Abstract
Fn14, the sole known signaling receptor for the TNF family member TWEAK, is inducibly expressed in the central nervous system (CNS) in endothelial cells, astrocytes, microglia, and neurons. There is increasing recognition of the importance of the TWEAK/Fn14 pathway in autoimmune neurologic conditions, including experimental autoimmune encephalomyelitis and neuropsychiatric lupus. Previously, we had found that Fn14 knockout lupus-prone MRL/lpr mice display significantly attenuated neuropsychiatric manifestations. To investigate whether this improvement in disease is secondary to inhibition of TWEAK/Fn14 signaling within the CNS or the periphery, and determine whether TWEAK-mediated neuropsychiatric effects are strain dependent, we performed intracerebroventricular (ICV) injection of Fc-TWEAK or an isotype matched control protein to C57Bl6/J non-autoimmune mice. We found that Fc-TWEAK injected C57Bl6/J mice developed significant depression-like behavior and cognitive dysfunction. Inflammatory mediators associated with lupus brain disease, including CCL2, C3, and iNOS, were significantly elevated in the brains of Fc-TWEAK treated mice. Furthermore, Fc-TWEAK directly increased blood brain barrier (BBB) permeability, as demonstrated by increased IgG deposition in the brain and reduced aquaporin-4 expression. Finally, Fc-TWEAK increased apoptotic cell death in the cortex and hippocampus. In conclusion, TWEAK can contribute to lupus-associated neurobehavioral deficits including depression and cognitive dysfunction by acting within the CNS to enhance production of inflammatory mediators, promote disruption of the BBB, and induce apoptosis in resident brain cells. Our study provides further support that the TWEAK/Fn14 signaling pathway may be a potential therapeutic target for inflammatory diseases involving the CNS.
Keywords: neuropsychiatric lupus, TWEAK
1. Introduction
Patients with depression and/or cognitive dysfunction suffer remarkably reduced quality of life compared with other chronic illnesses (Bonicatto et al., 2001; Whalley and McKenna, 1995). Cytokines have been implicated in the pathogenesis of mood disorders as well as in cognitive impairment (Dowlati et al., 2010). Various studies have established a significant correlation between neuropsychiatric syndromes and dysregulation of particular inflammatory cytokines, including TNF, IL-1, and IL-6 (Dowlati et al., 2010; Miller et al., 2009; Raison et al., 2006).
The mechanisms involved in cytokine-induced neuropsychiatric deficits, including depression and cognitive abnormalities, are however not well understood. Nonetheless, emerging evidence has suggested an interaction between the neurological and immune systems (Dowlati et al., 2010). Cytokines can alter neuroendocrine activity, leading to changes in the release of neurotransmitters including serotonin, dopamine, and noradrenaline (Dowlati et al., 2010). Furthermore, inflammatory mediators may also promote blood brain barrier disruption, neuroinflammation, and neurodegeneration, thus negatively impacting cognitive function (Dowlati et al., 2010; Wilson et al., 2002).
TNF-like weak inducer of apoptosis (TWEAK) is a member of the TNF family of cytokines, and its sole known signaling receptor is Fn14 (Winkles, 2008). In the central nervous system (CNS), Fn14 is inducibly expressed in endothelial cells, astrocytes, microglia, and neurons (Yepes, 2013). Depending on the specific cell type and downstream signaling pathways activated, the engagement of Fn14 by TWEAK can modulate cell proliferation or apoptosis, and promote inflammation (Winkles, 2008). Indeed, there is mounting evidence that TWEAK plays an important role in the pathogenesis of various CNS diseases. TWEAK mRNA is upregulated in the spinal cord of mice with experimental autoimmune encephalomyelitis (EAE), while anti-TWEAK monoclonal antibody treatment decreases immune cell infiltration into the CNS and attenuates the severity of disease in this model (Desplat-Jego et al., 2002). Similarly, blockade of TWEAK/Fn14 interactions reduces infarct volume in an ischemic stroke model (Yepes et al., 2005).
Recently, we reported that knocking out the TWEAK receptor Fn14 in the MRL/lpr strain, a widely used murine model for the study of neuropsychiatric lupus (NPSLE), significantly ameliorates neurologic manifestations. Fn14 knock-out MRL/lpr mice demonstrated less depression-like behavior and improved cognitive function, especially spatial memory (Wen et al., 2013). To establish whether this improvement in disease is attributable to the ablation of TWEAK/Fn14 signaling within the CNS or the periphery, and determine whether TWEAK-mediated neuropsychiatric symptoms are strain dependent, we performed intracerebroventricular injection of Fc-TWEAK into non-autoimmune C57Bl6/J mice.
2. Materials and Methods
2.1 Mice
Female C57Bl6/J (B6) mice were purchased from Jackson Laboratory (Bar Harbor, ME) at 7 weeks of age, and allowed to acclimate for one week. A single guide cannula (Plastics One, Roanoke, VA) was implanted into the left lateral ventricle according to the stereotaxic coordinates derived from The Mouse Brain in Stereotaxic Coordinates (Franklin, 2001) as follows: anteroposterior (AP): −0.34 mm; mediolateral (ML): −1.0 mm; dorsal ventricular (DV): 2 mm. Each guide cannula was encased with a dummy cannula to help maintain sterility. Five days after surgery, intracerebroventricular (ICV) injection of murine Fc-TWEAK (2 µg, n=19, Biogen Idec, Cambridge, MA) or an isotype control (P1.17, 2 µg, n=18, Biogen Idec) was performed twice a week starting at 9 weeks of age, for a total of 5 injections. That is, both “Fc-TWEAK” and “isotype control” mice were cannulated and injected. Both injections and the behavior tests were performed during daytime hours. Three separate cohorts of mice (n=4–7 in each of the Fc-TWEAK and P1.17 groups) were cannulated and tested under precisely the same conditions for several reasons. First, this was a logistically reasonable number of animals that could be surgically implanted, infused, and tested. Second, each cohort represented an independent replication. The experimental results in each independent cohort were similar, and thus the mice were pooled for the purpose of the neurobehavioral analyses and graphs.
For the histological and immunofluorescence studies an additional control group of age, sex and strain matched mice (“B6” group; n=4) that were not cannulated or injected were included, to provide the baseline/background for the strain and control for any effects of the cannulation and injection. All animals were handled according to the approved animal protocol at the Albert Einstein College of Medicine.
Fc-TWEAK was generated and gifted by scientists at Biogen Idec and is not a commercial product, while P1.17 is a purified monoclonal antibody from a commercially available myeloma cell line obtained from ATCC (Manassas, VA). Addition of the murine Fc portion to the murine TWEAK protein was employed to increase protein stability. P1.17, a murine IgG2a monoclonal antibody with an identical Fc fragment as Fc-TWEAK, has been previously extensively used as an isotype control for Fc-TWEAK (Campbell et al., 2006; Perper et al., 2006; Stephan et al., 2013; Wu et al., 2013). Fc-TWEAK and P1.17 were reconstituted in PBS. There were no preservatives in either the stock Fc-TWEAK or P1.17 preparations, or in the solutions injected into the mice.
2.2 Behavioral testing
Comprehensive behavior testing, including the open field test, anhedonia, object placement, object recognition, and the Porsolt swim test were conducted following procedures described previously (Gao et al., 2010), and further detailed below. When technical considerations precluded testing the entire cohort, randomly selected subgroups of mice were tested as specified.
2.2.1 Open field test
The open field test was conducted in an arena (40 cm × 40 cm × 40 cm) on the day following the 3rd injection. General locomotor activities, including total track length, center track length, center time, and center entries, were tracked by Viewer III software (Biobserve, Bonn, Germany) for 9 minutes.
