Abstract
Human immunodeficiency virus type 1 (HIV) infection of the brain produces cognitive and motor disorders. In addition, HIV positive individuals exhibit behavioral alterations, such as apathy, and a decrease in spontaneity or emotional responses, typically seen in anxiety disorders. Anxiety can lead to psychological stress, which has been shown to influence HIV disease progression. These considerations underscore the importance of determining if anxiety in HIV is purely psychosocial, or if by contrast, there are the molecular cascades associated directly with HIV infection that may mediate anxiety. The present study had two goals: 1) to determine if chronic exposure to viral proteins would induce anxiety-like behavior in an animal model and 2) to determine if this exposure results in anatomical abnormalities that could explain increased anxiety. We have used gp120 transgenic mice, which display behavior and molecular deficiencies similar to HIV positive subjects with cognitive and motor impairments. In comparison to wild type mice, 6 months old gp120 transgenic mice demonstrated an anxiety like behavior measured by open field, light/dark transition task, and prepulse inhibition tests. Moreover, gp120 transgenic mice have an increased number of spines in the amygdala, as well as higher levels of brain-derived neurotrophic factor and tissue plasminogen activator when compared to age-matched wild type. Our data support the hypothesis that HIV, through gp120, may cause structural changes in the amygdala that lead to maladaptive responses to anxiety.
Keywords: BDNF, gp120 transgenic mice, IL-1β, light-dark transition, open field, prepulse inhibition, tPA, TNFα
1. Introduction
Human immunodeficiency virus (HIV) infection of the brain often leads to neurological deficits characterized by cognitive impairments and motor deficits, termed HIV-associated neurological disorders (HAND) (Chan and Brew, 2014; Heaton et al., 2011; Tan and McArthur, 2012). Psychiatric and anxiety symptoms are also very common in individuals with HIV (Bing et al., 2001; Kemppainen et al., 2013). Moreover, people living with HIV are vulnerable to suicidal ideation and behavior. Indeed, the suicide rates are three times higher than in the general population (Carrico, 2010). In addition, anxiety-induced stress has also been shown to promote viral replication, reduce immune responses and interfere with antiretroviral therapy [reviewed in (Riley and Kalichman, 2015)]. Individuals with anxiety and psychiatric comorbidities have lower adherence to HIV medications, decreased quality of life, greater health care utilization, and poorer prognosis when compared to individuals without psychiatric disorders (Cohen et al., 2007; Weaver et al., 2005). Thus, the importance of understanding how HIV causes anxiety syndrome is paramount.
Anxiety symptoms are usually attributed to preexisting predisposition, trauma or abuse, or to the diagnosis itself. Initial diagnosis with HIV is associated with significant stress, as is the prospect of disclosure of diagnosis and having to live with HIV (Hult et al., 2012; Olley et al., 2005). While the general diagnosis and prevalence of anxiety and depression vary across cultures (Lewis-Fernandez et al., 2010), these symptoms have been widely reported in HIV-positive patient cohorts across the globe (Kee et al., 2015; Mutumba et al., 2015; Tesfaye and Bune, 2014). This cross-cultural prevalence raises the possibility that in addition to psychosocial stressors, a component of HIV infection may trigger anxiety symptoms.
Unfortunately, data on the efficacy of anxiolytic medications in HIV subjects are still inconclusive. This is partly due to the potential harmful drug interaction with antiretroviral therapy (Owe-Larsson et al., 2009). The effects of herbal supplements or psycho-spiritual interventions that are commonly used for anxiety in the general population have not been studied [reviewed in (Kemppainen et al., 2013)]. Moreover, past research has mostly focused on documenting associations between HIV and psychological disorders, but very little experimental work has been done to address explanatory processes that may underlie such associations. This is particularly important in view of the fact that anxiety-like behavior is also observed in a mouse model of HIV infection, in which the translational activator of HIV (Tat) is overexpressed (Paris et al., 2014). Thus, there could be a biological basis to explain anxiety in HIV subjects aside from the infection or the knowledge of HIV-related stigma. Revealing the biological causes of anxiety symptoms in HIV subject is vital for identifying effective therapies.
Anxiety, in addition to a wide range of autonomic and somatic symptoms, can also manifest with a variety of psychological problems including fear, impaired concentration and memory. The amygdala is a core component of neural circuits that mediate processing of emotions, particularly anxiety and fear-related stimuli across species (Becker et al., 2012; Rogan et al., 1997). Alterations in amygdala responses are found in neuropsychiatric disorders, especially those precipitated or sustained by stressors, such as anxiety disorders (Bickart et al., 2011). Increased amygdala size and a correlation between the volume of amygdala, hyperactivity and anxiety behavior has also been reported in HIV positive individuals (Ances et al., 2012; Clark et al., 2012). However, little is know about the biological substrate for these effects. Thus, in this study, we have tested whether amygdala function and plasticity are impaired in an animal model of HIV neurotoxicity. We have used mice that overexpress the HIV envelope protein gp120 in astrocytes and exhibit synaptic simplification in the cortex and hippocampus (Toggas et al., 1994). Moreover, these animals display an impaired hypothalamic-pituitary adrenal (HPA) axis (Raber et al., 1996). We report an anxiety behavior in gp120 transgenic (tg) mice which correlates with increased synaptic spines as well as levels of neurotrophic factors in the amygdala.
