized the membrane potential (from −51.76 ± 1.01 to −44.80 ± 1.07 mV) and increased the firing activity of all 13 labeled MVN neurons tested (Fig. 4, A and B). The effect of oxotremorine-M on spontaneous activity occurred within 2–3 min of drug application, and the firing frequency returned to the baseline in ~10 min after washout. In all 5 labeled MVN neurons examined, the stimulatory effect of 2 μM oxotremorine-M on firing activity was completely blocked by 2 μM atropine, a specific mAChR antagonist (Fig. 4C).
Fig. 4. Oxotremorine-M increases the firing activity of cerebellum-projecting MVN neurons.
A: Representative traces show the effect of 2 μM oxotremorine-M (Oxo) on discharges of a labeled MVN neuron. B: Summary data show the effect of oxotremorine-M on the firing frequency of 13 labeled MVN neurons. C: Group data show that atropine blocked the stimulatory effect of 2 μM oxotremorine-M on the firing frequency of 5 labeled MVN neurons. Data are presented as means ± SEM. *P < 0.05, compared with baseline.
Relative expression levels of mAChR subtypes in the MVN
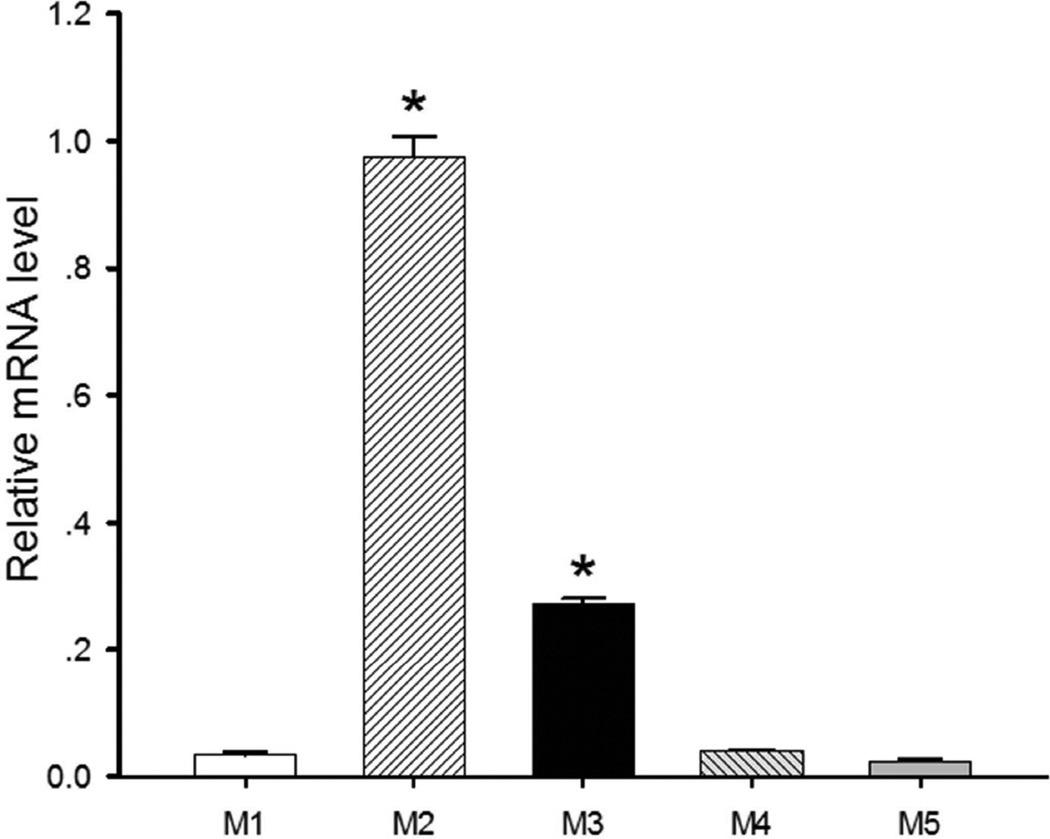
Because there are no highly specific antibodies against all mAChR subtypes, we used the quantitative PCR approach to estimate the relative expression levels of the M1, M2, M3, M4, and M5 subtypes in the MVN. Although the mRNAs of all five mAChR subtypes were detectable in MVN tissues, M2 was the most abundant subtype expressed in the MVN. Also, the level of M3 mRNA was much higher than those of M1, M4, and M5 mRNAs. With the mRNA level of M2 considered to be 100% (the quantitative PCR efficiency was close to 100%), the relative levels of M1, M3, M4, and M5 mRNAs were 3.3%, 27.7%, 3.8%, and 2.4%, respectively (Fig. 5). These results suggest that of the five mAChR subtypes, M2 and M3 may be the most highly expressed in the rat MVN.
Fig. 5. Expression levels of mAChR subtypes in the MVN.
Quantitative PCR data show the relative mRNA levels of the M1, M3, M4 and M5 subtypes compared with the M2 subtype (n = 6 rats). Data are presented as means ± SEM. *P < 0.05, compared with the M1 subtype.