2.2.2 Anhedonia
Anhedonia, a depression-associated behavior manifesting as a lack of preference for sweetened fluid, was performed on the second day after the 3rd injection (day +2). Animals were pre-exposed to increasing concentrations of saccharin starting at 0.02% saccharin (day −1), followed by 1% (day 0) and 2% (day +1), respectively. Starting from the evening of day +1, mice were fluid deprived overnight. On the day of testing (day +2), mice were provided with water and 2% saccharin for an hour to test their saccharin preferences in a standard 2 bottle choice test. Since the locations of the sweetened and non-sweetened water bottles are switched regularly over the duration of the test, preference for drinking from the bottle containing sweetened fluid is not dependent on intact spatial memory nor is confounded by any other reason by a side preference. The preference of saccharin was defined as the consumed volume of saccharin/the total consumed volume of the fluid. The cutoff for saccharin-sweetened water preference was set at 50% (dotted line) (Bueter et al., 2011).
2.2.3 Object placement (OP) and object recognition (OR) test
Object placement and object recognition tests were used to evaluate spatial and recognition memory, respectively, and are based on rodents’ innate preferential exploration of novel places/objects. Object placement was performed the day after the 4th injection, while object recognition was done on the second day after the 4th injection.
In both tests, mice underwent two trials: a training trial (trial 1) and a testing trial (trial 2). In the object placement test, animals were first exposed to two identical objects in two separate locations for 5 minutes (trial 1). Following a 30 minute retention interval, mice were tested in the same arena where one object was left in the same place, while the other object was moved to a new location (trial 2). In the object recognition test, animals were first allowed to explore two identical objects for 3 minutes (trial 1). After a 90 minute retention interval, animals were tested in the same arena where one object was replaced with a novel object (trial 2). Exploration, defined as any type of physical contract with an object (whisking, sniffing, rearing on or touching the object) (Gulinello et al., 2010), was recorded manually. Experimenters were blinded to the experimental assignment of the tested mice.
The preference score (%) for object recognition and object placement tasks was calculated as ([exploration time of the novel object]/[exploration time of both objects])×100. Mice with total exploration times of less than 2 seconds in either trial were excluded from analysis of preference scores, since this was insufficient time for animals to become familiar with both objects during the training trial, or to accurately score a preference during the testing trial. In OP, one mouse in the Fc-TWEAK group was excluded; in OR, two mice in the isotype treated group were excluded. The cutoff to define “pass” in the object placement and object recognition tests was 53%. During extensive validation of these tasks in the Einstein Behavioral Core Facility, it was determined that rodents with preference scores higher than 53% consistently displayed robust OR/OP preferences when re-tested (unpublished data); few animals had scores between 53% and 55%, and the use of a stricter criterion (>55%) makes little difference to the analysis (Cole et al., 2013; Li et al., 2010).
2.2.4 Porsolt swim test
The Porsolt swim test was performed on the day following the 5th injection to assess depression-like behavior. Mice with depression-like behavior exhibit immobility, while normal animals swim. In the forced swim test, each mouse was placed into a transparent cylindrical tank with water at 25°C. Animals were allowed to adjust to the environment for one minute and then the behavior during the following 3 minutes was digitally recorded as the test session using Viewer III software. Total immobility (in %) was calculated by the ratio of ([total time immobile in the water]/[total time scored]) ×100. The scorers were blind to the experimental groups.
2.3 Brain histology
After behavioral testing, mice were sacrificed and extensively perfused with cold PBS. Brains were dissected into right and left hemispheres. The left hemisphere of the brain was fixed in 4% paraformaldehyde for 24 hours at 4°C, and embedded in paraffin for sagittal sections. The right brain hemisphere was snap-frozen for RNA extraction.
2.4 Immunofluorescence
For immunofluorescence staining, paraffin sections were incubated with one of the following antibodies: rabbit anti-mouse Iba-1 (1:250, WAKO Chemicals, Mountain View, CA), rabbit anti-mouse aquaporin 4 (1:100, Santa Cruz, Dallas, TX), mouse anti-glial fibrillary acidic protein (GFAP) (1:300, Millipore, Billerica, MA), and goat anti-mouse C3 (1:250, MP Biomedicals, Santa Ana, CA), followed by the secondary antibodies donkey anti-rabbit Alexa Fluor 594, donkey anti-rabbit IgG Alexa Fluor 488, donkey anti-mouse IgG Alexa Fluor 594, or donkey anti-goat IgG Alexa Fluor 594, respectively (1:200 for all secondary antibodies, Jackson ImmunoResearch, West Grove, PA). IgG staining was performed by incubating slides directly with goat anti-mouse IgG Alexa Fluor 594 (1:100, Jackson Immunoresearch). Sections were analyzed under a fluorescence microscope (Zeiss AxioObserver CLEM or Digital Station II). For Iba-1 staining, a representative image was taken from the cortex and hippocampus in each section, and quantitation of the fluorescence intensity in each image was performed using Image J. For AQP4 and C3 staining, the slides were scored from 0–3 according to the staining intensity (negative staining=0, minimal staining intensity=1, moderate staining intensity=2, maximal staining intensity=3). For GFAP staining, GFAP+ cells were counted in each representative image. For all immunofluorescent stains, two sections from each mouse brain were averaged for a single data point to represent that individual mouse. All quantifications were done in a blinded manner.
2.5 TUNEL staining
TUNEL staining was performed using an in situ cell death detection (Fluroscein) kit (Roche Diagnostic, Indianapolis, IN), according to the manufacturer’s instructions. Slides were analyzed under a fluorescence microscope (Zeiss AxioObserver CLEM), and the number of TUNEL positive cells counted in each section in a blinded manner. Two sections from each mouse brain were averaged for a single data point to represent that individual mouse.
2.6 Real-time qPCR
RNA was isolated from the brain of the mice using a RNEASY midi kit (Qiagen, Valencia, CA). cDNA synthesis was performed with the superscript III first strand synthesis kit (Invitogen, Carlsbad, CA). Real-time qPCR was performed in triplicate for CCL2, CCL5, C3, and iNOS, as previously described (Wen et al., 2013). The primer sequences were as follows: GAPDH: (forward) 5-TGTGATGGGTGTGAACCACGAGAA-3, (reverse) 5-GAGCCCTTCCACAATGCCAAAGTT-3; CCL2: (forward): 5-TAAAAACCTGGATCGGAACCAA-3, (reverse) 5-GCATTAGCTTCAGATTTACGGG-3; CCL5: (forward) 5-GCAAGTGCTCCAATCTTGC-3, (reverse) 5-CTTCTTCTCTGGTTGGCAC-3; C3: (forward) 5-AAGCATCAACACACCCAA-3, (reverse) 5-CTTGAGCTCCATTCGTGAC-3; iNOS: (forward) 5-GGAGTGACGGCAAACATGACT-3, (reverse), 5-TCGATGCACAACTGGGTGAAC-3. The relative expression of each gene was normalized to GAPDH, and the mean expression of each gene in the control group was set to 1.
2.7 Statistical analysis
Graphpad Prism 6 software was used to perform the statistical analysis. Each graph depicts the mean ± standard error of the mean. Since the primary comparison was between cannulated mice groups that were injected either with Fc-TWEAK or P1.17, the unmanipulated B6 mice were only used as a reference for the strain baseline and thus not included in the statistical analysis. In other words, statistical analysis was only carried out between Fc-TWEAK and isotype control P1.17 treated group. Data was first analyzed by D’Agostino-Pearson test to assess whether the data was normally distributed. The differences between two groups were calculated by a two-tailed unpaired t test (for normally distributed data) or Mann-Whitney U test (non-normally distributed data). Chi-square test was used to analyze the differences in the two categories (preference or non-preference) in the behavioral testing between the two groups. P values <0.05 were considered significant.