2. Materials and Methods
2.1. Animals
A breeding colony of gp120tg mice were obtained from the University of California San Diego, CA. These mice have been previously characterized for their expression of gp120 under a glial fibrillary acid protein promoter (Toggas et al., 1994) and their deficiency in long-term potentiation (Krucker et al., 1998). Mice were bred and maintained in a temperature-controlled vivarium at Georgetown University Medical Center, on a standard 12h light-dark cycle (lights on 0600–1800h), to generate up to 12 months old gp120tg as well as wild type (WT) littermates. Food and water were available ad libitum. Both male and females were used for these studies. Pain and distress (e.g. weight loss, self-mutilation, decreased food and water consumption, abdominal breathing, abnormal posture, vocalization, ruffled fur) were monitored by research staff and veterinarians. None of these signs were present at any time. Prior each test, mice were transported from the vivarium to the testing rooms and allowed to acclimate for one hour before testing commenced. 24 hr after the conclusion of behavioral studies, mice were euthanized either by intracardial exsanguination for histological analysis of spines or cervical dislocation for biochemical determinations (see below). All studies were carried out following the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health and approved by Georgetown University Animal Care and Use Committee. Efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Light-dark test
The light box apparatus consisted of a standard mouse cage (18 cm × 28 cm) with an insert dividing the apparatus into two chambers: one light and one dark, which were connected by a small opening (6 × 6 cm). At the start of the five-minute test, animals were placed in the light box, next to entrance to the dark box. The time spent in the light versus the dark box as well as the number of entries into the light box versus the dark box were tracked in real time using ANYmaze software (Stoelting Co, Wood Dale, IL). Average duration of visits to the light zone was also examined as a measure of anxiety, as it integrates across both total entries and time in the light zone, providing a more sensitive measure than either parameter alone. A light box visit was recorded when the mouse moved at least half of its body into the light box.
2.3. Open field
Animals were placed in an enclosure (37 cm × 30.5 cm) with 770-lux illumination over the center of the arena. Animals were allowed to explore for 60 min, during which total distance traveled, number of entries into the center zone, and time spent in the center zone were recorded by using ANYmaze software.
2.4. Prepulse Inhibition of the acoustic startle reflex (PPI)
Mice were tested for PPI using SR-LAB system (San Diego Instruments, CA), as previously described (Forcelli et al., 2012). 70dB background noise was present throughout the test session, which consisted of “pulse alone” trials (presentation of a 120 dB, 30 msec broadband noise burst) and “prepulse” trials (prepulse of 3, 6, or 9 dB presented 130msec prior to the onset of the 120dB pulse). 5 pulse alone trials were presented to habituate the animal to the testing apparatus, followed by 10 pulse alone trials and 5 pulse trials to each prepulse intensity. Inter-trial intervals ranged from 5–25 s. Startle magnitude was calculated as the average of the startle responses to the pulse-alone trials. PPI was calculated according to the formula: % PPI= [1−(startle response for prepulse + pulse trials/startle response for pulse alone trials)] × 100.
2.5. Golgi-Cox staining and spine count
Brains were impregnated with Golgi-Cox solution (FDNeurotech Inc, Columbia, MD) for two weeks then stained and sectioned (100 μM) according to the manufacturer instructions. Neurons were imaged in the basolateral, medial, and lateral amygdala. Neurons were selected based on the following criteria: 1) being located within the boundaries of the aforementioned areas and relatively distant from the borders; 2) being relatively isolated from the neighboring impregnated cells to avoid “tangled” dendrites; 3) the dendrites should have well-impregnated and defined borders; and 4) the spines should be obviously distinguishable from the background. Because the number of impregnated neurons varied between sections, both sides of the brain were used. Two separate counts were performed dendritic spines on basal shaft dendrites, and dendritic spines on the apical oblique dendrites. Spines in the basal shaft dendrites, which project directly off the cell soma, were counted along a 30 μM section of the shaft between 30–100 μM away from soma. Spines in the primary apical oblique dendrites, which project off the apical dendrite, were counted in a 30 μM section of the primary apical oblique. Spines were counted in a blinded fashion using ImageJ software (National Institutes of Health, Bethesda, MD) and combined. We averaged a total of 110 neurons in each group (n=6 mice each group).
2.6. Enzyme-linked immunosorbent assay (ELISA)
The amygdala was dissected on ice. Levels of brain derived neurotrophic factor (BDNF), tissue plasminogen activator (tPA), interleukin-1β (IL-1β), and tumor necrosis factor α (TNFα) in the amygdala were determined using ELISA according to the manufacturer instructions with minor modifications described elsewhere (Bachis et al., 2012; Bachis et al., 2010). IL-1β, and TNFα DuoSet ELISA kits were from R&D System (Minneapolis, MN); ELISA for BDNF was from Promega Corp., ELISA for tPA was from Molecular Innovations, Inc. (Peary Court, Novi, MI).
2.7. Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc.). Results are presented as mean ± standard error of mean. For a comparison of more than two groups, an ANOVA test, followed by a proper post-hoc test for multiple comparisons, was applied. P values of <0.05 indicate statistical significance.
3. Results
3.1. Gp120 mice exhibit anxiety behavior
Gp120tg mice develop neurodegeneration and display increased numbers of microglia in the cerebral cortex and hippocampus (Toggas et al., 1994), and are, therefore considered an important animal model to study molecular mechanisms of HIV-mediated neurological disorders. These animals exhibit loss of synaptic plasticity when are 6 months old (Krucker et al., 1998; Toggas et al., 1994). Thus, we used 6 months old mice to analyze their behavior impairment connected to anxiety.
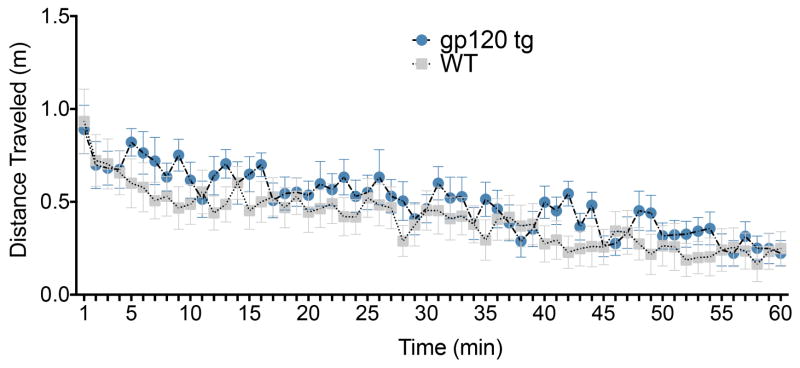
We first characterized the activity of gp120tg mice in the open field. We found that total distance traveled was equivalent between gp120tg and WT mice (Fig. 1). This was revealed by ANOVA which failed to find a main effect of genotype [F(1,28)=0.57, P=0.45], but did find a significant main effect of time since the start of the test [F(59,1652)=13.47, P<0.0001)] and a significant time by genotype interaction [F(59,1652)=1.45, P=0.015]. Post-hoc tests failed to identify any time points where genotypes differed significantly. Thus, overall locomotor activity did not differ between groups.