The M2 mAChR mediates the inhibitory effect of oxotremorine-M on glutamatergic EPSCs of cerebellum-projecting MVN neurons
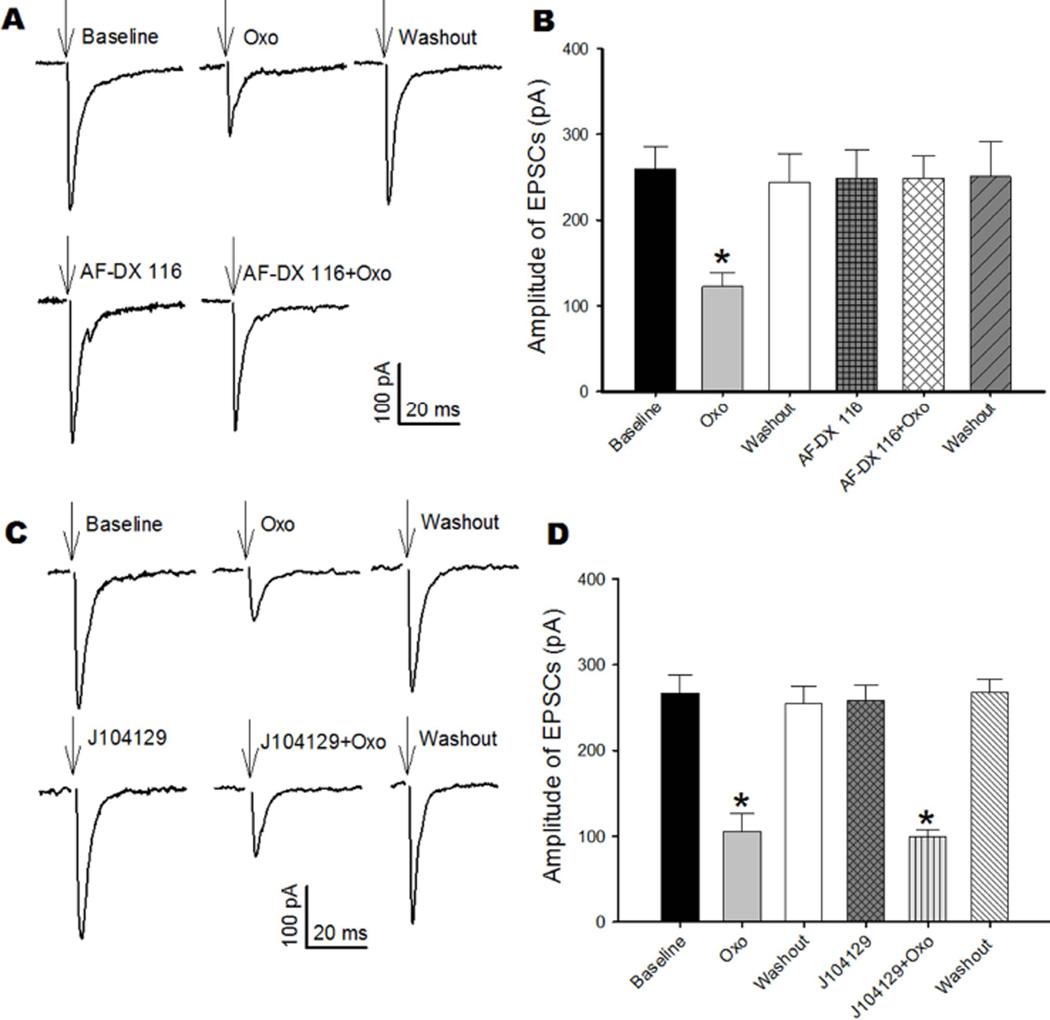
We next sought to identify the mAChR subtypes involved in regulating synaptic input and excitability of cerebellum-projecting MVN neurons by using selective mAChR subtype antagonists that have been validated previously in mAChR subtype knockout mice (Chen et al. 2010, Chen et al. 2014, Zhang et al. 2006). Because the M2 mAChR is coupled to inhibitory Gi/o proteins and highly expressed in the MVN, we determined whether this subtype mediates the inhibitory effect of oxotremorine-M on synaptic glutamate release to cerebellum-projecting MVN neurons. We recorded EPSCs of labeled MVN neurons monosynaptically evoked from vestibular afferent terminals. Bath application of 2 μM AF-DX 116, an M2 subtype-preferring antagonist (Yarosh et al. 1988, Zhang et al. 2006, Zhang et al. 2007), alone did not alter the amplitude of evoked EPSCs. AF-DX 116 completely blocked the inhibitory effect of oxotremorine-M on the amplitude of evoked EPSCs in all 6 labeled MVN neurons examined (Fig. 6, A and B). In contrast, the inhibitory effect of oxotremorine-M on the amplitude of evoked EPSCs was not affected by 50 nM J104129, an M3 subtype-preferring antagonist (Chen et al. 2010, Mitsuya et al. 1999), in another 5 labeled MVN neurons (Fig. 6, C and D). These data indicate that glutamatergic input from vestibular primary afferents to cerebellum-projecting MVN neurons is regulated by presynaptic M2 subtype.
Fig. 6. Oxotremorine-M-induced inhibition of glutamatergic input to cerebellum-projecting MVN neurons is mediated by the M2 mAChR.
A: Representative current traces show the effects of oxotremorine-M (Oxo) and AF-DX 116 on evoked monosynaptic EPSCs of a labeled MVN neuron. Note that the stimulating artifact was removed for clarity, and arrows indicate the original location of the stimulating artifact. B: Summary data show that 2 μM AF-DX 116 abolished the inhibitory effect of oxotremorine-M on the amplitude of evoked EPSCs in 6 labeled MVN neurons. C: Original recordings show the effects of oxotremorine-M (Oxo) on evoked monosynaptic EPSCs of a labeled MVN neuron before and during J104129 application. D: Group data show that J104129 (50 nM) did not alter the inhibitory effect of oxotremorine-M on the amplitude of evoked EPSCs in 5 labeled MVN neurons. Data are presented as means ± SEM. *P < 0.05, compared with baseline.
