Abstract
Background
Both self-rated health (SRH) and inflammation are implicated in chronic diseases and premature mortality. Better SRH is associated with lower proinflammatory cytokines, but there is little evidence about whether this relationship is more stable or dynamic.
Objective
To study the between- and within-person associations between SRH and IL-6.
Methods
Older adults (N = 131; Mage = 75 years) rated their health and provided blood samples for analysis of IL-6 at separate occasions every 6 months over a period up to 5 years. Age, sex, BMI, neuroticism, and statin use were examined as covariates in multilevel models.
Results
In bivariate models, better SRH, lower BMI, younger age, and female sex correlated with lower IL-6. In multilevel models, stable SRH (between-person differences; p < .001) but not dynamic SRH (within-person changes; p = .93) correlated with IL-6. The stable relationship persisted with demographic and health covariates in the model.
Conclusions
Better stable SRH but not dynamic SRH was robustly associated with lower IL-6 among older adults, lending support to previous cross-sectional findings on the relation between inflammatory markers and SRH. The findings suggest that trait-like mechanisms, rather than changes over a time scale of 6-month waves, govern this association. To further investigate the mechanisms behind the SRH–IL-6 association, studies with different measurement frequencies, higher within-person variability, and experimental approaches are warranted.
Keywords: Self-rated health, interleukin-6, cytokines, longitudinal study
1. Introduction
Self-rated health (SRH) predicts future objective health risks and summarizes health information in a way that goes beyond the biomedical model (Ganna & Ingelsson, 2015; Idler & Benyamini, 1997). It is not yet understood how this one subjective rating can explain outcomes such as cardiovascular events and mortality even after accounting for other risk factors. Inflammation is also implicated in premature mortality (Baune, Rothermundt, Ladwig, Meisinger, & Berger, 2011; Volpato et al., 2001) and may be a key biological corollary of SRH. Inflammation induces sickness behavior, including behavioral withdrawal, nonspecific symptoms of weakness, listlessness, changed sleep patterns, hyperalgesia and decreases in motivation and appetite (Dantzer & Kelley, 2007). These symptoms may affect subjective appraisals of health, even among generally healthy adults.
Indeed, low-grade inflammation as measured by elevated levels of pro-inflammatory cytokines, especially interleukin-6 (IL-6), correlates with poorer SRH (Andreasson et al., 2013; Cohen, Pieper, Harris, Rao, & Currie, 1997; Janszky, Lekander, Blom, Georgiades, & Ahnve, 2005; Lekander, Elofsson, Neve, Hansson, & Undén, 2004). However, these cross-sectional findings cannot distinguish to what extent changes in SRH and inflammation are correlated within individuals over time. Some authors have stressed the importance of changes in SRH (Gerber, Benyamini, Goldbourt, Drory, & Israel Study Group on First Acute Myocardial Infarction, 2009; Lekander et al., 2013; Lyyra, Leskinen, Jylha, & Heikkinen, 2009). For example, prediction of mortality from SRH improved when changes in health ratings were included (Gerber and et al., 2009). Because factors that co-vary with subjective health, such as disease, energy, sleep, and cytokines, are not stable over time (Jylhä, 2009; Lekander et al., 2013; Lekander et al., 2004), it is reasonable to assume that health is actively appraised in a responsive and dynamic manner and therefore follows changes in its presumed determinants. Short-term changes in symptoms and affect were related to SRH in a group of older adults (Winter, Lawton, Langston, Ruckdeschel, & Sando, 2007), and within-person changes in affect were likewise associated with changes in SRH in the present sample of older adults (Segerstrom, 2014). Similarly, experimental sleep restriction to 4h/night for five nights caused gradually poorer SRH in healthy young adults (Lekander et al., 2013). However, that study assessed current rather than general SRH. Studies of the link between changes in inflammation and SRH over time are generally lacking: The sole cross-sectional study found that retrospective perceived change in health in the past year was unrelated to IL-6 and did not influence the significant cross-sectional relationship between current SRH and IL-6 (Christian et al., 2011).
Longitudinal research can elucidate the nature of relationships between SRH and inflammatory markers in ways that cross-sectional research cannot (Ryu, West, & Sousa, 2012). Ryu and colleagues (2012) point out that in longitudinal health research, often “each person’s mean level and the fluctuations from the mean (chronic) level are the important data of interest” (p. 330), a distinction that can only be made when people are measured repeatedly over time. SRH and inflammation are likely to be related over a very long time course (e.g., over years as a consequence of aging), creating relationships that emerge as between-person differences in studies with shorter time frames as well as cross-sectional designs. They also appear to be related over a short time course, e.g., within hours to days as a consequence of sleep restriction (Lekander et al., 2013) or injected endotoxins (Lekander et al., 2012; Lidberg et al., 2013). However, no studies to date have examined natural covariation between them over intermediate time frames (e.g., over months).
