Abstract
Alzheimer’s disease, the most prevalent form of dementia in the elderly, is characterized by progressive memory loss and cognitive dysfunction. It has become increasingly clear that while neuronal cell loss in the entorhinal cortex and hippocampus occurs in Alzheimer’s disease, it is preceded by a long period of deficits in the connectivity of the hippocampal formation that contributes to the vulnerability of these circuits. Hippocampal neurogenesis plays a role in the maintenance and function of the dentate gyrus and hippocampal circuitry. This review will examine the evidence suggesting that hippocampal neurogenesis plays a role in cognitive function that is affected in Alzheimer’s disease, will discuss the cognitive assessments used for the detection of Alzheimer’s disease in humans and rodent models of familial Alzheimer’s disease, and their value for unraveling the mechanism underlying the development of cognitive impairments and dementia.
Keywords: ccc, ccc, bb, nnnn
Graphical Abstract
1. Introduction
Adult neurogenesis is instrumental for hippocampal function
Deficits in neurogenesis may cause/exacerbate Alzheimer’s disease cognitive failure
Enhancing adult neurogenesis could be a therapeutic target for Alzheimer’s disease
Alzheimer’s disease is characterized by a progressive loss of memory, the failure to learn or retain new information and the deterioration of cognitive function. Increasing evidence suggests that memory deficits develop over decades before they are detectable as mild cognitive impairments (MCI). As aging continues some of these MCI patients will progress into Alzheimer’s disease (AD) dementia. While extensive research in the last three decades unraveled the genetic constituents that are linked to familial Alzheimer’s disease, little is known about how learning and memory impairments develop, and the molecular substrates underlying the vulnerability of the entorhinal-hippocampal circuitry.
The generation of new neurons and glia in the adult hippocampus is increasingly implicated in forms of learning and memory. New neurons are added to the granular cell layer of the dentate gyrus throughout life and are being recruited in greater amounts following experience, learning and exercise (Deng et al., 2010). In turn, deficits in neurogenesis over time may compromise hippocampal function, gradually leading to memory deficits. Numerous studies suggest that neurogenesis is impaired in mouse models of familial AD (for review (Lazarov and Marr, 2010)). However it is not clear how hippocampal neurogenesis and its gradual decline with age contribute to the cognitive dysfunction in AD. In search for a connection between AD and adult hippocampal neurogenesis one may struggle with comparative behavioral assessment in humans and mouse models. This review will attempt to connect our basic understanding of mechanisms of learning and memory with memory dysfunction in Alzheimer’s disease. In addition, it will critically consider the current evidence concerning the role of neurogenesis in the development of cognitive deficits and Alzheimer’s disease.
2. The role of the hippocampus in learning and memory
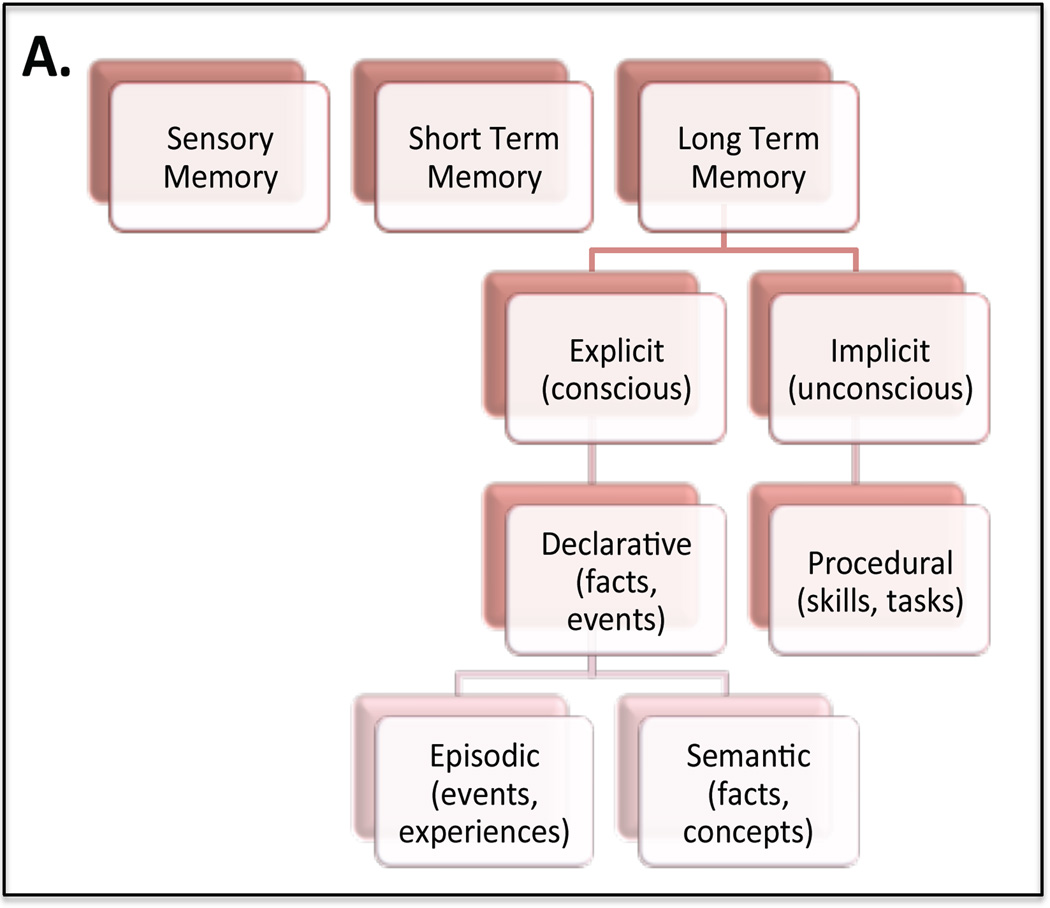
Memory and learning are essential components of human existence; they provide the framework for everyday activities and invest long-term meaning into significant events. Understanding the mechanism behind how memory works gives us insight into the fundamental nature of humanity. Extensive study has revealed that memory can be divided into functional segments; sensory memory, short term memory (working memory) and long term memory (Fig. 1). Chosen short term memory traces are converted to long-term memory in a process called memory consolidation (McGaugh, 1966, Dudai, 2012, Kitamura and Inokuchi, 2014). How the brain determines that some information is necessary and should be stored, while other can be discarded, is a fascinating enigma. Such long-term memory for facts (semantic memory) and events (episodic memory) is referred to as explicit (declarative) memory. This is in contrast to implicit (procedural) memory, which is required for perceptual and motor skills that are completed without conscious thought. Implicit memory relies mostly on brain areas implicated in motor function, such as, the cerebellum and the striatum, while explicit memory involves the hippocampus and related cortices. Studying patients with amnesic symptoms first hypothesized the role of the hippocampus in explicit memory, the most famous patient being Henry Molaison (H.M). Henry Molaison had a portion of his medial temporal lobe removed, including the hippocampus, in an effort to suppress epilepsy symptoms. This procedure left him unable to store or retrieve new memories (Scoville and Milner, 1957). Inspired by this early tragedy, extensive research has confirmed an essential role for the hippocampus in both storing and retrieving new memories as well as in spatial navigation. However the exact mechanisms and functions of individual parts of the hippocampal circuitry are still under debate.
Fig. 1. Human Memory Domains.
(a) Memory is divided into three primary functional domains; sensory memory, short-term memory and long-term memory. Long-term memory in turn can be divided into explicit, or conscious memory, and implicit, or unconscious memory. Implicit memory deals with procedural activities, like walking or tying ones shoe that are performed without conscious thought. In contrast explicit, or declarative, memory is memory for events (episodic memory) and facts (semantic memory).
1.1.1. Hippocampal Circuitry
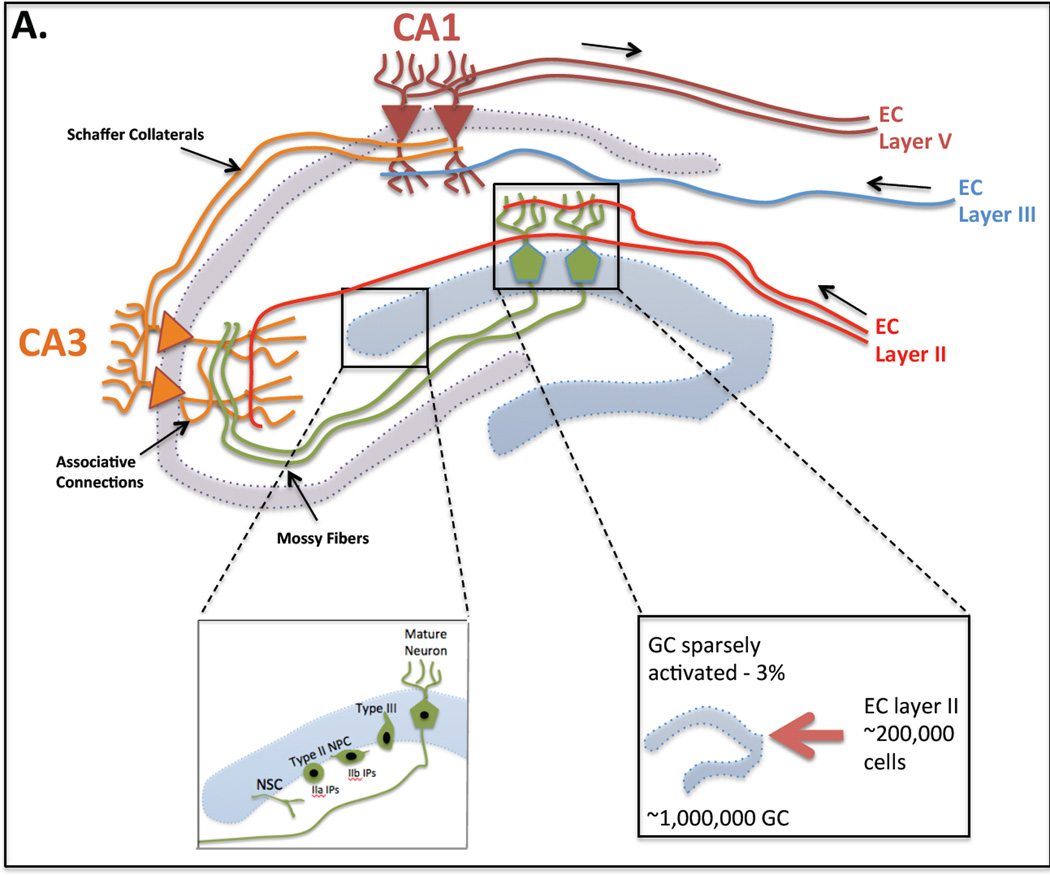
Sensory information is fed into the dentate gyrus (DG) of the hippocampus by projections from the entorhinal cortex, which in turn receives input from multiple cortical and sensory areas (Fig. 2). Medial perforant path input from the entorhinal cortex into the DG mediates spatial information, via activation of NMDA receptors, while the lateral perforant path mediates visual object information (e.g. odors or objects) through activation of opioid receptors (Hunsaker et al., 2007).
Fig. 2. Hippocampal Circuitry.
(a) Perforant path axons, extending from layer II of the entorhinal cortex (EC), make excitatory synaptic contacts with the dendrites of younger and older granule cells (GC). GCs send mossy fiber projections to the CA3 pyramidal cells, which, in turn send Schaffer collaterals to CA1 pyramidal cells. In addition CA3 cells on the same side form a dense associative network interconnecting with each other. CA3 pyramidal cells are also innervated by a direct input from layer II cells of the EC and CA1 pyramidal neurons receive a direct input from layer III cells of the EC. CA1 pyramidal neurons send axons out of the hippocampus innervating layer V of the EC. (b) Adult neurogenesis occurs in the subgranular layer (SGL) of the dentate gyrus (DG). Type I neural stem cells (NSC) give rise to intermediate progenitors (IP) called type IIa IP and type IIb IP cells. Type IIb cells are early committed neural progenitor cells (NPCs) and give rise to type III neuroblasts. Neuroblasts migrate into the granular layer where they mature into neurons. (c) In rats the number of GCs in the DG (∼1,000,000) is significantly larger than the number of EC cells projecting onto the DG (∼200,000). Thus the DG is sparsely activated (3% of granule cells activated) in response to stimuli from these inputs. Small changes in entorhinal input results in distinct, non-overlapping activation of the DG.
In rats the number of granule cells in the dentate gyrus of the hippocampus (∼1,000,000) is significantly larger than the number of entorhinal cells projecting onto the DG (∼200,000) (Amaral et al., 1990). Thus the DG is sparsely activated (3% of granule cells activated) in response to stimuli from these inputs (Chawla et al., 2005) and as a result small changes in entorhinal input results in distinct, non-overlapping activation of the DG (O'Reilly and McClelland, 1994). In the DG interneurons also provide contribution to the circuitry; granular cells are interconnected by excitatory interneurons located in the hilus and a layer of inhibitory interneurons provides recurrent inhibition (Lisman et al., 2005, Myers and Scharfman, 2011, Scharfman and Myers, 2012). In contrast to the DG, whose primary input is the entorhinal cortex, the CA3 region of the hippocampus, receives multiple excitatory inputs; from granule cell mossy fibers in the DG (Blackstad et al., 1970, Swanson et al., 1978), directly from layer II of the entorhinal cortex (via the perforant path) (Witter, 1993) and through recurrent collateral input from CA3 neurons themselves (Amaral, 2007). The recurrent collateral input in the CA3 suggests that this region has auto-associative properties (for review (Rolls, 2013)). This means that groups of coactive CA3 neurons could have increasingly strengthened connections as a result of synaptic plasticity.
The unique circuitry of the hippocampus has lead to several hypotheses regarding its specific role in explicit memory, many of these arising from quantitative computational theories that have then been tested through genetic and molecular means (for review (Kesner and Rolls, 2015)).
