Abstract
Background
Ethanol feeding in mice activates complement via C1q binding to apoptotic cells in the liver; complement contributes to ethanol-induced inflammation and injury. Despite the critical role of C1q in ethanol-induced injury, the mechanism by which ethanol activates C1q remains poorly understood. Secretory IgM (sIgM), traditionally considered to act as an anti-microbial, also has critical housekeeping functions, facilitating clearance of apoptotic cells, at least in part through activation of C1q. Therefore, we hypothesized that 1) ethanol-induced apoptosis in the liver recruits sIgM, facilitating the activation of C1q and complement and 2) C1INH (C1 esterase inhibitor), which inhibits C1 functional activity, prevents complement activation and decreases ethanol-induced liver injury.
Methods
Female C57BL/6 wild-type, C1qa−/−, BID−/− and sIgM−/− mice were fed ethanol containing liquid diets or pair-fed control diets. C1INH or vehicle was given via tail vein injection to ethanol- or pair-fed wild-type mice at 24 and 48 hours prior to euthanasia.
Results
Ethanol exposure increased aptoptosis in the liver, as well as the accumulation of IgM in the liver. In the early stages of ethanol feeding, C1q co-localized with IgM in the peri-sinusoidal space of the liver and accumulation of IgM and C3b was dependent on ethanol-induced BID-dependent apoptosis. sIgM−/− mice were protected from both ethanol-induced activation of complement and early ethanol-induced liver injury when compared to wild-type mice. Treatment with C1INH also decreased hepatic C3b deposition and ethanol-induced injury.
Conclusion
These data indicate that sIgM contributes to activation of complement and ethanol-induced increases in inflammatory cytokine expression and hepatocyte injury in the early stages of ethanol-induced liver injury.
Keywords: alcoholic liver disease, inflammation, complement, apoptosis, hepatocytes
1. Introduction
1.1 The burden of alcoholic liver disease
The spectrum of alcohol-related liver injury varies from simple steatosis to alcoholic hepatitis (ASH) to cirrhosis (Lieber 2000). ASH is a major health burden in the US (Peery et al. 2012). Despite increasing awareness, it is estimated that approximately 18 million people abuse alcohol and 10 million people suffer from ASH and its complications (Mandayam et al. 2004). Many patients with ASH have co-existing liver fibrosis or cirrhosis (Lieber 2000). ASH contributes to more than 20,000 deaths annually in the US (Peery et al. 2012). There are limited treatment options for this patient population and the outcome remains poor (Joshi-Barve et al. 2015). Available medical treatments focus on suppression of inflammation; however, the response to current regimens is particularly ineffective in patients with severe ASH (Joshi-Barve et al. 2015).
1.2. Innate immunity and alcoholic liver disease
The progression of ethanol-induced liver injury results from both the direct effects of ethanol metabolism and indirect effects via interactions with innate immunity. Ethanol metabolism, via the alcohol dehydrogenase and cytochrome P450 2E1 (CYP2E1) pathways, generates acetaldehyde, as well as increases the production of reactive oxygen species (Nagy 2004); these reactive products result in oxidative stress and lipid peroxidation. Over time, the accumulation of oxidized macromolecules contributes to a loss of hepatocellular function (Barnes et al. 2014). Ethanol exposure increases gut-derived endotoxin/lipopolysaccharide (LPS) in the portal circulation. LPS, through binding with toll-like receptor 4 (TLR4) on Kupffer cells, activates multiple signaling cascades, resulting in an increase in the production of pro-inflammatory mediators, such as TNF-α production (Wang et al. 2012). The contribution of Kupffer cells and TNFα to ethanol-induced liver injury is well established (Wang et al. 2012).
1.2.1 The role of complement in ethanol-induced liver injury
The complement system is an important part of the innate immune system that is activated by three distinct activation pathways: the classical, lectin and alternative pathways (Gasque 2004). Complement is comprised of more than 30 proteins and the liver is the primary source of complement production. Activation cascades result in cleavage of C3 and C5 into C3a and C5a fragments, respectively; these anaphylatoxins then interact with their cognate receptors (Gasque 2004). Kupffer cells and hepatic stellate cells express the C3a receptor (C3aR) and C5a receptor (C5aR) under basal condition (Das et al. 2014; Qin and Gao 2006). C5aR expression is induced in proliferating hepatocytes or in response to inflammatory cytokines (Qin and Gao 2006). Macrophages also express complement receptor (CR) 1, CR3 (CD11b) and CR4 (CD11c), with CR1 binding to C1q, C3b and C4b, all associated with opsonization of cells and particles (Bohlson et al. 2014). Interestingly, C1q is suggested to shift macrophage polarization from the inflammatory M1 phenotype to a pro-resolution M2 phenotype, highly poised for phagocytosis (Bohlson et al. 2014).
Emerging evidence indicates that complement activation plays a significant role in the innate immune response to ethanol (Wang et al. 2012). Ethanol activates the classical complement pathway via C1q binding to apoptotic cells in the liver (Cohen et al. 2010). Chronic ethanol feeding increases activation of C3 in mice (Pritchard et al. 2007) and rats (Jarvelainen et al. 2002). Mice deficient in C3 or C5 are protected against ethanol-induced increases in hepatic triglycerides and circulating alanine aminotransferase (ALT) (Pritchard et al. 2007; Bykov et al. 2007). Further, chronic ethanol-induced liver injury is exacerbated in mice lacking CD55/decay accelerating factor (DAF), a complement regulatory protein (Pritchard et al. 2007). Increased immunoreactivity for C1q, C3 and C5, as well as C5aR, is observed in liver biopsies of patients with alcoholic hepatitis (Shen et al. 2014). In mice, evidence of complement activation is present early in the process of ethanol-induced liver injury, prior to detectable increases in ALT or accumulation of hepatic triglycerides (Roychowdhury et al. 2009). Early activation of complement contributes to increased inflammatory cytokine expression in Kupffer cells, mediated via the activation of C3aR and C5aR (Sebastian et al. 2011). On the other hand, C5aR is up-regulated during liver regeneration and C5-deficient mice have impaired liver regeneration after partial hepatectomy and carbon-tetrachloride (CCl4)-induced liver injury (Daveau et al. 2004; Mastellos et al. 2001). Binding of C5a to C5aR increases hepatocyte growth factor and c-Met mRNA levels (Daveau et al. 2004), suggesting a critical role of complement in hepatocyte proliferation and liver regeneration.