Figure 1. Locomotor activity is not altered in gp120tg mice.
Gp120tg and age-matched WT were tested in a 60 min open field task. Data show the total distance traveled (m) over the course of the 60 min test in 5 min time bins. Data are presented as the mean ± SEM (gp120tg mice, 7 females, 6 male; WT, 9 females, 8 male).
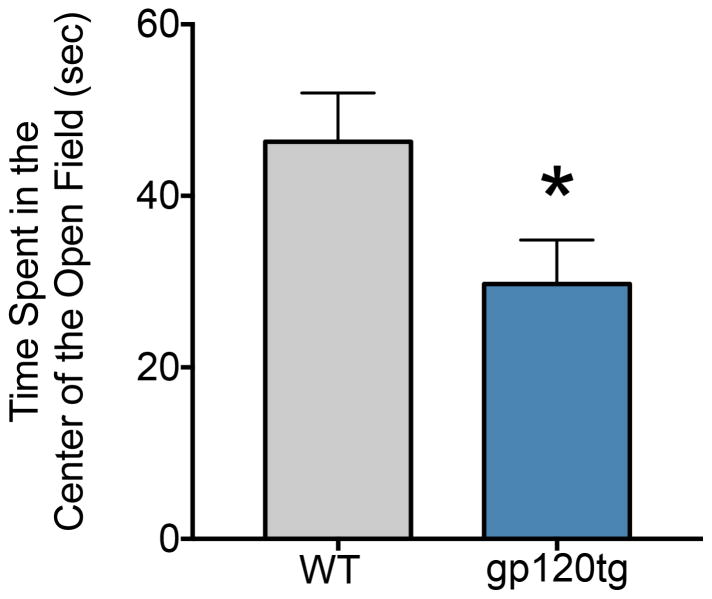
Because our central hypothesis is concerned with the effect of gp120 on anxiety-like behavior, we analyzed the time spent in the center of the open field (unprotected center area) during the first five minutes in the arena. Increased time in the center of the open field is often taken as a measure of less anxiety-like behavior. We found that gp120tg mice (Fig. 2) spent significantly less time in the center of the open field (a mean of 29.7 sec) than WT (46.3; unpaired t-test with Welch’s correction, t=2.16, df=27.9, P=0.039), consistent with an anxiety-like behavior.
Figure 2. Gp120tg mice show increased anxiety-like behavior in the open field.
Animals tested for locomotor activity were also recorded for the time spent in the center of the open field, using ANYmaze software. Data, expressed as mean ± SEM, show that gp120tg mice spent significantly less time in the center of the open field than did WT mice. *p<0.05 vs WT (t-test).
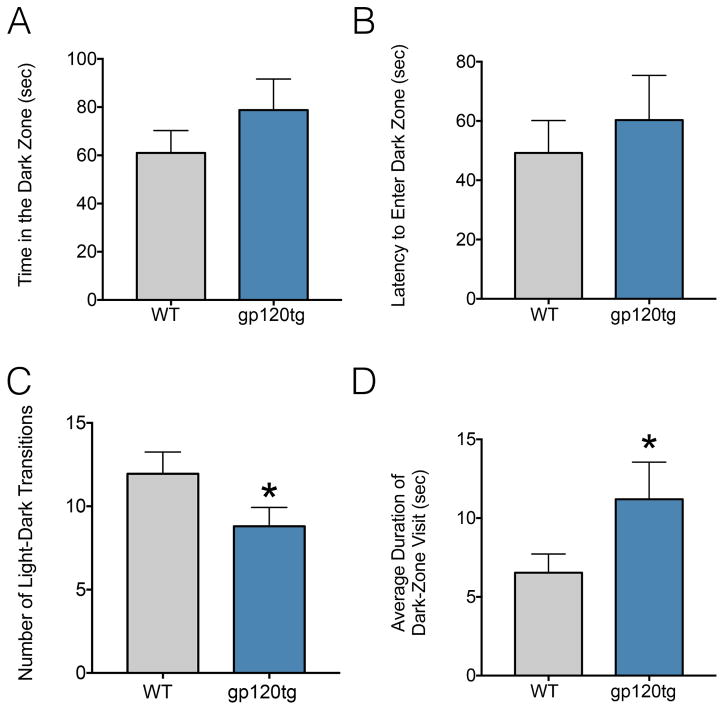
To further assess anxiety-like behavior in gp120tg mice, we turned to the light-dark transition task. Increased time spent in the brightly lit side of the apparatus is taken as an indicator of less anxiety-like behavior. The time spent in the dark side of the apparatus (Fig. 3A) as well as the latency to enter the dark zone (Fig. 3B) did not differ significantly between groups (t=1.1, df=41.39, P=0.27); however, there was a significant decrease in the number of light/dark zone entries (Fig. 3C) made by gp120tg mice (t=1.82, df=40.29, P=0.0375, one-tailed). When we calculated the average duration of a visit to the dark zone (Fig. 3D), we again found a significant increase in dwelling in the dark zone by gp120tg mice (t=1.76, df=33.46, P=0.043, one-tailed). These data are consistent with an increase in anxiety-like behavior in these animals.