3. Results
3.1 Fc-TWEAK treated mice display depression-like behavior
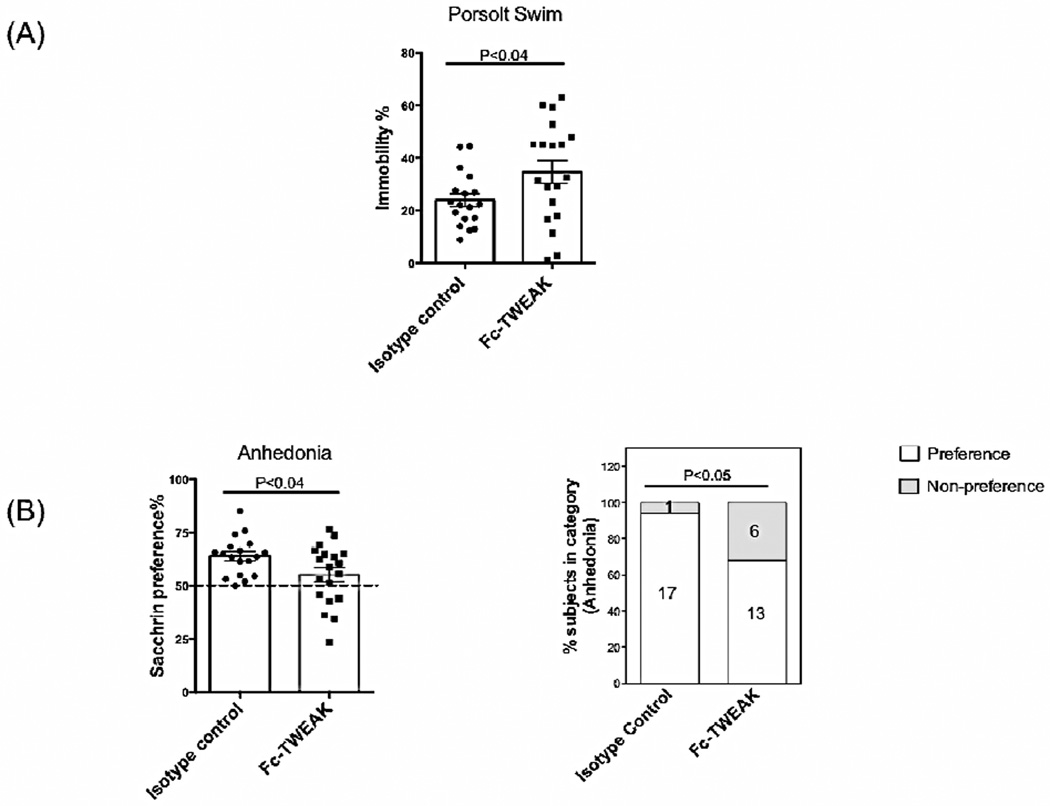
To evaluate whether locally elevated levels of TWEAK within the CNS can induce depression, we conducted the Porsolt swim test, a reliable and widely-used method to evaluate depression-like states in rodents (Can et al., 2012). Generally, normal mice swim, whereas mice exhibiting depression-like behavior become immobile (Can et al., 2012). Fc-TWEAK injected mice displayed a significant increase in immobility compared to the isotype control (isotype control vs Fc-TWEAK injected mice, t=2.1, df=35, P<0.04, Figure 1A).
Figure 1. B6 mice treated with Fc-TWEAK display depression-like behavior.
(A) Porsolt swim tests were performed after the 5th injection of Fc-TWEAK, and the floating time for the first 3 minutes was recorded. (B) The assay for anhedonia was conducted after the 3rd injection of Fc-TWEAK. The dotted line specifies the cutoff for saccharin-sweetened water preference. The percentage of mice in each group that showed the normal “preference”, or “no preference”, in the anhednonia assay is shown in (B, right panel), with the number of mice in each category provided in the columns. The number of mice in the istoype control and Fc-TWEAK treated group was 18 and 19, respectively, in both tests.
Another behavioral test commonly used to assess depression-like behavior is anhedonia, which refers to a reduced response to stimuli with positive emotional valence (Gorwood, 2008). In this test, the mice were provided with free access to both plain (unsweetened) and saccharin-sweetened water. Generally, there is a preference for sweetened fluid, which is lost however in rodents with depression-like behavior (Gorwood, 2008). Fc-TWEAK treated mice showed a significantly decreased preference for sweetened water compared with the isotype control (isotype control vs Fc-TWEAK injected mice, t=2.2, df=35, P<0.04, Figure 1B, left panel), consistent with Fc-TWEAK promoting depression-like behavior. In addition, Fc-TWEAK treated mice had a significantly increased incidence of anhedonia: 6 out of 19 mice in the Fc-TWEAK treated group displayed lack of preference for sweetened fluid, as compared to only 1 out of 18 in the isotype control injected group (Chi-square=4.1, df=1, P<0.05, Figure 1B, right panel).
3.2 Fc-TWEAK administration induces learning/memory deficits
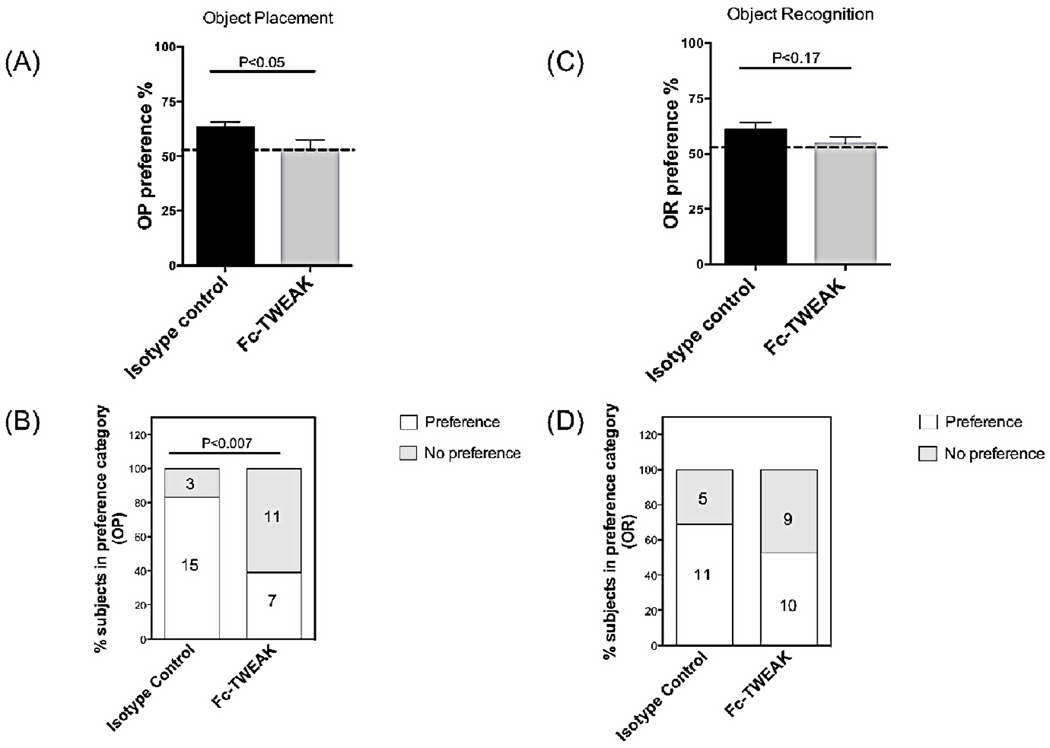
To assess whether Fc-TWEAK administration modulates cognitive function, object placement (OP) and object recognition (OR) tests were conducted. The object placement test is a robust assay to evaluate the spatial memory of the rodents, during which the animals are examined for a preference for an object in a novel position (Antunes and Biala, 2012). Object recognition, on the other hand, is applied for the evaluation of recognition memory, where mice are tested for the ability to distinguish a novel object from an object with which they had previously interacted (Rosenbaum et al., 2012). Spatial memory was impaired after Fc-TWEAK administration, as assessed by a significantly decreased preference score in the OP test (Figure 2A, isotype control vs Fc-TWEAK, t=2.0, df=34, P<0.05). Furthermore, we found that injection of Fc-TWEAK significantly increased the incidence of failure in the spatial memory test: 11 out of 18 mice failed to prefer exploration of the object in the new location, while only 3 out of 18 in the isotype control group exhibit spatial memory deficits (Chi-square=7.5, df=1, P<0.007, Figure 2B). Changes in object recognition induced by Fc-TWEAK administration, however, did not reach statistical significance (Figure 2C,D). There were no significant differences in object exploration times during trial 1 (training trial) in OP or OR between isotype control and Fc-TWEAK injected mice: mean exploration times for the isotype control and Fc-TWEAK groups were 37.8 ± 3.4 s and 37.7 ± 3.5 s, respectively, during the training session in OP, and 11.8 ± 1.8 s and 14.4 ± 1.8 s in OR.