This unique functional combination of the complement system to influence both inflammation and regeneration is critical for adequate repair of the liver in response to injury. However, the role of complement in the coordination of these two processes remains largely unknown. This knowledge is key to understanding liver injury-repair mechanisms. Molecules targeting the classical pathway to decrease inflammation would be expected to preserve complement activation via the lectin and alternative pathways, thus maintaining both C5a-mediated cell regeneration, but also maintaining the role of complement in resistance to infections.
1.3 Secretory IgM and complement in alcoholic liver disease
Secretory IgM (sIgM) is the primary isotype of natural antibodies with germline origin that arise in the absence of previous exposure to foreign antigens (Tsiantoulas et al. 2015). In addition to the anti-microbial functions, there is accumulating evidence that sIgM has critical housekeeping functions by facilitating clearance of apoptotic cells through activation of C1q (Zwart et al. 2004). Indeed, the presence of IgM antibodies has been shown to be a pre-requisite for the activation of the classical pathway and promotion of C3 binding to apoptotic cells (Quartier et al. 2005). Natural IgM against oxidized phospholipids exposed on the surface of dying cells contributes to complement activation and timely clearance (Kim et al. 2002). Therefore, we hypothesized that ethanol exposure activates C1q through sIgM binding to apoptotic hepatocytes in the liver. Making use of sIgM−/− mice fed ethanol as part of a complete liquid diet, we find that sIgM is required for the activation of complement during early stages of ethanol-induced liver injury. Further, we find that inhibition of C1 activity with C1 inhibitor (C1INH, Cinryze) also prevented ethanol-induced liver injury.
2. Experimental Procedures
2.1 Materials and animals
sIgM−/− mice on a C57BL/6 background were a gift from Jay Kolls (Boes et al. 1998) and BID−/− a gift to Dr. A. Feldstein from X. Yin (Yin et al. 1999). Lieber-DeCarli ethanol and control diets were purchased from Dyets (Bethlehem, PA). Antibodies were purchased from the sources indicated below: CYP2E1 (Abcam, Cambridge MA), F4/80 (AbD Serotec, Raleigh, NC), C3b/C3c/iC3b (C3b) (mAb 2/11) and C1q (Hycult, Plymouth Meeting, PA, IgM from Southern Biotech (Birmingham, AL), and TNFα (Fitzgerald, Acton, MA). Alexa fluor-488 and -568 conjugated secondary antibodies were purchased from Invitrogen (Carlsbad, CA). TUNEL assay kit Apop Tag @ Plus in Situ apoptosis detection kit was purchased from Millipore (Billerica, MA, cat. no. S7111).
2.2 Mouse models
All procedures using animals were approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Female mice were housed in shoe-box cages (2 animals/cage) with microisolator lids. Standard microisolator handling procedures were used throughout the study. Age-matched, 8–10 weeks old female mice were randomized into ethanol-fed and pair-fed groups and then adapted to a control liquid diet for 2 days.
Two models of ethanol feeding were utilized. The first was a dynamic model of chronic ethanol-induced liver injury in which mice in the ethanol-fed groups were allowed free access to an ethanol-diet containing 1% (vol/vol) ethanol for 2 days followed by 2% ethanol for 2 days (a model of early ethanol exposure; termed 2% d4) and then 4% ethanol for 1 week (a model of moderate ethanol exposure; termed EtOH-fed, 4% d11), then 5% for 1 week and finally 6% ethanol for an additional week (Heavy, chronic ethanol exposure; termed EtOH-fed, 6% d25). The second model was considered a binge model of ethanol exposure was used for the C1INH study. Mice were allowed free access to an ethanol-diet containing 1% for 2 days, followed by 2 days at 6% ethanol (termed EtOH-binge). Ethanol provided a final 22% of calories in the diet in the EtOH-fed model at 4% d11 and 32% of calories in the EtOH-binge model. Control mice received a control pair-food diet which iso-calorically substituted maltose dextrins for ethanol.
2.2.1 C1INH treatment
Mice on the EtOH-binge protocol were treated with or without C1INH (Cinryze, human purified C1INH, ViroPharma Inc, Downingtown, PA) according to the following scheme: 1000U/kg in sterile NaCl was provided via tail vein injection 24 and 48 h before euthanasia. Untreated control mice were injected with an equivalent volume of 0.9% sterile saline.
After the ethanol-feeding protocol, mice were anesthetized, blood samples taken into non-heparinized syringes from the posterior vena cava, livers blanched with saline via the portal vein and then excised. Portions of each liver were then either fixed in 10% formalin or frozen in optimal cutting temperature (OCT) compound (Sakura Finetek U.S.A., Inc., Torrance, CA) for histology, preserved in RNAlater (Qiagen, Valencia, CA) or flash frozen in liquid nitrogen and stored at −20 or 80°C until further analysis.
2.3 Immunohistochemistry/immunofluorescence
Frozen liver sections were mounted on glass slides, fixed with paraformaldehyde and washed three times in PBS. Sections were then blocked with 2% bovine serum albumin (diluted in PBS) with 0.1% Triton-X100 for 1 hour followed by overnight incubation with the primary antibody (1:50 dilution for C3b/iC3b/C3c (abbreviated here as C3b), 1:100 for IgM and 1:50 dilution for C1q) at 4°C in a humidified, light-protected chamber. All sections were then washed in PBS (3 times 5 min each), incubated with the fluorochrome-conjugated secondary antibody (Alexa fluor-488 donkey anti-rat or -568 labeled donkey anti-goat IgG, 1:250 diluted in blocking buffer) for 2 hr in the dark at room temperature. All slides were washed again in PBS and mounted with VECTASHIELD containing anti-fade reagent and DAPI (Vector Laboratories, Inc., Burlingame, CA). Fluorescent images were acquired using a LEICA confocal microscope. No specific immunostaining was seen in sections incubated with PBS rather than the primary antibody.