Figure 3. Gp120tg mice display increased anxiety-like behavior by the light/dark test.
Gp120tg (10 each gender) and age-matched WT mice (12 females, 13 male) were tested for: (A) Time spent in the dark zone; (B) Latency to enter the dark zone; (C) Number of light dark transitions and (D) average duration of visits to dark zone. Data are expressed by means ± SEM. *p<0.05 vs WT, t-test (one-tailed).
3.2. Gp120 and prepulse inhibition
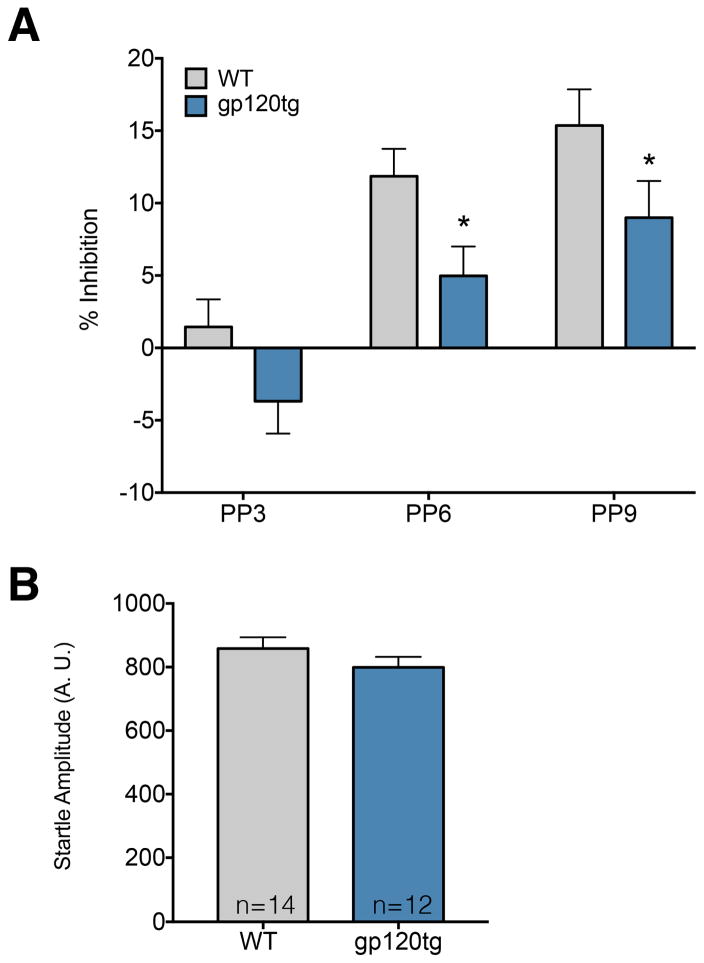
The amygdala, and in particular its basolateral nucleus (BLA), plays a crucial role in processing sensory-motor information (Wan and Swerdlow, 1997). In animals, this process is measured by the prepulse inhibition of startle reflex (PPI). Impairment of the function of the BLA disrupts PPI (Forcelli et al., 2012; Wan and Swerdlow, 1997). Moreover, a previous study has shown PPI deficits in gp120tg mice after methamphetamine treatment (Henry et al., 2014). Thus, we examined PPI in WT and gp120tg mice (Fig. 4). While we hypothesized that gp120tg animals would display enhanced startle, mean startle amplitude in these animals (Fig. 4B) did not differ from WT (t=1.21, df=24, P=0.24). However, with respect to PPI, we found a significant main effect of genotype [F(1,24)=6.84, P=0.015; tg less than WT] and a significant main effect of prepulse intensity [F(2,48)=30.07, P<0.0001] but no genotype-by-prepulse intensity interaction [F(2,48)=0.13, P=0.88]. Holm-Sidak corrected planned comparisons revealed significant (P<0.05, one-tailed) reduction in PPI in gp120tg mice compared to WT mice at prepulse intensities 6 and 9 dB above background (Fig. 4A).
Figure 4. Gp120tg mice exhibit impaired sensorimotor gating, but normal acoustic startle response.
(A) Prepulse inhibition (PPI) of the acoustic startle response as a function of prepulse intensity. Gp120tg (6 each gender) and matched WT (7 each gender) were tested. Data are the mean ± SEM. Gp120tg mice showed decreased PPI at prepulses 6 and 9 dB above background. *p<0.05 (Holm-Sidak post-hoc test, one-tailed). (B) Acoustic startle (in arbitrary units) was unaffected by genotype.
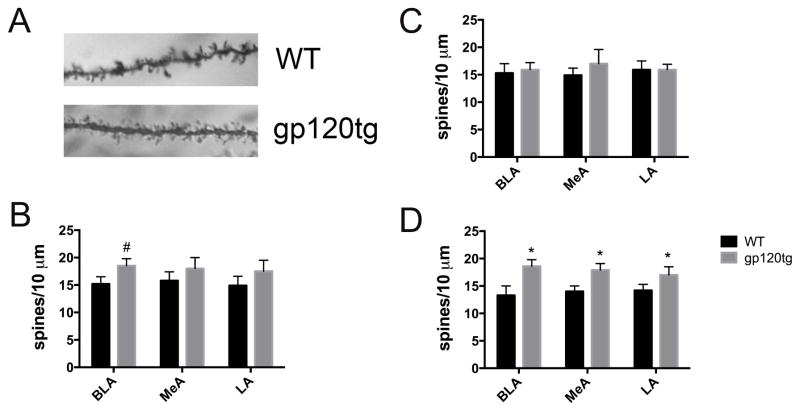
3.3. Dendritic spines in the amygdala are increased in gp120tg mice
The BLA participates in the storage for memories of fearful experience and it is involved in long-term consolidation of emotional memories (Gale et al., 2004; Schafe et al., 2001). Hyperactivation of the amygdala in anxiety has been show to be associated with increased spine count and dendritic arborization (Mitra et al., 2005). Therefore, we used Golgi staining to measure the number of spines in WT and gp120tg mice (Fig. 5A). The analysis was carried out in sections containing various amygdala nuclei, including the BLA, medial (MeA), and lateral amygdala (LA). There was a significant increase in the number of spines in the BLA in 6 months old gp120tg mice when compared to WT mice (Fig. 5B). No changes were observed in the MeA and LA (Fig. 5B).