1.1.1. Conjunctive encoding
In particular, the DG is believed to perform conjunctive encoding, integrating both the visual object and spatial information supplied by the perforant pathway to create a memory of a ‘total’ spatial representation (for review (Kesner and Rolls, 2015)). The conjunctive encoding hypothesis is supported by a study by Morris et al. (2013). Following lesion of the DG rodents were not able to acquire the cue-context association in a contextual associative learning task, in which a spatial context was paired with olfactory information (Morris et al., 2013). This was despite the fact that in separate tests of odor and context discrimination rats were able to acquire discrimination at similar rates. Thus deficits were unlikely caused by a failure to discriminate in general and suggest that the DG does indeed play an important role in conjunctive encoding pairing spatial and olfactory information.
1.1.1. Pattern Separation
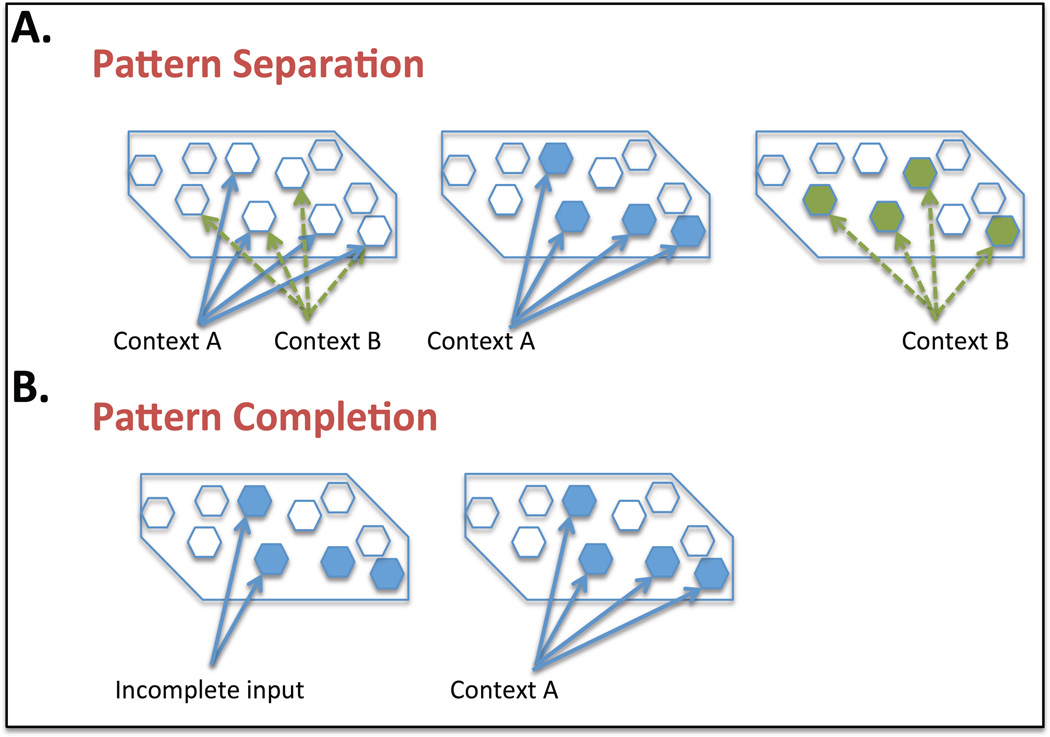
Along with conjunctive encoding the hippocampus has also been well studied in regards to its proposed role in in pattern separation, the ability to distinguish between two similar contexts or spatial locations (Fig. 3 A). Early behavioral studies that lesion the DG demonstrated deficits in pattern separation, in the water maze, radial arm maze or spatial contextual fear conditioning tasks (for review (Kesner and Rolls, 2015)). For example low dose X-irradiation of the DG in mice demonstrated a deficit in a delayed non-matching-to-place task in the radial arm maze. In particular mice showed deficits in closely placed arms but were unimpaired in widely separated arms, demonstrating a deficit in spatial pattern separation (Clelland et al., 2009). Similarly Lee and Kesner 2004 observed deficits in spatial memory when examining rats with lesioned dorsal DG twenty four hours after acquisition in the contextual fear conditioning task (Lee and Kesner, 2004). We will discuss further the role of the DG in regards to pattern separation later in the review. Particularly considering the impact that modulation of adult neurogenesis in this region has on this memory paradigm.
Fig. 3. Hippocampal Pattern Separation and Pattern Completion.
(a) Pattern separation. Overlapping inputs from the entorhinal cortex, conveying information regarding context A and context B, are coded separately by the dentate gyrus. (b) Pattern completion. When a partial subset of cues for context A are presented, pattern completion induces retrieval of the whole memory.
1.1.1. Pattern Completion
The CA3 region of the hippocampus is also believed to be important for pattern completion or the retrieval of a memory when only partial input cues are presented (Fig. 3 B). As mentioned previously the CA3 is known for having recurrent collateral inputs which suggests that this region has auto-associative properties (for review (Rolls, 2013)). Thereby when one part of a group of CA3 neurons is activated the whole set becomes activated resulting in pattern completion. Lesion studies of the CA3 demonstrated deficits in a pattern completion test using rats (Gold and Kesner, 2005). This was a spatial task where extra maze cues were used to train the rats on location of a food well. Ability to remember the spatial location was analyzed with decreasing number of cues to demonstrate impairment in pattern completion following CA3 lesions. A similar study in which an µ opiod receptor antagonist (naloxone) was injected into CA3 produced the same pattern completion deficit (Kesner and Warthen, 2010).
Several papers support the mechanistic role of the DG in pattern separation and the CA3 in pattern completion using electrophysiology. Leutgeb et al 2007 performed in vivo recordings in rats demonstrating that individual granular cells change their firing maps in response to small contextual variations (Leutgeb et al., 2007). Similarly a recent paper by Neunuebel et al. 2014 provided quantitative electrophysical evidence supporting both the role of the DG in pattern separation and the CA3 in pattern completion (Neunuebel and Knierim, 2014). Single unit and population level activity was simultaneously recorded from the DG and the CA3 in behaving rats when local and distal spatial cues were manipulated in the “double rotation task.” As predicted the DG pattern of input to the CA3 degraded after alteration of the environment (pattern separation), while the CA3 produced an output pattern more similar to the originally stored representation (pattern completion). Thus, providing strong support for the long-standing hypothesis that these areas perform separate functions of storage and retrieval of memories.
1.1.1. Episodic Memory
The DG also plays an important role in temporal separation of events, an essential hallmark of episodic memory. Rangel et al (2014) showed through in vivo electrophysiological recordings that cells in the DG selectively encode environments that are temporally distinct (Rangel et al., 2014). Significantly, as the timeline between experiences/contexts was reduced, and as adult neurogenesis was reduced, the proportion of cells with activity selective to a particular context was also reduced, indicating a clear temporal component of DG function.
1.1.1. Spatial Navigation
The hippocampus has also been well studied in relation to its functional role in spatial navigation. The hippocampus contains pyramidal neurons called place cells, located in CA1 and CA3, that fire in stereotypic patterns only when an animal moves through a specific region of its environment and hence are believed to be encoding for the spatial map of that region ( (O'Keefe and Dostrovsky, 1971), for review (Moser et al., 2008)). Significantly place cells are not topographically organized and different populations of cells can be recruited for the same environment by different individuals (Redish et al., 2001, Dombeck et al., 2010). To establish that these place cells do indeed regulate spatial navigation multiple transgenic animal lines were created to lesion specific cell populations in the hippocampus (Dupret et al., 2008, Imayoshi et al., 2008, Zhang et al., 2008). Using various spatial learning tests including the Barnes maze task and the Morris water maze (MWM) task these studies demonstrate that the hippocampus has an important role in allocentric spatial mapping, where the location of objects is defined relative to other objects locations, but that loss of hippocampal neurons did not disrupt egocentric mapping, where spatial location is determined in relation to the body axis.
2.1. Hippocampal neurogenesis
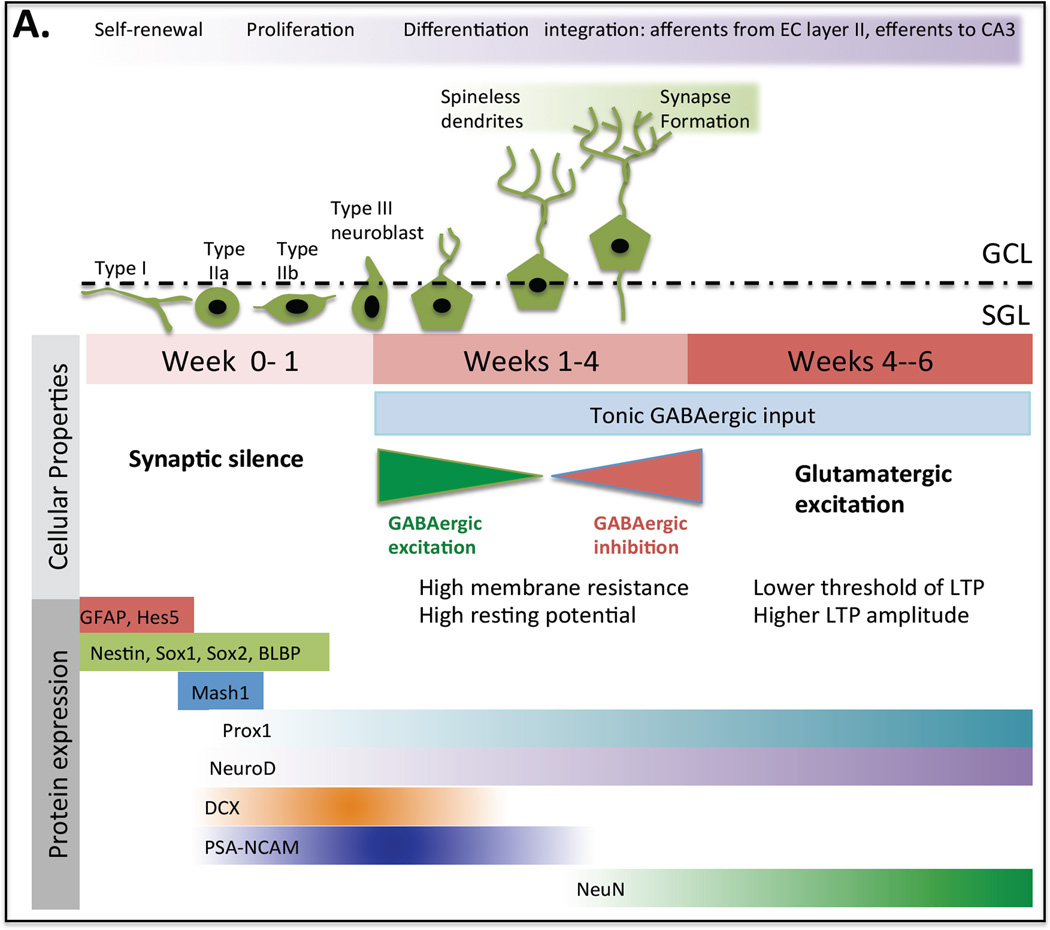
The hippocampus is one of only a few regions in the adult brain, including the sunventricular zone (in rodents) and, recently shown in humans, the striatum, that continues to produce new neurons (Ernst et al., 2014). In the bottom third of the DG of the hippocampus, the subgranular layer (SGL), type I neural stem cells (NSC) give rise to type IIa, type IIb and type III neural progenitor cells (NPC), and neuroblasts that mature into neurons and migrate into the granule cell layer (GCL) (Fig. 4). These various cell types can be identified by a stereotypic pattern of protein expression (for review (Lazarov et al., 2010, Kempermann et al., 2015)).
Fig. 4. Maturation of Granule Cells.
(a) In the first week after birth neural progenitor cells are distinguished by their irregular shape, immature spikes and synaptic silence. In week two they have migrated into the granule cell layer, developed spineless dendrites and slow GABAergic synaptic inputs. By the third week they start to form afferent connections from the perforant pathway of the entorhinal cortex and efferent connections to the CA3. Also a transition from GABAergic to glutamatergic synaptic inputs takes place. At this stage the developing neurons are highly excitable with high membrane resistance and high resting potential. Finally between weeks four and six these immature neurons exhibit stronger synaptic plasticity than mature dentate granule cells. They have a lower threshold for induction of LTP and higher LTP amplitude.
Adult neurogenesis is a form of hippocampal plasticity with NPCs bearing little resemblance to their mature counterparts. Maturation from the NPC stage to mature neurons follows a specific pattern of events, with developing neurons having distinct firing and physical characteristics from their mature counterparts (for review (Aimone et al., 2010, Deng et al., 2010, Aimone et al., 2014), Fig. 4). In the first week after birth NPCs are distinguished by their irregular shape, immature spikes and synaptic silence. In week two they have migrated into the GCL, developed spineless dendrites and slow GABAergic synaptic inputs. By the third week they start to form afferent connections from the perforant pathway of the entorhinal cortex and efferent connections to the CA3. Also a transition from GABAergic to glutamatergic synaptic inputs takes place. At this stage the developing neurons are highly excitable with high membrane resistance and high resting potential. Finally between weeks four and six these immature neurons exhibit stronger synaptic plasticity than mature dentate granule cells. They have a lower threshold for the induction of LTP and higher LTP amplitude.