1.1 The present study
In the present study, we applied this longitudinal framework of stable mean levels and dynamic fluctuations to potential associations between SRH and inflammation in a sample of healthy older adults. The association between SRH and inflammatory cytokines is thought to be stronger with advancing age: partly because lower levels of inflammatory cytokines in younger adults (Knudsen et al., 2008) restrict the range and limit the ability to test relationships, and also perhaps due to an increased sensitivity to these cytokines with age (Unden et al., 2007). Studying older adults thus provides an excellent research model. Among proinflammatory cytokines, IL-6 is a suitable target as it is, as noted above, often related to SRH in cross-sectional studies, distributed in detectable ranges, and increases with age. We hypothesized that worse SRH would correlate with higher levels of the inflammatory marker IL-6 both between people and within people over time, reflecting relationships at the levels of (1) stable individual differences that emerge over very long time frames and (2) dynamic relationships that emerge as people change over shorter time frames. Additional sensitivity analyses assessed the roles of demographic and health covariates (age, sex, statin use, and BMI) and blood sample timing relative to SRH assessment. Finally, negative dispositional factors such as neuroticism are linked with inflammatory markers (Marsland, Prather, Petersen, Cohen, & Manuck, 2008; Roy et al., 2010) and may confound a potential association between SRH and inflammatory cytokines. Therefore, neuroticism was also included among the covariates.
2. Methods
2.1 Participants
Study participants were 131 community-dwelling, married older adults over the age of 60 (Mage = 74 years; range: 60–93 at study entry). No dyads were included in the sample to avoid dyadic dependencies in the data. Consistent with the sex ratio in older age, 41% of the sample was male, and 59% was female. The majority of the sample was White (96%), and the remainder was African American (4%). Median annual household income was $57,000 (range: $12,000– $400,000), and median education was 16 years (range: 7–22).
Exclusion criteria at enrollment included self-reported (a) diseases or disorders affecting the immune system, (b) chemotherapy or radiation treatment within the past 5 years, (c) unwillingness to undergo vaccination or venipuncture, (d) immunomodulatory medications including opiates and steroids, and (e) more than two of the following classes of medications: psychotropics, antihypertensives, hormone replacement, or thyroid supplements. Based on the clinician’s judgement at screening and subsequent neuropsychological assessment, all participants were cognitively able to respond to questionnaires.
2.2 Procedure
Study participants were recruited from a volunteer subject pool maintained by the Sanders–Brown Center on Aging at the University of Kentucky. Prospective participants were contacted and screened by telephone. Those who were interested and eligible were enrolled and completed questionnaire measures verbally with the assistance of a research assistant and response cards. These interviews were undertaken at 6-month intervals over a period of up to 10 waves (5 years). Participants received a $20 gift card at each wave completed. Informed consent was obtained at the first interview, and all study procedures were approved by the University of Kentucky Institutional Review Board.
Blood samples were drawn in spring and fall. For the purposes of this study, we selected participants who had provided at least one valid IL-6 sample at any wave. In addition, twelve observations were excluded for elevated IL-6 values (range = 89-–2048 pg/mL) due to current/recent sickness. The final sample included 131 out of 150 participants in the parent study. Of these, 131 completed Wave 1, 128 completed Wave 2; 121, Wave 3; 116, Wave 4; 110, Wave 5; 108, Wave 6; 102, Wave 7; 92, Wave 8; 57, Wave 9; and 34, Wave 10. Because some participants enrolled in the study later than others, they completed fewer waves before the end of the study; lower N in wave 8, 9, and 10 are attributable to this mechanism. These missing data are missing completely at random and thus do not bias the parameter estimates (Fitzmaurice, Laird, & Ware, 2011). There were in total 999 observations of SRH and 775 of IL-6 (due to, e.g., missing values due to sickness) that in combination yielded a final sample of 769 observations included for analysis.
2.3 Measures
2.3.1 Demographics
Demographic information was collected at the first interview. Date of birth and interview date were used to calculate exact chronological age at each interview.
2.3.2 Self-rated health
SRH was measured using a single item from the Medical Outcomes Study Health-Related Quality of Life scale (Ware Jr & Sherbourne, 1992). The item reads: “In general, would you say your health is …” with responses excellent, very good, good, fair, poor. The variable was coded for analysis so that higher values represent better SRH.
2.3.3 Interleukin-6
Study nurses drew blood samples in fall and spring. The sample that was drawn closest to the interview was paired with that interview wave. The median interval from interview to blood draw was 40 days (M = 50, SD = 60, range = −86 to 279). Blood samples were not collected in the fasting state.
Blood draw was deferred if the participant was acutely ill. Sera were frozen at −80°C and later thawed for analysis at the University of Kentucky General Clinic Research Center. High-sensitivity ELISA kits (R&D Systems, Minneapolis, MN) were used according to the manufacturer’s specifications. The mean intra-assay coefficient of variance was 1.9% and the mean inter-assay coefficient of variance was 4.5%. Before analysis, IL-6 results were log10 transformed to achieve normality (for log IL-6, skew = 1.09 and kurtosis = 1.77) and Z-transformed to improve interpretation of coefficients.
2.3.4 Personality
At Wave 2, participants completed the NEO Five-Factor Inventory (Costa & McCrae, 1992). Scores for neuroticism (α = .79) were of primary interest and used herein. Neuroticism correlates highly with other measures of negative affectivity such as trait anxiety and depression and captures the general disposition to experience negative mood states including anxiety, depression, and hostility (Costa & McCrae, 1992; Watson & Clark, 1984).