To detect TNFα in mouse liver, formalin-fixed paraffin embedded liver sections (8 µm) were deparaffinized in Safeclear II™ xylene substitute (Protocol, Kalamazoo, MI), (3 times 3 min each) and hydrated consecutively in 100% (2 times), 95% and 70% ethanol followed by two washes in water. Following antigen retrieval using TRIS-EDTA buffer, pH 9.0, slides were blocked in 10% normal goat serum in PBS for 1 h at room temperature, then primary antibody overnight. Primary antibody was incubated overnight (1:300) at 4°C, and biotinylated anti-rabbit secondary antibody was applied for 30 min. Slides were developed with AEC substrate chromagen using avidin-biotin-peroxidase detection.
Images were analyzed and semi-quantified using Image Pro Plus software (Media Cybernetics, Bethesda, MD).
Apoptosis was detected using a TUNEL assay, as described previously (12).
2.4 Biochemical assays
Activities of liver-specific enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), were detected in plasma samples using commercially available enzymatic assay kits (Sekisui Diagnostics LLC, Lexington MA) as described in the manufacturer’s instructions. Total hepatic triglycerides were assayed using the Triglyceride Reagent Kit from Pointe Scientific Inc. (Lincoln Park, MI). IgM was measured in whole liver lysates and plasma using a commercially available kit (Abcam, Cambridge, MA).
2.5 Western blots
Liver lysates (20 µg protein) were loaded onto 8% reducing SDS-polyacrylamide gels. Antibodies CYP2E1 was from Abcam and HSC70 (used as a loading control for Western blots) was from Santa Cruz Biotechnology, Inc). Immunoreactive proteins were visualized using enhanced chemiluminescence, images were collected, and signal intensities were quantified using Eastman Kodak Co. Image Station 4000R.
2.6 Isolation of RNA and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated and reverse transcribed followed by amplification using qRT-PCR. The relative amount of target mRNA was determined using the comparative threshold (Ct) method by normalizing target mRNA Ct values to those of 18S.
2.7 Statistical analysis
Values shown in all figures represent means ± SEM, n=4 for pair-fed and n=6 for ethanol-fed. Data were analyzed by general linear models procedure (SAS, Carey, IN). Data were tested for normality using the Shapiro-Wilk test (SAS). Some data sets were log transformed, as needed, to obtain a normal distribution. Non-parametric analysis was used for analysis of the IgM ELISA data in liver lysates, as this data set was not normally distributed. Follow-up comparisons were made by least square means testing.
3. RESULTS and DISCUSSION
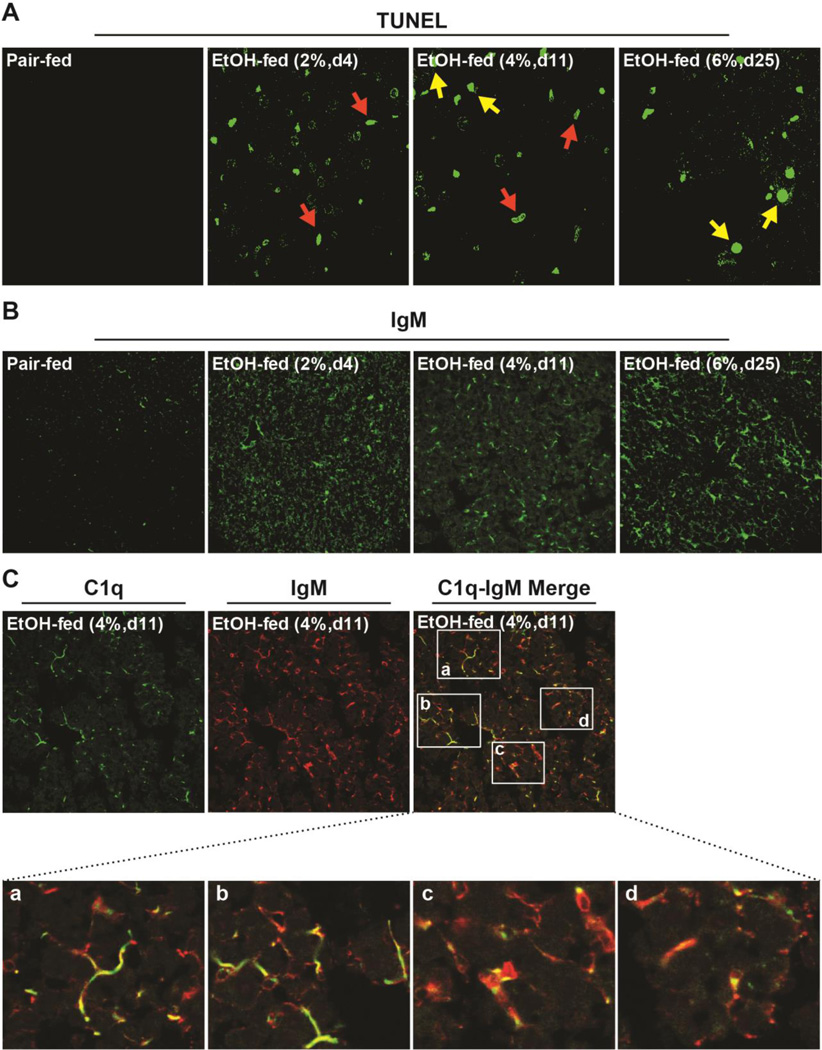
Apoptosis is associated with the progression of ethanol-induced liver injury (Barnes et al. 2014). Interestingly, the identity of cells in the liver undergoing apoptosis depends on the time and dose of exposure. Initially, at low concentrations of ethanol in the diet (2% d4), apoptosis is predominantly in sinusoidal cells (Figure 1A); we have previously identified these TUNEL positive cells as F4/80 positive macrophages (Cohen et al. 2010). Over time and with increased ethanol in the diet, apoptosis is also initiated in hepatocytes; at 4% d11, we observe a mixed population of macrophages and hepatocytes undergoing apoptosis, and by 6% d25 of ethanol feeding, hepatocyte apoptosis is predominant (Figure 1A).
Figure 1. Dynamic responses in the liver to ethanol feeding over time: hepatocellular apoptosis is associated with the accumulation of IgM in hepatic sinusoids.
Female C57BL/6 mice were fed a complete liquid diet with concentrations of ethanol ranging from 2–6% (vol/vol) over 25 days or pair-fed control diets. A) TUNEL positive cells were enumerated. Red arrows: non-parenchymal cells, yellow arrows: hepatocytes and B) accumulation of IgM assessed in livers by immunofluorescence. C) C1q (green) and IgM (red) were co-localized in hepatic sinusoids. Co-localization was semi-quantified using Image Pro Plus software. Images are representative of at least 3 images from n=4 pair-fed and n=6 EtOH-fed mice per group.