Newly born neurons can account for up to ten percent of the granule cell population in the hippocampus (Imayoshi et al., 2008). The role that new neurons play in the function of the dentate gyrus in memory formation remains controversial. Adult born neurons have been shown to modulate signal processing in the DG and to be necessary for pattern separation specifically (for review (Aimone et al., 2011, Sahay et al., 2011b, Kropff et al., 2015)). Using a combination of methods to decrease or enhance neurogenesis in behavioral tasks such as the RAWM, contextual fear discrimination learning and two-choice spatial discrimination, studies have shown that animals with ablated neurogenesis had deficits in their ability to discriminate between highly similar situations and animals with enhanced neurogenesis improved in such tasks (Clelland et al., 2009, Creer et al., 2010, Sahay et al., 2011a, Kheirbek et al., 2012, Nakashiba et al., 2012, Tronel et al., 2012). However, the changing characteristics of newly born neurons over time leaves the exact developmental phase at which they are required for pattern separation unclear, especially because in these studies alterations in neurogenesis were started weeks before training and lasted throughout the testing period. Similarly because testing and training are closely linked it is not possible to determine if adult neurogenesis is required for task acquisition, retrieval or both. A more detailed temporal analysis of adult neurogenesis in pattern separation will be important to understand the functional role of new neurosn in the DG in their entirety. In addition to their synaptic connection with EC layer II and CA3; new neurons indirectly activate contralateral granule neurons, via innervation of hilar mossy cells. In addition, they innervate basket interneurons, which consecutively inhibit granule neurons (Freund and Buzsaki, 1996). Depletion of new neurons results in enhanced spontaneous γ-frequency burst amplitude in the DG. This suggests that young neurons may inhibit or destabilize the hippocampal circuitry (Lacefield et al., 2012). Interestingly, recent evidence suggests that removal of NPCs also plays a role in hippocampal plasticity. For example, Dupret and colleagues (2007) suggest that spatial learning promotes the survival of relatively mature neurons, concomitantly with the apoptosis of more immature cells, as well as the proliferation of NPCs (Dupret et al., 2007). Thus, they suggest that blocking apoptosis impairs memory. Intriguingly, neurogenesis has been implicated in the modulation of forgetting and memory clearance as well (Feng et al., 2001, Akers et al., 2014).
Important key characteristic of NPCs and new neurons is their modulation by numerous local, systemic and environmental factors, including but not limited to neurotransmitters, hormones, cytokines, as well as learning, exercise, environmental enrichment and stress. Modulation by these factors will determine the extent of NPCs and of new neurons that will join the GCL, and play an important role in the resulting hippocampal structure and function. Specific factors may affect particular neurogenic populations and affect neurogenesis to a different extent. For example, it has been hypothesized that there is a critical period in which an enriched environment results in enhanced survival and population response of adult born neurons. However the exact window is controversial with some suggesting it lies at two to four weeks following birth of new neurons (Tashiro et al., 2007, Gu et al., 2012).
Significantly, activity-dependent promotion of neurogenesis has also been shown to occur following deep brain stimulation of the entorhinal cortex. Stimulation of the entorhinal cortex promotes proliferation in the SGL and NPCs mature and integrate into the circuitry of the hippocampus (Stone et al., 2011). These various forms of modulation impacting neurogenesis, including adaptation to novelty, learning and activity dependent stimulation, all demonstrate that adult neurogenesis has an important role in neural plasticity.
Importantly, neurogenesis is recruited for learning, and plays an important role in many aspects of hippocampus-dependent memory, as discussed in detail below. Learning in tasks such as the MWM task have been shown to promote survival of relatively mature neurons, apoptosis of more immature cells, and finally, proliferation of neural precursors. Blocking apoptosis impairs memory and inhibits learning-induced cell survival and cell proliferation (Dupret et al., 2007). Arruda-Carvaloho et al. (2011) utilized diphtheria toxin to selectively ablate predominantly mature, adult born neurons either before or after learning. They observed deficits in contextual fear and water maze tasks when ablation was performed following learning but not before. Taken together, the remarkable plasticity of new neurons allows for a dynamic system controlling learning and memory and it will be important to consider how such plasticity stands up to aging and AD.
3. Age related memory loss in the dentate gyrus
Normal aging is often characterized by some degree of cognitive decline and memory loss. Interestingly, this decline tends to be restricted to specific domains, with age related decline most often observed in episodic, spatial, attention and working memory (Kausler, 1994). These observations are supported by high resolution fMRI (Small et al., 2002, Small et al., 2004, Moreno et al., 2007) and cognitive studies (Toner et al., 2009, Stark et al., 2010, Brickman et al., 2011, Yassa et al., 2011) suggesting that the primary initial target of normal aging is the DG while the entorhinal cortex is relatively preserved (Small et al., 2011). Thus it is thought that age-related memory loss begins in the DG (Pavlopoulos et al., 2013). But what is the underlying structural basis for this loss?
Significantly, we and others have shown that there is a dramatic decrease in neurogenesis during adulthood and aging (Seki and Arai, 1995, Kuhn et al., 1996, Tropepe et al., 1997, Kempermann et al., 1998, Kempermann et al., 2002). This is observed in rodents by a reduction in rate of proliferation, total number of progenitor cells and reduced number of neuroblasts and immature neurons. The decline in neurogenesis is thought to result from age associated changes in the neurogenic niche, such as vascular factors, trophic signals and/or a change in progenitor cell ability to respond to these signals (for review (Binder, 2007)). This decline raises the possibility that reduced neurogenesis may compromise hippocampal function and account, at least in part, for impaired learning and memory and cognitive deterioration in the elderly While there are dramatic changes in neurogenesis with age, there are also fewer subtle changes in synaptic structure (for review (Burke and Barnes, 2006)). In particular it has been shown in rodents and humans that the density of synaptic contacts formed onto granule cells of the DG is reduced with age (Geinisman et al., 1992, Flood, 1993). In contrast, dendritic complexity and spine density of granule cells and pyramidal neurons of CA3 and CA1 is not changed with age (Geinisman et al., 1992).
Also, it is important to remember that, as stated above, levels of hippocampal neurogenesis are not static over rodent lifespans. They can be enhanced by environmental enrichment (van Praag et al., 2000), exercise (van Praag et al., 1999, van Praag et al., 2005), learning (Gould et al., 1999) and treatment with antidepressants (Warner-Schmidt and Duman, 2006), or they can be suppressed by conditions, such as stress (Kreisel et al., 2014), social isolation (Gould et al., 1997), or irradiation (Monje and Palmer, 2003). Enhancement of neurogenesis has been linked with improvement in behavioral learning and memory tasks, while reduction is linked to deficits in similar tasks. This dynamic nature of adult neurogenesis and its clear role in learning and memory suggests that modulation of neurogenesis could have significant impact on cognitive decline observed with aging.
4. Adult neurogenesis in humans
While neurogenesis has been well studied in rodents, elucidating its role in humans has been a more complex challenge. The first evidence for human neurogenesis was reported in 1998. Examination of post mortem tissue from the hippocampus of cancer patients who were treated once with 5-bromo-2'-deoxyuridine (BrdU) for diagnostic purposes demonstrated the presence of new neurons (Eriksson et al., 1998). However, this approach could not provide insight into the extent of neurogenesis and whether similar numbers of new cells were produced as in rodents. More recently Spalding and colleagues birth dated hippocampal cells retrospectively using the ratio of 14C to 12C in DNA of postmortem individuals exposed to nuclear testing to estimate a turnover rate of 700 new neurons a day in the DG (Spalding et al., 2013). Significantly, compared to rodents, which replace ten percent of the DG; in humans roughly thirty five percent of neurons are subject to exchange (Ninkovic et al., 2007, Imayoshi et al., 2008). Also unlike rodents, which have a dramatic drop in rate of neurogenesis with age, in humans there is only a modest decline in neurogenesis with aging. Another differentiating feature is that some evidence suggests that hippocampal neurogenesis in rodents is additive, resulting in a net increase in the number of dentate gyrus neurons with age, while in humans there is a net loss of dentate gyrus neurons during adult life and the generation of new neurons does not keep up with neuronal loss (Bayer, 1985, Kempermann et al., 2003, Ninkovic et al., 2007, Imayoshi et al., 2008). It remains to be seen how the decline in human adult neurogenesis with time impacts cognitive function and whether this causes the hippocampus to be more sensitive to memory impairment as a result of disease pathology such as AD. Also it is important to validate the role of adult neurogenesis in human memory and whether it is necessary for similar cognitive domains as in rodents. While identical gain and loss of function studies cannot be performed in humans as they were in rodents it may be possible to statistically correlate levels of neurogenesis with cognitive ability. However, elucidating this role will require a large cohort of patients, with varying medical histories, cognitive ability, lifestyles and activity level, since all these factors have been shown to modulate levels of neurogenesis in rodents.
5. Alzheimer’s disease
Disruption in learning and memory and cognitive deterioration leading to dementia has devastating effects on both the individual and their extended family. AD is the most prevalent form of dementia in the elderly and is characterized by progressive memory loss and cognitive dysfunction (Selkoe and Wolfe, 2007). Neuropathology initially appears in the transentorhinal cortex before encroaching on the entorhinal cortex and hippocampus. Eventually the temporal, frontal and parietal lobes exhibit neuronal loss and the gray mater of the brain decreases in size (Thompson et al., 2003, Thompson et al., 2007, Serrano-Pozo et al., 2011). In contrast to normal aging where the DG is primarily impacted, AD postmortem studies show that the CA1 subregion and the subiculum are the regions of the hippocampus that are most affected (Thompson et al., 2007). This has also been observed in vivo by fMRI studies (Mueller et al., 2010). Given the early spread of this disease to the hippocampus it is important to understand how AD related proteins impact adult neurogenesis and thus hippocampal control of memory. Notably, the vast majority of the AD cases are sporadic, late onset, and aging is the greatest risk factor for AD, suggesting that aging processes render the brain vulnerable to AD. Rare, Familail AD (FAD) is caused by mutations in the amyloid precursor protein (APP) and presenilin 1 and 2 (PS1,2) (Selkoe and Wolfe, 2007). Interestingly, there is significant cross talk between many of the important proteins that regulate the progression of FAD and adult neurogenesis (for review (Lazarov and Marr, 2010, 2013)). Of particular interest are PS1 and soluble amyloid percursor protein alpha (sAPPα), (Demars et al., 2011, Gadadhar et al., 2011, Demars et al., 2013). PS1 regulates NPC differentiation in vitro and in vivo (Demars et al., 2011), while sAPPα acts as a proliferation factor on NPCs in the adult brain (Demars et al., 2011, Baratchi et al., 2012). PS1 is also the catalytic core of the aspartyl protease γ-secretase, which is required for cleavage of APP to produce Aβ in familial AD. Importantly, PS1 and the γ-secretase also regulate the metabolism of important neurogenic signals, such as notch, EGF,β -catenin and the cAMP response element binding protein (CREB). Alterations in PS1 expression levels are likely to play a role in modulating neurogenesis through regulation of these proteins.
5.1. Alzheimer’s disease transgenic mouse models and neurogenesis
Could vulnerability to AD be enhanced by the decline in rate of neurogenesis seen in the elderly (Spalding et al., 2013)? Could dysfunction of neurogenesis lead to hippocampal impairments characterizing AD? Our studies and others have demonstrated impairments in both the proliferative capacity of NPCs and in neuronal differentiation in the SGL of FAD-linked APPswe/PS1 ΔE9 mice (Demars et al., 2010), as early as two months of age. Early neurogenic impairments were detected in other models of FAD as well, such as the 3XTg-AD mice (Rodriguez et al., 2008, Hamilton et al., 2010), preceding the hallmarks of the disease and cognitive deficits (for review (Lazarov and Marr, 2010, 2013)). In addition, NPCs isolated from the SGL of APPswe/PS1ΔE9 animals exhibited reduced proliferation and expressed hyperphosphorylated tau, suggesting that expression of APPswe/PS1ΔE9 intrinsically affects NPC function (Demars et al., 2010). Also, work by Hartl et al. (2008) analyzing protein changes in the proteomes of APP23 mice, demonstrated a disruption of hippocampal plasticity during adolescence in APP23 mice (Hartl et al., 2008). Combined, this data demonstrates that neurogenesis is impaired early in FAD mice, however it is reasonable to assume that the extent of neurogenesis may change with the progression of AD, as a response to brain pathology (for review (Lazarov and Marr, 2010, Lazarov et al., 2010, Lazarov and Marr, 2013)). This may underlie some controversy in AD transgenic mouse lines reported to exhibit both enhancement and reduction of adult neurogenesis (Chuang, 2010). Unfortunately, there is little information regarding neurogenesis in humans affected with AD, and the data that exists is inconclusive (Jin et al., 2004, Boekhoorn et al., 2006).
5.2. Cognitive domains impaired in Alzheimer’s disease
To fully understand the role of adult neurogenesis in AD it is critical to first understand what types of memory are disrupted in AD and how this analysis is performed. The National Institute on Aging and the Alzheimer’s Association have created a standardized criteria for the cognitive profile of the three stages of AD memory deterioration; (1) preclinical, (2) mild cognitive impairment (MCI) and (3) AD dementia (Jack et al., 2011, McKhann et al., 2011, Sperling et al., 2011) (Fig. 5). Studies that assess cognition at multiple time points prior to AD dementia (over a decade) have shown a decrease in episodic memory by impairment in one or more cognitive domains, with episodic memory impairments the most common in patients who progress to AD dementia. However, impairments can also be observed in executive function, attention, language and visuospatial skills. Patients who have AD dementia have impairments in at least two of the following; ability to acquire and remember new information, reasoning and handling of complex tasks, visuospatial abilities, language functions and personality (behavior or comportment) (McKhann et al., 2011). Many of these deficits can be seen in MCI as well; the difference between dementia and MCI are primarily based on whether deficits are so severe that the patient is not able to function at work or in their normal daily activities (Albert et al., 2011). Dementia is likely caused by AD when it has a gradual onset over months and years not hours and days. Amnesia, impairments in learning and recall of recently learned information, is the most common symptom of AD dementia. Non-amnestic deficits are also seen in language (word finding), visuospatial (spatial cognition, object recognition, face recognition, visual attention and written language) and executive dysfunction (impaired reasoning, judgment and problem solving).