M3 is the mAChR that plays the dominant role in mediating the excitatory effect of oxotremorine-M on the firing activity of cerebellum-projecting MVN neurons
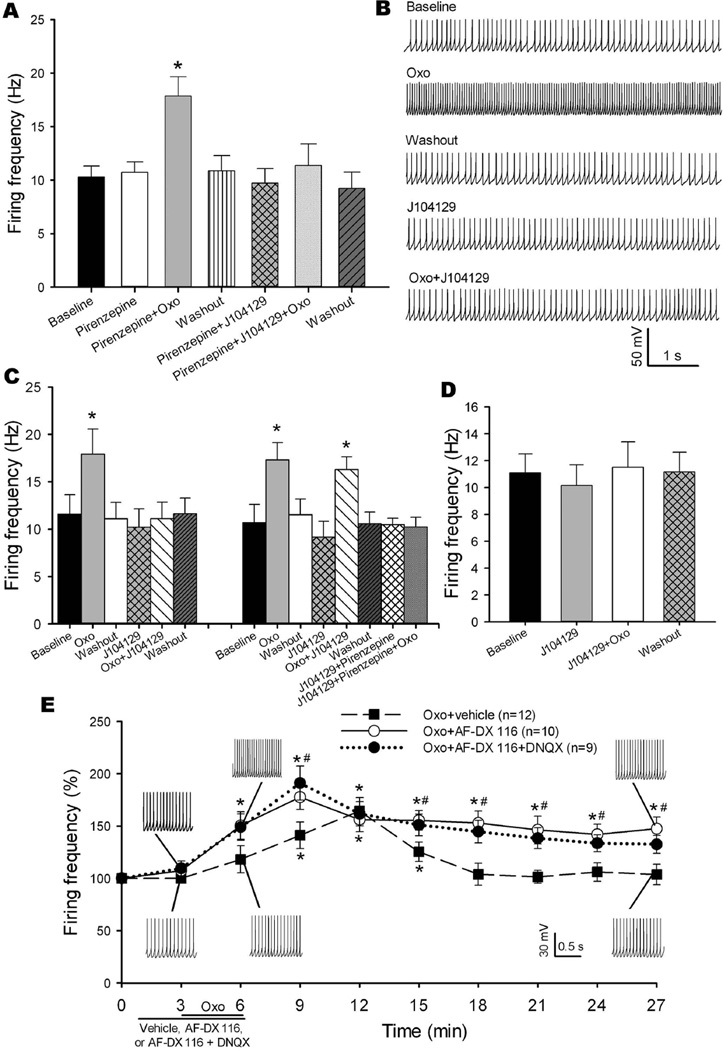
To determine which mAChR subtypes are involved in the excitatory effect of oxotremorine-M on the firing activity of cerebellum-projecting MVN neurons, we used pirenzepine, an M1 subtype-preferring antagonist (Fiszman et al. 1991, Stengel & Cohen 2003), and J104129. Due to the lack of M5-specific antagonists, the role of the M5 subtype was not examined in our study. In 11 labeled MVN neurons, bath application of pirenzepine at 10 μM alone had no significant effect on the baseline firing frequency (Fig. 7A). Bath application of 2 μM oxotremorine-M still significantly increased the firing frequency of labeled MVN neurons in the presence of pirenzepine. However, subsequent application of 50 nM J104129 abolished the stimulatory effect of oxotremorine-M on these neurons (Fig. 7A).
Fig. 7. Oxotremorine-M increases the firing activity of cerebellum-projecting MVN neurons predominantly via M3 subtype, and blocking the M2 mAChR prolongs the stimulatory effect of oxotremorine-M.
A: Summary data show the effects of oxotremorine-M (Oxo) on the firing activity of 11 labeled MVN neurons in the presence of pirenzepine alone or pirenzepine plus J104129. B: Representative current traces show the effect of oxotremorine-M on the firing activity of a labeled MVN neuron in the presence of J104129 (50 nM). C: Group data show that oxotremorine-M failed to increase the firing frequency of labeled MVN neurons in the presence of J104129 in 11 of 14 labeled MVN neurons. In another 3 labeled MVN neurons, oxotremorine-M still increased the firing frequency in the presence of J104129 but failed to increase the firing frequency in the presence of J104129 plus pirenzepine. D: Summary data show that oxotremorine-M failed to increase the firing frequency of 6 labeled MVN neurons in the presence of J104129. E: Time course of the excitatory effect of oxotremorine-M on the firing activity of labeled MVN neurons in the presence of 2 μM AF-DX 116 (n = 10), AF-DX 116 plus 20 μM DNQX (n = 9), or vehicle (n = 12). Data are presented as means ± SEM. *P < 0.05, compared with the respective baseline (time 0); #P < 0.05, compared with the corresponding value in the vehicle group at the same time point.
In another 14 labeled MVN neurons in which an initial bath application of oxotremorine-M potentiated the firing, J104129 had no significant effect on the baseline firing rate. In 11 of the 14 neurons, J104129 abolished the stimulatory effect of oxotremorine-M on the firing frequency (Fig. 7, B and C). In the remaining 3 neurons, repeated application of oxotremorine-M still significantly increased the firing in the presence of J104129 and pirenzepine (Fig. 7C).