Figure 2. Fc-TWEAK administration induces cognitive impairment.
Object placement (OP, spatial memory) (A, B) and object recognition (OR, recognition memory) (C, D) tests were used to evaluate cognitive function after the 4th injection of Fc-TWEAK. The dotted line in each panel specifies the cutoff for each test. The percentage of mice in each group that showed either a “preference”, or “no preference” in the OP and OR tests is shown in (B) and (D), with the number of mice in each category provided in the columns. For OP, the number of mice in the istoype control and Fc-TWEAK groups was 18 and 18, respectively; for OR, 16 and 19, respectively.
3.3 Fc-TWEAK administration does not alter locomotor activity
The open field test, which assesses locomotion and exploratory activity of rodents (Prut and Belzung, 2003), was performed after Fc-TWEAK administration. There were no differences in total track length among the isotype control (3818.3 ± 155.3 cm) and Fc-TWEAK treated groups (4089.7 ± 153.4 cm), indicating that locomotor activity was unaffected by the local increase in TWEAK levels within the CNS. In addition to locomotor activity, the open field test can also assess anxiety-like behavior by measuring relative center track length (the ratio of central/total track length, isotype control: 0.1 ± 0 and Fc-TWEAK: 0.1 ± 0), the time spent in the center (isotype control: 38.3 ± 6.1 s and Fc-TWEAK: 31.7 ± 2.9 s), and the number of center entries (isotype control: 31.9 ± 32 and Fc-TWEAK: 32.9 ± 2.3). There were no significant differences in relative center track length, center time, and center visits between the isotype control and Fc-TWEAK treated groups.
3.4 ICV injection of Fc-TWEAK permeabilizes the BBB
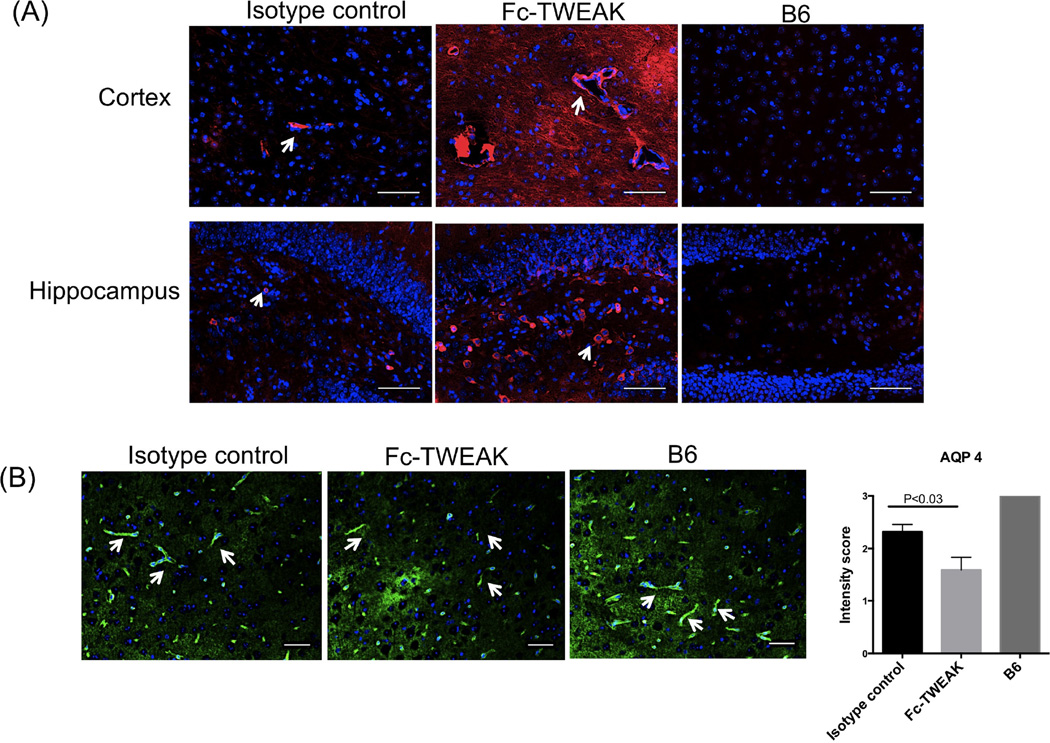
In vitro, we had found that TWEAK increased the permeability of a brain microvascular endothelial cell monolayer (Stephan et al., 2013). To investigate whether acute ICV administration of Fc-TWEAK might compromise the BBB in vivo, we assessed the brain levels of several proteins related to BBB function including IgG and aquaporin 4 (AQP 4) via immunofluorescence. Circulating IgG may penetrate through a compromised BBB, and deposit in brain tissue. We observed increased IgG leakage from the vessels into the cortical brain parenchyma, while in the hippocampus IgG deposition was mostly localized to the dentate gyrus region (Figure 3A). To further investigate the mechanism of the BBB breakdown, we also evaluated the expression of AQP4, a water channel protein located in astrocyte end feet which forms strong interactions with the endothelium - a specialized feature of the BBB (Nicchia et al., 2004). Staining for AQP4 indicated that Fc-TWEAK treated mice displayed significantly reduced cortical AQP4 expression compared with the isotype control injected mice (U=34.5, df=21, P<0.05, Figure 3B).
Figure 3. Fc-TWEAK treated mice exhibit increased BBB permeability.
(A) IgG staining was performed on brain sections from mice treated with isotype control, Fc-TWEAK, and untreated B6 mice, respectively. Arrows indicate blood vessels (cortex) or IgG staining cells (hippocampus). Immunofluorescent staining for AQP4 is shown in (B). Arrows indicate positive AQP4 staining (astrocyte end feet). Quantitation of AQP4 staining is shown on the right. The scale bar for all images equals 50 µm, unless otherwise indicated. For IgG and AQP4 staining, the number of mice in the istoype control, Fc-TWEAK, and unmanipulated B6 groups was 11, 12, and 4, respectively.
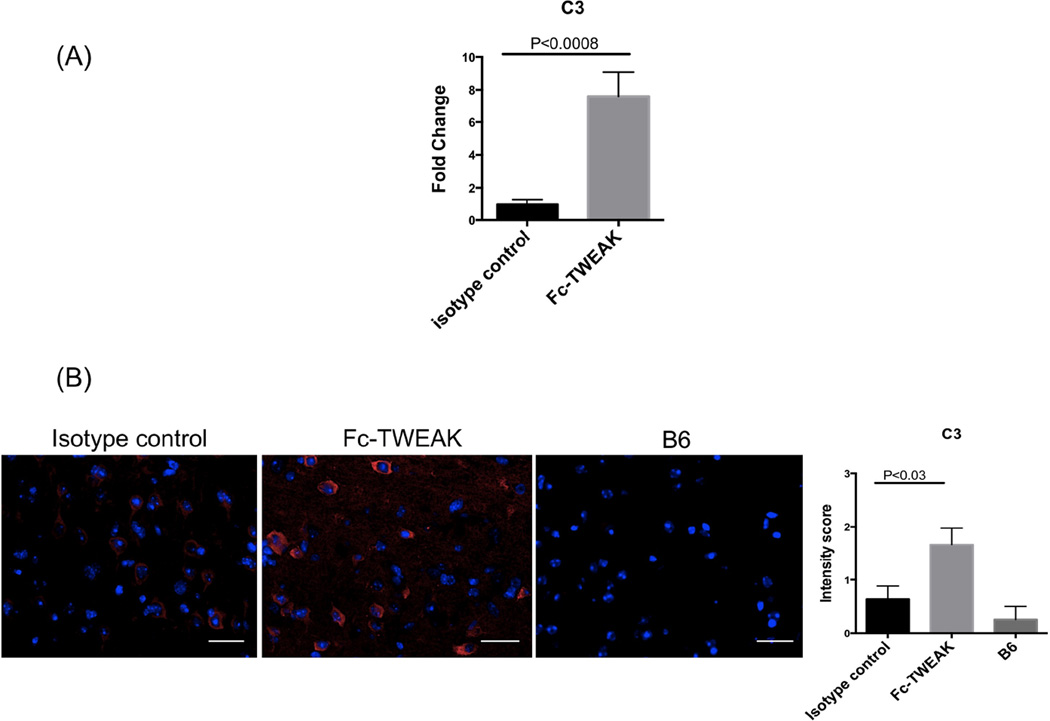
Since inflammation and antibody deposition can activate the complement system, we performed qRT-PCR and immunofluorescence staining for the complement component C3. Significantly elevated levels of C3 mRNA were found (t=4.1, df=20, P<0.0008, Figure 4A), consistent with the increased brain IgG deposition. Furthermore, Fc-TWEAK treated mice displayed significantly increased C3 deposition in cortical neurons (isotype control vs Fc-TWEAK injected mice, U=30.5, df=21, P<0.03, Figure 4B).