2.3.5 Body mass index
Body mass index (BMI) can be associated with SRH and IL-6 (Christian et al., 2011; Unden et al., 2007). To control for effects of adiposity on outcomes of interest, BMI was calculated (kg/m2) using height and weight reported at Wave 1. The original scale was used for descriptive statistics whereas the variable was log10 transformed before analysis to achieve normality.
2.3.6 Statin use
Statins have anti-inflammatory properties (Jain & Ridker, 2005). At each wave, participants provided a list of current medications. A study nurse coded all medications into classes. Statins were coded as either taken or not taken (1/0) at each wave.
2.4 Data Analysis
Initial data checks indicated that one participant with 8 waves of data was missing BMI and three participants with one wave of data each were missing neuroticism scores. Their missing values were replaced through stochastic regression imputation, Stochastic regression imputation is preferable to simple regression methods in that both methods predict missing values from observed values conditional on predictors whereas stochastic imputation also includes a random residual component in order not to artificially decrease variance which is the case in simple regression imputation (Little & Rubin, 2002). Missing values were thus drawn from a random normal distribution with mean and variance from the other participants’ values predicted by age and sex. There were 739 valid observations for statin use. Statin use was deemed unfit for imputation as a dichotomous variable and so the models that included statin were run on this slightly smaller sample.
Data were analyzed in linear mixed models as outlined by Singer and Willett (2003) using SPSS v22. Syntax for the models is provided in the supplemental online material. These models effectively use all available observations under the assumption that the distribution of the missing values of IL-6 depend on observed values and are similar to the observed data (i.e., missing at random; MAR). Note that most of the missing data are missing completely at random as a consequence of missing data at waves 8-10 and so meet an even stricter standard than MAR for valid inference with missing data. First, an unconditional means model with no predictors was fit to log IL-6 and SRH (Table 2, Model 1). This model provided estimates of the amount of variance due to stable individual differences between people and to dynamic changes within people over time and allowed for calculation of the intraclass correlation (ICC), which is the percent of variance due to stable individual differences.
Table 2.
Parameter estimates and standard errors for bivariate and final multivariate mixed models of predictors for IL-6.
| Model 1 |
Model 2 |
Model 3 |
Mode l 4 |
Model 5 |
Model 6 |
Mode l 7 |
Model 8 |
Model 9 |
||
|---|---|---|---|---|---|---|---|---|---|---|
|
Fixed
effects |
Predictor
units |
|||||||||
| Intercept | 0.035 (0.066) |
0.20 (0.074) |
0.035 (0.067) |
0.20 (0.10) |
−0.36 (0.17) |
0.028 (0.064) |
−0.20 (0.076 ) |
0.033 (0.064) |
−0.26 (0.20) |
|
| Wave | Each 6- month interval |
−0.05 (0.012) |
||||||||
| Neuroticis m |
Each point (possible range = 0.7 −3.6) |
−0.085 (0.13) |
||||||||
| Sex | Reference = female |
−0.29 (0.13) |
−0.22 (0.12) |
|||||||
| Age | Each year at study entry |
0.028 (0.011) |
0.027 (0.011) |
|||||||
| BMI | Each log unit |
2.62 (0.86) |
2.75 (0.86) |
|||||||
| Statin use | Reference = no |
0.16 (0.090 ) |
0.11 (0.087) |
|||||||
| Stable SRH |
Each unit change in scale (e.g., from good to fair) |
−0.29 (0.074) |
−0.21 (0.073) |
|||||||
| Dynamic SRH |
Each unit change in scale (e.g., from good to fair) |
0.006 (0.050) |
−0.004 (0.049) |
|||||||
|
Random
effects |
||||||||||
| Residual stable (between-person) variance |
0.46 (0.071) |
0.44 (0.072) |
0.46 (0.071) |
0.45 (0.069 ) |
0.44 (0.068) |
0.42 (0.067) |
0.46 (0.072 ) |
0.41 (0.064) |
0.35 (0.057) |
|
| Residual dynamic (within-person) variance |
0.54 (0.030) |
0.50a (0.030) |
0.54 (0.030) |
0.54 (0.030 ) |
0.54 (0.030) |
0.54 (0.030) |
0.53 (0.031 ) |
0.53b (0.031) |
0.54 (0.030) |
|
| Change in −2 log likelihoodc |
− 24.46** |
−0.44 | − 4.81* |
−6.13* | − 9.05** |
−2.97 | − 14.65** |
− 34.35** |
Note. Interleukin-6 (IL-6) was log-transformed and standardized to Z-scores before analysis. The models are based on 769 observations except for Model 7 and 9, which include 738 observations and were compared to a Model 1 fit onto these observations (−2 LL = 1854.87). SRH = Self-rated health. BMI = Body Mass Index.
p < .05
p < .001
Variance in the effect of wave was included in the model (estimate = 0.003, SE = 0.002) but was statistically nonsignificant (p = .12)
Variance in the effect of dynamic SRH was included in the model (estimate = 0.01, SE = 0.029) but was statistically nonsignificant (p = .72)
Change in −2 log likelihood compared to Model 1 (−2 LL = 1930.7) when models were refit with maximum likelihood.