C1q accumulates in the liver in response to ethanol-induced apoptosis, likely to facilitate the clearance of apoptotic cells (Cohen et al. 2010). In many conditions, C1q is assisted in the clearance of apoptotic bodies by the action of sIgM (Zwart et al. 2004). Dead or dying cells expose a number of self-altered molecules on their surface at early stages of death and/or soluble components, released during late phases of cell death, considered to be “eat me” signals that activate their clearance. Natural IgM recognize a number of such “eat me” signals, including oxidized lipids (Binder 2010). If IgM was involved with C1q in the clearance of ethanol-induced apoptotic or injured cells, then IgM should be detectable in the livers of ethanol-fed mice. Immunohistochemistry of mouse liver sections revealed the presence of IgM after ethanol feeding. IgM was increased as early as 2% d4 and remained elevated over time and at higher concentrations of ethanol (Figure 1B). IgM was distributed in a pattern typical of sinusoidal localization and co-localized with C1q in the sinusoids after moderate ethanol feeding (Figure 1C). When this co-localization was semi-quantified, approximately 4 ± 2% (n=6) of immunoreactive C1q was co-localized with IgM.
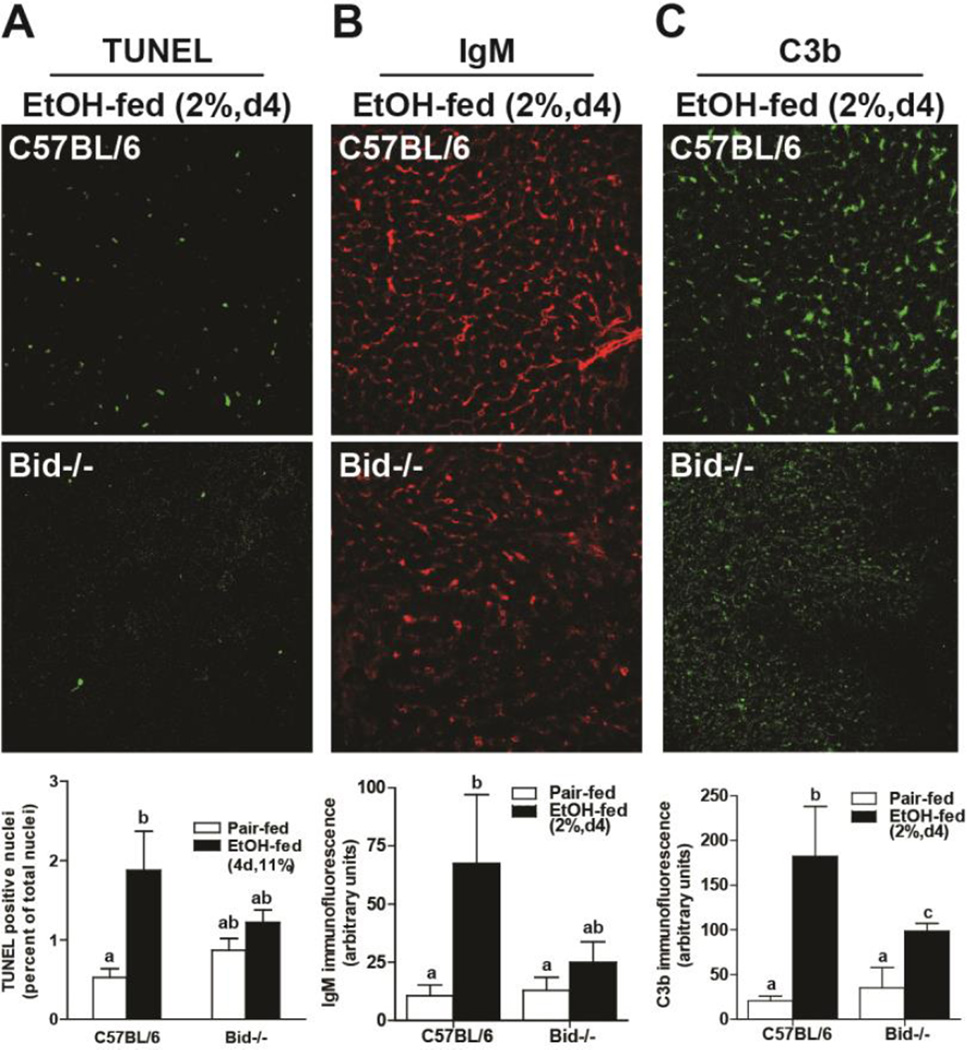
If ethanol-induced apoptosis was driving the recruitment of sIgM to the liver, then mice deficient in BID should not exhibit increased IgM binding in the liver after chronic ethanol exposure. We have previously reported that when BID−/− mice are fed ethanol containing diets (6% 25d), apoptosis is prevented (Roychowdhury et al. 2012). Ethanol-induced apoptosis, indicated by an increase in TUNEL-positive nuclei, was apparent as early as 2% d4 in wild-type mice (Figure 1A and 2A); this early phase of apoptosis was absent in BID−/− mice, indicating that early apoptosis was also BID-dependent (Figure 2A). While C57BL/6 mice accumulated IgM in response to 2% d4 ethanol feeding, BID−/− mice were protected (Figure 2B). Importantly, C3b deposition in the liver, an indicator of complement activation, was also prevented in BID−/− mice compared to wild-type mice (Figure 2C).
Figure 2. Accumulation of IgM and complement activation in liver is dependent on ethanol-induced hepatocellular apoptosis.
Female C57BL/6 and BID−/− mice were fed a complete liquid diet for 4 days at a maximal concentration of 2% (vol/vol) ethanol or pair-fed control diets. A) TUNEL positive cells were enumerated and accumulation of B) IgM and C) C3b assessed in livers by immunofluoresence. Values represent means ± SEM, P values are shown to indicate differences between wild-type and BID−/− mice after ethanol feeding. n=4 for Pair-fed and n=6 for EtOH-fed.