Figure 5. Spine density is increased in the amygdala of old gp120tg mice.
A. Photomicrograph of a Golgi stain-impregnated apical dendritic process in the BLA from WT and gp120tg mice. B–D. Spine density was determined by counting both basal shaft dendrites as well as apical oblique dendrites (see also Materials and Methods) in 6–7 (B), 3–4 (C) and 9–12 (D) months old mice. Data are the mean ± SEM (average number of spines per 10 μm) from a total of 110 neurons in each group (n=6 mice each group, 3 each gender). #p<0.05, *p<0.01 vs WT.
3.4. Dendritic spine increases are age-dependent
Aging appears to be an independent risk factor in the development of neurocognitive alterations in HIV positive subjects (Canizares et al., 2014). In addition, as gp120tg animals get older, they also display more synaptic abnormalities (Toggas et al., 1994) as well as production of neurotoxic processes (Bachis et al., submitted). To determine if the change in the number of spines was more prominent in aged mice, we euthanized young (3–4 months) and old (10–12 months) WT and gp120tg mice to count dendritic spines in several areas of the amygdala. No significant changes between WT and gp120tg mice were seen in any nuclei of the amygdala in young animals (Fig. 5C). In 10–12 months old WT mice, the number of spines was lower than young WT mice in several nuclei of the amygdala (Figs. 5C and D). However, in gp120tg mice, the number of spines was significantly increased in BLA as well as MeA and LA when compared to age-matched WT (Fig. 5D), suggesting that aging, which per se decreases spines in the amygdala, is exacerbating the effect of gp120.
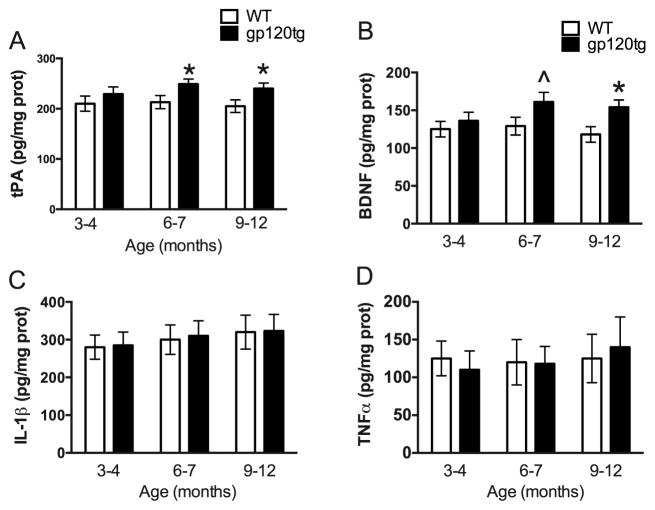
3.5. tPA and BDNF levels are increased in gp120tg mice
There are many mechanisms that could explain why gp120 causes an increase in the number of spines in the amygdala. Pathological anxiety-like behavior overlaps with an increase in the expression of tissue plasminogen activator (tPA) (Pawlak et al., 2003), a serine protease that plays a role in synaptic plasticity and spine morphology (Baranes et al., 1998; Li et al., 2004; Pang et al., 2004). Therefore, we measured tPA levels in the amygdala of gp120tg and WT mice. We found an aged-dependent increase in tPA levels in gp120tg animals when compared to WT, starting at 6–7 months (Fig. 6A).
Figure 6. gp120 increases tPA and BDNF levels in the amygdala.
Tissue lysates from the amygdala of gp120tg and age-matched WT were prepared. ELISAs were then used to determine the levels of tPA (A), BDNF (B) IL-1β (C) and TNFα (D). Data are the mean ± SEM of 6 mice per each group (3 per gender). ^p<0.05, *p<0.01 vs WT.
Evidence indicates that anxiety, which triggers spinogenesis in the amygdala (Mitra et al., 2005), promotes also the synthesis of BDNF (Lakshminarasimhan and Chattarji, 2012). Overexpression of BDNF has been shown to cause spinogenesis in the BLA (Govindarajan et al., 2006). Moreover, BDNF is up-regulated in the amygdala of stressed animals (Lakshminarasimhan and Chattarji, 2012). Thus, in an attempt to provide a correlation between increased spines and BDNF, we determined the levels of BDNF in WT and gp120tg mice. We found that 6 months old and older gp120tg mice exhibit higher levels of BDNF when compared to WT controls (Fig. 6B).
Microglia activation has been observed in the hippocampus and cortex of gp120tg mice (Toggas et al., 1994), suggesting that gp120 may promote an inflammatory response. To ascertain whether the increase in tPA and BDNF reflects a generalized augmentation of the inflammatory response, we measured the levels of pro-inflammatory cytokines IL-1β (Fig. 6C) and TNFα (Fig. 6D) in the amygdala. We found no significant changes in either IL-1β or TNFα levels between WT and gp120tg mice at any age examined.