Second, an unconditional growth model with only wave as a predictor was fit to estimate the degree to which dynamic changes within people were time-structured (i.e., systematically related to the passage of time) or time-unstructured (i.e., fluctuations not systematically related to time; Ram & Gerstorf, 2009) (Table 2, Model 2). Wave was centered at Wave 1.
Third, bivariate relationships were examined by adding individual time-invariant covariates to the model: Age (centered around the youngest age in the study, 60 years); log BMI (mean-centered); neuroticism (mean-centered); and sex (coded 0 = female, 1 = male) (Table 2, Models 3-6). Statin use (coded 0 = no, 1 = yes) as a time-varying covariate was also included.
Fourth, SRH was added to the model with and without covariates (Table 2, Models 8-9). To clearly specify stable and dynamic effects of SRH on log IL-6, SRH was partitioned into two orthogonal terms (Wang & Maxwell, 2015). The individual’s mean score (centered around the sample mean) reflected stable, between-person relationships between SRH and log IL-6. The individual’s deviation from his or her own mean at each wave reflected dynamic, within-person effects.
Finally, planned sensitivity analyses were performed to evaluate whether the order of, or time interval between, the SRH rating and IL-6 draw influenced their association. To this end, three models were assessed: one in which a dichotomous variable indicated whether SRH was assessed before or after the IL-6 draw at each wave; one that tested a linear effect of the difference in days between SRH assessment and IL-6 draw at each wave; and one in which the quadratic effect of time was tested, which would indicate that the length of the interval regardless whether SRH or IL-6 was measured first contributed to the association.
For each model, each parameter estimate is shown with its standard error. Parameters can be interpreted in the same manner as unstandardized beta weights (i.e., the amount of change expressed in standard deviations of log IL-6 for each unit change in the predictor). The residual (i.e., unpredicted) variance between and within people is reported for each model. Percent change in these estimates between models can be interpreted in the same manner as R2 change. Finally, change in the −2 log likelihood is provided for Models 2-8 compared with Model 1. The models that include statin use were compared to Model 1 fit on all observations with valid statin use data. Statistically significant change in this parameter (which has a chi-squared distribution) indicates statistically significant improvement in the model by inclusion of the model predictors.
3. Results
3.1 SRH and IL-6: Test of stable and dynamic associations
Table 1 summarizes descriptive data and between-subject correlations among study variables at Wave 1. As expected, IL-6 and SRH were inversely associated with each other. The intraclass correlation coefficient (ICC) for log IL-6 was 0.46, suggesting that nearly half of the total outcome variation was due to stable, between-person differences. The ICC for SRH was 0.66 and indicated that two-thirds of the total variation in SRH was due to stable, between-person differences. Expressed differently, the standard deviation of each participant’s SRH across assessments (within-person, between-waves variation) was on average M=0.44 whereas the standard deviation of all participants’ individual mean SRH across waves (between-person variation) was 0.77.
Table 1.
Between-subject bivariate correlations at wave 1 among study variables
| Variable | M (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| 1. Log IL-6 | 0.38 (0.020)a |
|||||||
|
2. Self-rated
health |
3.64 (0.065)a |
−.213* | ||||||
|
3. Statin use (1 =
yes) |
44%b | −.133 | .002 | |||||
| 4. Sex (1 = male) | 59% | −.059 | −.024 | .016 | ||||
| 5. Age at entry | 74.43 (6.02) |
.038 | −.097 | −.042 | −.164 | |||
| 6. Neuroticism | 1.84 (0.52) |
.053 | −.154 | .021 | .112 | −.134 | ||
| 7. BMI | 27.33 (4.94) c |
.191 | −.149 | .200* | .038 | −.255** | .101 | |
|
8. No. of waves
completed |
7.63 (2.48) |
.098 | .105 | .102 | −.291*** | −.203* | .055 | .243** |
N = 131 except for correlations with log IL-6 (n = 95).
Means and SDs represent the intercept and its SE as estimated from linear mixed models; these estimates are more accurate than simple means for unbalanced (e.g., different numbers of observations across people) multilevel designs.
At wave 1; 41% across all person-waves.
Mean and SD for BMI are presented untransformed.
p < .05
p < .01
p < .001
Table 2 shows results for the linear mixed models. Model 2 shows that there was a small average decrease in log IL-6 over time; however, this time-structured effect accounted for only 7% of the within-person variance ([0.54 – 0.50]/0.54). In other words, most of the within-person changes in log IL-6 were fluctuations that were not linearly related to time. Therefore, wave was not included in further models. Models 3 through 7 show that at the univariate level, higher BMI, female gender, and older age at baseline, but not neuroticism or statin use, were associated with higher log IL-6 (Table 2).
Model 8 shows the stable and dynamic relationships between IL-6 and SRH without including covariates. In this model, individual differences in mean SRH across waves were associated with individual differences in log IL-6 in the predicted direction (i.e., better SRH was associated with lower IL-6). SRH accounted for 11% of the stable between-person variance in log IL-6 ([0.46 – 0.41]/0.46). However, dynamic changes – fluctuations from wave to wave in SRH – were unrelated to changes in log IL-6. Exploratory models including quadratic effects of SRH indicated no firm support for a quadratic between-person effect. Although the slope was somewhat steeper from the lowest to middle values on SRH as compared to the decrease from middle to higher SRH values, the quadratic effect was not statistically significant, F = 3.53, p = .062. There was no evidence of a quadratic dynamic effect, F = 0.03, p = .86.