Figure 4. Fc-TWEAK administration increases brain C3 complement deposition.
Brain C3 gene expression as measured by qRT-PCR is shown in (A). (B) C3 staining was performed on brain sections from mice treated with isotype control, Fc-TWEAK, and unmanipulated B6 mice, respectively. Representative images of C3 staining in the cortex from each group were shown. Quantitation of C3 staining is shown on the right. The scale bar refers to 20 µm. For C3 qPCR, the number of animals in istoype control and Fc-TWEAK group was 10 and 12, respectively. For C3 staining, the number of mice in the istoype control, Fc-TWEAK, and unmanipulated B6 groups was 11, 12, and 4, respectively.
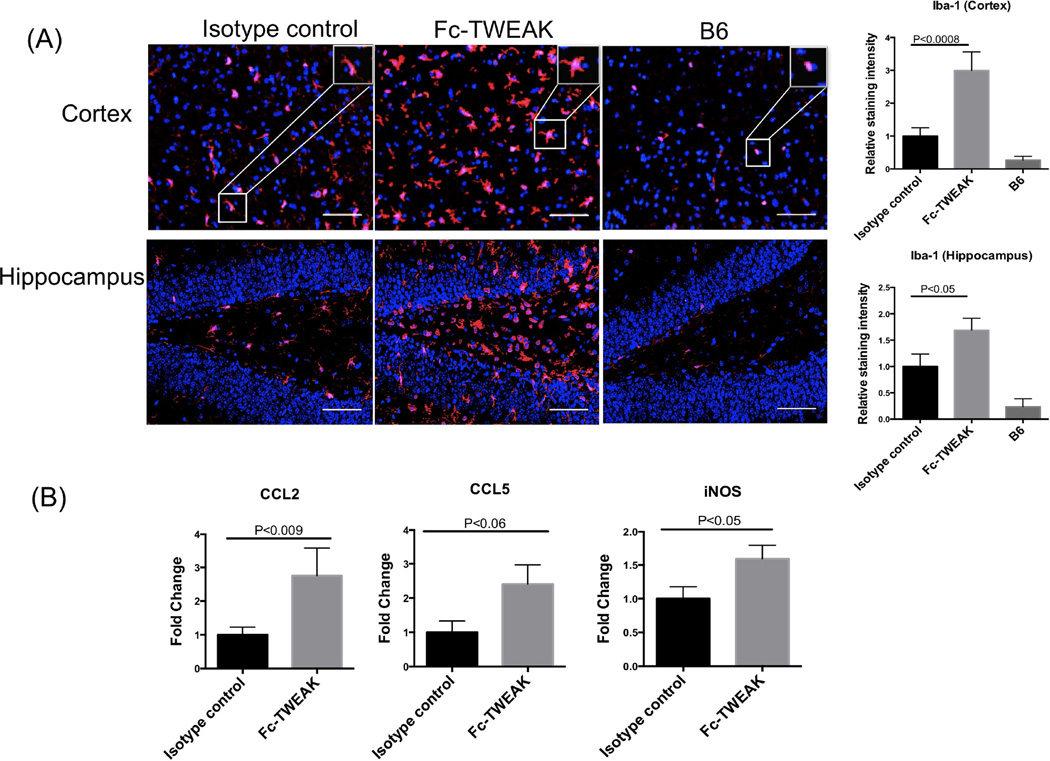
3.5 Fc-TWEAK administration enhances microglia/macrophage activation and neuroinflammation
As the major immune cell type resident in the brain, microglia play an important role in brain surveillance. Microglia become activated in response to various inflammatory stimuli, which may further lead to abundant cytokine production or phagocytosis of cellular debris. To evaluate whether Fc-TWEAK injection modulates the state of microglia/macrophage activation, Iba-1 staining was performed. Increased fluorescence intensity of Iba-1 staining was observed in the cortex and hippocampus of Fc-TWEAK treated mice (Figure 5A; U=14, df=21, P<0.0008 (cortex); t=2.2, df=21, P<0.05 (hippocampus)). Specifically, the dominant morphology of microglia observed in the Fc-TWEAK treated group was that of larger cell bodies and thicker processes, as compared with the isotype control group (Figure 5A, magnified white box). Furthermore, elevated expression of CCL2 (U=21, df=20, P<0.009), CCL5 (t=2.1, df=20, P<0.06), and iNOS (t=2.2, df=20, P<0.05), was detected after Fc-TWEAK administration (Figure 5B).
Figure 5. B6 mice treated with Fc-TWEAK demonstrate increased microglia/macrophage activation and neuroinflammation.
(A) Iba-1 staining was performed on brain sections from mice treated with isotype control, Fc-TWEAK, and untreated B6 mice. A representative Iba-1+ cell from the cortex is shown magnified in the white box in the right upper corner of the image. Quantitation of Iba-1 staining is shown on the right. (B) qRT-PCR for CCL2, CCL5, and iNOS was performed on brain mRNA from mice treated with isotype control and Fc-TWEAK. For Iba-1 staining, the number of mice in the istoype control, Fc-TWEAK, and unmanipulated B6 groups was 11, 12, and 4, respectively. For qRT-PCR, the number of mice in the istoype control and Fc-TWEAK groups was 10 and 12, respectively.
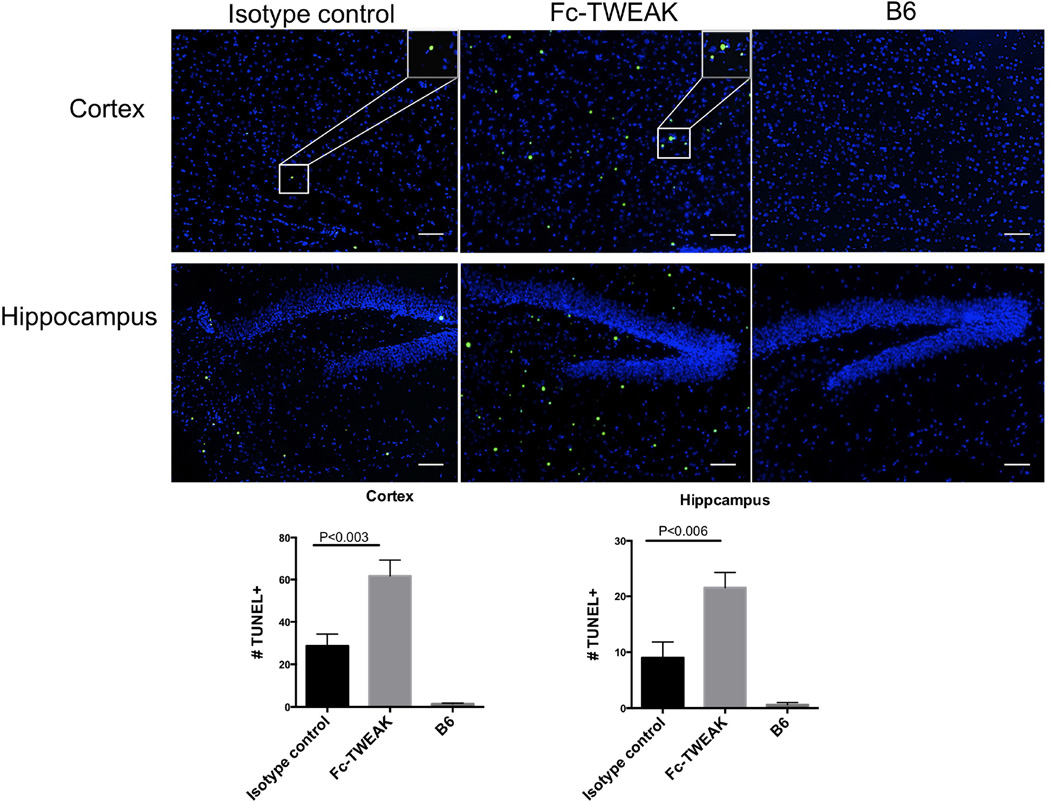
3.6 Fc-TWEAK treated mice display increased brain cell apoptosis
TWEAK/Fn14 interactions can lead to either cell apoptosis or cell proliferation, depending on the cellular context (Winkles, 2008). By TUNEL staining, we found markedly increased TUNEL positive cells in the cortex and hippocampus after Fc-TWEAK administration compared with the isotype control injected mice (Figure 6; isotype control vs Fc-TWEAK injected mice, t=3.4, df=21, P<0.003 (cortex); t=3.2, df=21, P<0.006 (hippocampus)).