In 8 additional labeled MVN neurons, we first blocked the M3 mAChR with 50 nM J104129 before bath application of oxotremorine-M. In 6 of the 8 neurons, bath application of 2 μM oxotremorine-M failed to increase the firing frequency in the presence of J104129 (Fig. 7D). In the remaining 2 neurons, oxotremorine-M still increased the firing frequency (from 11.53 to 19.43 Hz in one neuron, and from 15.59 to 19.86 Hz in another neuron) in the presence of J104129. These data suggest that postsynaptic M3 is the dominant mAChR involved in muscarinic excitation of cerebellum-projecting MVN neurons.
Role of the M2 mAChR in the excitatory effect of oxotremorine-M on spontaneous firing activity of cerebellum-projecting MVN neurons
Because the M2 mAChR is involved in inhibiting glutamatergic input to cerebellum-projecting MVN neurons, we next determined how presynaptic M2 influences the excitatory effect of oxotremorine-M on labeled MVN neurons. In 12 labeled neurons tested, bath application of 2 μM oxotremorine-M significantly increased the firing frequency, with an onset latency of 2.75 ± 0.49 min and a peak effect of 7.63 ± 0.72 min after oxotremorine-M application. The oxotremorine-M effect returned to baseline 10.00 ± 1.73 min after washout (Fig. 7E). In comparison, in another 10 labeled MVN neurons treated with AF-DX 116, oxotremorine-M increased the firing activity with a short latency (1.25 ± 0.23 min), and the peak effect was reached within 3.50 ± 0.38 min. Furthermore, the firing activity of these 10 neurons remained elevated for at least 18 min after drug washout (Fig. 7E). In addition, bath application of AF-DX 116 similarly potentiated the effect of oxotremorine-M on neuronal firing in the presence of 20 μM DNQX in another 9 labeled MVN neurons (Fig. 7E). These results suggest that presynaptic M2 mAChR-mediated inhibition of glutamatergic input counteracts the postsynaptic muscarinic excitation of cerebellum-projecting MVN neurons.
Discussion
Neurons in the vestibular nuclei are critically involved in the control of reflex, voluntary movements, and the construction of a sense of self-motion. The cerebellum receives input from MVN neurons and plays an important role in balance control (Kotchabhakdi & Walberg 1978, Barmack 2003, Shin et al. 2011). Because cerebellum-projecting neurons are scattered throughout the MVN and exhibit heterogeneous morphologies that are indistinguishable from those of other interneurons, we used a retrograde fluorescent tracer injected into lobules IX/X of the cerebellum in rats and then identified cerebellum-projecting MVN neurons for electrophysiological recordings in brain slices. The brain slice preparation is appropriate for our study, because it largely preserves the synaptic connection, native receptors and ion channels. High-resolution whole-cell recording of postsynaptic currents provides a sensitive and accurate measure of the synaptic release of glutamate, GABA and glycine to identified neurons (Chen et al. 2010, Li et al. 2002, Zhang et al. 2006). With pharmacological and ionic manipulations, information can be readily obtained about the types of presynaptic and postsynaptic receptors activated and the drug effects on synaptic transmission and neuronal excitability. Another advantage of this preparation is the absence of anesthetics. However, a major disadvantage is that many physiological inputs to MVN neurons are likely disrupted in the thin brainstem slices.
Using immunocytochemical labeling and confocal microscopy, we mapped the distribution of primary vestibular afferent terminals in the MVN and identified their spatial relationship to cerebellum-projecting MVN neurons. We observed a large number of cerebellum-projecting neurons, which were in close proximity with vestibular afferent nerve terminals in the MVN. This information was used to guide the placement of stimulating electrode for the study of synaptic input from primary vestibular afferent fibers. Vestibular primary afferent nerves make synapses with neurons in the vestibular nuclei, where they release glutamate (Doi et al. 1990, Takahashi et al. 1994, Sun et al. 2002). We found that EPSCs that were monosynaptically evoked from primary vestibular afferent fibers were blocked by a glutamate non-NMDA receptor antagonist, suggesting that cerebellum-projecting neurons receive excitatory glutamatergic input from vestibular afferent nerves. Although both GABA and glycine are the major inhibitory neurotransmitters in the brainstem, we found that all sIPSCs of labeled neurons were blocked by the GABAA, but not glycine, receptor antagonist. These results suggest that cerebellum-projecting MVN neurons predominantly receive GABAergic inhibitory input.
Our study provides new information suggesting that presynaptic M2 mAChR plays a critical role in regulating glutamatergic input from vestibular primary afferents to cerebellum-projecting MVN neurons. Five distinct mAChR subtypes are widely distributed in the central nervous system (James et al. 1983, Wess et al. 2003). However, the cellular locations and functional roles of these subtypes in regulating synaptic input to MVN neurons remain unclear. Our quantitative PCR data suggest that M2 and M3 are probably the dominant mAChRs expressed in the MVN. We found that oxotremorine-M significantly inhibited monosynaptic EPSCs and that this inhibitory effect persisted when the postsynaptic muscarinic effect was blocked by GDP-β-S. However, we found that oxotremorine-M had no effect on IPSCs in all labeled neurons, indicating that mAChRs are not involved in controlling GABAergic input to cerebellum-projecting MVN neurons. Furthermore, blocking M2 with AF-DX 116 completely blocked the inhibitory effect of oxotremorine-M on EPSCs in all neurons tested. The inhibitory effect of M2 activation is likely mediated by inhibition of voltage-gated Ca2+ channels (Cao et al. 2011). It has been shown that mRNA can be transported from cell bodies to axonal terminals for local protein synthesis (Jung et al. 2012), although the mRNA levels may not be consistent with the protein expression/abundance (Vogel & Marcotte 2012). It is possible that in addition to the presynaptic location on vestibular afferent terminals, M2 may be present in other neuronal types in the MVN that are not in direct contact with cerebellum-projecting neurons.