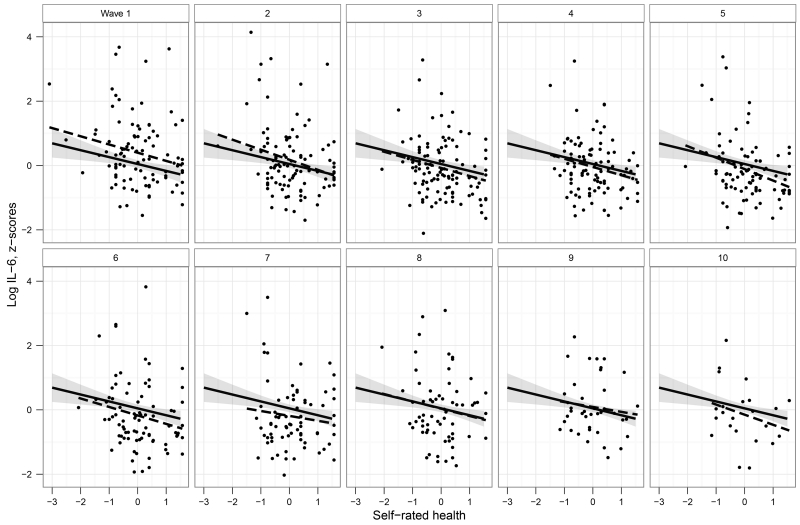
Model 9 shows the effect of adding demographic and health covariates to the model. This final model accounted for 24% of the stable between-person variance and 0% of the dynamic within-person variance in log IL-6. After including covariates, the relationship between SRH and log IL-6 persisted at the stable level with a slightly attenuated magnitude but remained statistically significant. Figure 1 shows the relationship between stable, between-person SRH and log IL-6 across all waves as well as at each individual wave, demonstrating that this relationship was relatively invariant at each wave.
Figure 1. Between-subject differences in self-rated health (SRH, mean centered) and interleukin-6 (IL-6).
SRH-values reflect each participant’s mean SRH rating across all waves. The solid line shows the linear mixed model overall estimate with 95% confidence interval after partialling out sex, age, and log BMI. Ordinary least squares regressions at each wave (dashed lines) are included for comparison purposes.
Additional exploratory models not shown tested interactions between the covariates (age, sex, BMI, neuroticism, statin use) and SRH to explore whether the SRH-IL-6 relationship might vary across levels of these covariates. There were no indications of any interactions either between or within people (.35 < p < .99).
3.2 Sensitivity analyses: Effects of timing of SRH and IL-6 measurement
The presence of a stable, between-person relationship and absence of a dynamic, within-person relationship between SRH and IL-6 suggests that the timing of measurement would make little difference, as the apparent time scale of the association (years to decades) would far exceed the time scale of measurement discrepancies (days to weeks). However, in order to rule out the possibility that measurement timing obscured the dynamic relationship, three models were fit. First, a model predicted regressed IL-6 on SRH, days between SRH assessment and blood draw, and their interactions (one each for stable and dynamic SRH). In this model, there was no significant main effect of timing (F = 0.42, p = .52), nor any moderation of the SRH effects by timing (stable, F = 0.01, p = .91; dynamic, F = 1.62, p = .20). In the second model, days between SRH assessment and blood draw was replaced with quadratic days, potentially showing variations related to time between measurements regardless of order. There was no significant effect of this operationalization of difference (F = 0.52, p = .47) and no moderation of SRH effects (stable, F = 0.11, p = .74; dynamic, F = 0.17, p = .68). In the third model, number of days was replaced with a dichotomous variable (before/after). There was no significant effect of this operationalization of difference (F = 0.48, p = 0.49) and no moderation of SRH effects (stable, F = 0.50, p = .48; dynamic, F = 1.74, p = 0.19).
4. Discussion
This study is the first to our knowledge to investigate the longitudinal association between SRH and a marker of systemic inflammation. This longitudinal design allowed the relationship between SRH and IL-6 to be characterized across two time scales: A very long time scale that results in stable individual differences and a medium time scale that reflects dynamic changes over 6-month waves. There was a robust association between SRH and IL-6 only at the level of stable individual differences, one that was consistent across all 10 waves of assessment and remained after adjusting for demographic and health covariates. There was little evidence that the association between SRH and IL-6 could be accounted for by sex, age, statin use, or BMI. The present data thus support a model in which the SRH–inflammation association has a dominant trait-like component in addition to a relationship over very short time scales (hours to days) that has been illustrated in experimental studies (Lekander et al., 2012; Lekander et al., 2013; Lidberg et al., 2013).