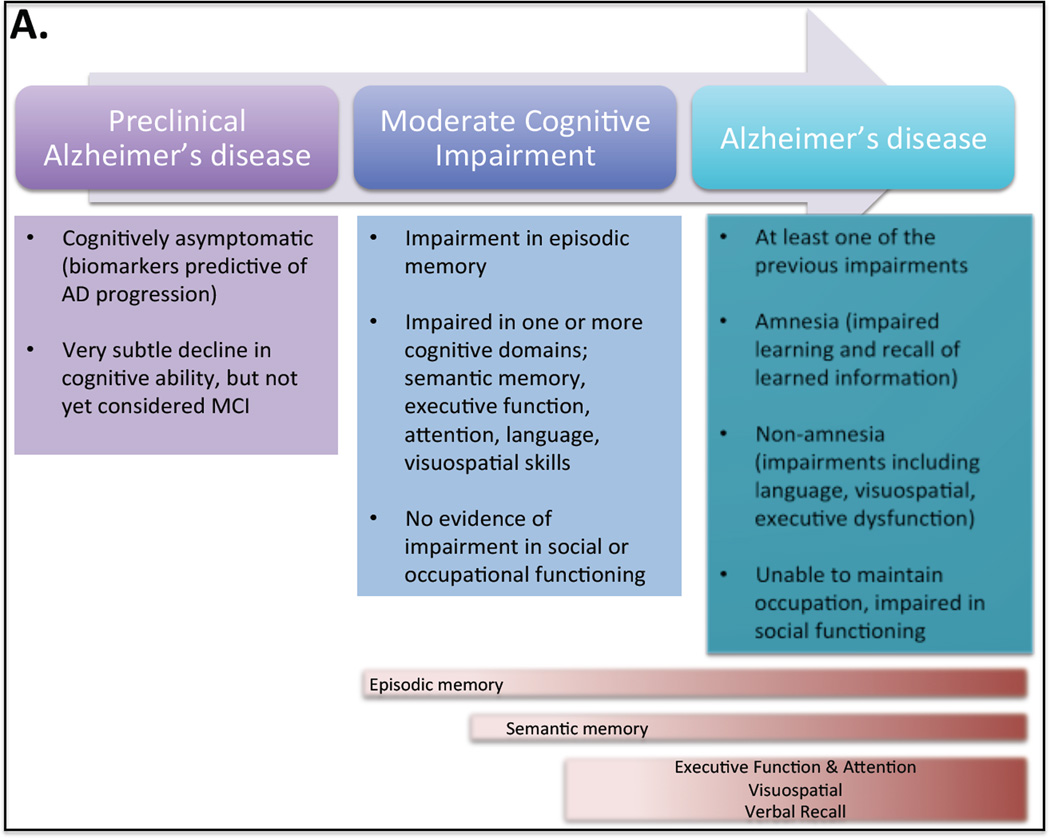
Fig. 5. Progression of Memory Impairments in preclinical, mild cognitive impairment and Alzheimer’s disease.
The National Institute on Aging and the Alzheimer’s Association have created a standardized criteria for the cognitive profile of the three stages of AD memory deterioration; (1) preclinical Alzheimer’s disease (AD), (2) mild cognitive impairment (MCI) and (3) AD dementia. Preclinical AD individuals commonly display biomarkers predictive of AD progression, but they are either asymptomatic or have very subtle cognitive deficits. MCI individuals that are likely to progress to AD dementia commonly have impairments in episodic memory. They can also be impaired in one or more cognitive domain including semantic memory, executive function, attention, language and visuospatial skills. AD dementia individuals will display deficits in one or more of the cognitive domains affected in AD as well as amnestic deficits that include impairment in learning and recall of recently learned information. Non-amnestic impairments may also be present in language, visuospatial and executive function. Commonly progression to AD consists of memory deficits in episodic memory followed by semantic memory and finally deficits in executive function, attention, visuospatial memory, and verbal recall. However there is variation among individuals in the exact progression of cognitive dysfunction.
5.3. Cognitive tests for Alzheimer’s disease dementia
There is no single cognitive test that is used to assess global cognitive function and AD dementia in humans. Instead, a battery of tests is required to assess a range of dissociable cognitive abilities. These tests are designed to be brief and to be easily used in a primary care physicians’ office. Examples include recall of a list of words or details of a story (i.e., The Word List Memory Test and Logical Memory test) (Welsh et al., 1994). There are many different cognitive battery tests, which assess a range of different cognitive domains, including the Wechsler Memory Scale Revised (WMS) or other versions, the Mini-Mental Status Examination, the Montreal Cognitive Assessment, the Short Blessed Test and the Alzheimer’s Disease Assessment Scale (for review (Webster et al., 2014)).
While most of the cognitive assessment tests are conversational in nature, patients with AD dementia also have difficulties with spatial orientation and navigation (Klein et al., 1999). This can progress to disorientation even in familiar locations such as their own neighborhood or home. Spatial memory is difficult to assess in a physicians’ office. In AD patients allocentric maps, in which the location of one object is defined relative to the location of other objects, have been examined by assessment of navigation inside a hospital (Cushman and Duffy, 2007, Cushman et al., 2008) or orientation in a circular arena (Hort et al., 2007). In contrast, egocentric analysis, determining the location of objects relative to the body axis of the individual, is often assessed using a table top Money Road Map test, which is a paper and pencil test to examine left right discrimination (Rainville et al., 2002).
In the best case scenario cognitive tests used in humans would also be used to study rodent behavior. However many of the tests performed in humans are verbal tests. It is important to consider whether a behavioral task is accurately analyzing the desired cognitive domain. Only in this way can a true comparison be made between humans and rodents so that we can understand the role of hippocampal neurogenesis in memory and AD in regards to specific aspects of memory dysfunction.
6. Behavioral Studies in Rodents
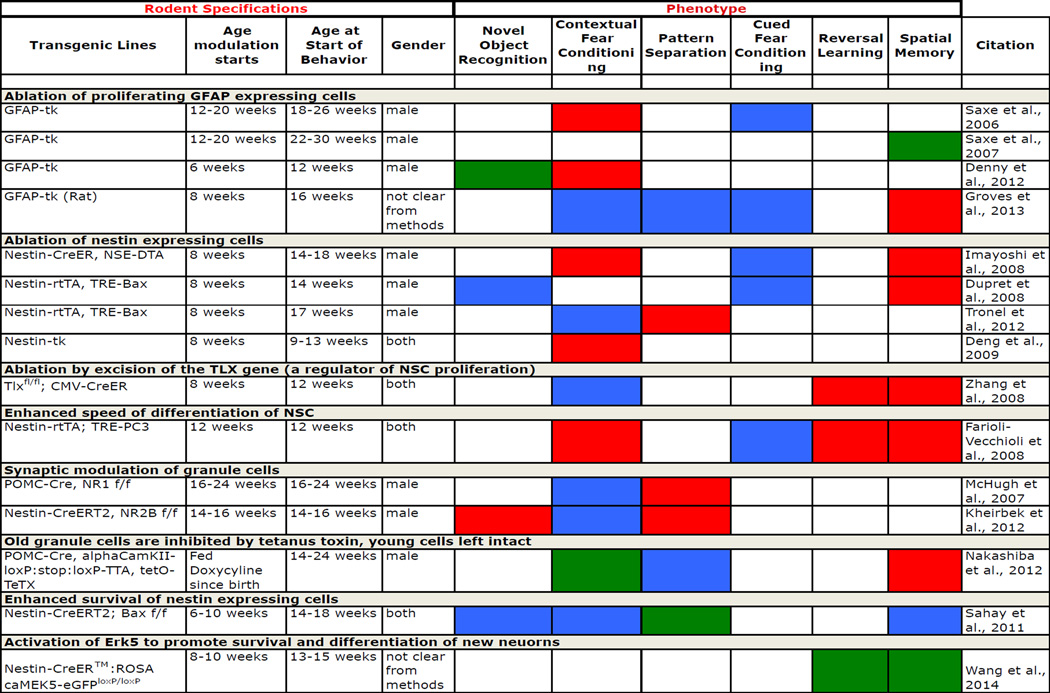
Specific manipulation of the extent of adult neurogenesis, either up or down, in rodents has been achieved through multiple means, including application of anti-mitotic agents, irradiation, environmental enrichment, anti-depressants and more recently creation of transgenic mice, in which neurogenesis is genetically modulated (van Praag et al., 2000, Warner-Schmidt and Duman, 2006, Deng et al., 2010, Koehl and Abrous, 2011, Sahay et al., 2011a, Marin-Burgin and Schinder, 2012). Utilizing differing techniques for modification of neurogenesis, differing behavioral tests as well as differing time lines for when neurogenesis was modulated and when the behavioral studies were administered makes comparison of the behavioral data a complicated challenge. Extensive review of hippocampal behavioral studies has been performed previously (for review (Deng et al., 2010, Koehl and Abrous, 2011, Marin-Burgin and Schinder, 2012)). For the purpose of this review we will highlight the role of adult neurogenesis only in cognitive domains that are affected in AD and describe results from recent genetic manipulations of neurogenesis as they provide the most specific analysis of how manipulating subpopulations of cells (i.e. type I NSC, type II NPC, type III NPC or new neurons) affects behavior (Table I). However it must be taken into consideration that even some of the genetic approaches to modulate production of new adult neurons have complications to their analysis. For example GFAP-TK (glial fibrillary acidic protein-driven herpes virus thymidine kinase) transgenic animals, in which ablation of GFAP expressing cells takes place following ganciclovir treatment, results in the ablation of both NSCs and mature astrocytes (Bush et al., 1999, Groves et al., 2013). This is due to GFAP expression in both type I NSCs and astrocytes. In that regard, Saxe et al 2007 suggested that ganciclovir treatment induces the selective death of dividing GFAP-positive cells, while nondividing astrocytes are spared (Saxe et al., 2007). Similarly many methods of reducing adult neurogenesis entail transgenic ablation of nestin expressing cells (Dupret et al., 2008, Imayoshi et al., 2008, Deng et al., 2009, Tronel et al., 2012). Nestin is expressed both in type I NSCs and Type IIa and IIb NPCs, with Type IIa NPCs producing glial cells and type IIb NPCs producing type III neuroblasts (Tobin et al., 2014). This means that both the neural population and the glial populations within the hippocampus will be reduced. To this point the specific impact of reduced gliogenesis in the adult hippocampus has not been examined. Teasing out the effect that this population has separate from neurogenesis will be necessary for the determination of glial contribution to the maintenance of the neurogenic niche and to hippocampal function.
Table I.
Genetic Modulation of Adult Neurogenesis and Resulting Behavioral Phenotypes
 No Impact
No Impact
 Impaired
Impaired
 Enhanced
Enhanced
Similarly it is important to note that many genetic efforts to ablate adult neurogenesis affect the neurogenic populations in both the SGL of the hippocampus and the SVZ. Modulation of the olfactory circuitry through the SVZ may have implications for learning and memory particularly in rodents who utilize the sense of smell to a far greater extent then humans. Interestingly, evidence from Imayoshi et al 2008 demonstrated that knockdown of adult neurogenesis does not inhibit olfactory learning. They show that following genetic ablation of nestin expressing cells in the SVZ and SGL of the hippocampus mice still had innate olfactory preference between two different odors (Imayoshi et al., 2008). In a more complex task mice with decreased adult neurogenesis were able to learn to associate a sugar reward with a particular odorant demonstrating acquisition of odor-associated memory. However, more studies are warranted for the understanding of the specific roles of the SVZ and SGL populations in different aspects of learning and memory.
6.1. Recognition memory (amnesia)
One of the primary cognitive deficits that underlie AD dementia is amnesia, which is a deficit in recognition memory. In humans the most commonly used test to assess this is the visual paired comparison task (Fig. 6). This task infers memory for familiar stimuli based on length of time spent looking at a novel or familiar stimuli. Other tasks, the behavioral pattern separation object task, the Modbent task or the three alternative forced choice task, are methods used in humans to assess recognition memory as it relates to pattern separation (Yassa et al., 2011, Brickman et al., 2014). In this task participants are shown a series of color pictures of objects. After the initial round new pictures of objects are displayed and the participants are asked if the object in the second round is the same as one seen before (old), a similar object (lure) or a new object (novel). Scoring a similar object (lure) as an old object suggests that the participants are biased towards pattern completion while scoring a similar object correctly indicates successful pattern separation.
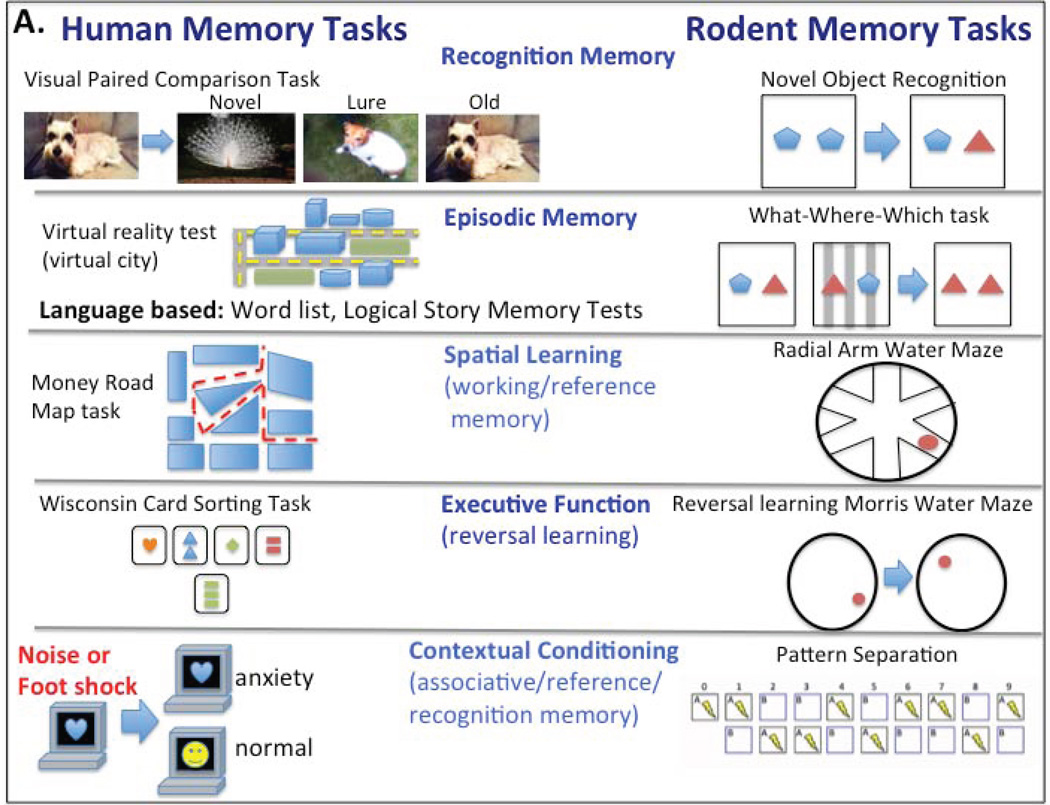
Fig. 6. Comparison of Human Versus Rodent Memory Assessment Methodologies.