Another major finding of our study is that postsynaptic M3 is the mAChR predominantly responsible for muscarinic excitation of cerebellum-projecting MVN neurons. Blocking of mAChRs reduces the firing activity of MVN neurons (Takeshita et al. 1999), whereas activation of mAChRs excites unidentified MVN neurons (Phelan & Gallagher 1992, Kirsten & Sharma 1976, Ujihara et al. 1988). We found that oxotremorine-M significantly increased the firing activity of all labeled MVN neurons, and this effect was abolished by atropine, a non-subtype-selective mAChR antagonist. Furthermore, we showed that the excitatory effect of oxotremorine-M on neuronal firing was blocked by J104129, an M3-preferring antagonist, in most neurons examined. However, the M1-preferring antagonist pirenzepine did not affect the firing activity increased by oxotremorine-M in all neurons tested. M3 activation-induced excitation of MVN neurons is likely mediated by phospholipase C stimulation and subsequent inhibition of KCNQ/Kv7 potassium channels (Oldfield et al. 2009, Perez et al. 2010). Nevertheless, we observed that oxotremorine-M still increased the firing of a few labeled MVN neurons in the presence of J104129. Because the stimulatory effect of oxotremorine-M was not affected by pirenzepine but was abolished by atropine, the remaining muscarinic effect in the presence of J104129 is probably mediated by M5. In the mouse spinal cord, M5 is a major subtype mediating the stimulatory effect of oxotremorine-M (Chen et al. 2010). Owing to the lack of ligands that can specifically activate or inhibit M5, the physiological role of M5 mAChR in the control of MVN neurons remains unclear. The possibility that postsynaptic M5 contributes to regulating the excitability of MVN neurons needs to be validated using M5 subtype-knockout mice in future studies.
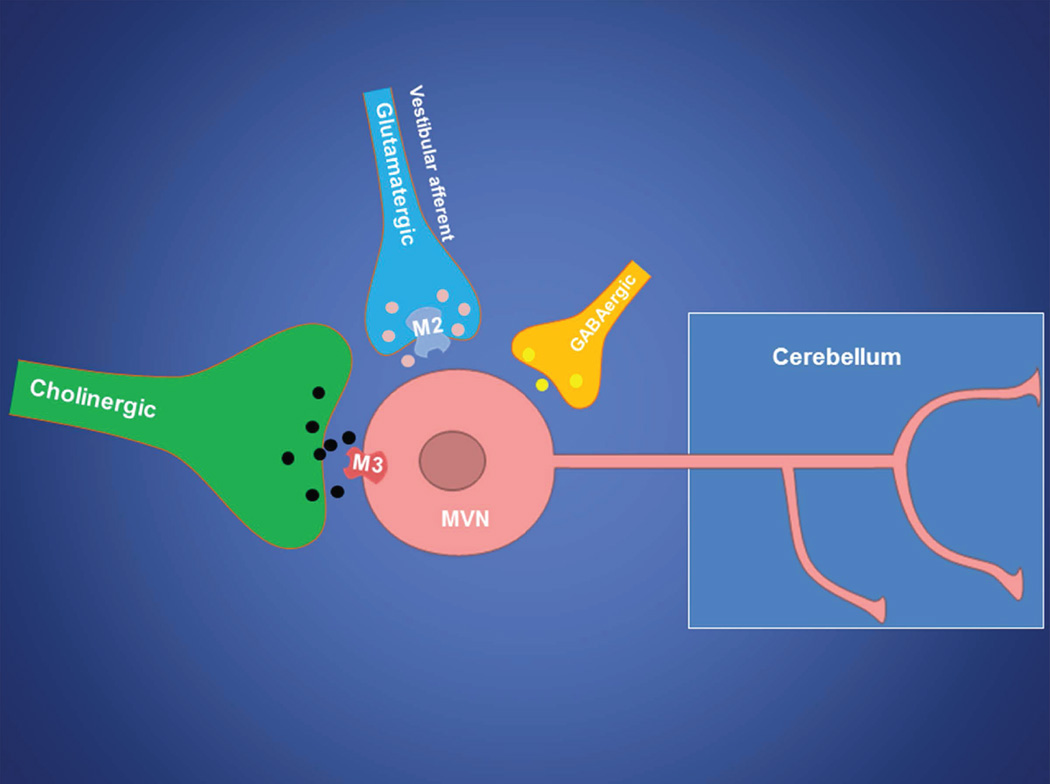
We found that the stimulatory effect of oxotremorine-M occurred more quickly and was prolonged when M2 was blocked with AF-DX 116. This finding is likely due to the dynamic interaction between M2 and M3 (and possibly M5 to a lesser extent). Within the MVN, M2 is located presynaptically and is coupled to inhibitory Gi/o proteins, whereas M3 is expressed postsynaptically and is coupled to excitatory Gq/11 proteins. Thus, blocking M2-mediated inhibition of glutamatergic input can enhance the excitatory effect of oxotremorine-M on MVN neurons (Fig. 8). A complex and dynamic interaction between the inhibitory (M2/M4) and excitatory (M3/M5) mAChR subtypes has been demonstrated in spinal dorsal horn neurons (Chen et al. 2010, Chen et al. 2014). Our findings suggest that activation of M2 mAChR could restrain muscarinic excitation of MVN neurons by reducing excitatory glutamatergic input.