These findings agree with those from cross-sectional studies linking SRH and low-grade systemic inflammation (e.g., Andreasson et al., 2013; Janszky et al., 2005; Lekander et al., 2004; Nakata, Takahashi, Otsuka, & Swanson, 2010). However, the present study extends these previous findings by demonstrating that the between-subject association was similar across up to 10 assessments over five years, which lends further weight to the link between SRH and inflammation. Furthermore, cross-sectional associations can be driven by either stable or momentary relationships; this study indicates that such associations are due primarily to stable relationships. In addition, it adds evidence to the conclusion that the previously demonstrated relationship between SRH and inflammation in patient populations (Lekander et al., 2004; Janszky et al., 2005) and representative population samples (Andreasson et al., 2013) is not an artifact of poor control over disease or medication, since such relations should likely be captured by the medium time scale changes analyses presented in the current study.
The observed association between SRH and inflammation is thus likely to be governed mainly by individual differences that are stable across time. Facets of personality and genes are perhaps the most apparent suggestions. In this sample, higher negative affectivity was related to poorer SRH (Segerstrom, 2014). Some evidence from diverse samples suggests that negative dispositions are also related to higher levels of inflammatory markers (Marsland et al., 2008; Roy et al., 2010). In this sample, however, we found no association between neuroticism and IL-6. The negativity–inflammation association seems to be influenced by lifestyle and sociodemographics (e.g., smoking; Marsland et al., 2008; Roy et al., 2010) but further explorations are needed to understand the conditions that underpin associatinos among affectivity, SRH, and inflammation. For example, health behaviors such as sleep, physical activity, and diet may simultaneously improve perceptions of one’s health and have anti-inflammatory effects. Another viable area for further study is immunomodulatory genes, which may influence factors relevant to health perception, and ultimately, health and mortality. For example, a polymorphism in the human μ-opioid receptor OPRM1 gene is implicated in proinflammatory cytokine levels and general health perception (Matsunaga et al., 2009), as well as in cortisol responses to a psychosocial stressor (Chong et al., 2006).
Contrary to our hypothesis, dynamic variation in SRH was unrelated to variation in IL-6. One possibility is that there was not enough dynamic variation in one or both variables to capture covariation across time. Only one-third of the variance in SRH was due to changes over the 5-year study period. With regard to IL-6, about half of the variance was due to changes over the 5-year study period, which were primarily fluctuations rather than systematic change over time. There appeared to be more dynamic variance in IL-6 than has been reported for other inflammatory markers, although existing studies are generally based on small samples, and their findings for long-term variability have been mixed (Cava, Gonzalez, Pascual, Navajo, & Gonzalez-Buitrago, 2000; Ho et al., 2005; Navarro et al., 2012; Picotte, Campbell, & Thorland, 2009).
Future research into the sources of SRH may benefit from, for example, using a more fine-grained response scale that could capture more subtle changes over time. Nonetheless, only marginal differences have been found between SRH measures with five and seven response alternatives (Eriksson, Undén, & Elofsson, 2001) and various measures of SRH are similar in predicting premature mortality (Idler & Benyamini, 1997).
The interval between SRH assessment and blood draw prevented the capture of more fine-grained (e.g., daily) covariation in SRH and IL-6. Had SRH been assessed on the same day as blood draw, for example, both daily and wave-level (biannual) variation would have influenced the estimate. The interval between assessments therefore has the advantage that the lack of a relationship within people can be more confidently attributed to the wave-level time frame of months, because it was not confounded with day-level covariation. Very short time scales (hours to days) that are consonant with a sickness behavior model of subjective health are not captured in this paradigm. Furthermore, the lack of dynamic, within-person relationships at the longer time scale limited the degree to which any direction in the SRH–IL-6 association could be tested. Future studies could impose a shorter time scale on the longer time scale (i.e., a longitudinal burst design) to further explore the levels at which SRH and IL-6 are related and the temporal precedence of changes in SRH and changes in IL-6. In addition, although single-item measures of SRH perform well as predictors of mortality independent of possible confounders and over several years of follow up (Benyamini, 2011), their performance is uncertain in longitudinal designs such as in the present study.
The present study extends the literature on the relationship between SRH and inflammatory markers by employing a longitudinal design. The large sample size and the many time points of assessment over a relatively long follow-up period are the major strengths of this study, as they provided opportunity to disentangle stable and dynamic partitions of the SRH–IL-6 association. However, the study would have benefited from including other inflammatory markers, as they may differ in their relationships to SRH (Lekander et al., 2004; Nakata et al., 2010).
Missing IL-6 data were more common than missing SRH data, and the greater attrition of older individuals yielded a small negative time slope for IL-6 (older age was associated with higher IL-6 at baseline). In addition to disproportionate retention of people with lower IL-6, this sample was from the outset composed of generally healthy individuals, which may have restricted the IL-6 range. On the other hand, inclusion of generally healthy participants minimized the confounding role of concurrent disease and medication.
Taken together, the present data support a robust stable, between-person association between SRH and IL-6. In other words, people with higher levels of circulating cytokines tend to give lower ratings of their health than those who have lower levels of cytokines. However, a change within one person on any of these variables is not reliably followed by changes in the other, at least in the present context of the months-long time scale of measurement. With some exceptions (e.g., Gerber et al., 2009), it has generally not been investigated whether the portion of SRH that predicts mortality is attributable to stable or dynamic SRH. To that end, we hope to see further longitudinal investigations and experimental designs that can shed further light on the causes behind the stable relationship between inflammation and SRH and what mechanisms explain the link between SRH and mortality.