There are many behavioral tasks that are used to study memory in humans and rodents. Unfortunatley the same exact task can not be used to study both species, making behavioral studies a challenge for comparing rodents and humans. Here are several examples of tasks in humans and rodents used to study recognition memory, episodic memory, spatial learning, executive function and contextual conditioning, highlighting the challenging nature of comparing behavior. Recognition memory: humans are analyzed with the visual paired comparison task (analysis for memory of a previously seen image, as compared to a similar image (lure), a different image and the old image) and rodents using the novel object recognition task (assessing memory of a previously seen object). Episodic Memory: humas are analysed using a virtual reality city test (driving through the city to create episodic memory) or language based tests such as the Word List or Logical Memory recall tests while rodent tests include the What-Where-Which task (a modification of the novel object recoginition task to include a contextual component). Spatial Learning: humans are analyzed with the Money Road Map test (a table top spatial test) and rodents using the radial arm water maze task (extra maze cues guide swimming to an escape platform). Executive Function: humans are analyzed using the Wisconsin Card Sorting Task (sorting cards by shape, color or number) and rodents using reversal learning in the morris water maze (changing the escape platform location). Contextual Conditioning: humans are analyzed with a contextual conditioning task (specific images are paired with a negative stimulus) while rodent studies utilize the pattern separation task (context is paired with a negative stimulus).
A limited number of studies have examined pattern separation in MCI and AD patients. Amnestic MCI patients have impairment in pattern separation meaning that they have difficulty discriminating the image of an old object from a similar but new lure object (Yassa et al., 2010, Stark et al., 2013). Similarly Brandon et al 2013 utilized a form of pattern separation test with increasing lag time between interfering objects to demonstrate that amnestic MCI patients are able to initially properly encode an object but quickly forget these memory representations (Ally et al., 2013). In contrast AD patients were unable to properly encode objects to begin with, regardless of lag time, showing an enhanced memory deficit as compared to amnestic MCI patients. Wesnes et al 2014 also recently examined the relationship between apolipoprotein E ε4 (ApoE4) status or cerebrospinal fluid (CSF) Aβ42 levels with performance in the pattern separation task (Wesnes et al., 2014). They observed deficits in pattern separation in ApoE4 homozygote patients and also saw that CSF Aβ42 levels correlated with poor performance in this task. Combined this data links poor performance in a hippocampal task to AD disease stage. Behavioral tests in rodents that analyze the ability of an animal to identify a previously presented object or context also assess recognition memory. The primary task for this in rodents is the Novel Object Recognition (NOR) task (Ennaceur and Delacour, 1988, Antunes and Biala, 2012). When rodents are presented with a familiar object and a novel object they display an unconditioned preference for the novel object (Ennaceur, 2010). This is interpreted to suggest that an encoded memory of the familiar object exists. The NOR test can be manipulated to examine short-term, and long-term memory simply by varying the length of time before exposure to the novel object (Taglialatela et al., 2009). Unlike NOR, pattern separation in rodents is commonly analyzed through a version of the contextual fear conditioning task (not cued). In this task rodents learn to associate an aversive stimulus, such as a foot shock, with a particular context, when each context, aversive and non-aversive, is very similar to each other. This task is more difficult to learn then when the two contexts are very different from each other and is thought to specifically evoke the hippocampal circuitry. Another method of examining pattern separation uses an alternative version of the radial arm water maze (RAWM) task that varies the distance between the goal arms (Groves et al., 2013). Noticeably, these tests involve a combination of cognitive domains and the participation of several brain areas. For example, the contextual fear conditioning-based pattern separation test involves the participation of the amygdala, and requires spatial reasoning, visual processing etc. Thus, favorably, the targeting of a specific form of memory should be done by using more than one task.
Analysis of recognition memory tasks in rodents has demonstrated an important role for adult neurogenesis in enhancing long-term memory recognition. Using an inducible MEK5 MAP kinase knockin that specifically activates extracellular-signal-regulated kinase 5 (ERK5), enhancement of adult neurogenesis was shown to extend memory for novel objects or novel object locations from one hour to forty-eight hours (Wang et al., 2014). ERK5 is critical for promoting survival and differentiation of new adult neurons (for review (Pan et al., 2013)). A different method of enhancing adult neurogenesis by excising the pro-apoptotic gene Bax in Nestin expressing cells, to enhance survival of new neurons, also examined the NOR task (Sahay et al., 2011a). However in this case they had an intertrial interval of only three minutes between the familiar objects and the novel object probe test. This study confirmed the previous result that no deficit in NOR was observed using a short-term memory assessment.
The specific electrophysical characteristics of adult born neurons have also been shown to play an important role in the NOR task. Impairment of LTP by genetic deletion of the NR2b–containing NMDA receptor in four to six week old adult born neurons results in decreased exploration in the NOR task (Kheirbek et al., 2012). As mentioned above, four to six week old neurons are known to exhibit heightened plasticity. Deficits in the NOR task when this specific population is modified suggests that the varying electrophysical characteristics of new neurons is essential for their contribution to functional outcomes in recognition memory.
Similar to the NOR task, probing recognition memory by analyzing pattern separation also shows an important role for adult neurogenesis. This is highlighted by the fact that ablation of nestin-expressing cells results in deficits in this task (Tronel et al., 2012). Interestingly probing the impact of heightened synaptic plasticity in young neurons by knockouts of either the NRI (in PomC expressing granule cells) or NR2B (in nestin expressing NSC) subunits of the NMDA receptor resulted in deficits in pattern separation, although no deficits were seen in contextual fear conditioning (McHugh et al., 2007, Kheirbek et al., 2012). This suggests an important role for young synaptically active neurons in pattern separation. Surprisingly contrasting analysis of ablation of mature granule cells resulted in enhancement of pattern separation. When transmission of mature granule cells (POMC expressing cells) is inhibited by expression of tetanus toxin mice are able to distinguish the context faster then controls (Nakashiba et al., 2012). Similarly Nakashiba et al 2012, observed enhancement in pattern separation when they ablated mature granule cells (Nakashiba et al., 2012). Combined this suggests an important role of young neurons in pattern separation and that in contrast removal of adult granule cells may be eliminating an inhibitory signal, which allows for the enhancement in learning. Given that ablation of adult neurogenesis has a negative impact on cognitive function the targeted enhancement of this population should increase cognitive performance. This has proved true in the case of pattern separation, enhancement of neurogenesis by increasing the rate of survival of NPCs through excision of the pro-apoptotic gene Bax resulted in mice that were more efficient in differentiating between overlapping contextual representations (Sahay et al., 2011a).
While these studies have demonstrated a role for adult neurogenesis in recognition memory there is some conflicting results. Ablation of GFAP expressing cells resulted in enhanced exploration in the NOR task, four to six weeks following ablation (Denny et al., 2012). This result conflicts with the decreased exploration observed when LTP is impaired in four to six week old hippocampal neurons. In addition to differences in the targeted populations, another major difference is that in the case of the GFAP ablated mice two animals were tested simultaneously in adjacent boxes. Potentially the addition of the second mouse in some way impacted the activity level of the animals, however one would expect a similar increase in controls if this were the case.
Deficits in recognition memory have also been observed in the majority of AD mouse models at multiple ages (for review (Webster et al., 2014)). For example 5xFAD animals exhibit impairment in the NOR task as early as three to five months of age (Shukla et al., 2013). In contrast 3x Tg-AD animals don’t exhibit deficits in the same task until nine to eleven months (Clinton et al., 2007). Cognitive dysfunction is observed with varying severity and timelines in these models, likely as the result of differences in the specific transgenic method and the different FAD forms that are expressed.
6.2. Episodic memory
One of the earliest cognitive domains disrupted in AD is episodic memory. In humans this has been assessed in MCI and AD patients by the use of virtual reality environments, such as a virtual city, in which the patient ‘drives’ around. Following the task, components of episodic memory such as factual, spatial, temporal, and binding ability, which is the linking of the multiple components of a memory, are analyzed (Plancher et al., 2012). Episodic memory is also tested in humans using several language tests, including the Word List Memory test, Word List Recall test and Word List Recognition test (Welsh et al., 1994). Also story based tests such as the Logical Memory Story A from the Wechsler Memory scale are often used (Weschler, 1987). In rodents the recently described “What-Where-Which” task (WWWhich task), is an episodic-like task, in which animals are tested for the ability to associate an object (what), with its location (where) and its visuospatial context (which occasion) (Davis et al., 2013a, Davis et al., 2013b). Here the context is being used to distinguish a distinct experience or event. It has been shown through lesion studies that the ability to successfully associate these three factors, what, where, which occasion, is dependent on an intact hippocampus (Eacott and Norman, 2004, Langston et al., 2010, Langston and Wood, 2010). Also using this task an age dependent decline can be observed in the 3xTg-AD mouse model (Davis et al., 2013a, Davis et al., 2013b). An alternate version of this task distinguishes the episodic event using a time component in place of the visuospatial context, the What-where-when task (WWWhen task), however it is thought that this version of the task does not truly probe episodic memory. Testing humans using similar versions of the WWWhich and WWWhen rodent specific tasks led to the suggestion that tasks using a time component could potentially be solved with non-episodic strategies, such as decaying trace strength (Easton et al., 2012). While tasks using contextual information to identify the event could only be accurately performed by using recollection, a key feature of episodic memory. It is important to consider how to accurately assess the role of episodic memory in rodents because of its early disruption in AD. Given the temporally regulated birth of groups of DG cells one possible role for adult neurogenesis could lie in encoding the time component of episodic memories (Aimone et al., 2006). However while this task has been examined in lesioned animals genetic methods of ablating adult neurogenesis have not yet been used. To understand the role of new neuronal populations and also their specific electrophysical characteristics in AD dysfunction it will be essential to probe episodic memory tasks, such as the WWWhich task, that accurately represent this memory domain.
6.3. Spatial learning (working memory, reference memory)
Spatial learning tasks are often used to analyze deficits in working memory (short term memory) and in reference memory in mice. Active spatial memory tests in humans include but are not limited to the Jigsaw Puzzle task, where a puzzle is solved by describing the order of the pieces not by moving them, the Active Visual Pattern task, where participants must fill in squares on a matrix matching a previously seen pattern, and the Pathway Span task, where participants must describe the final position of a man based on a series of turns (Cornoldi et al., 1995, Della Sala, 1997, Vecchi and Richardson, 2000, Mammarella et al., 2008). In contrast examples of passive spatial tasks include the Corsi Block-Tapping test, in which a researcher taps a sequence of blocks and the participant must repeat the sequences with the task gradually increasing in complexity, the Dots Reproduction task, testing remembrance of the location of dots in a white space, and the Static or Dynamic Maze tasks, in which a pathway out of a 2-D maze is remembered (Berch et al., 1998, Pickering et al., 1998, Mammarella et al., 2008). Another common human spatial task is the table top Money Road Map test, a paper and pencil assessment of left right discrimination (Vingerhoets et al., 1996).
One important consideration when analyzing spatial navigation is the size of the environment. Vista space, or space that can be seen all at one time (i.e. a room) requires less complex decisions and length of memory retention than large environmental spaces, or areas that can only be experienced through a great deal of movement (i.e. a city). In a larger environmental space the goal might be out of view, and complex routes may be needed to reach it, extending the length of time and the difficulty of the task (for review (Wolbers and Wiener, 2014)). This is an important consideration for behavioral testing since many rodent tests of spatial navigation are indeed vista space tests. Examples include the MWM, T-, Plus- or Y-maze spatial navigation tests. In each of these the entire test space, cues and goal are visible at all times by the animal. In contrast in the RAWM test the animal is not able to see the whole extent of the testing arena at all times and so this test can be used as an analysis of environmental space. In humans to examine spatial navigation in large environmental spaces virtual reality environments have been created such that the participant is able to ‘drive’ through a city thus imitating real complex activities that engage working and reference memory, as well as episodic memory (Plancher et al., 2013). When considering animal models and their relevance to human spatial navigation it is also important to consider the scale of the environment and thus the complexity of the task.
Adult hippocampal neurogenesis has been shown to play a role in spatial navigation. Ablation of nestin-expressing cells impaired relational memory specifically in allocentric (in the MWM task, the start position of the mice was varied but the platform location remained the same) rather than egocentric tasks (the start position of the mice and location of platform did not change) (Dupret et al., 2008). An alternative approach used upregulation of the PC3 (Tis21/BTG2) gene, known to induce terminal differentiation of NPCs, inappropriately in type I NSCs and type II NPCs. Shifting NSCs and NPCs from proliferation to differentiation resulted in impairment in the MWM and RAWM tasks (Farioli-Vecchioli et al., 2008). Deficits observed after speeding up of the differentiation process indicates that early stages of neurogenesis, when distinct morphological and electrophysical characteristics are present, are indeed necessary for the correct functional integration of these cells into the mature hippocampal circuitry (Farioli-Vecchioli et al., 2008).