Fig. 8. Schematic drawing illustrates that the cerebellum-projecting MVN neuron receives synaptic input from glutamatergic vestibular primary afferents, cholinergic neurons, and GABAergic interneurons.
The presynaptic M2 mAChR controls glutamatergic input from vestibular primary afferents, whereas the postsynaptic M3 mAChR predominantly excites cerebellum-projecting MVN neurons.
In conclusion, our study provides new evidence for cholinergic control of the central vestibular system. Because the mAChR is a major drug target in the treatment of vestibular disorders, our findings have important therapeutic implications. mAChR antagonists, such as scopolamine and atropine, are effective for treatment of vertigo in patients (Soto et al. 2013, Golding & Gresty 2015). However, the use of these non-subtype-selective mAChR antagonists is associated with various side effects, such as dryness of mouth, photophobia, blurred vision and tachycardia. Our study suggests that specific M2 agonists and M3 antagonists could be developed to treat vestibular disorders.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL077400) and by the N.G. and Helen T. Hawkins Endowment (to H.-L.P.).
List of abbreviations
- EPSCs
excitatory postsynaptic currents
- GABA
γ-aminobutyric acid
- IB4
isolectin-B4
- mAChRs
muscarinic acetylcholine receptors
- MVN
medial vestibular nucleus
- NMDA
N-methyl-D-aspartate
- sIPSCs
spontaneous inhibitory postsynaptic currents
- TBS
Tris-buffered saline
Footnotes
Conflict of interest disclosure
The authors have no conflict of interest to declare.
References
- Alvarez JC, Diaz C, Suarez C, Fernandez JA, Gonzalez del Rey C, Navarro A, Tolivia J. Neuronal loss in human medial vestibular nucleus. Anat Rec. 1998;251:431–438. doi: 10.1002/(SICI)1097-0185(199808)251:4<431::AID-AR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull. 2003;60:511–541. doi: 10.1016/s0361-9230(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Baughman RW, Errico P, Shojaku H. Vestibular primary afferent projection to the cerebellum of the rabbit. J Comp Neurol. 1993;327:521–534. doi: 10.1002/cne.903270405. [DOI] [PubMed] [Google Scholar]
- Bevan P. [3H]oxotremorine-M binding to membranes prepared from rat brain and heart: evidence for subtypes of muscarinic receptors. Eur J Pharmacol. 1984;101:101–110. doi: 10.1016/0014-2999(84)90035-9. [DOI] [PubMed] [Google Scholar]
- Cao XH, Byun HS, Chen SR, Pan HL. Diabetic neuropathy enhances voltage-activated Ca2+ channel activity and its control by M4 muscarinic receptors in primary sensory neurons. J Neurochem. 2011;119:594–603. doi: 10.1111/j.1471-4159.2011.07456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors--characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- Chen SR, Chen H, Yuan WX, Wess J, Pan HL. Dynamic control of glutamatergic synaptic input in the spinal cord by muscarinic receptor subtypes defined using knockout mice. J Biol Chem. 2010;285:40427–40437. doi: 10.1074/jbc.M110.176966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Chen H, Yuan WX, Wess J, Pan HL. Differential regulation of primary afferent input to spinal cord by muscarinic receptor subtypes delineated using knockout mice. J Biol Chem. 2014;289:14321–14330. doi: 10.1074/jbc.M114.550384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol. 2006;95:3086–3096. doi: 10.1152/jn.01343.2005. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Stephan T, Brandt T, Schwaiger M, Bartenstein P. Medial vestibular nucleus lesions in Wallenberg's syndrome cause decreased activity of the contralateral vestibular cortex. Ann N Y Acad Sci. 2005;1039:368–383. doi: 10.1196/annals.1325.035. [DOI] [PubMed] [Google Scholar]
- Doi K, Tsumoto T, Matsunaga T. Actions of excitatory amino acid antagonists on synaptic inputs to the rat medial vestibular nucleus: an electrophysiological study in vitro. Exp Brain Res. 1990;82:254–262. doi: 10.1007/BF00231245. [DOI] [PubMed] [Google Scholar]
- Felder CC. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. Faseb j. 1995;9:619–625. [PubMed] [Google Scholar]
- Fiszman ML, Barker JL, Jones SV. Electrophysiological responses to muscarinic receptor stimulation in cultured hippocampal neurons. Brain Res. 1991;557:1–4. doi: 10.1016/0006-8993(91)90108-8. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Kitahara T, Takeda N, Saika T, Uno A, Kubo T. Role of cholinergic mossy fibers in medial vestibular and prepositus hypoglossal nuclei in vestibular compensation. Neuroscience. 2001;102:159–166. doi: 10.1016/s0306-4522(00)00457-7. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Cullen KE. Vestibular control of the head: possible functions of the vestibulocollic reflex. Exp Brain Res. 2011;210:331–345. doi: 10.1007/s00221-011-2611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding JF, Gresty MA. Pathophysiology and treatment of motion sickness. Curr Opin Neurol. 2015;28:83–88. doi: 10.1097/WCO.0000000000000163. [DOI] [PubMed] [Google Scholar]