4. Discussion
Anxiety symptoms are commonly seen in HIV-infected individuals (Kemppainen et al., 2013). Nevertheless, little is know about the biological basis of anxiety disorders in HIV patients. Anxiety and stress, either acute or chronic, are the prime factors, which have experimentally and clinically been shown to modulate amygdala reactivity. To test experimentally whether the amygdala architecture could be compromised in HIV subjects with neurological impairment, we used mice overexpressing the viral protein gp120. These mice exhibit several cognitive and motor features of HIV dementia (Krucker et al., 1998; Toggas et al., 1994), as well as impaired HPA (Raber et al., 1996), frequently seen in HIV positive subjects (George and Bhangoo, 2013; Vago et al., 1994). Here we report that these mice exhibit a behavior consistent with anxiety-like syndrome, when they are six months old. The amygdala of these animals is characterized by an increased number of dendritic spines, suggesting spinogenesis and synaptic remodeling. Spinogenesis correlated with elevated levels of BDNF and tPA, two key players in structural plasticity as well as dendritic and spine architecture (Baranes et al., 1998; Govindarajan et al., 2006). Our data suggest that long-term exposure to gp120 may promote an environment that is conducive for morphological plasticity of dendrites that underlies increased anxiety behavior. Thus, gp120 could also be a key factor in the progression of HIV-induced anxiety.
We have found that gp120tg mice exhibit anxiety-related behavior, demonstrated by using several behavior tests. For instance, the open field test, which allows for an overall measure of exploratory drive, showed that gp120tg mice explore less the center of the arena. Moreover, they tend to prefer the dark zone as reflected in a decrease in the number of transitions between the light and dark compartment and increased visit duration to the dark zone. Although the data were obtained in an animal model of HIV infection of the brain, these findings have a clinical relevance. In fact, the incidence of generalized anxiety and panic disorder is substantially greater in HIV positive individuals compared to those that are negative (Atkinson et al., 2008; Bing et al., 2001), or subjects suffering from mood disorders comorbid with other terminal illnesses, such as cancer (Massie, 2004).
We also observed deficits in prepulse inhibition in gp120tg mice. This pattern is consistent with a prior report (Henry et al., 2014) showing PPI deficits in female, but not male, gp120 mice; these gender-related changes in PPI could be due to the fact that PPI varies according to the estrus cycle (Koch, 1998). In the present study, although we were not powered to examine sex differences, we have found that even with mixed sex groups, gp120 alters PPI. These data are also consistent with a report examining PPI in HIV-infected individuals, which found significant PPI impairments in HIV+ individuals with HAND, as compared to HIV+ individuals without HAND (Minassian et al., 2013). This finding further underscores the relevance of the gp120 mouse model as a tool for examining the neurobiology and neuropathology of HIV infection.
Changes in anxiety status of HIV subjects may be provoked by psychosocial factors as well as biological mechanisms. In this study, we show that the amygdala of gp120tg mice with an anxiety behavior undergoes dendritic spine remodeling. The increase in the density of spines in the amygdala observed in this study is in contrast with a reduction of dendritic spines that we have previously detected in the hippocampus of these animals (Bachis et al., submitted). However, such discrepancy is not unusual because it has been demonstrated that the hippocampus and amygdala respond differently to anxiety caused by chronic stress. In fact, while in chronically stressed rats hippocampal pyramidal neurons exhibit shorter dendritic length and overall atrophy, pyramidal neurons of the amygdala, and in particular in the BLA, undergo morphological changes consistent with increased dendritic arborization (Mitra et al., 2005; Vyas et al., 2002). There are several explanations for these contrasting effects, and among others, the disparate role of the hippocampus and amygdala in the control of stress and the HPA axis and in their expression profile of genes (Alfonso et al., 2005). In addition, we cannot ignore the role that the HPA axis and glucocorticoids play in synaptic remodeling (McEwen, 2007). Gp120tg mice exhibit impaired HPA axis and an increase in glucocorticoid levels (Raber et al., 1996). Thus, glucocorticoids, which have been shown to exacerbate the toxic effect of gp120 in the hippocampus (Yusim et al., 2000), could be responsible for the atrophy of neurons in this brain region. Chronic glucocorticoid elevation induces dendritic atrophy in hippocampus (Wood et al., 2004), which in turn reduces negative feedback of ACTH and glucocorticoid secretion prolonging the stress response. By contrast, glucocorticoid elevation is sufficient to induce dendritic hypertrophy in the amygdala (Mitra and Sapolsky, 2008).
Another important result presented in our study is the up-regulation of BDNF in six month old or older gp120tg mice. BDNF has been shown to be important in the plastic effect on dendrite growth and spine morphology (Chapleau and Pozzo-Miller, 2012; Horch, 2004). BDNF has both anxiogenic as well as antidepressant properties (Govindarajan et al., 2006). Moreover, BDNF reverses the impaired neurite outgrowth described in gp120tg mice (Lee et al., 2013). Thus, we were not surprised to discover that spinogenesis in the amygdala was associated with an increase in BDNF levels. However, our data seems to be at odds with earlier study showing that gp120 reduces BDNF expression in the hippocampus of gp120tg mice (Bachis et al., submitted). Moreover, gp120 evokes a loss of BDNF expression both in neurons in vitro (Bachis et al., 2012) as well as in the caudate nucleus in vivo (Nosheny et al., 2004). The decrease in BDNF levels is not an artifact of the rodent model because a reduction in BDNF levels in the caudate nucleus and frontal cortex has been also demonstrated in HIV subjects with dementia (Bachis et al., 2012). Thus, the increase in BDNF levels in the amygdala observed in this study appears to be uniquely associated with a possible activation of a neuronal circuitry within the amygdala.
Elevated levels of BDNF may be causing an increase in spine number. However, in addition to BDNF, six months old or older gp120tg mice present elevated levels of tPA. Such increase resembles that observed within the amygdala in rodents after prolonged stress (Pawlak et al., 2003), which is know to trigger pathological anxiety like behavior, and to cause neuronal remodeling within the amygdala (Vyas et al., 2002). Moreover, glucocorticoid signaling (which is elevated in gp120 mice) triggers increases in tPA mRNA (Medcalf et al., 1988). tPA could be facilitating dendrite/axonal plasticity by a number of mechanisms. For instance, tPA could potentiate N-methyl-d-aspartate (NMDA) receptor signaling (Nicole et al., 2001), which is know to facilitate spinogenesis (Liu et al., 2012). In addition, tPA is a serine endopeptidase that, upon release into the synaptic cleft, activates the protease plasmin, which cleaves the high molecular weight precursor proBDNF to mature BDNF (Mowla et al., 1999). proBDNF has the opposite effects of the mature BDNF. These include neuronal apoptosis (Lee et al., 2001), axonal degeneration in the developing as well as mature nervous system (Kaplan and Miller, 2003; Park et al., 2010; Singh et al., 2008), presynaptic terminal retraction (Yang et al., 2009) and long term depression (Woo et al., 2005). Thus, tPA by promoting cleavage of proBDNF into mature BDNF, may induce spinogenesis. This observation suggests that both BDNF and tPA are critical for the effect of spine number observed in our study.