Supplementary Material
Highlights.
First longitudinal study to model the link between self-rated health (SRH) and IL-6
Higher IL-6 was consistently related to poor SRH across 10 waves over 5 years
Within-person variation in SRH was unrelated to variation in IL-6
Acknowledgments
Funding
The work was supported by the Dana Foundation (to SCS), the National Institute on Aging (R01-AG026307 and K02-AG033629 to SCS, P30-AG028383 to Linda J. Van Eldik), and the National Center for Advancing Translational Sciences, (UL1TR000117). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasson AN, Szulkin R, Unden AL, von Essen J, Nilsson LG, Lekander M. Inflammation and positive affect are associated with subjective health in women of the general population. Journal of Health Psychology. 2013;18(3):311–320. doi: 10.1177/1359105311435428. doi: 10.1177/1359105311435428. [DOI] [PubMed] [Google Scholar]
- Baune B, Rothermundt M, Ladwig K, Meisinger C, Berger K. Systemic inflammation (Interleukin 6) predicts all-cause mortality in men: results from a 9-year follow-up of the MEMO Study. AGE. 2011;33(2):209–217. doi: 10.1007/s11357-010-9165-5. doi: 10.1007/s11357-010-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamini Y. Why does self-rated health predict mortality? An update on current knowledge and a research agenda for psychologists. Psychology & Health. 2011;26(11):1407–13. doi: 10.1080/08870446.2011.621703. [DOI] [PubMed] [Google Scholar]
- Cava F, Gonzalez C, Pascual MJ, Navajo JA, Gonzalez-Buitrago JM. Biological variation of interleukin 6 (IL-6) and soluble interleukin 2 receptor (sIL2R) in serum of healthy individuals. Cytokine. 2000;12(9):1423–1425. doi: 10.1006/cyto.2000.0714. doi: 10.1006/cyto.2000.0714. [DOI] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31(1):204–211. doi: 10.1038/sj.npp.1300856. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Christian LM, Glaser R, Porter K, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrinology. 2011;36(10):1495–1504. doi: 10.1016/j.psyneuen.2011.04.003. doi: 10.1016/j.psyneuen.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 1997;52(4):M201–208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- Costa PTJ, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) manual. Psychological Assessment Resources; Odessa, FL: 1992. [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson I, Undén A-L, Elofsson S. Self-rated health. Comparisons between three different measures. Results from a population study. International Journal of Epidemiology. 2001;30(2):326–333. doi: 10.1093/ije/30.2.326. doi: 10.1093/ije/30.2.326. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. 2nd John Wiley & Sons; Hoboken, NJ: 2011. [Google Scholar]
- Ganna A, Ingelsson E. 5 year mortality predictors in 498 103 UK Biobank participants: a prospective population-based study. The Lancet. 2015;386(9993):533–540. doi: 10.1016/S0140-6736(15)60175-1. doi: 10.1016/S0140-6736(15)60175-1. [DOI] [PubMed] [Google Scholar]
- Gerber Y, Benyamini Y, Goldbourt U, Drory Y, Israel Study Group on First Acute Myocardial Infarction Prognostic importance and long-term determinants of self-rated health after initial acute myocardial infarction. Medical Care. 2009;47(3):342–349. doi: 10.1097/MLR.0b013e3181894270. doi: 10.1097/MLR.0b013e3181894270. [DOI] [PubMed] [Google Scholar]
- Ho GY, Xue XN, Burk RD, Kaplan RC, Cornell E, Cushman M. Variability of serum levels of tumor necrosis factor-alpha, interleukin 6, and soluble interleukin 6 receptor over 2 years in young women. Cytokine. 2005;30(1):1–6. doi: 10.1016/j.cyto.2004.08.008. doi: 10.1016/j.cyto.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38(1):21–37. [PubMed] [Google Scholar]
- Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nature Reviews Drug Discovery. 2005;4(12):977–987. doi: 10.1038/nrd1901. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S. Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease. Brain, Behavior, and Immunity. 2005;19(6):555–563. doi: 10.1016/j.bbi.2005.01.001. doi: 10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science and Medicine. 2009;69(3):307–316. doi: 10.1016/j.socscimed.2009.05.013. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Knudsen LS, Christensen IJ, Lottenburger T, Svendsen MN, Nielsen HJ, Nielsen L, Johansen JS. Pre-analytical and biological variability in circulating interleukin 6 in healthy subjects and patients with rheumatoid arthritis. Biomarkers. 2008;13(1):59–78. doi: 10.1080/13547500701615017. doi: 10.1080/13547500701615017. [DOI] [PubMed] [Google Scholar]
- Lekander M, Andreasson AN, Kecklund G, Ekman R, Ingre M, Akerstedt T, Axelsson J. Subjective health perception in healthy young men changes in response to experimentally restricted sleep and subsequent recovery sleep. Brain, Behavior, and Immunity. 2013;34:43–46. doi: 10.1016/j.bbi.2013.06.005. doi: 10.1016/j.bbi.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Lekander M, Elofsson S, Neve I-M, Hansson L-O, Undén A-L. Self-rated health is related to levels of circulating cytokines. Psychosomatic Medicine. 2004;66(4):559–563. doi: 10.1097/01.psy.0000130491.95823.94. [DOI] [PubMed] [Google Scholar]