Figure 6. B6 mice injected ICV with Fc-TWEAK display increased brain cell apoptosis.
TUNEL staining was performed on brain sections from mice treated with isotype control, Fc-TWEAK, and unmanipulated B6 mice. Representative TUNEL positive cells from the cortex are shown magnified in the white box in the right upper corner of the image. Quantitation of the number of TUNEL positive cells is shown in the lower panel. For TUNEL staining, the number of mice in the istoype control, Fc-TWEAK, and unmanipulated B6 groups was 11, 12, and 4, respectively.
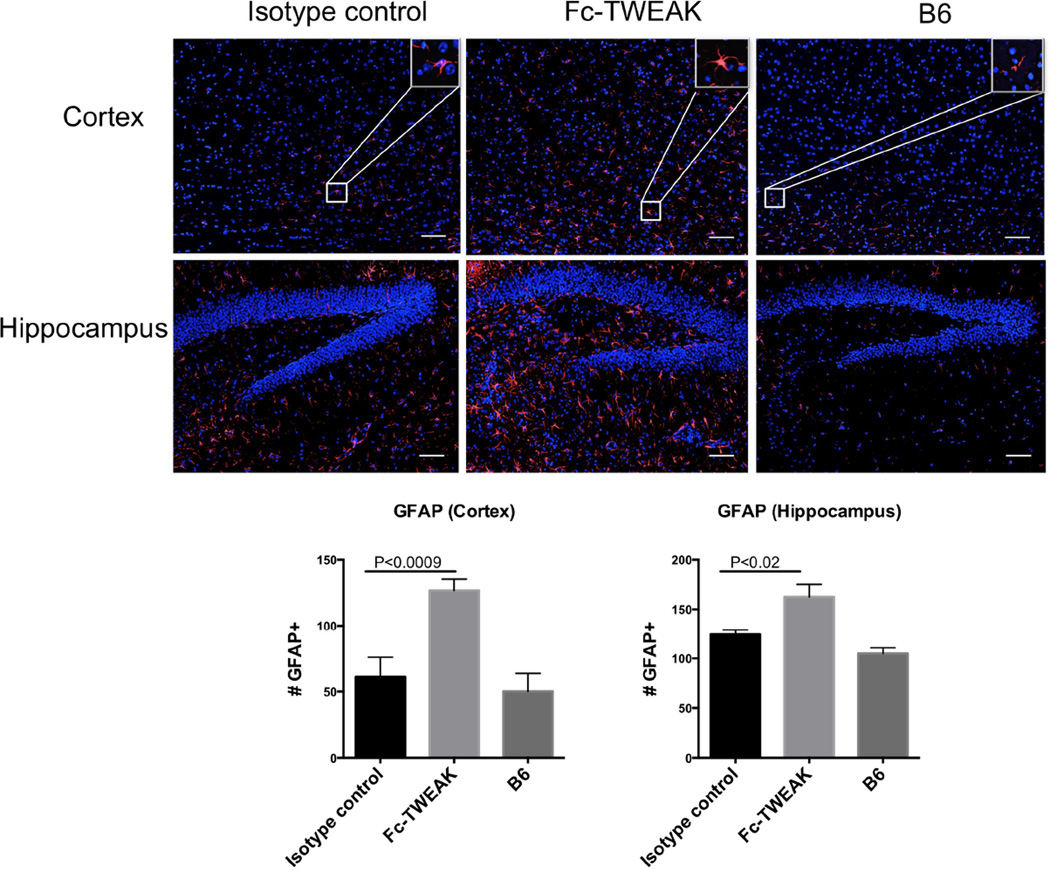
3.7 ICV injection of Fc-TWEAK promotes astrogliosis
Astrogliosis refers to the activation and/or proliferation of astrocytes which occurs in response to CNS injury, especially neurodegeneration. Since increased apoptotic cells were found after Fc-TWEAK administration and astrocytes are responsive to TWEAK stimulation in vitro (Desplat-Jego et al., 2002), we next conducted immunofluorescent staining for GFAP, an astrocyte marker, to assess the number of reactive astrocytes (Eng and Ghirnikar, 1994). As shown in Figure 7, we found that ICV injection of Fc-TWEAK led to increased numbers of GFAP+ cells in the cortex (t=4.0, df=21, P<0.0009) and hippocampus (t=2.8, df=21, P<0.02), when compared with isotype control injected mice.
Figure 7. Fc-TWEAK administration induces astrogliosis.
GFAP staining was performed on brain sections from mice injected ICV with isotype control or Fc-TWEAK and untreated B6 mice. A representative GFAP positive cell from the cortex is shown magnified in the white box in the right upper corner of the image. Quantitation of the number of GFAP positive cells is shown in the lower panel. For GFAP staining, the number of mice in the istoype control, Fc-TWEAK, and unmanipulated B6 groups was 11, 12, and 4, respectively.
4. Discussion
There is increasing recognition of the relevance of TWEAK to the pathogenesis of neurologic disease including NPSLE (Stock et al., 2013; Wen et al., 2015; Wen et al., 2013), EAE (Desplat-Jego et al., 2002), and ischemic stroke (Zhang et al., 2007). We report here that Fc-TWEAK given by ICV injection can induce significant depression-like behavior and learning/memory deficits in non-autoimmune mice. While the neurobehavioral assessments were performed in the daytime hours, this practice is common and the control mice were similarly tested. Furthermore, the primary objective of these studies was to determine if ICV TWEAK administration to B6 mice can induce features present in spontaneous neuropsychiatric disease in lupus-prone MRL/lpr mice, the latter of which were also tested in the daytime hours (Wen et al., 2013). The mechanisms of Fc-TWEAK mediated behavioral dysfunction may be attributed to a combination of factors including the compromised BBB, enhanced neuroinflammation, and increased brain cell apoptosis.
The behavioral phenotype was observed in B6 mice injected with Fc-TWEAK, a recombinant immunoglobulin fusion protein of the murine IgG2a subclass, but not P1.17, an intact myeloma protein of the same subclass. Recombinant fusion proteins built on an immunoglobulin scaffold are commonly employed as a means to increase the half-life and stability of otherwise short-lived molecules, and comparison to mice injected with the intact IgG of the same subclass suitably controls for Fc receptor interactions (both FcRn and FcγR mediated) (Campbell et al., 2006; Perper et al., 2006; Stephan et al., 2013). Unmanipulated B6 mice could not serve in our studies as a relevant reference, since any difference from Fc-TWEAK injected mice might be attributable to the cannulation and injection procedures or Fc-mediated effects, rather than a true effect of the injected cytokine. Finally, while a free Fc-fragment would be expected to have similar FcR interactions as Fc-TWEAK, such a reagent would not be the appropriate control due to its lower molecular mass and therefore more rapid renal clearance.