4.1. Conclusions
Morphometric and neurocognitive studies have revealed significant changes in the amygdala of HIV positive subjects (Clark et al., 2012). Moreover, HAND subjects demonstrate severe PPI compared to HIV+ individuals with no cognitive alterations (Minassian et al., 2013). In this study, we have attempted to provide the biological basis for the mechanisms underlying this anxiety-induced neuronal plasticity, and we have observed a significant increase in dendrite spines in the BLA of gp120tg mice, associated with an anxiety-like behavior. Both are consistent with the notion that the amygdala of these animals is undergoing structural plasticity. These data underscore the importance of future longitudinal examination of anxiety-like behavior in these mice. Moreover, they validate an animal model that may be used to screen the efficacy of therapeutic interventions for anxiety symptoms associated with HIV infection.
gp120 transgenic mice develop anxiety-like behavior when they are 6 months old.
Anxiety correlates with more spines in the amygdala.
spinogenesis occurs concomitantly with an increased expression of tPA and BDNF
Acknowledgments
This work was supported by HHS grants NS079172 and NS074916.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfonso J, Frasch AC, Flugge G. Chronic stress, depression and antidepressants: effects on gene transcription in the hippocampus. Rev Neurosci. 2005;16:43–56. doi: 10.1515/revneuro.2005.16.1.43. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59:469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JH, Heaton RK, Patterson TL, Wolfson T, Deutsch R, Brown SJ, Summers J, Sciolla A, Gutierrez R, Ellis RJ, Abramson I, Hesselink JR, McCutchan JA, Grant I, Group H. Two-year prospective study of major depressive disorder in HIV-infected men. J Affect Disord. 2008;108:225–234. doi: 10.1016/j.jad.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci. 2012;32:9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Boelk A, Tessarollo L, Mocchetti I. The neurotrophin receptor p75 mediates gp120-induced loss of synaptic spines in aging mice. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2016.07.001. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Cruz MI, Mocchetti I. M-tropic HIV envelope protein gp120 exhibits a different neuropathological profile than T-tropic gp120 in rat striatum. Eur J Neurosci. 2010;32:570–578. doi: 10.1111/j.1460-9568.2010.07325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- Becker B, Mihov Y, Scheele D, Kendrick KM, Feinstein JS, Matusch A, Aydin M, Reich H, Urbach H, Oros-Peusquens AM, Shah NJ, Kunz WS, Schlaepfer TE, Zilles K, Maier W, Hurlemann R. Fear processing and social networking in the absence of a functional amygdala. Biol Psychiatry. 2012;72:70–77. doi: 10.1016/j.biopsych.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, MABDL, et al. Psychiatric disorders and drug use among human immunodeficiency virus–infected adults in the united states. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Canizares S, Cherner M, Ellis RJ. HIV and aging: effects on the central nervous system. Semin Neurol. 2014;34:27–34. doi: 10.1055/s-0034-1372340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW. Elevated Suicide Rate Among HIV-Positive Persons Despite Benefits of Antiretroviral Therapy: Implications for a Stress and Coping Model of Suicide. American Journal of Psychiatry. 2010;167:117–119. doi: 10.1176/appi.ajp.2009.09111565. [DOI] [PubMed] [Google Scholar]
- Chan P, Brew B. HIV Associated Neurocognitive Disorders in the Modern Antiviral Treatment Era: Prevalence, Characteristics, Biomarkers, and Effects of Treatment. Current HIV/AIDS Reports. 2014;11:317–324. doi: 10.1007/s11904-014-0221-0. [DOI] [PubMed] [Google Scholar]
- Chapleau CA, Pozzo-Miller L. Divergent roles of p75NTR and Trk receptors in BDNF’s effects on dendritic spine density and morphology. Neural Plast. 2012;2012:578057. doi: 10.1155/2012/578057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Cohen RA, Sweet LH, Gongvatana A, Devlin KN, Hana GN, Westbrook ML, Mulligan RC, Jerskey BA, White TL, Navia B, Tashima KT. Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. J Int Neuropsychol Soc. 2012;18:657–668. doi: 10.1017/S1355617712000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Forcelli PA, West EA, Murnen AT, Malkova L. Ventral pallidum mediates amygdala-evoked deficits in prepulse inhibition. Behav Neurosci. 2012;126:290–300. doi: 10.1037/a0026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MM, Bhangoo A. Human immune deficiency virus (HIV) infection and the hypothalamic pituitary adrenal axis. Rev Endocr Metab Disord. 2013;14:105–112. doi: 10.1007/s11154-013-9244-x. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Geyer MA, Buell MR, Perry W, Young JW, Minassian A Translational Methamphetamine ARCG. Prepulse inhibition in HIV-1 gp120 transgenic mice after withdrawal from chronic methamphetamine. Behav Pharmacol. 2014;25:12–22. doi: 10.1097/FBP.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch HW. Local effects of BDNF on dendritic growth. Rev Neurosci. 2004;15:117–129. doi: 10.1515/revneuro.2004.15.2.117. [DOI] [PubMed] [Google Scholar]
- Hult JR, Wrubel J, Branstrom R, Acree M, Moskowitz JT. Disclosure and nondisclosure among people newly diagnosed with HIV: an analysis from a stress and coping perspective. AIDS Patient Care STDS. 2012;26:181–190. doi: 10.1089/apc.2011.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Axon growth inhibition: signals from the p75 neurotrophin receptor. Nat Neurosci. 2003;6:435–436. doi: 10.1038/nn0503-435. [DOI] [PubMed] [Google Scholar]