- Lekander M, Nixon Andreasson A, Karshikoff B, Lidberg L, Axelsson J, Olgart Höglund C. 171. Self-rated health in response to experimental manipulations of inflammation. Brain, Behavior, and Immunity. 2012;26(Supplement 1):S48. doi: 10.1016/j.bbi.2012.07.195. [Google Scholar]
- Lidberg L, Andreasson AN, Karshikoff B, Axelsson J, Olgart Höglund C, Lekander M. 117. Self-rated health in response to experimental manipulations of inflammation is mediated by sickness behavior as assessed by the sickness questionnaire. Brain, Behavior, and Immunity. 2013;32(Supplement):e34. doi: 10.1016/j.bbi.2013.07.129. [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. 2 Wiley; Hoboken, N.J.: 2002. [Google Scholar]
- Lyyra TM, Leskinen E, Jylha M, Heikkinen E. Self-rated health and mortality in older men and women: a time-dependent covariate analysis. Archives of Gerontology and Geriatrics. 2009;48(1):14–18. doi: 10.1016/j.archger.2007.09.004. doi: 10.1016/j.archger.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain, Behavior, and Immunity. 2008;22(5):753–761. doi: 10.1016/j.bbi.2007.11.008. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga M, Isowa T, Murakami H, Kasugai K, Yoneda M, Kaneko H, Ohira H. Association of polymorphism in the human mu-opioid receptor OPRM1 gene with proinflammatory cytokine levels and health perception. Brain, Behavior, and Immunity. 2009;23(7):931–935. doi: 10.1016/j.bbi.2009.03.007. doi: 10.1016/j.bbi.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Nakata A, Takahashi M, Otsuka Y, Swanson NG. Is self-rated health associated with blood immune markers in healthy individuals? International Journal of Behavioral Medicine. 2010;17(3):234–242. doi: 10.1007/s12529-010-9102-0. [DOI] [PubMed] [Google Scholar]
- Navarro SL, Brasky TM, Schwarz Y, Song X, Wang CY, Kristal AR, Lampe JW. Reliability of serum biomarkers of inflammation from repeated measures in healthy individuals. Cancer Epidemiology, Biomarkers and Prevention. 2012;21(7):1167–1170. doi: 10.1158/1055-9965.EPI-12-0110. doi: 10.1158/1055-9965.EPI-12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picotte M, Campbell CG, Thorland WG. Day-to-day variation in plasma interleukin-6 concentrations in older adults. Cytokine. 2009;47(3):162–165. doi: 10.1016/j.cyto.2009.05.007. doi: 10.1016/j.cyto.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Ram N, Gerstorf D. Time-structured and net intraindividual variability: tools for examining the development of dynamic characteristics and processes. Psychology and Aging. 2009;24(4):778–791. doi: 10.1037/a0017915. doi: 10.1037/a0017915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Diez-Roux AV, Seeman T, Ranjit N, Shea S, Cushman M. Association of optimism and pessimism with inflammation and hemostasis in the Multi-Ethnic Study of Atherosclerosis (MESA) Psychosomatic Medicine. 2010;72(2):134–140. doi: 10.1097/PSY.0b013e3181cb981b. doi: 10.1097/PSY.0b013e3181cb981b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu E, West SG, Sousa KH. Distinguishing between-person and within-person relationships in longitudinal health research: arthritis and quality of life. Annals of Behavioral Medicine. 2012;43(3):330–342. doi: 10.1007/s12160-011-9341-6. doi: 10.1007/s12160-011-9341-6. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Affect and self-rated health: a dynamic approach with older adults. Health Psychology. 2014;33(7):720–728. doi: 10.1037/a0033506. doi: 10.1037/a0033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- Unden AL, Andreasson A, Elofsson S, Brismar K, Mathsson L, Ronnelid J, Lekander M. Inflammatory cytokines, behaviour and age as determinants of self-rated health in women. Clinical Science. 2007;112(6):363–373. doi: 10.1042/CS20060128. doi: 10.1042/CS20060128. [DOI] [PubMed] [Google Scholar]
- Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, Harris TB. Cardiovascular Disease, Interleukin-6, and Risk of Mortality in Older Women: The Women’s Health and Aging Study. Circulation. 2001;103(7):947–953. doi: 10.1161/01.cir.103.7.947. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- Wang LP, Maxwell SE. On disaggregating between-person and within-person effects with longitudinal data using multilevel models. Psychological Methods. 2015;20(1):63–83. doi: 10.1037/met0000030. doi: 10.1037/met0000030. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992:473–483. [PubMed] [Google Scholar]
- Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychological Bulletin. 1984;96(3):465–490. [PubMed] [Google Scholar]
- Winter L, Lawton MP, Langston CA, Ruckdeschel K, Sando R. Symptoms, affects, and self-rated health: evidence for a subjective trajectory of health. Journal of Aging and Health. 2007;19(3):453–469. doi: 10.1177/0898264307300167. doi: 10.1177/0898264307300167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.