Further, adult neurogenesis is thought to regulate specific aspects of short- and long-term spatial memory, though the details of this are still controversial. Imayoshi et al. 2008 observed impairment in retention of long-term spatial memory (Barnes Maze probe test one week later) but not short-term memory (inter-trial memory during task) following the ablation of nestin-expressing cells (Imayoshi et al., 2008). However, interestingly, using transgenic deletion of the TLX (NR2E1) gene, a cell autonomous NSC regulator, Zhang et al 2008 observed deficits in short term memory (MWM probe test twelve hours later) while long term memory (three weeks later probe test) was not affected (Zhang et al., 2008). These differences could be related to differences in the length of time between the short or long term memory probe tests which varied significantly between the two investigators, or the respective contribution of the affected populations. Similar to these results Saxe et al 2007 observed differences in performance in the low memory load /limited interference (LML/LMI) version of the radial arm task with varying time lengths between trials and only two arm choices (Saxe et al., 2007). At thirty-five seconds there was no difference between groups but when the delay was increased to fifty-five seconds or above mice with ablated GFAP expressing cells had enhanced performance on the radial arm task, suggesting that in this case ablation of new neurons appeared to relieve an inhibitory constraint on working memory, potentially by eliminating irrelevant information from previous trials. In a separate study, enhancing neurogenesis by nestin-driven bax excision led to no improvement, or deficit, in the MWM task (Sahay et al., 2011a). However in MEK5 transgenic animals, enhanced neurogenesis results in enhanced learning on the MWM task, however only when shorter periods of training were used. Perhaps the discrepancy between these two results can be explained by the differing training regimes, with longer training periods the MEK5 animals also showed no difference compared to controls it was only when the test was made more challenging that the subtler differences were revealed.
An interesting study by Nakashiba et al. 2012 examined the contribution of older granule cells (six weeks of age and older) by specifically inhibiting them with tetanus toxin while leaving the younger highly excitable and synaptically plastic granule cells intact. Using this method they were able to probe the different roles of young and old granule cells. Interestingly ablation of old granule cells resulted in no impairments in the RAWM task. However, they did observe deficits in speed of pattern completion in the MWM task, but not in recall (Nakashiba et al., 2012), indicating that when mice are given sufficient time they are able to find the platform. This suggests interesting conceptual overlap with the previously discussed pattern separation studies. Here the pattern to be completed is the pathway to the goal platform. Their results suggest that old granule cells may favor pattern completion while young granule cells favor pattern separation. This highlights the difficulty of analyzing behavioral studies because varying approaches, spatial learning or pattern separation, can be used to solve for a single task. A similar approach was taken by Tronel et al. 2015 who utilized two thymidine analogs to analyze new neurons that are six weeks old compared to three months old (Tronel et al., 2015). They observed that both groups are equally activated during spatial learning. Taken together with the Nakashiba et al. 2012 study, this may suggest that while both populations are needed for spatial memory they are likely performing differing tasks. In contrast, reducing neurogenesis by ablating GFAP-expressing cells in rats did not affect their performance in the water maze or RAWM tasks (Groves et al., 2013). Beyond the discussion above about the ablation of populations other than NPCs in this approach, there might also be a species-related effect that is yet to be explored.
Analysis of mouse models of AD, using many forms of spatial tasks has demonstrated deficits in spatial learning (Dineley et al., 2002, Westerman et al., 2002, Trinchese et al., 2004, Comery et al., 2005, Jacobsen et al., 2006, Riddell et al., 2007, Saydoff et al., 2013). In particular the MWM is the most commonly assessed task with deficits observed as early as 3–5 months in the 5xFAD and 3xTg-AD and 6–8 months in the APP/PS1 familial AD mouse models (Lalonde et al., 2004, Ohno et al., 2006, Clinton et al., 2007). Beyond MWM, AD mouse models also show deficits in the RAWM, Barnes Maze, and Y/T-Maze tasks (Webster et al., 2014). Combined these results demonstrate a clear link between AD mouse models and deficits in spatial memory.
6.4. Executive function (reversal learning)
Another important cognitive function disrupted in AD is executive function. Executive function is higher cognitive reasoning such as cognitive flexibility, response inhibition, planning and abstract concept formation. In humans tests to examine this include the Wisconsin Card Sorting Task (Eling et al., 2008), a card sorting task in which the individual must adjust to changing criteria, and the intradimensional/extradimensional task (Owen et al., 1991), which examines attentional set-shifting and reversal learning. Other tests for executive function include the Standard Progressive Matrices test, Category Fluencey Test, Controlled Oral Word Association and Trial-Making test (Reitan, 1958, COWA: A.L. Benton, 1989). This type of memory is also analyzed in mice through a similar attentional set-shifting task, multiple choice serial reaction time task, and reversal learning tasks. Set shifting tasks require the mouse to shift between different types of stimuli to discriminate between bowls of food in which a reward is hidden. The multiple choice serial reaction time task models response inhibition and attention in rodents. In this task a five hole operant box is used and the mouse has to monitor the appearance of brief stimulus, such as light projected in one of the holes. Nose poke into the correct hole is met with a reward (de Bruin et al., 2006). In reversal learning mice are tested for cognitive flexibility and impulse control. They are trained to associate a particular stimulus with a reward, then the stimulus is reversed and the mouse must relearn the association of the reward with a previously unrewarded stimulus (Bussey et al., 1997). This task is also commonly used in water maze tasks where the goal or escape quadrant is reversed following training.
Studies for the assessment of the role of adult hippocampal neurons in executive function have primarily focused on reversal learning. Enhancing the speed of differentiation of NSCs and type II NPCs by expression of PC3 impairs reversal learning in the MWM task (Farioli-Vecchioli et al., 2008). Mice were also impaired in learning performance in previously experienced tasks. In this case mice that successfully completed the MWM were then subjected to inhibition of neurogenesis. These animals were not able to repeat the MWM task, meaning they could not learn a new location of the goal platform (Farioli-Vecchioli et al., 2008). Depletion of neurogenesis by genetic excision of TLX also produced deficits in reversal learning in the MWM (Zhang et al., 2008). Significantly, MEK5 transgenic mice exhibited improvement in reversal learning in the MWM task when neurogenesis was enhanced starting at eight to ten weeks and the MWM task was administered at thirteen to fifteen weeks (Wang et al., 2014). Combined this data shows that new neurons in the hippocampus are required for the use of previously consolidated memory in executive function tasks.
Many AD mouse models also display deficits in executive function. Marchese et al 2013 observed impairments in cognitive flexibility in 3xTg-AD mice when testing reversal learning in the MWM task (Bussey et al., 1997). Tg2576 mice also exhibit deficits in the attention set shifting task (Zhuo et al., 2007). At 6 months in two-choice compound discrimination the mice are able to learn the location of the reward but upon reversal continue digging in the previous reward location. The 3xTg-AD mouse model also has deficits in the multiple choice serial reaction time task, a task that analyzes response inhibition or the ability to appropriately respond to a stimulus (Romberg et al., 2011). Together these animal studies demonstrate a clear deficit in multiple aspects of executive function in AD mouse models.
6.5. Contextual conditioning (associative learning/memory, reference/recognition memory)
Contextual conditioning, also known as associative learning, is in simple terms the ability to learn to anticipate events. In humans contextual fear conditioning tasks are performed where participants are exposed randomly to two different context and an un-signaled aversive stimuli (foot shock or loud noise) is consistently associated with one. Skin conductance and anxiety ratings are assessed to determine whether the participant has learned to associate the aversive stimuli with the correct context (Alvarez et al., 2008).
Classic rodent behavioral tests of associative learning include contextual fear conditioning, cued fear conditioning, pattern separation and delay/trace conditioning (Peter Curzon, 2009). The hippocampus has been well studied in regards to its role in contextual conditioning and pattern separation (as discussed previously). It is important to remember that there is overlap for pattern separation in both contextual conditioning as well as recognition memory. For this review we discussed pattern separation in regards to recognition memory however it is important to remember that this task is based on a contextual fear conditioning protocol, it is simply the enhanced difficulty of the task (through the very similar contexts) that makes it specific for assessment of pattern separation. But a similar strategy of training rodents to associate an aversive stimulus with a specific context or cue is employed.
Deficits in contextual conditioning tasks have been observed in rodents by ablating adult neurogenesis in the DG, through several different transgenic methods. Reduction in the number of new neurons either by decrease in GFAP-expressing cells or decrease in Nestin-expressing cells resulted in deficits in contextual fear conditioning but not cued fear conditioning (Saxe et al., 2006, Imayoshi et al., 2008). Similarly by enhancing the maturation of granule cells, to decrease the pool of NSC, Farioll-Vecchioll et al. 2008 also observed deficits in contextual but not cued fear conditioning (Farioli-Vecchioli et al., 2008).
Similar to other behavioral tasks, the timing of ablation of adult neurogenesis impacts the ability of the rodents to successfully perform these tests. Denny et al 2012 observed deficits in contextual fear conditioning at six weeks following ablation of GFAP-expressing cells but not at four weeks (Denny et al., 2012), while Deng et al 2009 saw no affect on contextual conditioning at five weeks but did see a deficit in extinction (Deng et al., 2009). Given that enhanced synaptic plasticity is observed in new neurons that are four to six weeks old, this data suggests that complete elimination of this population by ablation for at least six weeks is necessary for a behavioral phenotype to be observed.
Some studies, however, report conflicting observations. For example, Deng et al 2009 observed no deficits in contextual conditioning but observed deficits in extinction, when ablating nestin expressing cells (Deng et al., 2009). Similarly ablation of the TLX-expressing cells had no effect on the performance of these mice in contextual conditioning (Zhang et al., 2008). Also Groves et al 2013 observed no deficits in contextual fear conditioning when ablating GFAP expressing cells in rats (Groves et al., 2013).
Multiple AD mouse models have also demonstrated deficits in contextual conditioning. For example, eleven-month-old APPswe/PS1ΔE9 animals spend less time freezing in response to the aversive shock context then control animals (Cramer et al., 2012). Similarly Tg2576 animals at three to five months of age displayed deficits in contextual conditioning (Dineley et al., 2002).
7. Means of enhancing adult neurogenesis
Given the role of adult neurogenesis in many of the memory domains affected in AD and the fact that upregulation of hippocampal neurogenesis enhances aspects of contextual conditioning, executive function, recognition and spatial memory, this may suggest that enhancement of neurogenesis may be a therapeutic target for AD (Sahay et al., 2011a, Wang et al., 2014). However, given the behavioral differences and different outcome among the different approaches described above, it should be cautiously considered what neurogenic population should be targeted or manipulated. Unfortunately, we are lacking approaches that exclusively and specifically modulate human neurogenesis. Thus, the information described here is of methods that enhance brain plasticity, and may directly or indirectly enhance neurogenesis.
Based on information from rodent studies, it is apparent that neurogenesis can be enhanced by lifestyle factors such as learning, enrichment or exercise. In particular exercise has been linked to increased cognitive performance in the elderly (Ahlskog et al., 2011). Even as brief an intervention as six to twelve months of enhanced physical activity can increase the volume of the hippocampus by up to two percent and improve spatial and episodic memory (Klusmann et al., 2010, Erickson et al., 2011, Ruscheweyh et al., 2011). These results suggest that exercise may be a potential lifestyle factor for the maintenance of high brain plasticity.
Another, indirect method of enhancing the function of the hippocampus is the recently described consumption of dietary flavanols. Oral consumption of the plant-derived epicatechin can enhance both capillary and dendritic spine density in the DG and combining this with exercise further improves this phenotype (van Praag et al., 2007). Recently Brickmann et al 2014 examined the effect of a high flavanols diet in human cognition (Brickman et al., 2014). They observed a significant improvement in a pattern separation task, the ModBent task, in participants who were fifty to sixty-nine years of age after twelve weeks of a 900 mg daily dose of flavanols. Demonstrating that enhancement of the hippocampal niche by improved blood flow can impact cognitive function.
It also has been demonstrated that chronic administration of anti-depressant drugs such as 5-HT or NE selective reuptake inhibitors enhance adult neurogenesis (Warner-Schmidt and Duman, 2006). Treatment with anti-depressants increases both proliferation and survival of newborn neurons (Malberg et al., 2000, Nakagawa et al., 2002). However using this approach as a means of enhancing neurogenesis and potentially cognitive function in AD is not so straightforward. Recent work suggests that the hippocampus is divided anatomically into functionally distinct regions, with the dorsal hippocampus required for learning and spatial memory while the ventral hippocampus is required to modulate emotion and reward behaviors (Fanselow and Dong, 2010). This hypothesis is supported by efferent connectivity from the ventral hippocampus to the nucleus accumbens, prefrontal cortex and amygdala, which regulate reward and emotional behavior (Sahay and Hen, 2007). Also treatment with the antidepressant agomelatine, a receptor agonist for both the 5-HT2C and melatonin receptors has been shown to increase neurogenesis specifically in the ventral hippocampus (Banasr et al., 2006). While there has been some evidence that antidepressants can suppress aging associated deficits in the MWM task in rats, the specific cognitive impact of antidepressants in AD animal models has not been demonstrated (Yau et al., 2002). Meta-analysis by Tiffany et al 2007 examining human clinical trials revealed that selective serotonin reuptake inhibitors (SSRIs) did increase cognitive performance in some AD patients, however it was not clear from their study if this improvement was simply the result of mood stabilization or a true cognitive enhancement (Chow et al., 2007). More recently Sepehry et al 2012 used a similar meta analysis approach and found no correlation between SSRI treatment and cognitive improvement in AD patients using the Mini Mental State examination method (Sepehry et al., 2012). From these results it is clear that the role of anti-depressants in cognitive performance in AD needs further analysis. While anti-depressants do indeed regulate adult neurogenesis they may be targeting a different functional domain and so potentially other pharmacological means should be explored.