- Kee MK, Lee SY, Kim NY, Lee JS, Kim JM, Choi JY, Ku NS, Kang MW, Kim MJ, Woo JH, Kim SW, Song JY, Baek JH, Choi BY, Kim SS. Anxiety and depressive symptoms among patients infected with human immunodeficiency virus in South Korea. AIDS Care. 2015;27:1174–1182. doi: 10.1080/09540121.2015.1035861. [DOI] [PubMed] [Google Scholar]
- Kemppainen JK, MacKain S, Reyes D. Anxiety symptoms in HIV-infected individuals. J Assoc Nurses AIDS Care. 2013;24:S29–39. doi: 10.1016/j.jana.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiol Behav. 1998;64:625–628. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Krucker T, Toggas SM, Mucke L, Siggins GR. Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience. 1998;83:691–700. doi: 10.1016/s0306-4522(97)00413-2. [DOI] [PubMed] [Google Scholar]
- Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One. 2012;7:e30481. doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, Nath A. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neurovirol. 2013;19:418–431. doi: 10.1007/s13365-013-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lewis-Fernandez R, Hinton DE, Laria AJ, Patterson EH, Hofmann SG, Craske MG, Stein DJ, Asnaani A, Liao B. Culture and the anxiety disorders: recommendations for DSM-V. Depress Anxiety. 2010;27:212–229. doi: 10.1002/da.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting Activation of AgRP Neurons Requires NMDA Receptors and Involves Spinogenesis and Increased Excitatory Tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Medcalf RL, Van den Berg E, Schleuning WD. Glucocorticoid-modulated gene expression of tissue- and urinary-type plasminogen activator and plasminogen activator inhibitor 1 and 2. J Cell Biol. 1988;106:971–978. doi: 10.1083/jcb.106.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, Perry W Translational Methamphetamine ARCG. Prepulse inhibition in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc. 2013;19:709–717. doi: 10.1017/S1355617713000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS, Murphy RA. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutumba M, Resnicow K, Bauermeister JA, Harper GW, Musiime V, Snow RC, Lepkowski JM. Development of a psychosocial distress measure for Ugandan adolescents living with HIV. AIDS Behav. 2015;19:380–392. doi: 10.1007/s10461-014-0973-y. [DOI] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Olley BO, Zeier MD, Seedat S, Stein DJ. Post-traumatic stress disorder among recently diagnosed patients with HIV/AIDS in South Africa. AIDS Care. 2005;17:550–557. doi: 10.1080/09540120412331319741. [DOI] [PubMed] [Google Scholar]
- Owe-Larsson B, Sall L, Salamon E, Allgulander C. HIV infection and psychiatric illness. Afr J Psychiatry (Johannesbg) 2009;12:115–128. doi: 10.4314/ajpsy.v12i2.43729. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology (Berl) 2014;231:2349–2360. doi: 10.1007/s00213-013-3385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KJ, Grosso CA, Aubert I, Kaplan DR, Miller FD. p75NTR-dependent, myelin-mediated axonal degeneration regulates neural connectivity in the adult brain. Nat Neurosci. 2010;13:559–566. doi: 10.1038/nn.2513. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- Raber J, Toggas SM, Lee S, Bloom FE, Epstein CJ, Mucke L. Central nervous system expression of HIV-1 Gp120 activates the hypothalamic-pituitary-adrenal axis: evidence for involvement of NMDA receptors and nitric oxide synthase. Virology. 1996;226:362–373. doi: 10.1006/viro.1996.0664. [DOI] [PubMed] [Google Scholar]
- Riley KE, Kalichman S. Mindfulness-based stress reduction for people living with HIV/AIDS: preliminary review of intervention trial methodologies and findings. Health Psychol Rev. 2015;9:224–243. doi: 10.1080/17437199.2014.895928. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11:649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- Tan I, McArthur J. HIV-Associated Neurological Disorders. CNS Drugs. 2012;26:123–134. doi: 10.2165/11597770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Tesfaye SH, Bune GT. Generalized psychological distress among HIV-infected patients enrolled in antiretroviral treatment in Dilla University Hospital, Gedeo zone, Ethiopia. Glob Health Action. 2014;7:23882. doi: 10.3402/gha.v7.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Vago T, Clerici M, Norbiato G. Glucocorticoids and the immune system in AIDS. Baillieres Clin Endocrinol Metab. 1994;8:789–802. doi: 10.1016/s0950-351x(05)80301-5. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR. The basolateral amygdala regulates sensorimotor gating of acoustic startle in the rat. Neuroscience. 1997;76:715–724. doi: 10.1016/s0306-4522(96)00218-7. [DOI] [PubMed] [Google Scholar]
- Weaver KE, Llabre MM, Duran RE, Antoni MH, Ironson G, Penedo FJ, Schneiderman N. A stress and coping model of medication adherence and viral load in HIV-positive men and women on highly active antiretroviral therapy (HAART) Health Psychol. 2005;24:385–392. doi: 10.1037/0278-6133.24.4.385. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci U S A. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Je HS, Ji Y, Nagappan G, Hempstead B, Lu B. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J Cell Biol. 2009;185:727–741. doi: 10.1083/jcb.200811147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusim A, Franklin L, Brooke S, Ajilore O, Sapolsky R. Glucocorticoids exacerbate the deleterious effects of gp120 in hippocampal and cortical explants. J Neurochem. 2000;74:1000–1007. doi: 10.1046/j.1471-4159.2000.0741000.x. [DOI] [PubMed] [Google Scholar]