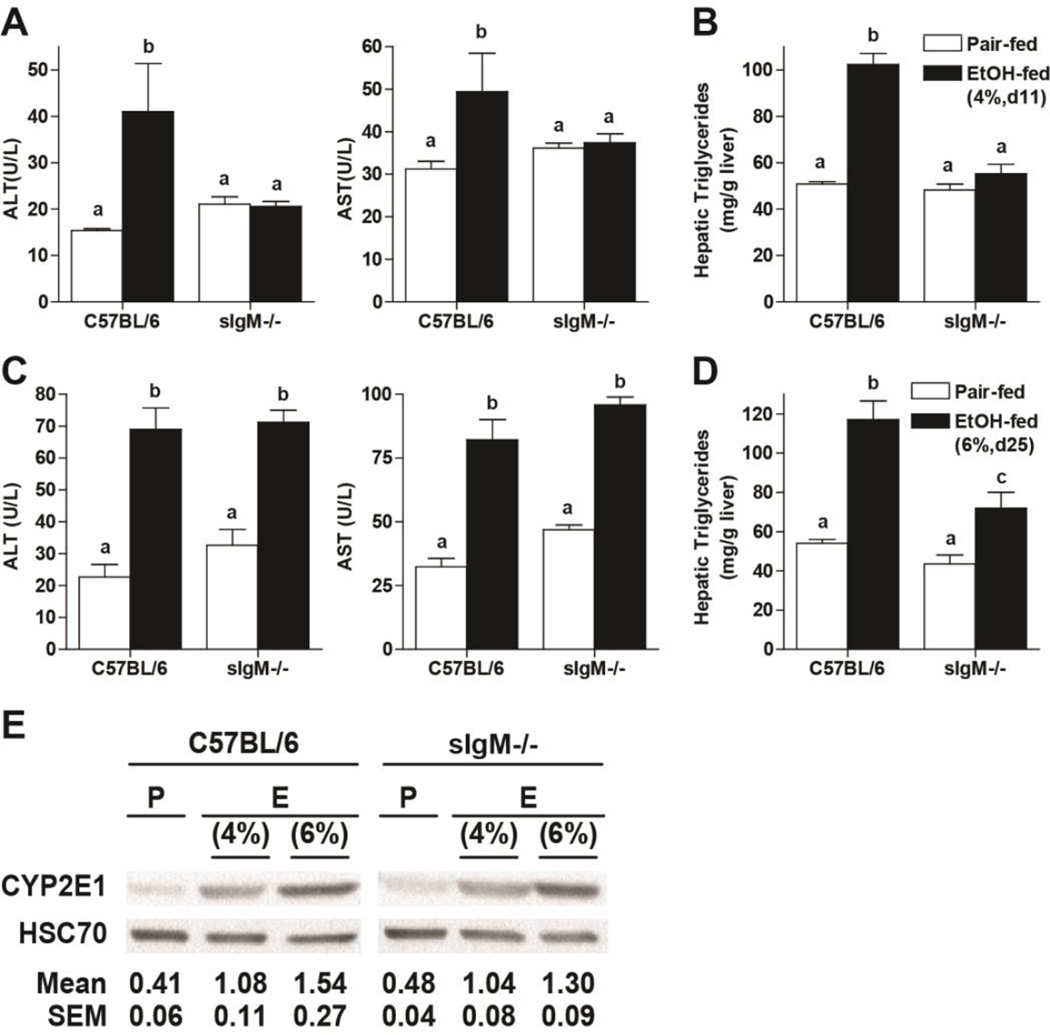
If IgM accumulation in the liver contributes to ethanol-induced complement activation, we would expect that mice deficient in sIgM would then be protected from ethanol-induced liver injury. sIgM−/− mice and C57BL/6 wild-type were allowed free access to an ethanol-containing diet (4% d11 or 6% d25) or pair-fed control diets. C57BL/6 mice in the 4% d11 moderate ethanol model had increased ALT/AST (Figure 3A) and hepatic triglyceride (Figure 3B); these indicators of injury were further increased in the 6% d25 heavy, chronic model (Figure 3C/D). At 4% d11, sIgM−/− mice were protected from ethanol-induced injury (Figure 3A/B); however, after 6% d25, sIgM−/− mice were no longer protected (Figure 3C/D). This lack of protection at higher levels of ethanol exposure is likely related to additional pathological mechanisms that contribute to liver injury at higher ethanol concentrations, such as increasing CYP2E1 expression and a contribution from LPS/TLR4 pathway, responses that are typically observed only at higher ethanol concentrations (Roychowdhury et al. 2009). Indeed, the concentration of CYP2E1 in the livers was higher at 6% d25 compared to 4% d11 (Figure 3E and (Roychowdhury et al. 2009)).
Figure 3. sIgM deficient mice are protected from liver injury in response to moderate, but not heavy, ethanol exposure.
Female C57BL/6 and sIgM−/− were fed a complete liquid diet for (A/B) 11 days at a maximal concentration of 4% (vol/vol) ethanol or (C/D) 25 days at a maximal concentration of 6% (vol/vol) ethanol. Pair-fed mice were fed control diets. A/C) ALT and AST activity was measured in plasma and B/D hepatic triglycerides were measured biochemically. E) Liver lysates were prepared and proteins separated by SDS-Polyacrylamide Electrophoresis. CYP2E1 and Hsc70 (loading control) quantity was measured by Western blot. Values represent means ± SEM, values with different superscripts are significantly different from each other, p<0.05. n=4 for Pair-fed and n=6 for EtOH-fed.