There are also a limited number of drug studies that are aiming to enhance adult neurogenesis. Isoxazole 9 (Isx-9) a synthetic small molecule enhances proliferation, differentiation and dendritic complexity of new neurons through a pathway involving the myocyte-enhancer family of proteins (Mef2) (Schneider et al., 2008, Petrik et al., 2012). Significantly treating mice for 12 days with Isx-9 followed by three weeks of recovery enhanced spatial learning (Petrik et al., 2012). They observed improvement in the MWM task during the probe test, but not during training. However in other tasks, contextual or cued fear conditioning, there was no difference between treated and control groups. Another chemical aminopropyl carbazole, designated P7C3, was shown to protect new neurons from apoptosis (Pieper et al., 2010). Analyzing eighteen-month-old rats they observed improvement in performance on the probe test for the MWM following two-month treatment with P7C3. Similarly Wang et al. demonstrated that metformin, a drug used to treat type 2 diabetes, also enhances neurogenesis and that treatment with metformin for thirty-eight days improved performance in mice in reversal learning in the MWM task (Wang et al., 2012). This enhancement of neurogenesis was shown to occur through the activation of the atypical protein kinase C (aPKC)-CBP pathway. Finally another small molecule (KHS101) has been shown to accelerate neuronal differentiation by interaction with TACC3 however the affect on cognitive performance has not yet been examined (Wurdak et al., 2010).
Many of these molecules were discovered using in vitro stem cell based screening methods that allow for high through put analysis of chemical libraries (Ding and Schultz, 2004, Lipinski and Hopkins, 2004). Instead of using a library designed to target a specific protein or pathway large diverse chemical libraries are examined for the ability to produce a specific phenotype in stem cells, such as differentiation into a neuron. This approach requires subsequent analysis of how such small molecules are acting mechanistically, however it has the added benefit of potentially identifying mechanisms of neural differentiation that have not yet been discovered.
8. Future perspectives
Adult neurogenesis is a highly dynamic process that occurs throughout life in both rodents and humans. NPCs and new neurons are highly plastic and are essential for learning and memory. Neurogenesis can be modulated by many environmental and lifestyle factors including but not limited to, diet, learning, environmental enrichment, exercise, antidepressants, depression, hormones, social isolation, aging or irradiation. It is becoming clear that adult neurogenesis can either be enhanced or suppressed at multiple time points over an individual’s lifespan and that changes in proliferation and incorporation into the mature circuitry of the hippocampus impact learning and memory and hippocampal function. Given the complimentary cognitive domains that are disrupted when lesioning or ablating adult neurogenesis in rodents, FAD models and humans, as well as the specific vulnerability of the hippocampus to normal aging and AD pathologic progression, this suggests that cognitive deficits observed in AD may be linked to hippocampal neurogenesis.
Current research has separately assessed adult neurogenesis and Alzheimer’s disease, moving forward it is important to consider the interplay between these two factors. Can we accelerate the cognitive decline observed in AD by ablating adult neurogenesis in familial AD mouse models or alternatively can enhancement of adult neurogenesis ameliorate cognitive deficits observed in AD models? Behavioral studies in rodents have designed several tasks that can be used to specifically probe aspects of AD cognitive decline. In these future studies it will be important to examine tasks that accurately reflect the progression of AD dementia. Unfortunately many studies examining supposedly similar animal behavior tasks utilize different procedures. This is particularly true regarding timelines for what is considered short or long term memory probe tests. Difficulties in comparison are also further compounded by differences in animal model, which must be considered. Appropriately timing the ablation of neurogenesis (what cell type is ablated and at what age), the assessment of the task (either before or after cognitive dysfunction arises) and choosing the correct behavioral paradigm for analysis of AD cognitive deficits, will be essential for meaningful conclusions.
Recent studies have confirmed the presence of adult neurogenesis in humans however little is known about its role in AD and it remains to be seen whether alterations in adult neurogenesis have similar impacts on human learning and memory as is observed in rodents. While identical gain and loss of function studies cannot be performed in humans as they are in rodents, it is possible to statistically correlate levels of neurogenesis with cognitive ability using postmortem analysis of aged patients. However, elucidating this role will require a large cohort of patients, with varying medical histories, cognitive ability, lifestyles and activity level, since all these factors have been shown to modulate levels of neurogenesis in rodents. While challenging establishing this correlation will be essential to demonstrating in humans the importance of modulating adult neurogenesis for learning and memory and will further validate this key therapeutic target for amelioration of cognitive deficits observed in AD.
A number of limited studies in rodents have examined pharmacological means of enhancing adult neurogenesis. These studies show promising results but must be expanded to include a larger battery of behavioral studies and analysis of the cognitive effects on familial AD mouse models. It will be essential to determine to what extent modulating neurogenesis can rescue cognitive dysfunction in AD models. This will be particularly important to analyze in regards to timing of intervention. Given the decline in neurogenesis prior to AD pathological hallmarks, including amyloid deposition and hyperphosphorylated tau, treatment to enhance neurogenesis may have to be given in the asymptomatic phase, or during MCI, to be effective. This is an important consideration for clinical trial design given that humans undergo a long preclinical stage prior to MCI and AD. It is clear that a growing body of work supports the hypothesis that impaired hippocampal adult neurogenesis underlies cognitive dysfunction observed in AD. It is an exciting time when we might finally achieve the therapeutic means to prevent or delay cognitive impairments, the most devastating characteristics of this disease.
Acknowledgments
The authors would like to acknowledge financial support from NIH/NIA R01AG033570 and RC1AG036208-01.
This study was supported by: NIH/NIA R01AG033570 and RC1AG036208-01.
Abbreviations
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- ApoE4
apolipoprotein E ε4
- aPKC
atypical protein kinase C
- BLBP
brain lipid-binding protein
- BrdU
5-bromo-2'-deoxyuridine
- CREB
cAMP response element binding protein
- CSF
cerebrospinal fluid
- DG
dentate gyrus
- DCX
doublecortin
- ERK5
extracellular-signal-regulated kinase 5
- FAD
familail AD
- GFAP
glial fibrillary acidic protein
- GCL
granule cell layer
- Isx-9
Isoxazole 9
- LML/LMI
low memory load /limited interference
- Hes5
mammalian hairy and enhancer-of-split homologs
- Ascl1
mash1
- MAM
methylazoxy-methanol acetate
- MCI
mild cognitive impairments
- MWM
Morris water maze
- Mef2
myocyte-enhancer family of proteins
- NPC
neural progenitor cells
- NSC
neural stem cells
- NeuroD1
neurogenic differentiation1
- PSA-NCAM
polysialated neural cell-adhesion molecule
- PS1
presenilin 1
- PS2
presenilin 2
- Prox1
prospero homeobox protein 1
- RAWM
radial arm water maze
- sox1 and sox2
sex-etermining region Y-box 1,2
- sAPPα
soluble amyloid percursor protein alpha
- SGL
subgranular layer
- SVZ
subventricular zone
- WWWhen task
What-where-when task
- WWWhich task
“What-Where-Which” task
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest on the content of this review.
References
- Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical Exercise as a Preventive or Disease-Modifying Treatment of Dementia and Brain Aging. Mayo Clinic Proceedings. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14:325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Hussey EP, Ko PC, Molitor RJ. Pattern Separation and Pattern Completion in Alzheimer's Disease: Evidence of Rapid Forgetting in Amnestic Mild Cognitive Impairment. Hippocampus. 2013;23:1246–1258. doi: 10.1002/hipo.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci. 1990;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- Amaral DGaL P. The Hippocampus Book. Oxford University press; 2007. [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087–1096. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Baratchi S, Evans J, Tate WP, Abraham WC, Connor B. Secreted amyloid precursor proteins promote proliferation and glial differentiation of adult hippocampal neural progenitor cells. Hippocampus. 2012;22:1517–1527. doi: 10.1002/hipo.20988. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Neuron production in the hippocampus and olfactory bulb of the adult rat brain: addition or replacement? Ann N Y Acad Sci. 1985;457:163–172. doi: 10.1111/j.1749-6632.1985.tb20804.x. [DOI] [PubMed] [Google Scholar]
- Berch DB, Krikorian R, Huha EM. The Corsi block-tapping task: methodological and theoretical considerations. Brain Cogn. 1998;38:317–338. doi: 10.1006/brcg.1998.1039. [DOI] [PubMed] [Google Scholar]
- Binder DK. The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications. 2007 [Google Scholar]
- Blackstad TW, Brink K, Hem J, Jeune B. Distribution of hippocampal mossy fibers in the rat. An experimental study with silver impregnation methods. J Comp Neurol. 1970;138:433–449. doi: 10.1002/cne.901380404. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24:1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Khan UA, Provenzano FA, Yeung LK, Suzuki W, Schroeter H, Wall M, Sloan RP, Small SA. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. 2014;17:1798–1803. doi: 10.1038/nn.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Stern Y, Small SA. Hippocampal subregions differentially associate with standardized memory tests. Hippocampus. 2011;21:923–928. doi: 10.1002/hipo.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Chow TW, Pollock BG, Milgram NW. Potential cognitive enhancing and disease modification effects of SSRIs for Alzheimer's disease. Neuropsychiatr Dis Treat. 2007;3:627–636. [PMC free article] [PubMed] [Google Scholar]
- Chuang TT. Neurogenesis in mouse models of Alzheimer's disease. Biochim Biophys Acta. 2010;1802:872–880. doi: 10.1016/j.bbadis.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, McGaugh JL, LaFerla FM. Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol Dis. 2007;28:76–82. doi: 10.1016/j.nbd.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, Zhou H, Kreft AF, Pangalos MN, Sonnenberg-Reines J, Jacobsen JS, Marquis KL. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2005;25:8898–8902. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornoldi C, Dalla Vecchia R, Tressoldi PE. Visuo-spatial working memory limitations in low visuo-spatial high verbal intelligence children. J Child Psychol Psychiatry. 1995;36:1053–1064. doi: 10.1111/j.1469-7610.1995.tb01350.x. [DOI] [PubMed] [Google Scholar]
- COWA: A.L. Benton KDH. Multilingual Aphasia Examination Iowa City: AJA Associates; 1989. [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman LA, Duffy CJ. The sex specificity of navigational strategies in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:122–129. doi: 10.1097/WAD.0b013e318047df2f. [DOI] [PubMed] [Google Scholar]
- Cushman LA, Stein K, Duffy CJ. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology. 2008;71:888–895. doi: 10.1212/01.wnl.0000326262.67613.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KE, Eacott MJ, Easton A, Gigg J. Episodic-like memory is sensitive to both Alzheimer's-like pathological accumulation and normal ageing processes in mice. Behav Brain Res. 2013a;254:73–82. doi: 10.1016/j.bbr.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Davis KE, Easton A, Eacott MJ, Gigg J. Episodic-like memory for what-where-which occasion is selectively impaired in the 3xTgAD mouse model of Alzheimer's disease. J Alzheimers Dis. 2013b;33:681–698. doi: 10.3233/JAD-2012-121543. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, Fransen F, Duytschaever H, Grantham C, Megens AA. Attentional performance of (C57BL/6Jx129Sv)F2 mice in the five-choice serial reaction time task. Physiol Behav. 2006;89:692–703. doi: 10.1016/j.physbeh.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Gray C, Baddeley A, Wilson L. The Visual Patterns Test: A new test of short-term visual recall Feltham. Sufolk: Thames Valley Test Company; 1997. [Google Scholar]
- Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J Neurosci Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars MP, Bartholomew A, Strakova Z, Lazarov O. Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell Res Ther. 2011;2:36. doi: 10.1186/scrt77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars MP, Hollands C, Zhao Kda T, Lazarov O. Soluble amyloid precursor protein-alpha rescues age-linked decline in neural progenitor cell proliferation. Neurobiol Aging. 2013;34:2431–2440. doi: 10.1016/j.neurobiolaging.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22:1188–1201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Xia X, Bui D, Sweatt JD, Zheng H. Accelerated plaque accumulation, associative learning deficits, and up-regulation of alpha 7 nicotinic receptor protein in transgenic mice co-expressing mutant human presenilin 1 and amyloid precursor proteins. J Biol Chem. 2002;277:22768–22780. doi: 10.1074/jbc.M200164200. [DOI] [PubMed] [Google Scholar]
- Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22:833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci. 2010;13:1433–1440. doi: 10.1038/nn.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The restless engram: consolidations never end. Annu Rev Neurosci. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci. 2004;24:1948–1953. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A, Webster LA, Eacott MJ. The episodic nature of episodic-like memories. Learn Mem. 2012;19:146–150. doi: 10.1101/lm.025676.112. [DOI] [PubMed] [Google Scholar]