Since lupus-like neurobehavioral abnormalities (including depression), to the best of our knowledge, have never been previously induced by cytokine injection through the ICV route, this mode of disease induction is likely very challenging especially in non-autoimmune mice. Therefore, also considering the significant technical difficulty in the experimental setup which limited the number of mice that could reasonably be tested, we examined both the average scores as well as the incidence of anhedonia. Indeed, both types of analyses yielded similar results, which were also consistent with the increased immobility seen with Fc-TWEAK injection. Another important point is that while the behavioral assessments were conducted on different days following a different cumulative number of injections (from 3rd to 5th), we found that time after injections or number of infusions were not a critical factor in determination of the phenotype. It is also essential to consider whether anti-TWEAK antibodies, or immune complexes, were involved in any of the observed effects. However, it is unlikely that limited exposure to murine TWEAK, administered by ICV injection and without adjuvant, would be immunogenic enough to induce generation of meaningful titers of antibodies within the short time frame of these studies. Indeed, we found no significant differences in serum IgG anti-TWEAK antibodies between Fc-TWEAK and isotype control injected mice (data not shown).
Object placement and object recognition were performed 24 and 48 hours after the 4th injection. Although with separate cohorts of mice both tests could have been done at the same time point, such an experimental design would have prevented simultaneous analysis of all behavioral phenotypes, as well as direct comparison to the immunofluorescence and immunohistochemical studies, in the same mice. Furthermore, we can be reasonably confident that defective object placement in the face of normal object recognition is not an experimental artifact since this dichotomy is also present spontaneously in lupus prone MRL/lpr mice (Wen et al., 2013).
TWEAK/Fn14 interactions have pleiotropic effects in the CNS. In vitro, TWEAK can promote the proliferation of brain microvascular endothelial cells and increase the permeability of endothelial cell monolayers by decreasing the expression of the tight junction protein ZO-1, upregulating the adhesion molecules VCAM-1 and ICAM-1, and enhancing CCL-2, IL-6, and IL-8 production (Stephan et al., 2013). Additionally, TWEAK promotes astrocyte proliferation and secretion of IL-6 and IL-8, and triggers neuronal apoptosis via the NF-kB pathway (Desplat-Jego et al., 2002). In vivo, activation of the TWEAK/Fn14 pathway leads to BBB compromise and neurodegeneration and facilitates the transmigration of neutrophils into the CNS in an ischemic stroke model (Polavarapu et al., 2005; Yepes et al., 2005; Zhang et al., 2007). In EAE, the severity of the disease is enhanced in soluble TWEAK-overexpressing mice and anti-TWEAK monoclonal antibodies reduce the CNS leucocyte infiltration (Desplat-Jego et al., 2005; Desplat-Jego et al., 2002). Similarly, TWEAK is upregulated in the CSF of patients with NPSLE, and prevention of TWEAK/Fn14 signaling in murine NPSLE attenuates disease (Wen et al., 2013).
In our study, we found that ICV administration of Fc-TWEAK significantly upregulates CCL2, CCL5, and iNOS, inflammatory mediators which have been implicated in neuropsychiatric disease. CCL2 levels are significantly higher in patients with major depressive disorder compared with healthy controls (Sutcigil et al., 2007). CCL5 may induce behavioral abnormalities by modulating neuron survival and differentiation via the neuronal CCR5 receptor (Valerio et al., 2004). Furthermore, elevated expression of iNOS in the CNS is associated with various inflammatory conditions such as viral infection as well as autoimmune diseases, including EAE (Koprowski et al., 1993). Although it is possible that TWEAK can act directly on neurons independently of other cytokines to promote neurobehavioral deficits, the various mechanisms determined above to be operative in Fc-TWEAK injected mice including permeabilized BBB, neuroinflammation, and brain cell apoptosis more likely involve several cells types and mediators, acting in concert, in producing the phenotype observed in this study.
Cytokines can contribute to cognitive dysfunction through induction of neurodegeneration. Cytokines such as IL-1 can lead to an increase in cortical release of the inhibitory neurotransmitter gamma amino butyric acid (Casamenti et al., 1999), leading to an imbalance between excitatory and inhibitory neurotransmitters and subsequently neuronal death (Nava-Mesa et al., 2014). Additionally, the induction of nitric oxide by cytokines may result in neuronal apoptosis. Furthermore, inflammatory mediators may also exert direct cytotoxic effects by binding to their own receptors on neurons. In our study, we found that Fc-TWEAK upregulated iNOS expression and increased brain cell apoptosis. Whether these apoptotic cells are indeed neurons and/or other cell types will need to be determined in future studies.
Generally, TWEAK is secreted by activated monocytes and macrophages. The source of TWEAK that mediates neurobehavioral abnormalities in NPSLE can either be from the periphery or the CNS itself, from infiltrating cells or resident microglia. Our study found that elevated levels of Fc-TWEAK within the CNS were sufficient to mediate negative behavioral outcomes (even) in a non-autoimmune mouse strain. However, we acknowledge that this does not yet conclusively exclude a potential role of TWEAK coming from the periphery. For example, circulating TWEAK may theoretically compromise the BBB and penetrate into the CNS, thereby leading to neuropsychiatric deficits. Additionally, cytokines hyperexpressed systemically may signal through the vagus nerve to trigger CNS responses even without crossing the BBB (Maier et al., 1998). At this time therefore the cellular source of TWEAK in murine NPSLE remains to be determined. Future experiments using bone marrow chimera approaches might help discriminate whether the neurobehavioral effects of TWEAK injected ICV are solely mediated by local engagement of brain Fn14 receptors, or if there is any contribution of peripheral (immune or hematologic) mechanisms as well.
Several hypotheses have been advanced to explain cytokine-induced depression. The monoamine hypothesis refers to the reduction of neurotransmitters such as serotonin, dopamine, and norepinephrine in the brain, which are regarded as important regulators of mood. Cytokines may alter the release of excitatory neurotransmitters through the activation of indolamine-2,3-dioxygenase activity, which is the key enzyme in the breakdown of tryptophan (Heyes et al., 1992), a precursor for serotonin synthesis (Mellor and Munn, 1999). Cytokines may also contribute to depression through an inhibitory effect on hippocampal neurogenesis (Malberg et al., 2000). Additionally, during a stress response, elevated levels of cytokines such as TNF and IL-6 can also affect the hypothalamic-pituitary-adrenal axis (Chrousos, 1995; Dantzer et al., 1999; Vallieres and Rivest, 1999) and induce cortisol, which further modulates neurogenesis (Cameron and Gould, 1994). Which of these cellular pathways are most modulated by ICV Fc-TWEAK and are directly instrumental in the depression-like behavior observed in Fc-TWEAK injected mice will need to be further investigated.
5. Conclusions
In conclusion, Fc-TWEAK injected ICV into non-autoimmune mice can promote BBB disruption, elicit neuroinflammation, and trigger brain cell apoptosis, leading to depression-like behavior and cognitive dysfunction reminiscent of spontaneous neuropsychiatric manifestations in lupus-prone MRL/lpr mice. Whether TWEAK administration can modulate neurotransmitter pathways, and whether the intravenous administration of TWEAK can cause a similar pattern of neurobehavioral deficits, are interesting questions which will need to be further addressed in future studies. Thus, the TWEAK/Fn14 signaling pathway may be a potential therapeutic target for CNS inflammatory diseases, including NPSLE.
Highlights.
TWEAK given ICV causes depressive-like behavior and cognitive dysfunction in B6 mice;
TWEAK injection disrupts the blood brain barrier and promotes neuro-inflammation;
TWEAK-injected mice exhibit increased brain cell apoptosis;
Increased brain TWEAK is sufficient to induce features of murine neuropsychiatric lupus.