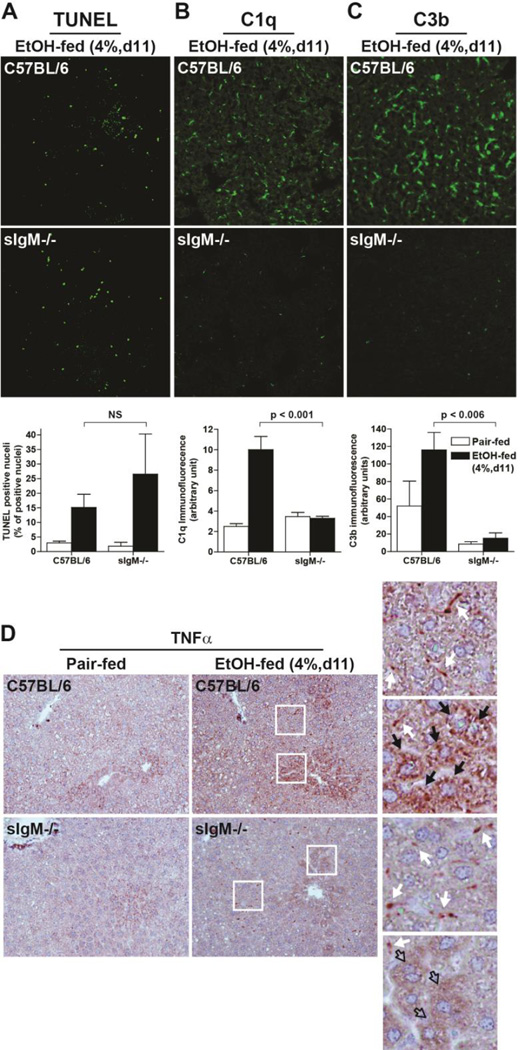
Exposure to moderate ethanol (4% d11) also increased TUNEL positive cells in liver of C57BL/6 mice; this increase was maintained in the sIgM−/− mice (Figure 4 A). Moderate ethanol exposure increased the accumulation of C1q and C3b within the liver of C57BL/6 mice (Figure 4B/C). Consistent with sIgM acting as the link between apoptotic cells and recruitment of C1q and subsequent activation of C3 in the liver, mice lacking sIgM did not accumulate C1q or deposit C3b in response to moderate ethanol exposure (4% d11). Similarly, TNFα protein (Figure 4D) was increased by moderate ethanol exposure in wild-type, but not sIgM−/− mice.
Figure 4. Moderate ethanol-induced complement activation, but not hepatocellular apoptosis, is dependent on sIgM.
Female C57BL/6 and sIgM−/− were fed a complete liquid diet for 11 days at a maximal concentration of 4% (vol/vol) ethanol or pair-fed control diets. A) TUNEL positive cells were enumerated and accumulation of B) C1q and C) C3b assessed in livers by immunofluorescence. D) Accumulation of immunoreactive TNFα was visualized by immunohistochemistry. Insets: white arrows: non-parenchymal cells; Black arrows: Hepatocytes. Filled arrows indicate cells with positive staining; Open arrows indicate cells without staining. Values represent means ± SEM, individual P values are shown for differences between C57BL/6 and sIgM−/− after ethanol feeding. NS: not signficant. n=4 for Pair-fed and n=6 for EtOH-fed.
In order to begin to translate these data obtained with genetic manipulation of sIgM and complement into a more clinically-relevant experiment, we next tested the hypothesis that a pharmacological inhibitor of C1 (C1INH) would prevent ethanol-induced injury to the liver. C1INH is a single 105 kDa polypeptide chain that belongs to the superfamily of serine-protease inhibitors (serpins) (Davis et al. 2010). C1INH consists of a serpin domain that contains the protease recognition region that irreversibly binds and inhibits proteases including C1r, C1s, MASP1 and MASP2 of the complement system; factor XII and plasma kallikrein of the contact system; factor XI and thrombin of the coagulation system; and plasmin and tissue plasminogen activator of the fibrinolytic system (Davis et al. 2010). C1INH has also been tested in a variety of animal models of inflammatory conditions including sepsis, ischemia-reperfusion injury (cardiac, cerebral, muscle, liver, gastrointestinal), hyper-acute transplant rejection and angioedema with beneficial effects (Cicardi et al. 2005). Further, C1INH has been approved for prophylaxis and treatment of hereditary angioedema attacks in patients (Cugno et al. 2009). In addition to the protease inhibition function, C1INH protects against endotoxin shock in animal models, at least in part through direct binding to the LPS molecule and preventing its interaction with, and activation of, macrophages and endothelial cells (Liu et al. 2003).
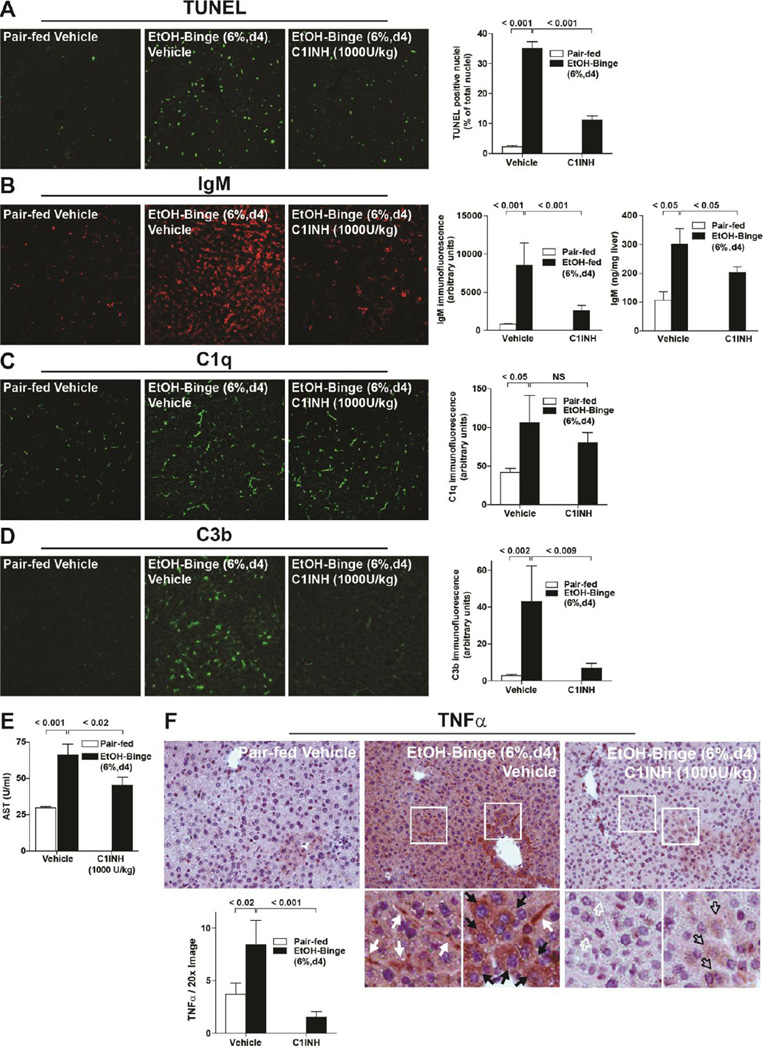
Because the short half-life of C1INH required twice daily injections, the effectiveness of this drug was tested in a short term binge model of ethanol exposure in which mice are exposed to 6% ethanol for 2 days. As in the early, moderate and chronic models used in earlier studies (Figure 1A), binge alcohol increased TUNEL positive cells (Figure 5A), IgM accumulation, assessed both by immunohistochemistry and ELISA assay (Figure 5B), as well as C1q (Figure 5C) and C3b (Figure 5D) deposition in the liver. Circulating IgM concentrations were not affected by ethanol exposure (Pair-fed vehicle= 3.95 ± 0.62µg/ml, EtOH-fed vehicle=3.39 ± 0.48µg/ml and EtOH-fed C1INH=3.67 ± 0.75µg/ml, n=6 per group), consistent with a previous report showing that ethanol feeding over 4 weeks did not change circulating concentrations of IgM in a number of mouse strains (Alonso et al. 2012). When mice were treated with 1000 U/kg C1INH during binge ethanol exposure, TUNEL remained moderately elevated (Figure 5A) and both IgM (Figure 5B) and C1q (Figure 5C) accumulated in the liver, albeit at slightly reduced levels. Importantly, C1INH blocked the progression of this injury pathway at the step of C3 activation: ethanol-induced accumulation of C3b was substantially reduced in mice treated with C1INH (Figure 5D). Consistent with the role for complement activation in the progression of ethanol-induced liver injury, ethanol-fed mice treated with C1INH had lower AST activity (Figure 5E) and TNFα expression (Figure 5F) compared to vehicle treated controls.