- Gottesman-Davis A, Shao M, Hirsch JC, Peusner KD. Electrophysiological properties of morphologically-identified medial vestibular nucleus neurons projecting to the abducens nucleus in the chick embryo. Neuroscience. 2011;172:494–509. doi: 10.1016/j.neuroscience.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi S, Della Torre G, Zampolini M, Pettorossi VE. Gaba mediated long-term depression (LTD) in the rat medial vestibular nuclei. Acta Otolaryngol Suppl. 1995;520:164–169. doi: 10.3109/00016489509125218. [DOI] [PubMed] [Google Scholar]
- James WM, Cheatham MA, Klein WL. Muscarinic acetylcholine receptor binding in the guinea pig cochlea. Hearing Res. 1983;9:113–121. doi: 10.1016/0378-5955(83)90139-9. [DOI] [PubMed] [Google Scholar]
- Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13:308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten EB, Sharma JN. Characteristicas and response differences to iontophoretically applied norepinephrine, D-amphetamine and acetylcholine on neurons in the medial and lateral vestibular nuclei of the cat. Brain Res. 1976;112:77–90. doi: 10.1016/0006-8993(76)90335-8. [DOI] [PubMed] [Google Scholar]
- Kitchener PD, Wilson P, Snow PJ. Selective labelling of primary sensory afferent terminals in lamina II of the dorsal horn by injection of Bandeiraea simplicifolia isolectin B4 into peripheral nerves. Neuroscience. 1993;54:545–551. doi: 10.1016/0306-4522(93)90274-j. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Matsumura G. Central projections of primary afferent fibers from the rat trigeminal nerve labeled with isolectin B4-HRP. Neurosci Lett. 1996;217:89–92. [PubMed] [Google Scholar]
- Kolkman KE, Moghadam SH, du Lac S. Intrinsic physiology of identified neurons in the prepositus hypoglossi and medial vestibular nuclei. J Vestib Res. 2011;21:33–47. doi: 10.3233/VES-2011-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchabhakdi N, Walberg F. Cerebellar afferent projections from the vestibular nuclei in the cat: an experimental study with the method of retrograde axonal transport of horseradish peroxidase. Exp Brain Res. 1978;31:591–604. doi: 10.1007/BF00239814. [DOI] [PubMed] [Google Scholar]
- Langer T, Fuchs AF, Scudder CA, Chubb MC. Afferents to the flocculus of the cerebellum in the rhesus macaque as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. 1985;235:1–25. doi: 10.1002/cne.902350102. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Finnegan TF, Pan HL. Signalling pathway of nitric oxide in synaptic GABA release in the rat paraventricular nucleus. J Physiol. 2004;554:100–110. doi: 10.1113/jphysiol.2003.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan YZ, Levey AI, Pan HL. Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol. 2002;543:807–818. doi: 10.1113/jphysiol.2002.020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Luan H. Signal processing of semicircular canal and otolith signals in the vestibular nuclei during passive and active head movements. Ann N Y Acad Sci. 2003;1004:169–182. doi: 10.1196/annals.1303.015. [DOI] [PubMed] [Google Scholar]
- Mitsuya M, Kawakami K, Ogino Y, Miura K, Mase T. Stereoselective synthesis of a new muscarinic M3 receptor antagonist, J-104129. Bioorg Med Chem Lett. 1999;9:2037–2038. doi: 10.1016/s0960-894x(99)00327-3. [DOI] [PubMed] [Google Scholar]
- Modianos DT, Pfaff DW. Brain stem and cerebellar lesions in female ratsITests of posture and movement. Brain Res. 1976;106:31–46. doi: 10.1016/0006-8993(76)90071-8. [DOI] [PubMed] [Google Scholar]
- Oldfield S, Hancock J, Mason A, Hobson SA, Wynick D, Kelly E, Randall AD, Marrion NV. Receptor-mediated suppression of potassium currents requires colocalization within lipid rafts. Mol Pharmacol. 2009;76:1279–1289. doi: 10.1124/mol.109.058008. [DOI] [PubMed] [Google Scholar]
- Perez C, Vega R, Soto E. Phospholipase C-mediated inhibition of the M-potassium current by muscarinic-receptor activation in the vestibular primary-afferent neurons of the rat. Neurosci Lett. 2010;468:238–242. doi: 10.1016/j.neulet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Phelan KD, Gallagher JP. Direct muscarinic and nicotinic receptor-mediated excitation of rat medial vestibular nucleus neurons in vitro. Synapse. 1992;10:349–358. doi: 10.1002/syn.890100410. [DOI] [PubMed] [Google Scholar]