Acknowledgments
These studies were supported by a research grant from the NIH (R01 AR065594) to C. Putterman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: None.
References
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonicatto SC, Dew MA, Zaratiegui R, Lorenzo L, Pecina P. Adult outpatients with depression: worse quality of life than in other chronic medical diseases in Argentina. Soc Sci Med. 2001;52:911–919. doi: 10.1016/s0277-9536(00)00192-1. [DOI] [PubMed] [Google Scholar]
- Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, le Roux CW. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav. 2011;104:709–721. doi: 10.1016/j.physbeh.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Campbell S, Burkly LC, Gao HX, Berman JW, Su L, Browning B, Zheng T, Schiffer L, Michaelson JS, Putterman C. Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells. J Immunol. 2006;176:1889–1898. doi: 10.4049/jimmunol.176.3.1889. [DOI] [PubMed] [Google Scholar]
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp. 2012:e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamenti F, Prosperi C, Scali C, Giovannelli L, Colivicchi MA, Faussone-Pellegrini MS, Pepeu G. Interleukin-1beta activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: implications for Alzheimer's disease. Neuroscience. 1999;91:831–842. doi: 10.1016/s0306-4522(98)00680-0. [DOI] [PubMed] [Google Scholar]
- Cole PD, Vijayanathan V, Ali NF, Wagshul ME, Tanenbaum EJ, Price J, Dalal V, Gulinello ME. Memantine protects rats treated with intrathecal methotrexate from developing spatial memory deficits. Clin Cancer Res. 2013;19:4446–4454. doi: 10.1158/1078-0432.CCR-13-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplat-Jego S, Creidy R, Varriale S, Allaire N, Luo Y, Bernard D, Hahm K, Burkly L, Boucraut J. Anti-TWEAK monoclonal antibodies reduce immune cell infiltration in the central nervous system and severity of experimental autoimmune encephalomyelitis. Clin Immunol. 2005;117:15–23. doi: 10.1016/j.clim.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Desplat-Jego S, Varriale S, Creidy R, Terra R, Bernard D, Khrestchatisky M, Izui S, Chicheportiche Y, Boucraut J. TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity. J Neuroimmunol. 2002;133:116–123. doi: 10.1016/s0165-5728(02)00368-5. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Gao HX, Sanders E, Tieng AT, Putterman C. Sex and autoantibody titers determine the development of neuropsychiatric manifestations in lupus-prone mice. J Neuroimmunol. 2010;229:112–122. doi: 10.1016/j.jneuroim.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008;10:291–299. doi: 10.31887/DCNS.2008.10.3/pgorwood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Acquarone M, Kim JH, Spray DC, Barbosa HS, Sellers R, Tanowitz HB, Weiss LM. Acquired infection with Toxoplasma gondii in adult mice results in sensorimotor deficits but normal cognitive behavior despite widespread brain pathology. Microbes Infect. 2010;12:528–537. doi: 10.1016/j.micinf.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H, Zheng YM, Heber-Katz E, Fraser N, Rorke L, Fu ZF, Hanlon C, Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci U S A. 1993;90:3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Vijayanathan V, Gulinello ME, Cole PD. Systemic methotrexate induces spatial memory deficits and depletes cerebrospinal fluid folate in rats. Pharmacol Biochem Behav. 2010;94:454–463. doi: 10.1016/j.pbb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava-Mesa MO, Jimenez-Diaz L, Yajeya J, Navarro-Lopez JD. GABAergic neurotransmission and new strategies of neuromodulation to compensate synaptic dysfunction in early stages of Alzheimer's disease. Front Cell Neurosci. 2014;8:167. doi: 10.3389/fncel.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchia GP, Nico B, Camassa LM, Mola MG, Loh N, Dermietzel R, Spray DC, Svelto M, Frigeri A. The role of aquaporin-4 in the blood-brain barrier development and integrity: studies in animal and cell culture models. Neuroscience. 2004;129:935–945. doi: 10.1016/j.neuroscience.2004.07.055. [DOI] [PubMed] [Google Scholar]
- Perper SJ, Browning B, Burkly LC, Weng S, Gao C, Giza K, Su L, Tarilonte L, Crowell T, Rajman L, Runkel L, Scott M, Atkins GJ, Findlay DM, Zheng TS, Hess H. TWEAK is a novel arthritogenic mediator. J Immunol. 2006;177:2610–2620. doi: 10.4049/jimmunol.177.4.2610. [DOI] [PubMed] [Google Scholar]
- Polavarapu R, Gongora MC, Winkles JA, Yepes M. Tumor necrosis factor-like weak inducer of apoptosis increases the permeability of the neurovascular unit through nuclear factor-kappa B pathway activation. J Neurosci. 2005;25:10094–10100. doi: 10.1523/JNEUROSCI.3382-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DA, Chapman KM, Weigelt M, Weiss DJ, van der Wel R. Cognition, action, and object manipulation. Psychol Bull. 2012;138:924–946. doi: 10.1037/a0027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz AB, Justo P, Sanchez-Nino MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol. 2008;19:695–703. doi: 10.1681/ASN.2007050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber E, Semra YK, Gregson NA, Sharief MK. Intrathecal production of IgG antibodies to neurofilament-light (NF-L) is elevated in patients with primary and secondary progressive multiple sclerosis. Neurology. 2000;54:A274–A274. doi: 10.1212/wnl.58.9.1372. [DOI] [PubMed] [Google Scholar]
- Stephan D, Sbai O, Wen J, Couraud PO, Putterman C, Khrestchatisky M, Desplat-Jego S. TWEAK/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier. J Neuroinflammation. 2013;10:9. doi: 10.1186/1742-2094-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AD, Wen J, Putterman C. Neuropsychiatric lupus, the blood brain barrier, and the TWEAK/Fn14 pathway. Front Immunol. 2013;4:484. doi: 10.3389/fimmu.2013.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, Ferrario M, Martinez FO, Locati M, Ghisi V, Bresciani LG, Mantovani A, Spano P. Gene expression profile activated by the chemokine CCL5/RANTES in human neuronal cells. J Neurosci Res. 2004;78:371–382. doi: 10.1002/jnr.20250. [DOI] [PubMed] [Google Scholar]
- Wen J, Doerner J, Weidenheim K, Xia Y, Stock A, Michaelson JS, Baruch K, Deczkowska A, Gulinello M, Schwartz M, Burkly LC, Putterman C. TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice. J Autoimmun. 2015;60:40–50. doi: 10.1016/j.jaut.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Xia Y, Stock A, Michaelson JS, Burkly LC, Gulinello M, Putterman C. Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway. J Autoimmun. 2013;43:44–54. doi: 10.1016/j.jaut.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley D, McKenna SP. Measuring quality of life in patients with depression or anxiety. Pharmacoeconomics. 1995;8:305–315. doi: 10.2165/00019053-199508040-00005. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008;7:411–425. doi: 10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Guo L, Jakubowski A, Su L, Li WC, Bonner-Weir S, Burkly LC. TNF-like weak inducer of apoptosis (TWEAK) promotes beta cell neogenesis from pancreatic ductal epithelium in adult mice. PLoS One. 2013;8:e72132. doi: 10.1371/journal.pone.0072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M. TWEAK and Fn14 in the neurovascular unit. Front Immunol. 2013;4:367. doi: 10.3389/fimmu.2013.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Brown SA, Moore EG, Smith EP, Lawrence DA, Winkles JA. A soluble Fn14-Fc decoy receptor reduces infarct volume in a murine model of cerebral ischemia. Am J Pathol. 2005;166:511–520. doi: 10.1016/S0002-9440(10)62273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Winkles JA, Gongora MC, Polavarapu R, Michaelson JS, Hahm K, Burkly L, Friedman M, Li XJ, Yepes M. TWEAK-Fn14 pathway inhibition protects the integrity of the neurovascular unit during cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:534–544. doi: 10.1038/sj.jcbfm.9600368. [DOI] [PubMed] [Google Scholar]