Figure 5. Treatment of mice with C1INH during an ethanol-binge decreased complement activation and hepatocellular injury.
Female C57BL/6 mice were treated with 1000U/kg C1INH or 0.9% sterile saline via tail vein injection 24 and 48 h before euthanasia. Mice were fed a complete liquid diet for 4 days at a maximal concentration of 6% (vol/vol) ethanol or pair-fed control diets. A) TUNEL positive cells were enumerated and accumulation of B) IgM, C) C1q and D) C3b assessed in livers by immunofluorescence. B) Liver IgM was also measured by ELISA in whole liver lysates. sIgM−/− mice were used as a negative control in this assay and had concentrations of IgM of less than 20 ng/mg protein. E) Plasma AST activity was measured and F) immunoreactive TNFα assessed by immunohistochemistry. Values represent means ± SEM, individual P values are shown for differences between pair-fed and ethanol-fed mice treated with vehicle and between ethanol-fed mice treated with vehicle or C1INH. NS: not signficant. n=4 for Pair-fed and n=6 for EtOH-fed.
4. Conclusion
Apoptosis has been associated with the progression of alcoholic liver disease. In mice, ethanol feeding results in a dynamic process of apoptosis. Apoptosis of F4/80 positive macrophages is observed early in the response to ethanol, followed by hepatocyte apoptosis at later times and higher concentrations of ethanol (Figure 1 and (Cohen et al. 2010)). Here we identify sIgM as an important link between apoptosis resulting from moderate ethanol exposure and subsequent activation of complement and development of inflammation and early stages of liver injury. Recognition of apoptotic cells by defense collagens, such as C1q, has been considered a mechanism to activate anti-inflammatory processes; essentially clearing out debris and quickly resolving inflammation (Litvack and Palaniyar 2010; Fraser and Tenner 2008). If this anti-inflammatory response is lost, pathology can result (Zipfel and Skerka 2009; Sjoberg et al. 2009). Indeed, this response seems to be dysregulated during chronic ethanol exposure. Importantly, here we demonstrate that inhibition of C1 esterase activity during binge ethanol exposure can break the link between ethanol-induced apoptosis-sIgM/C1q recognition and subsequent inflammatory injury.
Highlights.
The progression of ethanol-induced liver injury was studied in a mouse model
IgM co-localizes with C1q in the liver in response to ethanol feeding; accumulation of IgM and C3b at early stages of ethanol feeding is dependent on apoptosis
sIgM−/− mice are protected from early stages of ethanol-induced liver injury
C1INH, an inhibitor of C1 activity, protected from ethanol-induced liver injury
Acknowledgments
This work was supported in part by NIH grant P20 AA17069, U01AA021890 and R37 AA011876 (LEN); ABMRF/The Foundation for Alcohol Research (DC); NIH grant R21AA020941 (SR); T32DK007319 (RLM) and the Case Western Reserve University/Cleveland Clinic CTSA UL1RR024989. These funding sources had no involvement in the conduct of this research investigation.