- Eling P, Derckx K, Maes R. On the historical and conceptual background of the Wisconsin Card Sorting Test. Brain Cogn. 2008;67:247–253. doi: 10.1016/j.bandc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cina I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol. 2008;6:e246. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- Flood DG. Critical issues in the analysis of dendritic extent in aging humans, primates, and rodents. Neurobiol Aging. 1993;14:649–654. doi: 10.1016/0197-4580(93)90058-j. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gadadhar A, Marr R, Lazarov O. Presenilin-1 regulates neural progenitor cell differentiation in the adult brain. J Neurosci. 2011;31:2615–2623. doi: 10.1523/JNEUROSCI.4767-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15:808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves JO, Leslie I, Huang GJ, McHugh SB, Taylor A, Mott R, Munafo M, Bannerman DM, Flint J. Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS Genet. 2013;9:e1003718. doi: 10.1371/journal.pgen.1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci. 2012;15:1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LK, Aumont A, Julien C, Vadnais A, Calon F, Fernandes KJ. Widespread deficits in adult neurogenesis precede plaque and tangle formation in the 3xTg mouse model of Alzheimer's disease. Eur J Neurosci. 2010;32:905–920. doi: 10.1111/j.1460-9568.2010.07379.x. [DOI] [PubMed] [Google Scholar]
- Hartl D, Rohe M, Mao L, Staufenbiel M, Zabel C, Klose J. Impairment of adolescent hippocampal plasticity in a mouse model for Alzheimer's disease precedes disease phenotype. PLoS One. 2008;3:e2759. doi: 10.1371/journal.pone.0002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hort J, Laczo J, Vyhnalek M, Bojar M, Bures J, Vlcek K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci U S A. 2007;104:4042–4047. doi: 10.1073/pnas.0611314104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav Neurosci. 2007;121:742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausler DH. Learning and Memory in Normal Aging. the University of Michigan: Academic Press; 1994. [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Med. 2015;5:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci Biobehav Rev. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Warthen DK. Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus. 2010;20:550–557. doi: 10.1002/hipo.20676. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. NR2B–dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci. 2012;32:8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Inokuchi K. Role of adult neurogenesis in hippocampal-cortical memory consolidation. Mol Brain. 2014;7:13. doi: 10.1186/1756-6606-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DA, Steinberg M, Galik E, Steele C, Sheppard JM, Warren A, Rosenblatt A, Lyketsos CG. Wandering behaviour in community-residing persons with dementia. Int J Geriatr Psychiatry. 1999;14:272–279. doi: 10.1002/(sici)1099-1166(199904)14:4<272::aid-gps896>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Klusmann V, Evers A, Schwarzer R, Schlattmann P, Reischies FM, Heuser I, Dimeo FC. Complex Mental and Physical Activity in Older Women and Cognitive Performance: A 6-month Randomized Controlled Trial. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2010;65:680–688. doi: 10.1093/gerona/glq053. [DOI] [PubMed] [Google Scholar]
- Koehl M, Abrous DN. A new chapter in the field of memory: adult hippocampal neurogenesis. Eur J Neurosci. 2011;33:1101–1114. doi: 10.1111/j.1460-9568.2011.07609.x. [DOI] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- Kropff E, Yang SM, Schinder AF. Dynamic role of adult-born dentate granule cells in memory processing. Curr Opin Neurobiol. 2015;35:21–26. doi: 10.1016/j.conb.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, Kim HD, Fukuchi K. Exploratory activity, anxiety, and motor coordination in bigenic APPswe + PS1/DeltaE9 mice. Neurosci Lett. 2004;369:156–161. doi: 10.1016/j.neulet.2004.07.069. [DOI] [PubMed] [Google Scholar]
- Langston RF, Stevenson CH, Wilson CL, Saunders I, Wood ER. The role of hippocampal subregions in memory for stimulus associations. Behav Brain Res. 2010;215:275–291. doi: 10.1016/j.bbr.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20:1139–1153. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Marr RA. Neurogenesis and Alzheimer's disease: at the crossroads. Exp Neurol. 2010;223:267–281. doi: 10.1016/j.expneurol.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Marr RA. Of mice and men: neurogenesis, cognition and Alzheimer's disease. Front Aging Neurosci. 2013;5:43. doi: 10.3389/fnagi.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33:569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14:301–310. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lipinski C, Hopkins A. Navigating chemical space for biology and medicine. Nature. 2004;432:855–861. doi: 10.1038/nature03193. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Talamini LM, Raffone A. Recall of memory sequences by interaction of the dentate and CA3: a revised model of the phase precession. Neural Netw. 2005;18:1191–1201. doi: 10.1016/j.neunet.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammarella IC, Pazzaglia F, Cornoldi C. Evidence for different components in children's visuospatial working memory. British Journal of Developmental Psychology. 2008;26:337–355. [Google Scholar]
- Marin-Burgin A, Schinder AF. Requirement of adult-born neurons for hippocampus-dependent learning. Behav Brain Res. 2012;227:391–399. doi: 10.1016/j.bbr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- Moreno H, Wu WE, Lee T, Brickman A, Mayeux R, Brown TR, Small SA. Imaging the Abeta-related neurotoxicity of Alzheimer disease. Arch Neurol. 2007;64:1467–1477. doi: 10.1001/archneur.64.10.1467. [DOI] [PubMed] [Google Scholar]
- Morris AM, Weeden CS, Churchwell JC, Kesner RP. The role of the dentate gyrus in the formation of contextual representations. Hippocampus. 2013;23:162–168. doi: 10.1002/hipo.22078. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2010;31:1339–1347. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CE, Scharfman HE. Pattern separation in the dentate gyrus: a role for the CA3 backprojection. Hippocampus. 2011;21:1190–1215. doi: 10.1002/hipo.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81:416–427. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Mori T, Gotz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Ohno M, Chang L, Tseng W, Oakley H, Citron M, Klein WL, Vassar R, Disterhoft JF. Temporal memory deficits in Alzheimer's mouse models: rescue by genetic deletion of BACE1. Eur J Neurosci. 2006;23:251–260. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Pan YW, Storm DR, Xia Z. Role of adult neurogenesis in hippocampus-dependent memory, contextual fear extinction and remote contextual memory: new insights from ERK5 MAP kinase. Neurobiol Learn Mem. 2013;105:81–92. doi: 10.1016/j.nlm.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlopoulos E, Jones S, Kosmidis S, Close M, Kim C, Kovalerchik O, Small SA, Kandel ER. Molecular mechanism for age-related memory loss: the histone-binding protein RbAp48. Sci Transl Med. 2013;5:200ra115. doi: 10.1126/scitranslmed.3006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter Curzon NRR, and Kaitlin E. Browman. Methods of Behavior Analysis in Neuroscience. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- Petrik D, Jiang Y, Birnbaum SG, Powell CM, Kim MS, Hsieh J, Eisch AJ. Functional and mechanistic exploration of an adult neurogenesis-promoting small molecule. FASEB J. 2012;26:3148–3162. doi: 10.1096/fj.11-201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering SJ, Gathercole SE, Peaker SM. Verbal and visuospatial short-term memory in children: evidence for common and distinct mechanisms. Mem Cognit. 1998;26:1117–1130. doi: 10.3758/bf03201189. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, Capota M, Britt JK, Kotti T, Ure K, Brat DJ, Williams NS, MacMillan KS, Naidoo J, Melito L, Hsieh J, De Brabander J, Ready JM, McKnight SL. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plancher G, Barra J, Orriols E, Piolino P. The influence of action on episodic memory: a virtual reality study. Q J Exp Psychol (Hove) 2013;66:895–909. doi: 10.1080/17470218.2012.722657. [DOI] [PubMed] [Google Scholar]
- Plancher G, Tirard A, Gyselinck V, Nicolas S, Piolino P. Using virtual reality to characterize episodic memory profiles in amnestic mild cognitive impairment and Alzheimer's disease: influence of active and passive encoding. Neuropsychologia. 2012;50:592–602. doi: 10.1016/j.neuropsychologia.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Rainville C, Marchand N, Passini R. Performances of patients with a dementia of the Alzheimer type in the Standardized Road-Map test of Direction Sense. Neuropsychologia. 2002;40:567–573. doi: 10.1016/s0028-3932(01)00133-6. [DOI] [PubMed] [Google Scholar]
- Rangel LM, Alexander AS, Aimone JB, Wiles J, Gage FH, Chiba AA, Quinn LK. Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nat Commun. 2014;5:3181. doi: 10.1038/ncomms4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Battaglia FP, Chawla MK, Ekstrom AD, Gerrard JL, Lipa P, Rosenzweig ES, Worley PF, Guzowski JF, McNaughton BL, Barnes CA. Independence of firing correlates of anatomically proximate hippocampal pyramidal cells. J Neurosci. 2001;21:RC134. doi: 10.1523/JNEUROSCI.21-05-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Riddell DR, Zhou H, Comery TA, Kouranova E, Lo CF, Warwick HK, Ring RH, Kirksey Y, Aschmies S, Xu J, Kubek K, Hirst WD, Gonzales C, Chen Y, Murphy E, Leonard S, Vasylyev D, Oganesian A, Martone RL, Pangalos MN, Reinhart PH, Jacobsen JS. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol Cell Neurosci. 2007;34:621–628. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS One. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The mechanisms for pattern completion and pattern separation in the hippocampus. Front Syst Neurosci. 2013;7:74. doi: 10.3389/fnsys.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg C, Mattson MP, Mughal MR, Bussey TJ, Saksida LM. Impaired attention in the 3xTgAD mouse model of Alzheimer's disease: rescue by donepezil (Aricept) J Neurosci. 2011;31:3500–3507. doi: 10.1523/JNEUROSCI.5242-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, Volker K, Ho HV, Mooren F, Knecht S, Floel A. Physical activity and memory functions: an interventional study. Neurobiol Aging. 2011;32:1304–1319. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011a;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011b;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A. 2007;104:4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydoff JA, Olariu A, Sheng J, Hu Z, Li Q, Garcia R, Pei J, Sun GY, von Borstel R. Uridine prodrug improves memory in Tg2576 and TAPP mice and reduces pathological factors associated with Alzheimer's disease in related models. J Alzheimers Dis. 2013;36:637–657. doi: 10.3233/JAD-130059. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Myers CE. Hilar mossy cells of the dentate gyrus: a historical perspective. Front Neural Circuits. 2012;6:106. doi: 10.3389/fncir.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JW, Gao Z, Li S, Farooqi M, Tang TS, Bezprozvanny I, Frantz DE, Hsieh J. Small-molecule activation of neuronal cell fate. Nat Chem Biol. 2008;4:408–410. doi: 10.1038/nchembio.95. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Wolfe MS. Presenilin: running with scissors in the membrane. Cell. 2007;131:215–221. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Sepehry AA, Lee PE, Hsiung GY, Beattie BL, Jacova C. Effect of selective serotonin reuptake inhibitors in Alzheimer's disease with comorbid depression: a meta-analysis of depression and cognitive outcomes. Drugs Aging. 2012;29:793–806. doi: 10.1007/s40266-012-0012-5. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla V, Zheng YL, Mishra SK, Amin ND, Steiner J, Grant P, Kesavapany S, Pant HC. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer's disease phenotypes in model mice. FASEB J. 2013;27:174–186. doi: 10.1096/fj.12-217497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CE. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Dutton RA, Chiang MC, Leow AD, Sowell ER, De Zubicaray G, Becker JT, Lopez OL, Aizenstein HJ, Toga AW. Tracking Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:183–214. doi: 10.1196/annals.1379.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22:292–298. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- Tronel S, Charrier V, Sage C, Maitre M, Leste-Lasserre T, Abrous DN. Adult-born dentate neurons are recruited in both spatial memory encoding and retrieval. Hippocampus. 2015 doi: 10.1002/hipo.22468. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant-derived flavanol (−)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi T, Richardson JTE. Active processing in visuo-spatial working memory. Cahiers De Psychologie Cognitive-Current Psychology of Cognition. 2000;19:3–32. [Google Scholar]
- Vingerhoets G, Lannoo E, Bauwens S. Analysis of the Money Road-Map Test performance in normal and brain-damaged subjects. Archives of Clinical Neuropsychology. 1996;11:1–9. [Google Scholar]
- Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD. Metformin Activates an Atypical PKC-CBP Pathway to Promote Neurogenesis and Enhance Spatial Memory Formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Wang W, Pan YW, Zou J, Li T, Abel GM, Palmiter RD, Storm DR, Xia Z. Genetic activation of ERK5 MAP kinase enhances adult neurogenesis and extends hippocampus-dependent long-term memory. J Neurosci. 2014;34:2130–2147. doi: 10.1523/JNEUROSCI.3324-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model Alzheimer's dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet. 2014;5:88. doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- Weschler D. Wechsler Memory Scale -revised manual. San Antonio: Psychological Corp; 1987. [Google Scholar]
- Wesnes KA, Annas P, Basun H, Edgar C, Blennow K. Performance on a pattern separation task by Alzheimer's patients shows possible links between disrupted dentate gyrus activity and apolipoprotein E in4 status and cerebrospinal fluid amyloid-beta42 levels. Alzheimers Res Ther. 2014;6:20. doi: 10.1186/alzrt250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;(3 Spec No):33–44. [PubMed] [Google Scholar]
- Wolbers T, Wiener JM. Challenges for identifying the neural mechanisms that support spatial navigation: the impact of spatial scale. Front Hum Neurosci. 2014;8:571. doi: 10.3389/fnhum.2014.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdak H, Zhu S, Min KH, Aimone L, Lairson LL, Watson J, Chopiuk G, Demas J, Charette B, Halder R, Weerapana E, Cravatt BF, Cline HT, Peters EC, Zhang J, Walker JR, Wu CL, Chang J, Tuntland T, Cho CY, Schultz PG. A small molecule accelerates neuronal differentiation in the adult rat (vol 107, pg 16542, 2010) Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22360–22360. doi: 10.1073/pnas.1010300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Noble J, Hibberd C, Rowe WB, Meaney MJ, Morris RG, Seckl JR. Chronic treatment with the antidepressant amitriptyline prevents impairments in water maze learning in aging rats. J Neurosci. 2002;22:1436–1442. doi: 10.1523/JNEUROSCI.22-04-01436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhuo JM, Prescott SL, Murray ME, Zhang HY, Baxter MG, Nicolle MM. Early discrimination reversal learning impairment and preserved spatial learning in a longitudinal study of Tg2576 APPsw mice. Neurobiol Aging. 2007;28:1248–1257. doi: 10.1016/j.neurobiolaging.2006.05.034. [DOI] [PubMed] [Google Scholar]