- Pyykko I, Enbom H, Magnusson M, Schalen L. Effect of proprioceptor stimulation on postural stability in patients with peripheral or central vestibular lesion. Acta Otolaryngol. 1991;111:27–35. doi: 10.3109/00016489109137351. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh AG. Motion perception without Nystagmus--a novel manifestation of cerebellar stroke. J Stroke Cerebrovasc Dis. 2014;23:1148–1156. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Shin M, Moghadam SH, Sekirnjak C, Bagnall MW, Kolkman KE, Jacobs R, Faulstich M, du Lac S. Multiple types of cerebellar target neurons and their circuitry in the vestibulo-ocular reflex. J Neurosci. 2011;31:10776–10786. doi: 10.1523/JNEUROSCI.0768-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto E, Vega R, Sesena E. Neuropharmacological basis of vestibular system disorder treatment. J Vestib Res. 2013;23:119–137. doi: 10.3233/VES-130494. [DOI] [PubMed] [Google Scholar]
- Stengel PW, Cohen ML. M1 receptor-mediated nitric oxide-dependent relaxation unmasked in stomach fundus from M3 receptor knockout mice. J Pharmacol Exp Ther. 2003;304:675–682. doi: 10.1124/jpet.102.042283. [DOI] [PubMed] [Google Scholar]
- Sun Y, Waller HJ, Godfrey DA, Rubin AM. Spontaneous activity in rat vestibular nuclei in brain slices and effects of acetylcholine agonists and antagonists. Brain Res. 2002;934:58–68. doi: 10.1016/s0006-8993(02)02361-2. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Tsumoto T, Kubo T. N-methyl-D-aspartate receptors contribute to afferent synaptic transmission in the medial vestibular nucleus of young rats. Brain Res. 1994;659:287–291. doi: 10.1016/0006-8993(94)90895-8. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Sasa M, Ishihara K, Matsubayashi H, Yajin K, Okada M, Izumi R, Arita K, Kurisu K. Cholinergic and glutamatergic transmission in medial vestibular nucleus neurons responding to lateral roll tilt in rats. Brain Res. 1999;840:99–105. doi: 10.1016/s0006-8993(99)01775-8. [DOI] [PubMed] [Google Scholar]
- Tseng GF, Parada I, Prince DA. Double-labelling with rhodamine beads and biocytin: a technique for studying corticospinal and other projection neurons in vitro. J Neurosci Methods. 1991;37:121–131. doi: 10.1016/0165-0270(91)90122-g. [DOI] [PubMed] [Google Scholar]
- Ujihara H, Akaike A, Sasa M, Takaori S. Electrophysiological evidence for cholinoceptive neurons in the medial vestibular nucleus: studies on rat brain stem in vitro. Neurosci Lett. 1988;93:231–235. doi: 10.1016/0304-3940(88)90087-0. [DOI] [PubMed] [Google Scholar]
- Ujihara H, Akaike A, Sasa M, Takaori S. Muscarinic regulation of spontaneously active medial vestibular neurons in vitro. Neurosci Lett. 1989;106:205–210. doi: 10.1016/0304-3940(89)90227-9. [DOI] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nature Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackym PA, Chen CT, Ishiyama A, Pettis RM, Lopez IA, Hoffman L. Muscarinic acetylcholine receptor subtype mRNAs in the human and rat vestibular periphery. Cell Biol Int. 1996;20:187–192. doi: 10.1006/cbir.1996.0023. [DOI] [PubMed] [Google Scholar]
- Walberg F, Ottersen OP, Rinvik E. GABA, glycine, aspartate, glutamate and taurine in the vestibular nuclei: an immunocytochemical investigation in the cat. Exp Brain Res. 1990;79:547–563. doi: 10.1007/BF00229324. [DOI] [PubMed] [Google Scholar]
- Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol Sci. 2003;24:414–420. doi: 10.1016/S0165-6147(03)00195-0. [DOI] [PubMed] [Google Scholar]
- Wess J, Duttaroy A, Gomeza J, Zhang W, Yamada M, Felder CC, Bernardini N, Reeh PW. Muscarinic receptor subtypes mediating central and peripheral antinociception studied with muscarinic receptor knockout mice: a review. Life Sci. 2003;72:2047–2054. doi: 10.1016/s0024-3205(03)00082-1. [DOI] [PubMed] [Google Scholar]
- Yarosh CA, Olito AC, Ashe JH. AF-DX 116: a selective antagonist of the slow inhibitory postsynaptic potential and methacholine-induced hyperpolarization in superior cervical ganglion of the rabbit. J Pharmacol Exp Ther. 1988;245:419–425. [PubMed] [Google Scholar]
- Zanni M, Giardino L, Toschi L, Galetti G, Calza L. Distribution of neurotransmitters, neuropeptides, and receptors in the vestibular nuclei complex of the rat: an immunocytochemical, in situ hybridization and quantitative receptor autoradiographic study. Brain Res Bull. 1995;36:443–452. doi: 10.1016/0361-9230(94)00193-5. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Matsui M, Gautam D, Wess J, Pan HL. Opposing functions of spinal M2, M3, and M4 receptor subtypes in regulation of GABAergic inputs to dorsal horn neurons revealed by muscarinic receptor knockout mice. Mol Pharmacol. 2006;69:1048–1055. doi: 10.1124/mol.105.018069. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Pan HL. Regulation of glutamate release from primary afferents and interneurons in the spinal cord by muscarinic receptor subtypes. J Neurophysiol. 2007;97:102–109. doi: 10.1152/jn.00586.2006. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. The glutamatergic nature of TRPV1-expressing neurons in the spinal dorsal horn. J Neurochem. 2009;108:305–318. doi: 10.1111/j.1471-4159.2008.05772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci. 2010;30:4460–4466. doi: 10.1523/JNEUROSCI.5857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]