Abbreviations
- ALT
alanine aminotransferase activity
- ASH
alcoholic hepatitis
- C1INH
C1 inhibitor (Cinryze)
- C3
third component of the complement system
- C3b
C3b-iC3b/C3c
- C5
fifth component of the complement system
- C3aR
C3a receptor
- C5aR
C5a receptor
- CR
complement receptor
- CYP2E1
cytochrome P450 2E1
- DAF
decay accelerating factor
- FBS
fetal bovine serum
- IL-6
interleukin 6
- IFNγ
interferon γ
- LPS
lipopolysaccharide
- OCT
optimal cutting temperature
- PBS
phosphate buffered saline
- ROS
reactive oxygen species
- SEM
standard error of the mean
- TLR-4
toll-like receptor 4
- TNF-α
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Alonso M, Gomez-Rial J, Gude F, Vidal C, Gonzalez-Quintela A. Influence of experimental alcohol administration on serum immunoglobulin levels: contrasting effects on IgE and other immunoglobulin classes. Int J Immunopathol Pharmacol. 2012;25:645–655. doi: 10.1177/039463201202500311. [DOI] [PubMed] [Google Scholar]
- 2.Barnes MA, Roychowdhury S, Nagy LE. Innate immunity and cell death in alcoholic liver disease: role of cytochrome P4502E1. Redox Biol. 2014;2:929–935. doi: 10.1016/j.redox.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder CJ. Natural IgM antibodies against oxidation-specific epitopes. J Clin Immunol. 2010;30(Suppl 1):S56–S60. doi: 10.1007/s10875-010-9396-3. [DOI] [PubMed] [Google Scholar]
- 4.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 5.Bohlson SS, O'Conner SD, Hulsebus HJ, Ho MM, Fraser DA. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front Immunol. 2014;5:402. doi: 10.3389/fimmu.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bykov I, Jauhiainen M, Olkkonen VM, Saarikoski ST, Ehnholm C, Junnikkala S, Vakeva A, Lindros KO, Meri S. Hepatic gene expression and lipid parameters in complement C3(−/−) mice that do not develop ethanol-induced steatosis. J Hepatol. 2007;46:907–914. doi: 10.1016/j.jhep.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Cicardi M, Zingale L, Zanichelli A, Pappalardo E, Cicardi B. C1 inhibitor: molecular and clinical aspects. Springer Semin Immunopathol. 2005;27:286–298. doi: 10.1007/s00281-005-0001-4. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139:664–674. 674.e1. doi: 10.1053/j.gastro.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cugno M, Zanichelli A, Foieni F, Caccia S, Cicardi M. C1-inhibitor deficiency and angioedema: molecular mechanisms and clinical progress. Trends Mol Med. 2009;15:69–78. doi: 10.1016/j.molmed.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Das D, Barnes MA, Nagy LE. Anaphylatoxin C5a modulates hepatic stellate cell migration. Fibrogenesis Tissue Repair. 2014;7:9. doi: 10.1186/1755-1536-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daveau M, Benard M, Scotte M, Schouft MT, Hiron M, Francois A, Salier JP, Fontaine M. Expression of a functional C5a receptor in regenerating hepatocytes and its involvement in a proliferative signaling pathway in rat. J Immunol. 2004;173:3418–3424. doi: 10.4049/jimmunol.173.5.3418. [DOI] [PubMed] [Google Scholar]
- 12.Davis AE, 3rd, Lu F, Mejia P. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb Haemost. 2010;104:886–893. doi: 10.1160/TH10-01-0073. [DOI] [PubMed] [Google Scholar]
- 13.Fraser DA, Tenner AJ. Directing an appropriate immune response: the role of defense collagens and other soluble pattern recognition molecules. Curr Drug Targets. 2008;9:113–122. doi: 10.2174/138945008783502476. [DOI] [PubMed] [Google Scholar]
- 14.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Jarvelainen HA, Vakeva A, Lindros KO, Meri S. Activation of complement components and reduced regulator expression in alcohol-induced liver injury in the rat. Clin Immunol. 2002;105:57–63. doi: 10.1006/clim.2002.5267. [DOI] [PubMed] [Google Scholar]
- 16.Joshi-Barve S, Kirpich I, Cave MC, Marsano LS, McClain CJ. Alcoholic, Nonalcoholic, and Toxicant-Associated Steatohepatitis: Mechanistic Similarities and Differences. Cellular and Molecular Gastroenterology and Hepatology. 2015;1:356–367. doi: 10.1016/j.jcmgh.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J Exp Med. 2002;196:655–665. doi: 10.1084/jem.20020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieber CS. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J. Hepatol. 2000;32(Suppl 1):113–128. doi: 10.1016/s0168-8278(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 19.Litvack ML, Palaniyar N. Review: Soluble innate immune pattern-recognition proteins for clearing dying cells and cellular components: implications on exacerbating or resolving inflammation. Innate Immun. 2010;16:191–200. doi: 10.1177/1753425910369271. [DOI] [PubMed] [Google Scholar]
- 20.Liu D, Cai S, Gu X, Scafidi J, Wu X, Davis AE., 3rd C1 inhibitor prevents endotoxin shock via a direct interaction with lipopolysaccharide. J Immunol. 2003;171:2594–2601. doi: 10.4049/jimmunol.171.5.2594. [DOI] [PubMed] [Google Scholar]
- 21.Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004;24:217–232. doi: 10.1055/s-2004-832936. [DOI] [PubMed] [Google Scholar]
- 22.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 23.Nagy LE. Molecular mechanisms of alcohol metabolism. Ann. Rev. Nutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- 24.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, Dibonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187. e1–e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Medof ME, Nagy LE. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132:1117–1126. doi: 10.1053/j.gastro.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin X, Gao B. The complement system in liver diseases. Cell Mol Immunol. 2006;3:333–340. [PubMed] [Google Scholar]
- 27.Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol. 2005;35:252–260. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- 28.Roychowdhury S, Chiang DJ, Mandal P, McMullen MR, Liu X, Cohen JI, Pollard J, Feldstein AE, Nagy LE. Inhibition of apoptosis protects mice from ethanol-mediated acceleration of early markers of CCl4 -induced fibrosis but not steatosis or inflammation. Alcohol Clin Exp Res. 2012;36:1139–1147. doi: 10.1111/j.1530-0277.2011.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roychowdhury S, McMullen MR, Pritchard MT, Hise AG, van Rooijen N, Medof ME, Stavitsky AB, Nagy LE. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology. 2009;49:1326–1334. doi: 10.1002/hep.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebastian BM, Roychowdhury S, Tang H, Hillian AD, Feldstein AE, Stahl GL, Takahashi K, Nagy LE. Identification of a cytochrome P4502E1/Bid/C1q-dependent axis mediating inflammation in adipose tissue after chronic ethanol feeding to mice. J Biol Chem. 2011;286:35989–35997. doi: 10.1074/jbc.M111.254201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen H, French BA, Liu H, Tillman BC, French SW. Increased activity of the complement system in the liver of patients with alcoholic hepatitis. Exp Mol Pathol. 2014;97:338–344. doi: 10.1016/j.yexmp.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjoberg AP, Trouw LA, Blom AM. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30:83–90. doi: 10.1016/j.it.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Tsiantoulas D, Perkmann T, Afonyushkin T, Mangold A, Prohaska TA, Papac-Milicevic N, Millischer V, Bartel C, Horkko S, Boulanger CM, Tsimikas S, Fischer MB, Witztum JL, Lang IM, Binder CJ. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J Lipid Res. 2015;56:440–448. doi: 10.1194/jlr.P054569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 36.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 37.Zwart B, Ciurana C, Rensink I, Manoe R, Hack CE, Aarden LA. Complement activation by apoptotic cells occurs predominantly via IgM and is limited to late apoptotic (secondary necrotic) cells. Autoimmunity. 2004;37:95–102. doi: 10.1080/0891693042000196183. [DOI] [PubMed] [Google Scholar]